Abstract
We previously reported that mutations occurred in the gene myosin5 were responsible for resistance to the fungicide phenamacril in Fusarium graminearum. Here, we determined whether there is a functional link between phenamacril resistance and the myosin proteins FaMyo2B and Famyo2 in Fusarium asiaticum, which is the major causal agent of Fusarium head blight in China. We found that FaMyo2B acts jointly with FaSmy1 to affect resistance to phenamacril in F. asiaticum. We also found that FaMyo2B disruption mutant and Famyo2 deletion mutant were defective in hyphal branching, conidiation, and sexual reproduction. ΔFamyo2 also had an enhanced sensitivity to cell wall damaging agents and an abnormal distribution of septa and nuclei. In addition, the FaMyo2B and Famyo2 mutants had reduced pathogenicity on wheat coleoptiles and flowering wheat heads. Taken together, these results reveal that FaMyo2B and Famyo2 are required for several F. asiaticum developmental processes and activities, which help us better understand the resistance mechanism and find the most effective approach to control FHB.
Introduction
Fusarium graminearum sensu lato (teleomorph Gibberella zeae (Schwein.) Petch) is the main pathogens of Fusarium head blight (FHB) of wheat and other small cereal grains, which can produce harmful mycotoxins in infected grain and threat animals and human [1–4]. So far, altogether 11 phylogenetic species were the causal agent of FHB disease and these species belong to the Fusarium graminearum Schwabe species complex of B-trichothecene toxin producers [5–9]. In China, since 1936 when FHB was first reported, FHB disease have become more and more severe in the middle and lower reaches of the Yangtze River, southern winter wheat region, and the northeastern spring wheat region [10]. Zhang et al. [11] reported that 77.3% of the 299 isolates collected from different places experiencing FHB epidemics in China were identified as F. asiaticum and another 68 isolates were confirmed to be F. graminearum. So F.asiaticum is the major pathogen of FHB epidemics in China.
Owing to few crop cultivars with natural resistance to Fusarium are available, the control of FHB has been depended on application of fungicides during wheat anthesis in the past few decades [10, 12, 13]. The use of a novel cyanoacrylate compound, phenamacril, reduced both the mycotoxin level and FHB index by 80% [14–17]. Phenamacril-resistant mutants have been readily obtained in vitro by fungicide domestication and ultra-violet (UV) irradiation. Most of the resistant mutants were moderately to highly resistant. We recently reported that mutations occurred in the gene myosin5 were responsible for resistance to phenamacril in F. graminearum [18] and Zhang et al. [19] found that JS399-19 (phenamacril) inhibited ATPase activity of FgMyo1 (myosin5) motor domain and JS399-19 (phenamacril) had a serious impact on localization of the wild-type FgMyo1 at the tips of germlings. In F. graminearum, there are three types of myosins: class II myosin myo2 (FGSG_08719.1) [20], class V myosin myosin2B (FGSG_07469.1), and class I myosin myosin-5 (FGSG_01410.1). In this paper, we used gene heterokaryotic disruption and gene deletion to determine whether the myosin proteins myosin-2B and myo2 could affect resistance to phenamacril in F. asiaticum.
Myosins are ATPase-dependent molecular motors that are responsible for an interaction with actin filaments. Generally, all of the myosins have a highly conserved ~80-kDa motor domain which generate chemo-mechanical, unidirectional force. So far, 31 myosin family classes have been discovered due to phylogenetic analyses and genomic survey [21]. Class I myosins myosins-I are common actin-dependent motor proteins. Besides the actin-activated ATPase that translocates actin filaments, the myosins-I can trigger Arp2/3 complex dependent actin polymerization by a Tail Homology 2 (TH2) domain, which binds to microfilamentous actin [22]. In addition, The yeast myosin-I Myo5, and its homologue Myo3, were involved in the formation of vesicles at the plasma membrane [23]. Class V myosins are processive motor proteins that transport their cargo toward the barbed (+) ends of actin filaments and they participate in multiple membrane trafficking events [24, 25]. Saccharomyces cerevisiae has two class V myosins, the nonessential Myo4 and the essential Myo2. While Myo4 mediates the movement of ER tubules and the transport of mRNAs, Myo2 plays a significant role in the segregation of membrane-bound organelles including peroxisomes, vacuoles, and other organelles of the secretory pathway and in the transport of secretory vesicles [26–29]. The class II myosin Myo1 is either nonessential or essential for viability. However, Myo1 is important for cell wall maintenance in yeast cells [30]. In S. cerevisiae, the myosin passenger-protein Smy1 transported by myosin V is involved in a negative feedback mechanism which prevents overgrowth and detects cable length [31]. The coiled-coil interaction (CCI) network reveals that Myo1 and Myo2 are interacting proteins that regulate Smy1p; when overexpressed, Smy1p can partially restore for defects, in the Myo2 mutant, compensating polarized growth and overcoming lethality at a restrictive temperature [32–35].
In F. graminearum, Song et al. [20] identified myo2, a class II myosin gene; they further demonstrated that myo2 is required for septation, conidiation, and sexual reproduction, and is important for pathogenesis and mycotoxin production. In this paper, we found that the class V myosin gene, FaMyo2B, in F. asiaticum affects asexual and sexual development, reduces virulence, and acts jointly with the myosin passenger protein gene FaSmy1 to affect resistance to phenamacril. Our data suggest that FaMyo2B and Famyo2 help us better understand the resistance mechanism and find an effective approach for FHB control.
Materials and Methods
Growth, conidiation, and perithecial assays of strains and mutants
The strains and mutants used in this paper are listed in Table 1. The wild-type phenamacril-sensitive strain 2021 and phenamacril-resistant strain Y2021A with induced phenamacril resistance were used for transformation. For analysis of growth phenotype and growth rate, all the strains were grown at 25°C on PDA (200 g of potato, 20 g of glucose, 15 g of agar, and 1 L of water) for 3 days. To assay the ability of the mutants in response to the cell wall damaging agents, mycelial growth was measured after incubation at 25°C for 3–8 d on PDA plates containing 5 mM caffein and 0.05% (w/v) Congo red. The percentage of mycelial growth inhibition (RGI) was calculated using the formula RGI = ((A-B)/(A-5)) *100, where A is the colony diameter of the control, and B is that of a treatment.
Table 1. Fusarium asiaticum strains used in this study.
| Strain | Genotype | Reference |
|---|---|---|
| 2021 | Wild type | [15] |
| D2021FaMyo2B-93 | FaMyo2B disruption mutant in 2021 genetic background | This study |
| Δ2021Famyo2-7 | Famyo2 deletion mutant in 2021 genetic background | This study |
| Δ2021FaSmy1-19 | FaSmy1 deletion mutant in 2021 genetic background | This study |
| Y2021A | Isolate resistant to phenamacril; generated from the wild-type strain 2021 by fungicide treatment | [36] |
| DY2021AFgMyo2B-3 | FaMyo2B disruption mutant in Y2021A genetic background | This study |
| DY2021AFgMyo2B-11 | FaMyo2B disruption mutant in Y2021A genetic background | This study |
| DY2021AFgMyo2B-12 | FaMyo2B disruption mutant in Y2021A genetic background | This study |
| DY2021AFgMyo2B-3C | FaMyo2B complement mutant in Y2021A genetic background | This study |
| DY2021AFgMyo2B-11C | FaMyo2B complement mutant in Y2021A genetic background | This study |
| DY2021AFgMyo2B-12C | FaMyo2B complement mutant in Y2021A genetic background | This study |
| ΔY2021AMyo2-8 | Famyo2 deletion mutant in Y2021A genetic background | This study |
| ΔY2021AMyo2-8C | Famyo2 complement mutant in Y2021A genetic background | This study |
| ΔY2021AFaSmy1-14 | FaSmy1 deletion mutant in Y2021A genetic background | This study |
| ΔY2021AFaSmy1-15 | FaSmy1 deletion mutant in Y2021A genetic background | This study |
| ΔY2021AFaSmy1-40 | FaSmy1 deletion mutant in Y2021A genetic background | This study |
For sporulation production assays, 10 fresh mycelial plugs taken from the periphery of a 3-day-old colony of each strain and mutant were added to a 200-ml flask containing 100 ml of MBL medium (MBL; 30 g of mung beans were boiled in 1 L of water for 15 min, and the mixture was then filtered through cheesecloth). Each strain and mutant was represented by three flasks. The flasks were incubated at 25°C for 7 days with shaking (185 rpm). The number of conidia in the MBL medium in each flask was measured with a hemacytometer and microscope. Sexual reproduction on carrot agar plates were assayed as previously described [37]. All the experiments were performed three times.
