Abstract
Salinity and drought severely affect both plant growth and productivity, making the isolation and characterization of salinity- or drought-inducible promoters suitable for genetic improvement of crop resistance highly desirable. In this study, a 1468-bp sequence upstream of the translation initiation codon ATG of the promoter for ZmGAPP (maize Type-II H+-pyrophosphatase gene) was cloned. Nine 5´ deletion fragments (D1–D9) of different lengths of the ZmGAPP promoter were fused with the GUS reporter and translocated into tobacco. The deletion analysis showed that fragments D1–D8 responded well to NaCl and PEG stresses, whereas fragment D9 and CaMV 35S did not. The D8 segment (219 bp; -219 to -1 bp) exhibited the highest promoter activity of all tissues, with the exception of petals among the D1–D9 transgenic tobacco, which corresponds to about 10% and 25% of CaMV 35S under normal and NaCl or PEG stress conditions, respectively. As such, the D8 segment may confer strong gene expression in a salinity and osmotic stress inducible manner. A 71-bp segment (-219 to -148 bp) was considered as the key region regulating ZmGAPP response to NaCl or PEG stress, as transient transformation assays demonstrated that the 71-bp sequence was sufficient for the salinity or osmotic stress response. These results enhance our understanding of the molecular mechanisms regulating ZmGAPP expression, and that the D8 promoter would be an ideal candidate for moderating expression of drought and salinity response genes in transgenic plants.
Introduction
Water deficiency and salinity levels affect plant growth and crop yields, as they induce osmotic stress and ionic toxicity, issues that are becoming increasingly serious problems in agricultural areas worldwide [1–4]. Manipulation of plant genomes has the potential to promote revolutionary changes in crops [5], with translocated genes playing important roles in determining the phenotypic expression of transgenic plants [6]. Selection of appropriate promoters allows transgenes to be expressed at desired levels, thereby providing more precise control of transgenic plants [7–9]. The availability of various promoters that differ in their ability to regulate transgenic expression patterns can greatly facilitate the successful application of transgenic techniques [8]; as such, isolation and validation of the various promoters suitable for plant genetic transformation is therefore, necessary.
At present, the promoters used in the genetic transformation of plants are generally divided into three categories, consisting of constitutive, tissue- or stage-specific and inducible promoters. Constitutive promoters, such as the CaMV 35S promoter and the maize ubiquitin promoter, are widely used to direct transgene expression in almost all plant tissues at all development stages [10, 11], which frequently causes additional metabolic burden or toxic effects that result in morphological and physiological dysfunctions in plants [12, 13]. For example, both 35S::DREB1A and rd29A::DREB1A transgenic tobacco plants displayed enhanced cold and drought tolerances than did control plants, but the 35S::DREB1A transgenic plants experienced much more severe growth retardation than did the rd29A::DREB1A transgenic plants. The results showed that the stress-inducible rd29A promoter minimized negative growth effects on plant growth while conferring higher tolerance to adverse environmental conditions [14]. Indeed, the use of a tissue-specific or inducible promoter has been shown to be an ideal strategy for the elimination of negative effects resulting from constitutive overexpression of transgenes [14, 15]. Many tissue-specific (such as seed [16–19], anther [20–22], root [23–26] and green tissue-specific [27–29]) and inducible (such as drought [3, 30–32], salinity [3, 24, 30, 31, 33–35], pathogen [36] and hormone [3, 37, 38] induced) promoters have been described [6, 8, 39]. However, the majority of the reported tissue-specific or inducible promoters display weak ability in directing gene expression, which restricts their application. A shortage of available promoters with the desired expression profile limits the fine-tune control of transgene expression in plants.