Construction of vectors for the disruption and deletion mutants and complementation using double-joint PCR technique and transformation
The construction of gene heterokaryotic disruption and gene deletion vectors of F. asiaticum were carried out using the methods as previously described [17]. The primers used to amplify the flanking sequences for each gene are listed in Supporting Information S1 Table. Putative gene heterokaryotic disruption and gene deletion mutants were identified by PCR assays with primers (Supporting Information S1 Table) and Southern blot assays.
One of the Famyo2 deletion mutants (ΔY2021AFamyo2-8) was complemented with the full length Famyo2 gene to confirm that the phenotypic changes of the Famyo2 deletion mutant were due to the deletion of the gene. The vector for the complementation of Famyo2 was amplified from the genomic DNA of strain Y2021A using primers P9/P12 (S1 Table). Before this vector was transformed into strain Y2021A, Famyo2 in the vector was sequenced to ensure the flawlessness of the sequence. The complemented strain was designated ΔY2021AFamyo2-8C.
To complement FaMyo2B heterokaryotic disruption mutants, The FaMyo2B complement plasmid pNEO-FaMyo2B-Com was constructed using the plasmid pNEO [38]. The full-length FaMyo2B gene, including the 1757-bp upstream and 150-bp terminator regions, was amplified from genomic DNA of strain Y2021A with primer P15/P16 (S1 Table), and cloned into the XbaI-SbfI site of pNEO to generate the complement plasmid pNEO-FaMyo2B-Com. Transformation of DY2021AFgMyo2B-3, DY2021AFgMyo2B-11 and DY2021AFgMyo2B-12 with the complement plasmid pNEO-FaMyo2B-Com as well as other deletion and disruption vectors were conducted as described previously [17], except that neomycin (100 mg/mL) was used as a selection agent.
Microscopic examination of mycelial and ascospores
For investigation of the morphology of conidia, ascospores, and hyphae, freshly harvested conidia, 2-week-old perithecia, and fresh mycelial taken from colonies of each strain and mutant that had been grown on a thin layer of water agar for 36 h was examined with an Olympus IX-71 microscope (Tokyo, Japan). Young hyphae that grew from conidia in YEPD liquid medium (w/v, 1% peptone, 0.3% yeast extract, 2% glucose) for 12 h were collected for microscopy by staining with 5 μg ml-1 DAPI and 10 μg ml-1 CFW (both from Sigma) for 5 min. Images were taken from two independent experiments.
Sensitivity to phenamacril
Sensitivity to phenamacril (experiment code JS399-19), which was provided by the Jiangsu Pesticide Institute Co. and had been recognized by International Fungicide Resistance Action Committee (FRAC) in 2015, was assessed for all the strains and mutants listed in Table 2. Mycelial plugs (5 mm in diameter) taken from the periphery of a 3-day-old colony were placed on the centre of PDA plates amended with phenamacril at: 0, 0.025, 0.05, 0.1, 0.2, or 0.4 μg/mL for sensitive strains; 5, 10, 25, 50, or 100 μg/mL for strains with intermediate sensitivity; or 25, 50, 100, 200, or 400 μg/mL for resistant strains. Three replicates for each concentration were used for each strain and mutant. After cultures were incubated at 25°C for 3 to 8 days, colony diameters were measured; the diameter (5 mm) of the original mycelial plugs were subtracted from each measurement. the 50% effective concentration (EC50) values of strains and mutants were calculated by regressing percentage growth inhibition against the log of fungicide concentration with DPS software. The experiment was performed twice.
Table 2. Phenotypes of Fusarium asiaticum wild-type strain 2021, resistant strain Y2021A, and mutants in terms of growth, conidiation, and pathogenicity.a.
| Strain | Growth rateb (cm/day) | Conidia produced (×105/ml) | Percentage of diseased spikelets (%)c | Lesion length on stem (cm)d |
|---|---|---|---|---|
| 2021 | 2.55±0.02A | 44.75±6.72A | 94.55±1.20A | 2.61±0.28A |
| D2021FaMyo2B-93 | 2.31±0.05B | 3.47±1.03C | 17.59±6.68B | 0.52±0.19B |
| Δ2021Famyo2-7 | 1.36±0.08D | 0.25±0.07D | 2.84±0.40C | 0.06±0.07C |
| Y2021A | 2.58±0.03A | 42.00±8.49A | 96.58±1.30A | 2.78±0.26A |
| DY2021AFaMyo2B-3 | 2.21±0.05C | 6.15±1.48D | 19.56±5.87B | 0.43±0.16B |
| DY2021AFaMyo2B-3C | 2.52±0.04A | 31.62±6.24B | NA | NA |
| ΔY2021AFamyo2-8 | 1.43±0.01D | 0.18±0.04C | 2.92±0.18C | 0.06±0.08C |
| ΔY2021AFamyo2-8C | 2.49±0.06A | 40.05±5.37A | NA | NA |
aValues are means and standard deviations. Means in a column followed by the same letter are not significantly different (P = 0.01).
bGrowth rate and conidiation were measured after incubation of three replicates for 3 and 7 days, respectively. Growth rate was measured on PDA plates. Conidiation was measured in flasks containing MLB.
cPercentage of diseased spikelets per spike 14 days after inoculation. Thirty spikes were inoculated for each strain.
dThe length of brown lesions on diseased stems 14 days post inoculation. Ten coleoptiles were inoculated for each strain. NA, not analysis.
Plant infection and pathogenicity
Flowering wheat heads of cultivar Zhenmai 5 (which is sensitive to F. asiaticum and is widely cultured in China) were inoculated with 10 μl of conidia suspensions (1.0 ×105 conidia ml-1) as previously described [39]. Thirty replicate wheat heads were inoculated for each strain and mutant. After inoculation, each wheat head was covered with a plastic bag for 2 days to maintain moisture, and scab symptoms were examined at 15 d post-inoculation (dpi).
For wheat coleoptiles assays, 3-day-old seedlings of wheat cultivar Zhenmai 5 were used according to previous method with modifications [40]. 3 days after seeds were sown, the top 2 to 3 mm of the coleoptiles were cut, and the tip was injected 10μl conidial suspension. For each strain and mutant, 10 coleoptiles were inoculated and maintained in the growth chamber at 25°C and with 95% relative humidity. The experiment was repeated twice. The pathogenicity of the strains and mutants were assessed by measuring the length of brown lesions on diseased stems at 14 dpi.
Quantitative RT-PCR (qRT-PCR)
RNA was extracted with the RNAsimple kit (Tiangen) from germ-tubes grown for 18 h in YEPD liquid medium. The cDNAs were amplified with the PrimeScript® RT reagent kit (TaKaRa). Quantitative PCR experiments were performed using an ABI 7500 real-time detection system (Applied Biosystems, USA) with an SYBR Green reaction mix containing 10 μL of 2 × SYBR green premix, 2 μL of template, 0.4 μL of forward primer (10 mM) and reverse primer (10 mM), and 7.2 mL of nucleotide-free water. The PCR program included an initial denaturation step at 95°C for 30 s and then 40 amplification cycles at 95.0°C for 5 s and 60°C for 34 s. To ensure specificity, only primers that generated a single peak in the melting curve were selected. Primers used for qRT-PCR analysis are listed in S1 Table and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a reference. Each experiment included two technology replicates and was repeated at least three times.
Yeast two-hybrid analysis
The coding sequence of each tested gene was amplified from the cDNA of F. asiaticum strain 2021 with primer pairs for constructing yeast two-hybrid plasmids. The cDNA fragment of FaSmy1 encoding 933 amino acids was inserted into the yeast GAL4-binding domain vector pGBKT7 and the cDNA fragment of FaMyo2B and Famyo2 encoding the tail domain (790 amino acids and 743 amino acids, respectively) were inserted into GAL4 activation domain vector pGADT7. The pairs of yeast two-hybrid plasmids were transformed into S. cerevisiae strain AH109 together following the LiAc/SS-DNA/PEG (lithium acetate/single-stranded DNA/ polyethylene glycol) transformation protocol [41]. The plasmid pair pGADT7 and pGBKT7-53 served as a positive control, and the plasmid pair pGADT7 and pGBKT7-Lam served as a negative control. Transformants were cultured at 30°C for 3 days on synthetic medium (SD) lacking Trp and Leu and then were transferred to SD lacking of Leu, His, and Trp. Three independent experiments were conducted to confirm the yeast two-hybrid assay results.