H+-translocating pyrophosphatases (H+-PPase) activate proton transport across membranes by catalytic hydrolysis of inorganic pyrophosphate to provide energy [40, 41]. Higher plants have two distinct H+-PPase subclasses: Type I is stimulated by K+, whereas Type II is hypersensitive to Ca+ instead of K+ [42]. Several studies have shown that the transcriptional expression of genes encoding Type I H+-PPase in a number of plant species (such as Salicornia europaea, Suaeda corniculata, wheat, Suaeda salsa, and Thellungiella halophila) was induced by drought or salt stress [43–47]. Overexpression of Type I H+-PPase in Arabidopsis [44, 46, 48], tobacco [43, 45], wheat [47], maize [49], sugar beet [50], cotton [51–53], tomato [54], alfalfa [55] and creeping bentgrass [56] increased salt or drought tolerance of the transgenic plants. Sun et al. (2010) found that the TsVP1 (Type I H+-PPase gene) promoter from Thellungiella halophila was highly active in leaves and roots, and could be induced by NaCl treatment, with a 130-bp segment identified as the key region for salt-stress response [34]. In contrast with Type I H+-PPase, only two Type II H+-PPase genes have been identified on higher plants (AVP2 from Arabidopsis and ZmGAPP from maize). Drozdowicz et al. (2000) cloned an Arabidopsis Type II H+-PPase gene AVP2, the amino acid sequence of which is 36% identical to that of the Type I H+-PPase encoded by AVP1 [57]. Mitsuda et al. (2001) demonstrated that AVP2 is localized primarily in the Golgi apparatus; these same authors also investigated the tissue-specific expression patterns of AVP2 using a promoter–GUS reporter system [58], finding that AVP2 differed from AVP1 in that it is highly expressed in the trichome and the stamen filaments [58]. Our laboratory previously isolated the cDNA sequence of a Type II H+-PPase gene in maize (ZmGAPP; GenBank accession no. EF051578). The full-length cDNA sequence of ZmGAPP is 2974 bp including 2400 bp protein coding sequence, 215 bp 5' UTR and 359 bp 3' UTR [59]. Based on the length of 5' UTR, we speculated the transcription start stie of ZmGAPP in maize may be located in the translation initiation codon ATG upstream of approximately 215 bp. The transcription of ZmGAPP is enhanced in response to dehydration, cold, and salt stresses [59], but the promoter of ZmGAPP has thus far not been well defined. Thus, isolation and characterization of the ZmGAPP promoter will provide novel insights into understanding the transcriptional regulation of ZmGAPP and the promoter resources for plant genetic transformation.
Materials and Methods
Isolation of ZmGAPP promoter from Zea mays L.
The 5' flanking sequence of ZmGAPP was retrieved from the NCBI High Throughput Genomic Sequences Database of Zea mays using its full-length cDNA sequence (GenBank accession no. EF051578) as query. The forword and reverse primers (named pZmGAPPFR, Table 1) were designed according to the ZmGAPP sequence and its 5' flanking sequence. The 1584-bp fragment (–1468 to +116 bp; the “A” of the translation start codon “ATG” of ZmGAPP was designated as “+1”) was amplified from maize genomic DNA with the pZmGAPPFR primers (Table 1). The PCR conditions were as follows: initial denaturation at 95°C for 5 min followed by 35 cycles of 95°C 1 min, 55°C 1 min, and 72°C 2 min, and then final extension at 72°C for 7 min. The PCR products were excised from a 1% agarose gel and purified by AxyPrepTM DNA Gel Extraction Kit (Axygen Scientific, Inc, China). Then the fragment (–1468 to +116 bp) were cloned in the pGEM-T® Easy cloning vector (Promega, USA) following the manufacturer's instructions and confirmed by sequencing. Finally, a 1468-bp fragment upstream of the translation start codon of ZmGAPP was isolated by PCR amplification using D1 primers (Table 1) and considered as the full-length promoter.
Table 1. PCR primers used in the current study.
| Name | Forward (5’ to 3’) | Reverse (5’ to 3’) |
|---|---|---|
| pZmGAPPFR | cctgacttaatcgcacccat | ggagaaagattagcgaaagcc |
| D1 | cccaagcttcctgacttaatcgcac | ccggaattcgatggaatatgagtttg |
| D2 | cccaagctttttgttgggcttagtg | ccggaattcgatggaatatgagtttg |
| D3 | cccaagcttgcttcgttgctgcctt | ccggaattcgatggaatatgagtttg |
| D4 | cccaagctttcgtgaaatcaagtgg | ccggaattcgatggaatatgagtttg |
| D5 | cccaagctttagaatcgctacttgc | ccggaattcgatggaatatgagtttg |
| D6 | cccaagcttctactgccattgtcac | ccggaattcgatggaatatgagtttg |
| D7 | cccaagcttagaaggtgtctgggta | ccggaattcgatggaatatgagtttg |
| D8 | cccaagcttgtaggcttgacggcaa | ccggaattcgatggaatatgagtttg |
| D9 | cccaagcttgtgtttaacttttagg | ccggaattcgatggaatatgagtttg |
| p35SFR | aatggatccaagtctcaatagcccttt | tgagaattccgtattggctagagcagc |
| p71bpFR | taaggatccgtaggcttgacggca | aaactgcaggtaaacacatccaga |
| HPTFR | cgtctgctgctccatacaa | tgtcctgcgggtaaatagc |
| GUSFR | acggatggtatgtccaaagc | aacgtatccacgccgtattc |
| Ntα-Tub1FR | atgagagagtgcatatcgat | ttcactgaagaaggtgttgaa |
The underlined sites are the sites for the digestion of restriction enzymes HindШ. The underlined italicized sites are the sites for the digestion of restriction enzymes EcoR1.