Results
Sequence analysis and characterization of FaMyo2B and Famyo2 in F. asiaticum
We identified the F. asiaticum other myosin genes FaMyo2B (FGSG_07469) and Famyo2 (FGSG_08719) by a BLASTP search of the Fusarium genome (http://www.broadinstitute.org/annotation/genome/fusarium_group/MultiHome.html) using the S. cerevisiae Myo1 and Myo2 proteins as queries. FaMyo2B (GenBank accession no. ESU13735.1) was predicted to encode a 1583 amino acid protein which shares 46% similarity with S. cerevisiae class V myosin Myo2. Famyo2 (GenBank accession no. ESU14588.1) was predicted to encode a 2342 amino acid protein which shares 24% similarity with S. cerevisiae class II myosin Myo1.
The DNA sequences of an FaMyo2B locus (ranging from 234 bp upstream to 150 bp downstream of the FaMyo2B coding region) and an Famyo2 locus (ranging from 281 bp upstream to 318 bp downstream of the Famyo2 coding region) from strains 2021 and Y2021A of F. asiaticum were retrieved by PCR amplification using primers 07469F/07469R and 08719F/08719R (S1 Table). FaMyo2B has an open reading frame (ORF) of 5,095 bp and six introns, and Famyo2 has an ORF of 7,300 bp and four introns. In addition, multiple sequence alignment of FaMyo2B and Famyo2 revealed that their motor domains are conserved to those of other myosin orthologues (S1–S3 Figs). This region comprises the myosin superfamily domain.
Disruption of FaMyo2B and deletion of Famyo2 and complementation of both of the mutants
For a detailed functional analysis of FaMyo2B and Famyo2, we generated deletion mutants or heterokaryotic disruption mutants by transformation of the gene replacement cassette HPH-HSV-tk in a phenamacril-sensitive strain (2021) and phenamacril-resistant strain (Y2021A) of F. asiaticum (Table 1). Putative deletion strains were determined by PCR amplification using different primer pairs and southern blot analysis using genomic DNA of the parental strain and mutants (S4A–S4D Fig). When we targeted FaMyo2B for gene deletion, we recovered 217 HPH-resistant and HSV-sensitive transformants, but all were ectopic mutants, perhaps because FaMyo2B encodes an essential gene in F. asiaticum as it does in S. cerevisiae citation. In spite of the highly efficient homologous integration events in F. asiaticum, the failure to obtain an FaMyo2B null mutant strongly revealed a lethal impact of FaMyo2B deletion in this fungi. However, when we identified these transformants by PCR and southern blot, we found that some transformants indicated gene replacement cassette HPH-HSV-tk integration at the left junction and right junction but the results of southern blot indicated two bands, one is wild-type band and another is deletion mutant band. So we thought we got FaMyo2B heterokaryotic disruption mutants and we used these mutants for further functional study.
To complement the Famyo2 deletion mutants, One of the ΔFamyo2 mutants (ΔY2021AFamyo2-8) was complemented with the parental gene Famyo2. The putative complementations were examined by PCR and southern blot analysis (S4B and S4D Fig). And to complement the FaMyo2B disruption mutants, DY2021AFgMyo2B-3, DY2021AFgMyo2B-11 and DY2021AFgMyo2B-12 were complemented with the complement plasmid pNEO-FaMyo2B-Com. The putative complementation were selected by neomycin and examined by southern blot analysis (S4D Fig).
FaMyo2B and Famyo2 are involved in regulating hyphal growth and conidiation
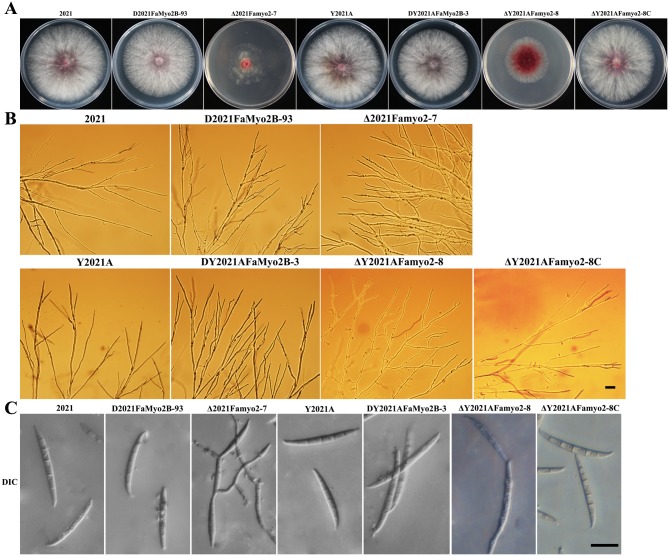
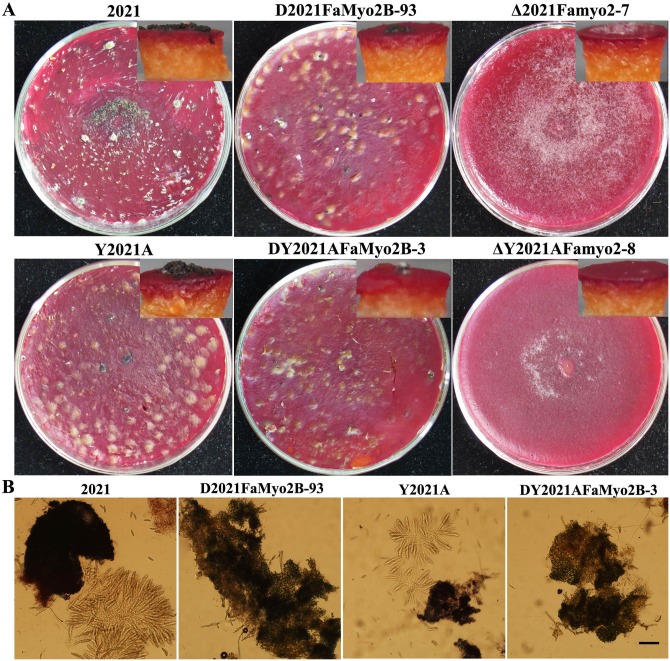
As shown in Fig 1, mycelial growth on potato dextrose agar (PDA) was slightly reduced by disruption of FaMyo2B but greatly reduced by deletion of Famyo2. ΔFamyo2 mutant grew prominently slower than the progenitor strains 2021 and Y2021A on PDA plates and had a distinctive colony morphology but the complementation of Famyo2 deletion mutants restored the morphology (Fig 1A). Microscopic examination indicated that the hyphae of the FaMyo2B heterokaryotic disruption mutant generally branched at a narrower angle than the parental strains (Fig 1B), and that the hyphae of the Famyo2 deletion mutant were distorted, suggesting that FaMyo2B and Famyo2 play a major role in vegetative development.
Fig 1. Colony and conidia morphology and hyphal tip growth and branching patterns of the wild-type 2021, the FaMyo2B disruption mutant, the Famyo2 deletion mutant and the Famyo2 complement mutant.
(A) Colonies were photographed after 3 days at 25°C on PDA. (B) The branching angles of the hyphae were reduced and distorted in the extension zone of the mutant colonies that had grown on a thin layer of water agar for 36 h. Bar = 200 μm. (C) Photographs of conidia obtained in the conidiation assay (see text). Bar = 20 μm.
To assay the effects of FaMyo2B and Famyo2 on conidiation, the strains were incubated in MBL medium with shaking. After 7 days, the FaMyo2B and Famyo2 mutants had produced significantly fewer conidia than the progenitor strains or the complemented strains (Table 2). Moreover, the deletion mutant formed conidia in chains whereas the parental strain formed separate, canoe-shaped conidia (Fig 1C). Disruption of FaMyo2B did not significantly affect conidial morphology (Fig 1C).