Analysis of the ZmGAPP promoter sequence
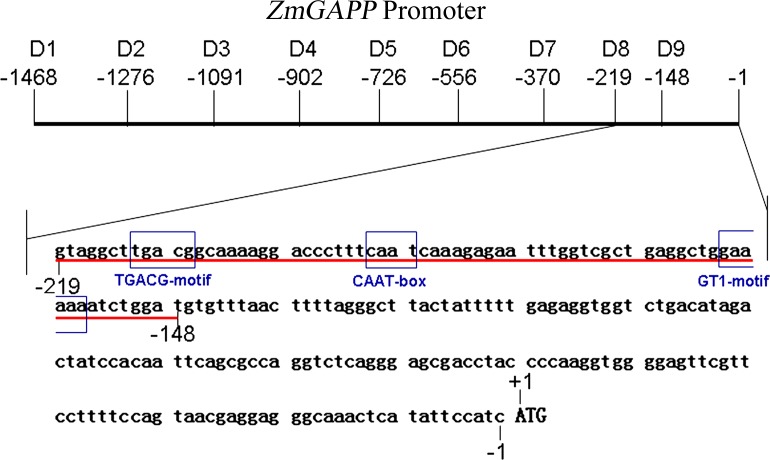
The 1468-bp (–1468 to –1 bp) sequence of the ZmGAPP promoter was searched to locate the potential cis-acting elements using PLACE (http://www.dna.affrc.go.jp/PLACE/) and PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) [60, 61]. The position and description of the predicted cis-acting elements are listed in Fig 1 and Table 2.
Fig 1. Nucleotide sequence of the ZmGAPP promoter.
The “A” of the translation initiation code “ATG” of ZmGAPP was designated as “+1”. Putative cis-acting elements underlined or shown in the border. See Table 2 for descriptions of the elements. The arrow above the sequence indicates the start point of different deletion fragments (D1–D9).
Table 2. Identification of cis-acting elements in the ZmGAPP promoter sequence using the PLACE and PlantCARE databases.
| Cis-elements | Description | Position from ATG | No. |
|---|---|---|---|
| Box E | Cis-acting element for induction upon fungal elicitation | -1454 | 1 |
| CuRE-motif | Copper-response element | -1389, -278 | 2 |
| LTRE | Low temperature responsive element | -1370, -710 | 2 |
| TATA-box | Core promoter element around -30 of transcription start | -1310, -756, -750, -599, -538, -521, -504, -375, -336, -248, -139 | 11 |
| GTGA-motif | Cis-acting element involved in late pollen development and pectate lyase | -1240, -1016, -900, -887 | 4 |
| GAGAC-motif | Sulfur-responsive element | -1217 | 1 |
| G-Box | Cis-acting element involved in light responsiveness | -1207 | 1 |
| MBS | MYB binding site involved in drought-inducibility | -1185, -990, -798, -630, -602, -329 | 6 |
| Sp1 | Light responsive element | -1137, -960, -938, -879, -866, -585 | 6 |
| motif IIb | Abscisic acid responsive element | -1066 | 1 |
| GCC-box | Cis-acting element involved in ethylene, jasmonate and defence responsiveness | -973, -970 | 2 |
| GC-motif | Enhancer-like element involved in anoxic specific inducibility | -865 | 1 |
| GARE-motif | Gibberellin-responsive element | -771 | 1 |
| Pollen1lelat52 | Cis-acting element required for pollen specific expression | -672 | 1 |
| O2-site | Cis-acting regulatory element involved in zein metabolism regulation | -666 | 1 |
| TGTCACA motif | Enhancer element necessary for fruit-specific expression | -546 | 1 |
| CAAT-box | Common cis-acting element in promoter and enhancer regions | -545, -514, -406, -192, -92 | 5 |
| GCN4-motif | Cis-acting element involved in endosperm expression | -411 | 1 |
| Gap-box | Part of a light responsive element | -404 | 1 |
| Skn-1_motif | Cis-acting element required for endosperm expression | -227 | 1 |
| TGACG-motif | Cis-acting element involved in the MeJA-responsiveness | -212 | 1 |
| GT-1 motif | Cis-acting element involved in pathogen and NaCl induced expression | -162 | 1 |
| ACE | Cis-acting element involved in light responsiveness | -66 | 1 |
Construction of the promoter::GUS plasmids
For functional validation of the ZmGAPP promoter, nine 5′ deleted fragments (D1–D9) of different lengths (-1468 bp, -1276 bp, -1091 bp, -902 bp, -726 bp, -556 bp, -370 bp, -219 bp and -148 bp to -1 bp; Fig 1 and S1A Fig) were amplified by PCR from the 1468-bp promoter sequence of ZmGAPP using the primers listed in Table 1. To construct the ZmGAPP promoter::GUS plasmids, each amplified fragment was subsequently ligated into the vector pCAMBIA1391Z (Cambia, Australia) with HindШ/EcoRI restriction sites, and confirmed by restriction digestion analysis (S1B Fig) and sequencing. The resulting constructs were used for the tobacco transformation.