Combined effects of FaMyo2B and the myosin passenger protein FaSmy1 on the phenamacril resistance in F. asiaticum
To determine whether FaMyo2B and Famyo2 affect the level of phenamacril resistance in F. asiaticum, we conducted fungicide resistance and sensitivity tests. As indicated in Table 3, disruption of FaMyo2B significantly reduced phenamacril EC50 values for the phenamacril-resistant strain Y2021A but not for the phenamacril-sensitive strain 2021. In contrast, deletion of Famyo2 did not affect phenamacril resistance for the resistant strain or for the wild-type sensitive strain. Interestingly, the complemented strains partially restored the resistance of the FaMyo2B disruption mutants to phenamacril (Table 3).
Table 3. Sensitivity of Fusarium asiaticum strains to phenamacril.a.
| Strain | EC50 (μg/ml) |
|---|---|
| 2021 | 0.19 |
| D2021FaMyo2B-93 | 0.20 |
| Δ2021Famyo2-7 | 0.16 |
| Δ2021FaSmy1-19 | 0.21 |
| Y2021A | 168.61 |
| DY2021AFaMyo2B-3 | 21.99 |
| DY2021AFaMyo2B-11 | 24.88 |
| DY2021AFaMyo2B-12 | 21.00 |
| DY2021AFgMyo2B-3C | 92.61 |
| DY2021AFgMyo2B-11C | 95.15 |
| DY2021AFgMyo2B-12C | 98.24 |
| ΔY2021AFamyo2-8 | 161.16 |
| ΔY2021AMyo2-8C | 166.30 |
| ΔY2021AFaSmy1-14 | 52.81 |
| ΔY2021AFaSmy1-15 | 62.32 |
| ΔY2021AFaSmy1-40 | 73.49 |
aValues are means of three experiments (differences among the experiments were not significant, i.e., P > 0.05, Fisher’s LSD test).
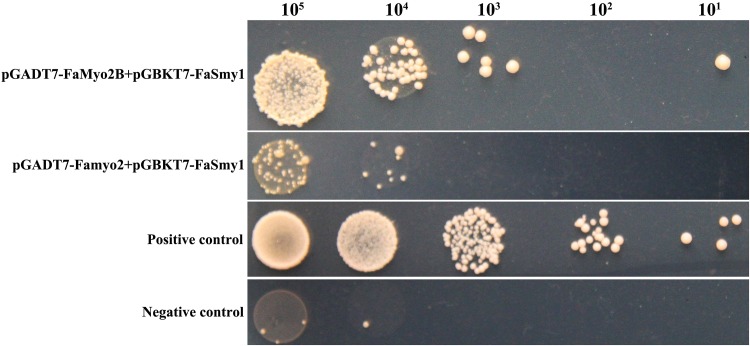
As the myosin passenger protein, Smy1p physically interacts with the class V myosin Myo2p in budding yeast. In this paper, yeast two-hybrid analysis revealed that FaSmy1 interacts with the tail portion of the FaMyo2B but not with Famyo2 (Fig 2). Furthermore, knockout of FaSmy1 significantly reduced phenamacril EC50 values in the phenamacril-resistant strain Y2021A but not in the sensitive strain (2021A) (Table 3).
Fig 2. Yeast two hybrid analyses of interactions between FaSmy1 and FaMyo2B and between FaSmy1 and Famyo2 of Fusarium asiaticum.
Serial dilutions of yeast cells (cells ml-1) that were transferred with the prey and bait constructs shown in the figure were determined for growth on yeast minimal synthetic defined base (SD) lacking tryptophan, leucine and histidine. The plasmid pairs pGADT7 and GBKT7-53 was used as a positive control, and the plasmid pairs pGADT7 and pGBKT7-Lam and was used as a negative control.
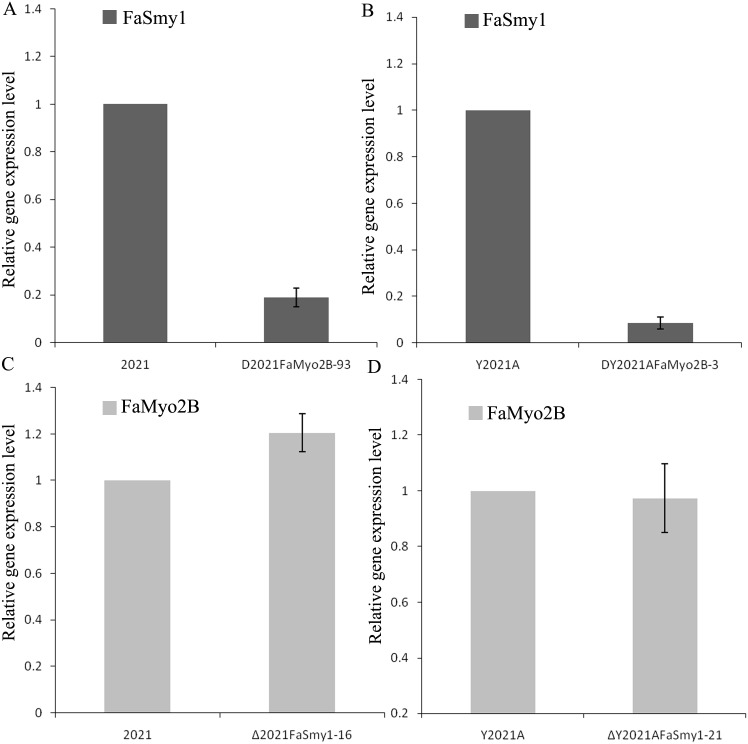
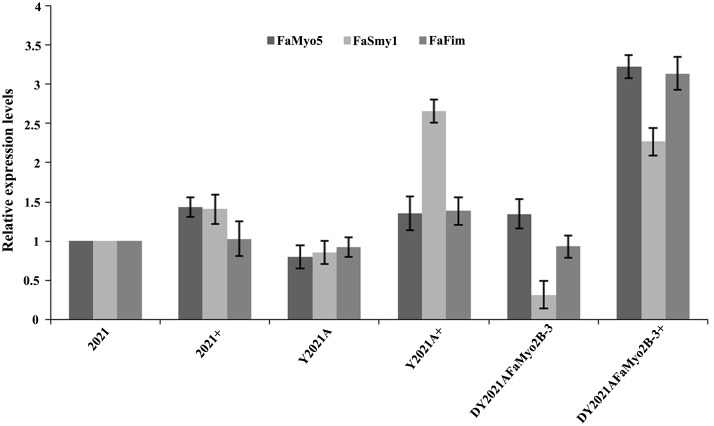
In S. cerevisiae, overexpression of Smy1p not only complements Myo2p localization in the deletion mutant, but it also increases the localization of wild-type Myo2p [37]. However, disruption of FaMyo2B significantly down-regulated the expression of FaSmy1 in the phenamacril-sensitive strain 2021 and in phenamacril-resistant strain Y2021A of F. asiaticum but deletion of FaSmy1 didn’t affect the expression of FaMyo2B (Fig 3). Our previous studies showed that FgFim is a key protein regulating phenamacril resistance and that mutations occurred in the gene myosin5 confer resistance to phenamacril in F. graminearum [17, 18]. When treated with 1 μg/ml phenamacril for 6 h, the FaMyo2B disruption mutant of the phenamacril-resistant strain Y2021A (DY2021AFaMyo2B-3) significantly up-regulated FaMyo5, FaFim, and FaSmy1 genes relative to the sensitive strain 2021. However, the phenamacril-resistant strain Y2021A significantly up-regulated only the FaSmy1 gene (Fig 4). Furthermore, the DY2021AFaMyo2B-3 mutant significantly up-regulated the resistance gene FaMyo5 relative to the resistant strain Y2021A. These results suggest that FaMyo2B acts jointly with the myosin passenger protein FaSmy1 to affect phenamacril resistance in F. asiaticum.
Fig 3. Expression levels of FaSmy1 and FaMyo2B in phenamacril-sensitive strain 2021, phenamacril-resistance strain Y2021A and mutants.