The minimal CaMV 35S promoter sequence (-46 to +10 bp) was amplified by PCR using the primer p35SFR (Table 1) and confirmed by sequencing; the fragment was then inserted into the PstI/SpeI sites upstream of the reporter gene GUSA in the vector pCAMBIA1304 (Cambia, Australia). The plasmid was designated as p-mini35S. The 71-bp fragment of the ZmGAPP promoter (-219 to -148 bp) was isolated by PCR using the primer p71bpFR (Table 1) and then confirmed by sequencing; the fragment was then inserted into the BamH1/Pst1 sites of the p-mini35S vector. The plasmid was given the name p-71bp-mini35S and used for the tobacco transient assay. The pCAMBIA1304 vector containing the CaMV 35S promoter upstream from the GUSA was used as a positive control.
Tobacco culture and genetic transformation
Tobacco (Nicotiana benthamiana) seeds were sterilized with 70% ethanol for 1 min, 10% NaClO for 8 min, and then washed 5–6 times with sterile water and allowed to germinate at 25°C for 1 week. The seedlings were then transferred into culture bottles containing 50% MS nutrient medium, sucrose (30 g/L) and 0.7% agar (pH 6.0), and grown in a tissue culture chamber at 25°C under a 16-h light (220–260 μmol m–2 s–1) regime daily for 6 weeks, until transformation.
The pCAMBIA1304 and D1–D9 plasmids were transferred into Agrobacterium tumefaciens strain GV3101 using a freeze–thaw method. The transformation of tobacco leaf discs was performed as described by Voelker et al. (1987), with minor modifications [62]. Transformed leaf discs were screened on an MS medium supplemented with 0.1 mg/L indole-3-acetic acid, 1.0 mg/L 6-benzylaminopurine, 15 mg/L hygromycin B and 400 mg/L cefotaxime. Regenerated shoots were rooted on an MS medium containing 15 mg/L hygromycin B and 200 mg/L cefotaxime. The transformed plants were grown in soil under day/night temperatures of 25–28°C (day)/19–22°C (night) and a 16-h light (220–260 μmol m–2 s–1) cycle. The T0 transgenic plants were screened out for propagation by PCR (S2A Fig) of the hygromycin resistant gene located in the pCAMBIA1304 and pCAMBIA1391Z vectors with the primer HPTFR (Table 1) and GUS staining (S2B Fig). The transgenic tobacco lines that displayed a Mendelian segregation ratio of 3:1 in T1-generation seedlings by GUS staining were selected for subsequent propagation. Finally, three homozygous transgenic lines, each containing a single copy of the promoter::GUS insert from the ZmGAPP promoter deletion construct D1–D9 and the CaMV 35S promoter, were selected for subsequent function analyses using T3-generation plants.