Expression levels of FaSmy1 in the phenamacril-sensitive strain 2021 and in the corresponding FaMyo2B mutant (A), and in the phenamacril-resistant strain Y2021A and in the corresponding FaMyo2B mutant (B). Expression levels of FaMyo2B in the phenamacril-sensitive strain 2021 and in the corresponding FaSmy1 mutant (C), and in the phenamacril-resistant strain Y2021A and in the corresponding FaSmy1 mutant (D).Values are the means ± standard error (SE) of three repeated experiments.
Fig 4. Expression level of FaMyo5, FaFim, and FaSmy1 genes in the phenamacril-sensitive strain 2021, the phenamacril-resistant strain Y2021A, and the phenamacril-resistant FaMyo2B disruption mutant DY2021AFaMyo2B-3.
+ represents strains treated with phenamacril at 1 μg/ml for 6 h. Values are the means ± standard error (SE) of three repeated experiments.
FaMyo2B and Famyo2 are essential for sexual reproduction
Because F. graminearum is a homothallic fungus, sexual reproduction plays an important role in its infection cycle [42, 43]. Previous study has shown that Δmyo2 mutant strains of F. graminearum produced no perithecia when cultured on wheat kernels for 14 days [20]. ΔFamyo2 mutants of F. asiaticum also failed to form perithecia on carrot agar plates (Fig 5A), which showed that the mutant may be defective in female fertility. However, the parental strains produced obvious perithecia and discharged ascospores on carrot agar plates. Interestingly, the FaMyo2B mutant produced normal perithecia but did not produce viable ascospores in 2-week-old perithecia (Fig 5B). Thus, we conclude that FaMyo2B and Famyo2 are essential for sexual reproduction.
Fig 5. Effects of FaMyo2B disruption and Famyo2 deletion on the sexual development of Fusarium asiaticum.
(A) Cultures were photographed after 2 weeks of growth on carrot agar. The insets show that the relative numbers of perithecia produced by each strain. (B) Morphology of ascospores in 2-week-old perithecia. Bar = 80 μm.
FaMyo2B and Famyo2 affect pathogenicity of F. asiaticum
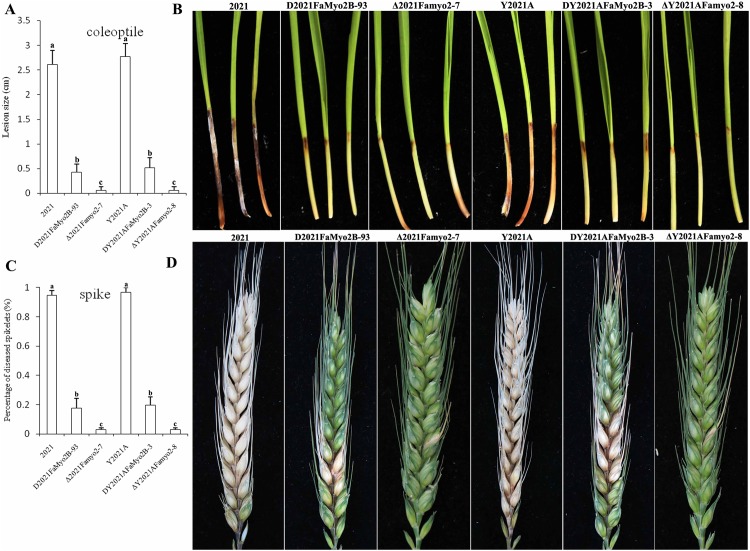
In infection assays with flowering wheat heads, the FaMyo2B disruption mutants caused obvious scab symptoms on the inoculated wheat kernels and were able to infect nearby spikelets (Table 2), but their virulence was reduced by ~80% relative to the virulence of the parental strains (Fig 6C and Table 2). In contrast, the two ΔFamyo2 mutants caused nearly no disease symptoms on most of the spikelets inoculated with Δ2021Famyo2-7 or ΔY2021AFamyo2-8 even 15 days post inoculation (DPI); the other inoculated spikelets showed only mild bleaching symptoms (Fig 6D). Fewer than 3% of spikelets were infected by the Δ2021Famyo2-7 and ΔY2021AFamyo2-8 mutants at 15 DPI.
Fig 6. Virulence of FaMyo2B and Famyo2 mutants.
(A) Three-day-old seedlings were inoculated with conidial suspensions of the wild-type 2021, the FaMyo2B mutant, and the Famyo2 mutant. The lengths of brown lesions of wheat coleoptiles were measured 14 days post inoculation. (B) Infected wheat coleoptiles were photographed 15 days after inoculation. (C) Percentage of diseased spikes on inoculated wheat heads. (D) Infected wheat heads were photographed 14 days after inoculation.
In infection assays with wheat coleoptiles, spore suspensions of F. asiaticum parental and mutant strains were injected on the cut tips of coleoptiles of 3-d-old wheat seedlings. Majority of the coleoptiles split naturally within 1 to 3 DPI, and the stems, which beginning from the inoculated apex, started to turn brown within 4 to 7 DPI. By 10 to 14 DPI, dark brown lesions were evident on the coleoptiles of the inoculated seedlings. Lesions caused by the FaMyo2B and Famyo2 mutants were obviously smaller than those caused by the parent strains (Fig 6A and 6B).
Because the trichothecene toxin deoxynivalenol (DON) is a major virulence factor for F. graminearum, we assayed the expression levels of two trichothecene biosynthesis genes, TRI5 and TRI6, using quantitative real-time PCR and RNA samples extracted from germ tubs grown in GYEP medium. The expression levels of TRI5 and TRI6 were obviously lower in ΔFamyo2 than in the parental strains (S5A Fig).
The MAP kinases Gpmk1 and Mgv1 are required for pathogenesis in F. graminearum, and previous studies demonstrated that Gpmk1 regulates the activities of xylanolytic, extracellular endoglucanase and proteolytic enzymes [44]. To determine whether FaMyo2B and Famyo2 participate in the regulation of Gpmk1 or Mgv1, we analyzed the expression levels of FaGpmk1 and FaMgv1 in the mutants and parental strains. The expression levels of FaGpmk1 and FaMgv1 were substantially down-regulated in ΔFamyo2 (S5B Fig). These results may explain the reduced pathogenicity of the Famyo2 deletion mutants.
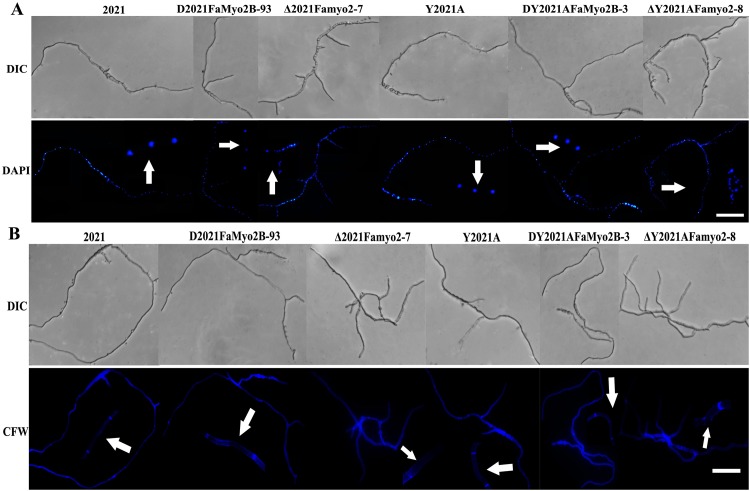
Famyo2 is required for nucleus and septum distribution and for cell wall integrity
Song et al. (2013) [20] showed that the Class II myosin myo2 is required for septation in F. graminearum. We investigated the role of FaMyo2B and Famyo2 in the distribution of nuclei and in septum development in F. asiaticum by staining hyphae of the parental strain and of the Famyo2 and FaMyo2B mutants with calcofluor white (CFW) and 4’,6-diamidino-2-phenylinodole (DAPI). Analysis revealed that the nuclei were regularly distributed in the FaMyo2B mutant hyphae and in the parental hyphae. In contrast, they were unevenly distributed and clustered in the hyphae of the ΔFamyo2 mutants (Fig 7A). Septum development also differed substantially between the parental and the ΔFamyo2 mutant strains. Septa in parental hyphae were complete and uniformly distributed. In contrast, septa in ΔFamyo2 mutant hyphae were often incomplete and irregularly distributed. Moreover, many of the ΔFamyo2 mutant septa revealed by CFW staining could not be detected by fluorescence microscopy (Fig 7B).
Fig 7. Distribution of nuclei and septa in hyphae of the wild-type strain 2021 and of the Famyo2 mutants.
(A) Nuclei were stained with DAPI after incubation for 12 h. (B) Septa were stained with CFW after incubation for 12 h. The arrow heads represent partial, enlarged results. Bar = 40 μm.
Because the myosin Class II gene Myo1 plays an important role in cell wall maintenance in yeast cells [30], we also determined the effects of the cell wall damaging agents caffeine and Congo red on the ΔFamyo2 mutant. Compared to the parental strain, the ΔFamyo2 mutant showed increased sensitivity to these compounds (S6 Fig). We also quantified the expression of FaMgv1, which is homologous to the S. cerevisiae cell wall integrity core element gene, Slt2. Expression levels of FaMgv1 were slightly down-regulated in ΔFamyo2 relative to the parental strains (S5B Fig). These results indicated that Famyo2 is required for nucleus and septum distribution and for cell wall integrity.
Discussion
Because the actin cytoskeleton and microtubules have important roles in key cellular events, they are attractive targets for drug design. The actin cytoskeleton is composed of polymers of actin (microfilaments) together with actin-binding and actin-associated proteins, such as cytoskeletal motor myosins [45, 46]. Myosins are important components of the eukaryotic cytoskeleton because they provide motility for many kinds of cargo. We previously reported that the actin-bundling protein fimbrin was important in regulating the level of phenamacril resistance in F. graminearum [17]. We then demonstrated that mutations in the class I myosin gene myosin5 is responsible for resistance to phenamacril [18]. In this paper, we demonstrated that FaMyo2B acts jointly with the myosin passenger protein FaSmy1 to affect phenamacril resistance in F. asiaticum.
Class V myosins have a myosin head domain and a tail domain with IQ and globular DIL domains. In budding yeast, the class V myosin Myo2p and the myosin passenger protein Smy1p have an intimate relationship, as determined by co-localization and physical interaction [21, 33, 34]. Furthermore, overexpression of Smy1p not only complements the localization of the mutated Myo2p but also enhances the localization of the wild-type Myo2p [34]. In this study, a yeast two hybrid assay revealed that FaSmy1 interacts with the FaMyo2B tail in F. asiaticum. To our surprise, disruption of either FaMyo2B or FaSmy1 in the phenamacril-resistant strain Y2021A significantly reduced phenamacril EC50 values. And when we complemented the FaMyo2B disruption mutants with full-length FaMyo2B gene, the complementations partially restored the resistance of the mutants, which indicated that the FaMyo2B protein played an important role in the resistance to phenamacril in F.asiaticum. In addition, disruption of FaMyo2B in F. asiaticum also significantly down-regulated the expression of FaSmy1 in the phenamacril-resistant strain Y2021A and in the phenamacril-sensitive strain 2021, which is consistent with previous studies with S. cerevisiae indicating that Smy1 accumulation in the bud tip needs Myo2 and that Smy1 is trafficked by Myo2 on actin cables [21, 31, 34, 47]. In the current study, treatment with 1 μg/ml phenamacril for 6 h caused the DY2021AFaMyo2B-3 disruption mutant to significantly up-regulate FaMyo5, FaFim, and FaSmy1 genes relative to the sensitive strain 2021. These results help us better understand the resistance mechanism.
In S. cerevisiae, Myo2 encodes an essential gene. The failure to obtain an FaMyo2B null mutant for F. asiaticum strongly indicated that deletion of FaMyo2B has a lethal effect in this fungus. However, we selected several FaMyo2B heterokaryotic disruption mutants for further study. We found that the FaMyo2B disruption mutant and the Famyo2 deletion mutant exhibited defective hyphal branching, reduced asexual reproduction, and no sexual reproduction. As we know, Fusarium species are mononuclear fungus, but the heterokaryotic mycelial of FaMyo2B disruption mutants have two types of genes, one is deletion type and another is wild-type. Maybe the lethal impact lead to this phenomenon in this fungus. Interestingly, the FaMyo2B heterokaryotic disruption mutants also caused the changes of phenotype like deletion mutants, which indicated that FaMyo2B has a direct or indirect impact on asexual or sexual reproduction.
Yeast cells carry on cell divisions by separating the daughter cells, while filamentous fungi form compartments divided by septa [48]. We discovered that septal formation in the hyphae of the Famyo2 mutants was seriously disrupted, which resulted in unevenly distributed and clustered nuclei. The Famyo2 deletion mutant also exhibited abnormal conidial development and enhanced sensitivity to cell wall damaging agents, which revealed that Famyo2 is essential for cell wall integrity. In addition, the production of perithecia was abolished in the Famyo2 mutants on carrot agar plates, indicating a major role of the Famyo2 protein in the development of F. asiaticum. Although the FaMyo2B mutant produced normal perithecia, the ascospores within those perithecia failed to germinate. Ascospores released by F. graminearum perithecia are required for the primary infection of wheat spikes during wheat flowering [49]. The defective sexual reproduction of the Famyo2 and FaMyo2B mutants suggests that Famyo2 and FaMyo2B or the proteins they encode could be the targets for new drugs that control FHB.
In the current study, the Famyo2 and FaMyo2B mutants were less pathogenic than the wild types on flowering wheat heads and wheat coleoptiles. In addition to having reduced septation and enhanced sensitivity to cell wall damaging agents, the ΔFamyo2 mutant had reduced expression levels of FaGpmk1, resulting in reduced penetration of host tissue. Expression levels of the trichothecene biosynthesis genes TRI5 and TRI6 were also reduced in the ΔFamyo2 mutant. These genes are responsible for the synthesis of DON, which is required for the spread of F. graminearum in rachis tissue [50]. The reduced expression levels of TRI5 and TRI6 are consistent with the failure of the ΔFamyo2 mutant to spread in planta. Although mycelial growth was only slightly reduced and TRI5 and TRI6 expression were normal in the FaMyo2B disruption mutant, its virulence was reduced on flowering wheat heads and wheat coleoptiles. We suspect that the disruption of FaMyo2B affects the transport of cargo along the actin cable, which results in downstream defects that reduce pathogenicity.
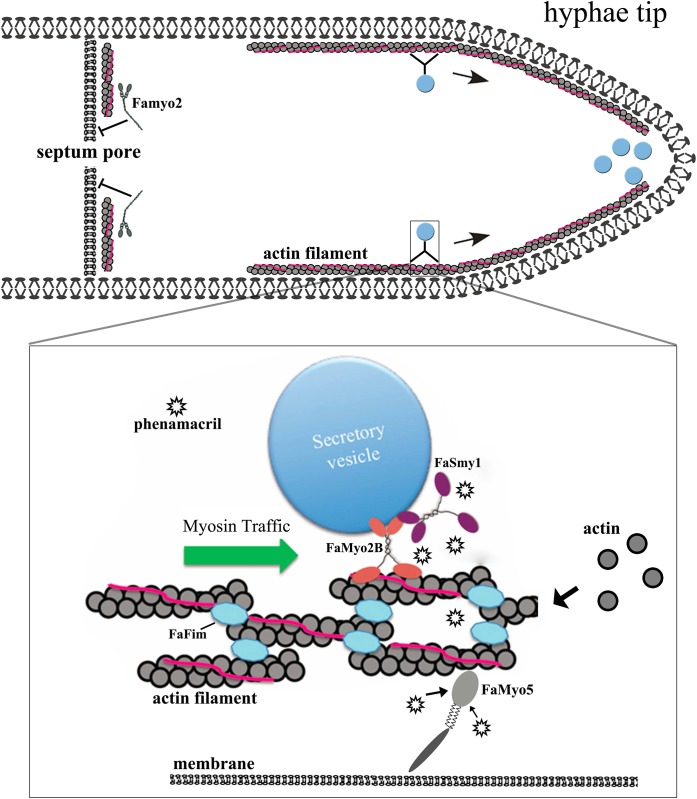
Identifying drug targets and drug resistance mechanisms is difficult because many proteins and complex and systemic pathways are involved. Finally, we present a model for phenamacril resistance in F. asiaticum (Fig 8). In the absence of phenamacril, the actin-bundling protein FaFim stables the actin cable; FaMyo5 always trigger Arp2/3 complex-dependent actin polymerization and travel toward the hyphae tips along the actin cable; FaMyo2B and FaSmy1 (the myosin passenger protein) transport secretory vesicles along the actin cable; and Famyo2 maintains cell wall integrity and controls septum development. When a phenamacril-sensitive strain is treated with the fungicide, phenamacril binds to FaMyo5 or inhibits ATPase activity of FaMyo5 motor domain and thereby reduces actin polymerization and the transport of secretory vesicles along the actin cable; this can greatly disrupt cell functions and hyphae growth. Resistance to phenamacril results from mutations in myosin-5, which apparently reduces the binding of the fungicide to FaMyo5. While mutations in FaMyo5 result in phenamacril resistance, the disruption of FaMyo2B and deletion of FaSmy1 significantly reduced phenamacril resistance for the phenamacril-resistant strain (Y2021A), which resulted from the disrupted transport of secretory vesicles. In summary, our results revealed a logical explanation for phenamacril resistance and we needed more experiments to prove.