NaCl and PEG stress treatments
D1–D9 and CaMV 35S promoter transgenic and non-transgenic (WT) tobacco plants were grown under conditions of 25/19°C ± 3°C (day/night temperatures), a 16-h light (220–260 μmol m–2 s–1) cycle and approximately 65% relative humidity for 2 months, then subjected to NaCl and PEG 6000 stress treatments. Two fully expanded leaves per 60-day-old plant were used for the detached-leaves treatments. Leaf discs of 0.5 cm in diameter were cut out and floated in a liquid 1/2 MS medium supplemented with either 200 mM NaCl (salt stress treatment) or 18% (w/v) PEG 6000 (osmotic stress treatment) at 25°C for 1, 3, 6, 12, 16, 24, 48, and 72 h. The leaf discs floated in 1/2 MS liquid medium were considered the control. For whole-plant treatments, 60-day-old tobacco plants were immersed in a liquid 1/2 MS medium supplemented with either 200 mM NaCl (salt stress treatment) or 18% (w/v) PEG 6000 (osmotic stress treatment) at 25°C for 24 h. The control plants were grown in 1/2 MS liquid medium. Leaf tissues were then immediately sampled for GUS histochemical staining, and frozen in liquid nitrogen and stored at -80°C in preparation for GUS fluorometric assays. All experiments were repeated in triplicate with independent samples.
qRT-PCR analysis
Total RNA was isolated from leaves of transgenic tobacco plants using the TRIzol reagent (Sangon, China) and then treated with RNase-free DNase (Takara, China). The cDNA synthesis was performed with the RT reagent kit (Takara, China) according to the manufacturer’s protocol. The qRT-PCR assays were performed using the SYBR Green RT-PCR Kit (Takara, China) on a ChromoTM 4 Gene Amplification System (MJ Research, USA), in a 10 μl reaction volume containing 5 μL of SYBR Green PCR mix, 0.2 μM of each forward and reverse primer, 1 μL of diluted cDNA template, and the appropriate amount of sterile ddH2O. The amplification conditions were as follows: 2 min at 95°C, 40 cycles of 15 s at 95°C, 30 s at 58°C, and 30 s at 72°C. The relative expression level of RNA transcripts were calculated by the 2-ΔΔCt method [63]. As the expression of tobacco α-tubulin (Ntα-Tub1; AJ421411) is known to be fairly uniform; it was used as an internal control to normalize the expression of GUS. The entire experiment was repeated three times with independent samples, and the primer sequences (GUSFR and Ntα-Tub1FR) are shown in Table 1.
GUS histochemical and fluorometric analysis
GUS histochemical staining and fluorometric assay were performed according to the methods described by Jefferson et al. (1987) with minor modifications [64]. The tissues were placed in GUS staining solution containing 50 mM sodium phosphate (pH 7.0), 0.5 mM potassium ferricyanide, 0.5 mM potassium ferrocyanide, 10 mM EDTA, 0.1% Triton X-100 and 1 mM X-Gluc (Sangon, Shanghai, China), then D1-D3 and D4-D9 fragments incubated at 37°C for 24 h and 6 h, respectively. Because of the original high GUS expression levels in D4-D9 transgenic tobacco plants, they require shorter incubation time in order to better evaluate intensity of the reaction. To make clear the difference of GUS staining between D4-D9 before and after stresses, GUS staining for 6 h was determined by the pre experiment. After staining, the tissues were bleached with 70% ethanol and photographed (Sony DSC-F828 digital camera).
Leaf tissues were homogenized in a 4°C extraction buffer containing 50 mM sodium phosphate (pH 7.0), 0.1% sodium lauryl sarcosine, 10 mM DTT, 0.1% Triton X-100 and 10 mM EDTA for GUS fluorometric assays. The samples were centrifuged for 15 min at 10000 g and 4°C, with supernatant activity detected via an assay buffer containing 1 mM 4-methylumbelliferyl-b-glucuronide (4-MUG, Sigma, USA) at 37°C. The reaction was terminated by the addition of 200 mM Na2CO3, to a final concentration of 180 mM. Fluorescence was measured with a fluorescence spectrophotometer (HITACHI F-4600, Japan) at the excitation and emission wavelengths of 365 nm and 455 nm, respectively. Protein concentration of the supernatant was determined using the Bradford method [65]. The GUS activity was calculated as nmol of 4-Methylumbelliferone (4-MU) per mg protein per minute under controlled conditions.
GUS transient expression assay
Transient expression of GUS activity was carried out using leaves of 60-day-old tobacco plants as described previously [66]. Agrobacterium tumefaciens GV3101 harboring p-mini35S, p-71bp-mini35S and pCAMBIA1304 plasmids were grown on YEP medium containing 50 mg/L Rif and 50 mg/L kanamycin at 28°C for 18 h. The Agrobacterium cultures were isolated by centrifugation for 15 min at 6000 g, resuspended in the infiltration medium containing 10 mm MES, 100 μm acetosyringone and 10 mm MgCl2 (pH 5.6) to an OD600 of 0.6, and incubated at room temperature for 3 h. The Agrobacterium cultures were then agro-injected into tobacco leaves at the abaxial surfaces using a needleless syringe, following which the agro-infiltrated plants were maintained in a moist chamber at 25°C for 48 h.
For NaCl and PEG stress treatments, the infiltrated leaf discs were cut out and floated on a liquid 1/2 MS medium supplemented with either 200 mM NaCl (salt stress treatment) or 18% (w/v) PEG 6000 (osmotic stress treatment) for 24 h. The infiltrated leaves incubated in the liquid 1/2 MS medium were considered the control. Leaf tissues from fifteen independently infiltrated plants were then used for GUS histochemical staining and GUS fluorometric assays. All experiments were repeated in triplicate.
Data analysis
Results were expressed as mean values ± SD (standard deviation). A Student’s t test (n = 3, P < 0.05; Sigmaplot 12.0) at a 95% confidence level was used to test for statistical significance.
Results
Isolation of ZmGAPP promoter from Zea mays L. and sequence analysis
Based on the public sequence from MaizeGDB (http://www.maizegdb.org/), the 1468-bp 5′ flanking sequence of ZmGAPP upstream of the start codon ATG was obtained from maize genomic DNA. The ZmGAPP promoter sequence was analyzed using the online software PlantCARE and PLACE. Twenty-three kinds of potential cis-acting elements were present in the 1468-bp region of the ZmGAPP promoter (Fig 1 and Table 2). Multiple core cis-acting elements, including 11 TATA and 5 CAAT boxes, were found at numerous positions. A series of putative cis-regulatory elements that enables the inducible or tissue-specific expression of ZmGAPP were identified, including four types of light-responsive elements (G-Box, Sp1, Gap-box and ACE), four kinds of hormone-responsive elements (motif IIb, GCC-box, GARE motif and TGACG motif), a copper-responsive element (CuRE motif), a sulfur-responsive element (GAGAC-motif), a cis-acting element involved in pathogen- and NaCl-induced expression (GT1), two low-temperature-responsive elements (LTRE), a fungal-inducible element (Box E), six MYB binding sites involved in drought-inducibility (MBS), an enhancer-like element involved in anoxic specific inducibility (GC motif), a zein-metabolism-related element (O2-site) and several elements required for tissue-specific expression (GTGA motif, Pollen1lelat52, TGTCACA motif, GCN4 motif and Skn-1 motif).