Fig 8. A hyphae tip cell uses bilayer membrane.
Myosin transports secretory vesicles to hyphae tips along the actin cables. Famyo2 controls septum development. Box, the actin filaments in cables contained actin bundling proteins (FaFim) and tropomyosin (pink), which maintain filament stability and organization. Myosin-V (FaMyo2B, red) transports FaSmy1 (purple) and secretory vesicles on actin cables to the hyphae tips. FaMyo5 (grey) trigger actin polymerization and travel toward the hyphae tips. By binding to FaMyo5 in phenamacril-sensitive strains (the fungicide is indicated by asterisks), the fungicide disrupts transport along the actin cables. In phenamacril-resistant strains, the binding of phenamacril to FaMyo5 is reduced, and the disruption in transport is therefore reduced.
Supporting Information
The conserved motor domain, myosin tail (TH1), and src homology domain 3 (SH3) are highlighted.
(DOC)
(DOC)
(DOC)
(A) Gene replacement strategy for FaMyo2B and Famyo2. The gene replacement cassette HPH-HSV-tk contains the hygromycin resistance gene and the herpes simplex virus thymidine kinase gene. Primer binding sites are indicated by arrows (see S1 Table for the primer sequences). (B) PCR analysis for identification of FaMyo2B and Famyo2 mutants. (C) Southern blot hybridization analysis of FaMyo2B mutants using the 576-bp upstream DNA fragment of FaMyo2B as a probe and genomic DNA are digested with Hind III. (D) Southern blot hybridization analysis of Famyo2 mutants using the 661-bp downstream DNA fragment of Famyo2 as probe and genomic DNA are digested with Cla I.
(DOC)
(A) Expression level of TIR5 and TRI6 in mutants relative to expression in strain 2021. (B) Expression level of FaMgv1 and FaGpmk1 in mutants relative to expression in strain 2021. Values are the means ± SE of three repeated experiments.
(DOC)
Values are the means ± SE of three repeated experiments.
(DOC)
(DOC)
Acknowledgments
This work was supported by the Chinese 973 Program (2012CB114000), the National Science Foundation of China (31201543), and The Ph.D. Programs Foundation of Ministry of Education of China (no. 20120097120009).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Chinese 973 Program (2012CB114000), the National Science Foundation of China (31201543), and The Ph.D. Programs Foundation of Ministry of Education of China (no. 20120097120009). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bai GH, Shaner G. Management and resistance in wheat and barley to Fusarium head blight. Annual Review of Phytopathology 2004. 42:135–161. [DOI] [PubMed] [Google Scholar]
- 2.Goswami RS, Kistler HC. Heading for disaster: Fusarium graminearum on cereal crops. Molecular Plant Pathology 2004. 5:515–525. 10.1111/j.1364-3703.2004.00252.x [DOI] [PubMed] [Google Scholar]
- 3.Desjardins AE. Fusarium mycotoxins: chemistry, genetics, and biology. American Phytopathological Society (APS Press) 2006. [Google Scholar]
- 4.Sutton J. Epidemiology of wheat head blight and maize ear rot caused by Fusarium graminearum. Canadian Journal of Plant Pathology 1982. 4:195–209. [Google Scholar]
- 5.O’Donnell K, Ward TJ, Geiser DM, Kistler HC, Aoki T. Genealogical concordance between the mating type locus and seven other nuclear genes supports formal recognition of nine phylogenetically distinct species within the Fusarium graminearum clade. Fungal genetics and biology 2004. 41:600–623. [DOI] [PubMed] [Google Scholar]
- 6.Starkey DE, Ward TJ, Aoki T, Gale LR, Kistler HC, Geiser DM, et al. Global molecular surveillance reveals novel Fusarium head blight species and trichothecene toxin diversity. Fungal genetics and biology 2007. 44:1191–1204. [DOI] [PubMed] [Google Scholar]
- 7.Tóth B, Mesterházy Á, Horváth Z, Bartók T, Varga M, Varga J. Genetic variability of central European isolates of the Fusarium graminearum species complex. European Journal of Plant Pathology 2005. 113:35–45. [Google Scholar]
- 8.Niessen L. PCR-based diagnosis and quantification of mycotoxin producing fungi. International journal of food microbiology 2007. 119:38–46. [DOI] [PubMed] [Google Scholar]
- 9.Xu XM, Parry D, Nicholson P, Thomsett M, Simpson D, Edwards S, et al. Within-field variability of Fusarium head blight pathogens and their associated mycotoxins. European Journal of Plant Pathology 2008. 120:21–34. [Google Scholar]
- 10.Chen L, Bai G, Desjardins A. Recent advances in wheat head scab research in China, p 258–273. In (ed), [Google Scholar]
- 11.Zhang JB, Li HP, Dang FJ, Qu B, Xu YB, Zhao CS, et al. Determination of the trichothecene mycotoxin chemotypes and associated geographical distribution and phylogenetic species of the Fusarium graminearum clade from China. Mycological research 2007. 111:967–975. [DOI] [PubMed] [Google Scholar]
- 12.Parry D, Jenkinson P, McLeod L. Fusarium ear blight (scab) in small grain cereals—a review. Plant pathology 1995. 44:207–238. [Google Scholar]
- 13.Windels CE. Economic and social impacts of fusarium head blight: changing farms and rural communities in the northern great plains. Phytopathology 2000. 90:17–21. 10.1094/PHYTO.2000.90.1.17 [DOI] [PubMed] [Google Scholar]
- 14.Li HK, Diao YM, Wang HX, Chen CJ, Ni JP, Zhou MG. JS399-19, a new fungicide against wheat scab. Crop Protection 2008. 27:90–95. [Google Scholar]
- 15.Chen Y, Zhou MG. Characterization of Fusarium graminearum Isolates Resistant to Both Carbendazim and a New Fungicide JS399-19. Phytopathology 2009. 99:441–446. 10.1094/PHYTO-99-4-0441 [DOI] [PubMed] [Google Scholar]
- 16.Zhang YJ, Zhang XA, Chen CJ, Zhou MG, Wang HC. Effects of fungicides JS399-19, azoxystrobin, tebuconazloe, and carbendazim on the physiological and biochemical indices and grain yield of winter wheat. Pesticide Biochemistry and Physiology 2010. 98:151–157. [Google Scholar]
- 17.Zheng ZT, Gao T, Zhang Y, Hou YP, Wang JX, Zhou MG. FgFim, a key protein regulating resistance to the fungicide JS399-19, asexual and sexual development, stress responses and virulence in Fusarium graminearum. Molecular Plant Pathology 2014. 15:488–499. 10.1111/mpp.12108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng Z, Hou Y, Cai Y, Zhang Y, Li Y, Zhou M. Whole-genome sequencing reveals that mutations in myosin-5 confer resistance to the fungicide phenamacril in Fusarium graminearum. Scientific Reports 2015. 5:82–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang C, Chen Y, Yin Y. A small molecule species specifically inhibits Fusarium myosin I. Environmental microbiology 2015. 17:2735–2746. 10.1111/1462-2920.12711 [DOI] [PubMed] [Google Scholar]
- 20.Song B, Li HP, Zhang JB, Wang JH, Gong AD, Song XS, et al. Type II myosin gene in Fusarium graminearum is required for septation, development, mycotoxin biosynthesis and pathogenicity. Fungal Genetics and Biology 2013. 54:60–70. 10.1016/j.fgb.2013.02.010 [DOI] [PubMed] [Google Scholar]
- 21.Sebe-Pedros A, Grau-Bove X, Richards TA, Ruiz-Trillo I. Evolution and Classification of Myosins, a Paneukaryotic Whole-Genome Approach. Genome Biology and Evolution 2014. 6:290–305. 10.1093/gbe/evu013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pollard TD, Doberstein SK, Zot HG Myosin-I. Annu Rev Physiol 1991. 53: 653–681. [DOI] [PubMed] [Google Scholar]