Expression patterns and activities of ZmGAPP promoter and its 5′ deletion segments in transgenic tobacco plants under normal conditions
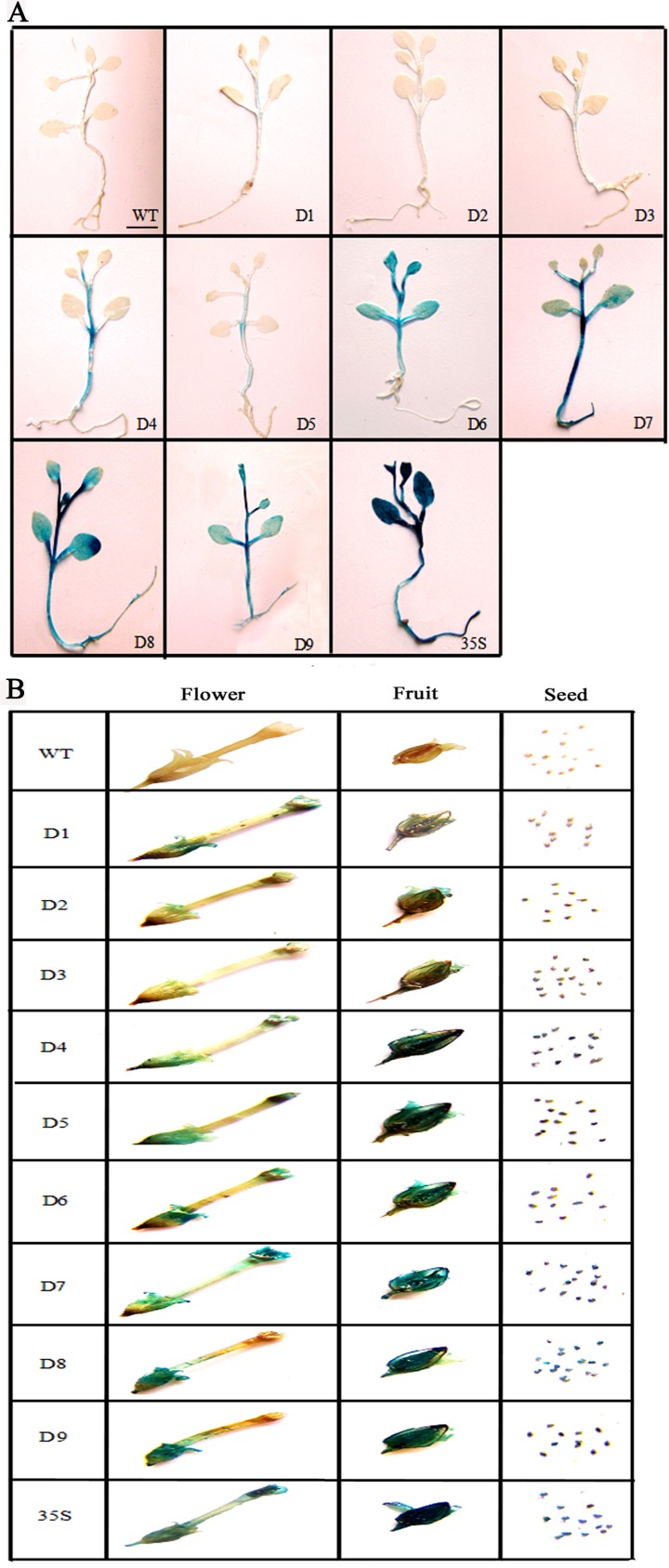
Our results suggest that all nine of the ZmGAPP promoter deletion segments (D1–D9) could direct GUS expression in transgenic tobacco, but they differed considerably in expression patterns and activities. To assess the expression patterns of both D1–D9 and the CaMV 35S promoter under normal conditions, the flowers, fruits, seeds, and 20-day-old seedlings of transgenic tobacco were tested via GUS histochemical staining (Fig 2).
Fig 2. GUS histochemical assays of tissues of D1–D9 and CaMV 35S transgenic tobacco plants.
Twenty-day-old seedlings (A), and flowers, fruits and seeds (B) were incubated in staining solution at 37°C. The D1–D3 and D4–D9 fragments were stained for 24 h and 6 h, respectively, following which the samples were observed and photographed after decolorization. Scale bar: 0.5 cm.
For 20-day-old seedlings (Fig 2A), weak GUS expression was detected in the stems of D1–D3 and D5, whereas GUS expression was virtually absent in the roots, cotyledons and leaves. GUS expression in the D4 seedlings was also detected only in the stems, and GUS-expression intensity was stronger than that in D1–D3 and D5 transgenic tobacco. GUS was strongly expressed in all tissues of D6–D9 seedlings, with the exception of the roots of D6 mutants. The deletion of the 912-bp (–1468 to –556 bp) promoter fragment located between D1 and D6 enhanced GUS activity in the leaves and cotyledons of the seedlings, suggesting that the 170-bp (–726 to –556 bp) segment between D5 and D6 may contain the cis-acting elements that inhibited gene expression in the leaves and cotyledons of 20-day-old tobacco seedlings. Three TATA boxes, a pollen-specific expression required element (Pollen1lelat52) and several elements responsive to low temperatures (LTRE), zein metabolism (O2-site), drought (MBS), light (Sp1) and gibberellin (GARE motif) were present in the 170-bp region (Fig 1 and Table 2). Given that no expected elements were detected, the 170-bp sequence may thus contain unknown elements that inhibit gene expression in the leaves and cotyledons of 20-day-old tobacco seedlings. Moreover, GUS expression was absent in the roots of D1–D6 seedlings, whereas strong GUS expression was detected in the roots of D7–D9 tobacco seedlings. We would expect cis-acting elements that inhibit gene expression in the roots of 20-day-old tobacco seedlings to be present in the 186-bp (–556 to –370 bp) region between D6 and D7, yet only a fruit-specific expression required element (TGTCACA motif), a GCN4 motif involved in endosperm expression, a light-responsive element (Gap box), three CAAT boxes and two TATA boxes were found in this sequence. Therefore, the 186-bp sequence may contain no reported elements that inhibit gene expression in the roots of 20-day-old tobacco seedlings.