- 23.Geli MI, Riezman H Role of type I myosins in receptormediated endocytosis in yeast. Science 1996. 272: 533–535. [DOI] [PubMed] [Google Scholar]
- 24.Beningo KA, Lillie SH, Brown SS. The yeast kinesin-related protein Smy1p exerts its effects on the class V myosin Myo2p via a physical interaction. Molecular biology of the cell 2000. 11:691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trybus KM. Myosin V from head to tail. Cellular and Molecular Life Sciences 2008. 65:1378–1389. 10.1007/s00018-008-7507-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsui Y. Polarized distribution of intracellular components by class V myosins in Saccharomyces cerevisiae. International review of cytology 2003. 229:1–42. [DOI] [PubMed] [Google Scholar]
- 27.Pruyne D, Legesse-Miller A, Gao L, Dong Y, Bretscher A. Mechanisms of polarized growth and organelle segregation in yeast. Annu Rev Cell Dev Biol 2004. 20:559–591. [DOI] [PubMed] [Google Scholar]
- 28.Weisman LS. Organelles on the move: insights from yeast vacuole inheritance. Nature Reviews Molecular Cell Biology 2006. 7:243–252. [DOI] [PubMed] [Google Scholar]
- 29.Fagarasanu A, Mast FD, Knoblach B, Rachubinski RA. Molecular mechanisms of organelle inheritance: lessons from peroxisomes in yeast. Nature Reviews Molecular Cell Biology 2010. 11:644–654. 10.1038/nrm2960 [DOI] [PubMed] [Google Scholar]
- 30.Díaz-Blanco NL, Rodríguez-Medina JR. Dosage rescue by UBC4 restores cell wall integrity in Saccharomyces cerevisiae lacking the myosin type II gene MYO1. Yeast 2007. 24:343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chesarone-Cataldo M, Guérin C, Jerry HY, Wedlich-Soldner R, Blanchoin L, Goode BL. The myosin passenger protein Smy1 controls actin cable structure and dynamics by acting as a formin damper. Developmental cell 2011. 21:217–230. 10.1016/j.devcel.2011.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Zhang X, Zhang H, Lu Y, Huang H, Dong X, et al. Coiled-coil networking shapes cell molecular machinery. Molecular biology of the cell 2012. 23:3911–3922. 10.1091/mbc.E12-05-0396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lillie SH, Brown SS. Suppression of a myosin defect by a kinesin-related gene 1992. [DOI] [PubMed] [Google Scholar]
- 34.Lillie S, Brown S. Immunofluorescence localization of the unconventional myosin, Myo2p, and the putative kinesin-related protein, Smy1p, to the same regions of polarized growth in Saccharomyces cerevisiae. The Journal of cell biology 1994. 125:825–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang H, Chen J, Wang Y, Peng L, Dong X, Lu Y, et al. A computationally guided protein-interaction screen uncovers coiled-coil interactions involved in vesicular trafficking. Journal of molecular biology 2009. 392:228–241. 10.1016/j.jmb.2009.07.006 [DOI] [PubMed] [Google Scholar]
- 36.Chen Y., Chen C., Wang J., Jin L. and Zhou M. Genetic study on JS399-19 resistance in hyphal fusion of Fusarium graminearum by using nitrate nonutilizing mutants as genetic markers. J. Genet. Genomics, 2007. 34, 469–476. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Liu W, Hou Z, Wang C, Zhou X, Jonkers W, et al. A novel transcriptional factor important for pathogenesis and ascosporogenesis in Fusarium graminearum. Molecular plant-microbe interactions 2011. 24:118–128. 10.1094/MPMI-06-10-0129 [DOI] [PubMed] [Google Scholar]
- 38.Duan Y, Ge C, Liu S, Wang J, Zhou M. A two-component histidine kinase Shk1 controls stress response, sclerotial formation and fungicide resistance in Sclerotinia sclerotiorum[J]. Molecular plant pathology 2013. 14(7): 708–718. 10.1111/mpp.12041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gale L, Ward T, Balmas V, Kistler H. Population subdivision of Fusarium graminearum sensu stricto in the upper Midwestern United States. Phytopathology 2007. 97:1434–1439. 10.1094/PHYTO-97-11-1434 [DOI] [PubMed] [Google Scholar]
- 40.Wu AB, Li HP, Zhao CS, Liao YC. Comparative pathogenicity of Fusarium graminearum isolates from China revealed by wheat coleoptile and floret inoculations. Mycopathologia 2005. 160:75–83. [DOI] [PubMed] [Google Scholar]
- 41.Schiestl RH, Gietz RD. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Current genetics 1989. 16:339–346. [DOI] [PubMed] [Google Scholar]
- 42.Min K, Shin Y, Son H, Lee J, Kim JC, Choi GJ, et al. Functional analyses of the nitrogen regulatory gene areA in Gibberella zeae. FEMS microbiology letters 2012. 334:66–73. 10.1111/j.1574-6968.2012.02620.x [DOI] [PubMed] [Google Scholar]
- 43.Trail F. For blighted waves of grain: Fusarium graminearum in the postgenomics era. Plant physiology 2009. 149:103–110. 10.1104/pp.108.129684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jenczmionka NJ, Schäfer W. The Gpmk1 MAP kinase of Fusarium graminearum regulates the induction of specific secreted enzymes. Current genetics 2005. 47:29–36. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt A, Hall MN. Signaling to the actin cytoskeleton. Annual review of cell and developmental biology 1998. 14:305–338. [DOI] [PubMed] [Google Scholar]
- 46.Moseley JB, Goode BL. The yeast actin cytoskeleton: from cellular function to biochemical mechanism. Microbiology and Molecular Biology Reviews 2006. 70:605–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hodges AR, Bookwalter CS, Krementsova EB, Trybus KM. A nonprocessive class V myosin drives cargo processively when a kinesin-related protein is a passenger. Current Biology 2009. 19:2121–2125. 10.1016/j.cub.2009.10.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walther A, Wendland J. Septation and cytokinesis in fungi. Fungal Genetics and Biology 2003. 40:187–196. [DOI] [PubMed] [Google Scholar]
- 49.Lu W, Chen S, Wang Y. Research on wheat scab. Sci Publ House, Beijing, China: 2001. [Google Scholar]
- 50.Bai G-H, Desjardins A, Plattner R. Deoxynivalenol-nonproducing Fusarium graminearum causes initial infection, but does not cause DiseaseSpread in wheat spikes. Mycopathologia 2002. 153:91–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The conserved motor domain, myosin tail (TH1), and src homology domain 3 (SH3) are highlighted.
(DOC)
(DOC)
(DOC)
(A) Gene replacement strategy for FaMyo2B and Famyo2. The gene replacement cassette HPH-HSV-tk contains the hygromycin resistance gene and the herpes simplex virus thymidine kinase gene. Primer binding sites are indicated by arrows (see S1 Table for the primer sequences). (B) PCR analysis for identification of FaMyo2B and Famyo2 mutants. (C) Southern blot hybridization analysis of FaMyo2B mutants using the 576-bp upstream DNA fragment of FaMyo2B as a probe and genomic DNA are digested with Hind III. (D) Southern blot hybridization analysis of Famyo2 mutants using the 661-bp downstream DNA fragment of Famyo2 as probe and genomic DNA are digested with Cla I.
(DOC)
(A) Expression level of TIR5 and TRI6 in mutants relative to expression in strain 2021. (B) Expression level of FaMgv1 and FaGpmk1 in mutants relative to expression in strain 2021. Values are the means ± SE of three repeated experiments.
(DOC)
Values are the means ± SE of three repeated experiments.
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.