For flowers, fruits and seeds (Fig 2B), GUS expression could be detected in the transgenic tobacco plants with the ZmGAPP promoter and its 5′ deletion segments, with the exception of the petals of D8 and D9, and the GUS-expression intensity of D1–D9 was similar to that in the 20-day-old tobacco seedlings. GUS expression activities in D1–D3 transgenic tobacco were weak in the flowers, fruits and seeds, whereas, with the exception of the petals of D8 and D9, GUS expression was stronger in D4–D9 than that in D1–D3. Notably, the deletion of a 151-bp (–370 to –219 bp) segment between D7 and D8 resulted in the loss of GUS expression capability in the petals of D8 and D9, implying that the 151-bp (–370 to –219 bp) segment may contain some cis-regulatory elements required for petal-specific expression. However, only two TATA boxes, an endosperm-specific expression required element (Skn-1 motif), a copper-responsive element (CuRE motif), and a MYB binding site involved in drought inducibility (MBS) were identified in the region. As such, the 151-bp (–370 to –219 bp) segment may therefore, contain no reported elements required for petal-specific expression.
In regard to controls, GUS expression analysis of WT (negative control) and CaMV 35S (positive control) were also carried out (Fig 2). GUS expression of WT was not detected in all tissues of 20-day-old seedlings, flowers, fruits and seeds, whereas the CaMV 35S transgenic plants displayed the highest GUS-expression intensity among the tested constructs, and GUS was expressed in various tissues.
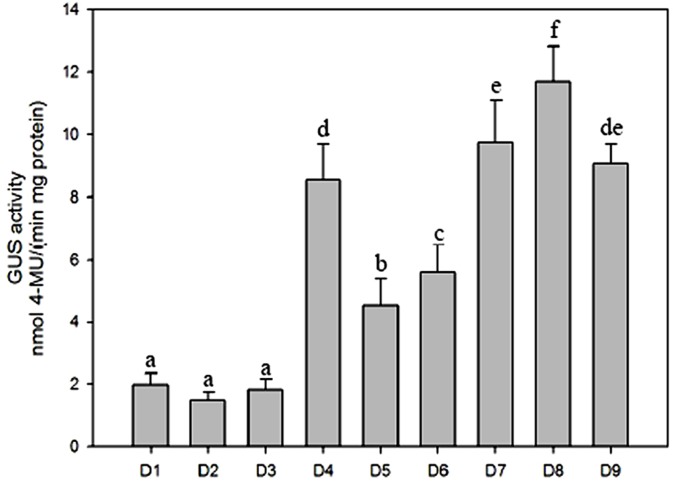
To further evaluate the contribution of different fragments of the ZmGAPP promoter to its expression activity under normal conditions and identify the core functional region, fluorometric GUS assays were performed on D1–D9 transgenic tobacco leaves of 60-day-old mature plants (Fig 3). The promoter activities of D1–D3 and D5 were relatively weak, whereas GUS expression was strong in D4 and D6–D9. The promoter activity of D4 was approximately 4.5-fold as high as that of D1–D3. A motif IIb (abscisic acid responsive element), a GTGA motif (late pollen development related element), a MBS (MYB binding site involved in drought-inducibility), two Sp1 light-responsive elements and two GCC boxes (Cis-acting element involved in ethylene, jasmonate and defense responsiveness) were found in the 189-bp (–1091 to –903 bp) region between D3 and D4 (Fig 1 and Table 2) by bioinformatics analysis. The results suggested that the 189-bp sequence may contain novel elements that inhibit ZmGAPP transcription. Moreover, GUS-expression intensity driven by D8 was considerably higher than that induced by D1–D7 and D9, which corresponds to approximately 6-fold of the full-length promoter (D1). The 219-bp (–219 to –1 bp) D8 segment may be the key sequence required for high-level expression of ZmGAPP, which contains the promoter core cis-acting elements CAAT and TATA boxes (Fig 1).
Fig 3. GUS activity assays of D1–D9 transgenic tobacco plants under normal conditions.
Values are means ± SD from 15 independent transgenic plants (5 individual plants/ line, 3 lines for each construct). Different lowercase letters above the bars indicate significant differences at P < 0.05.
Salinity and osmotic stress-induced activity analysis of the ZmGAPP promoter and its 5′ deletion segments in transgenic tobacco
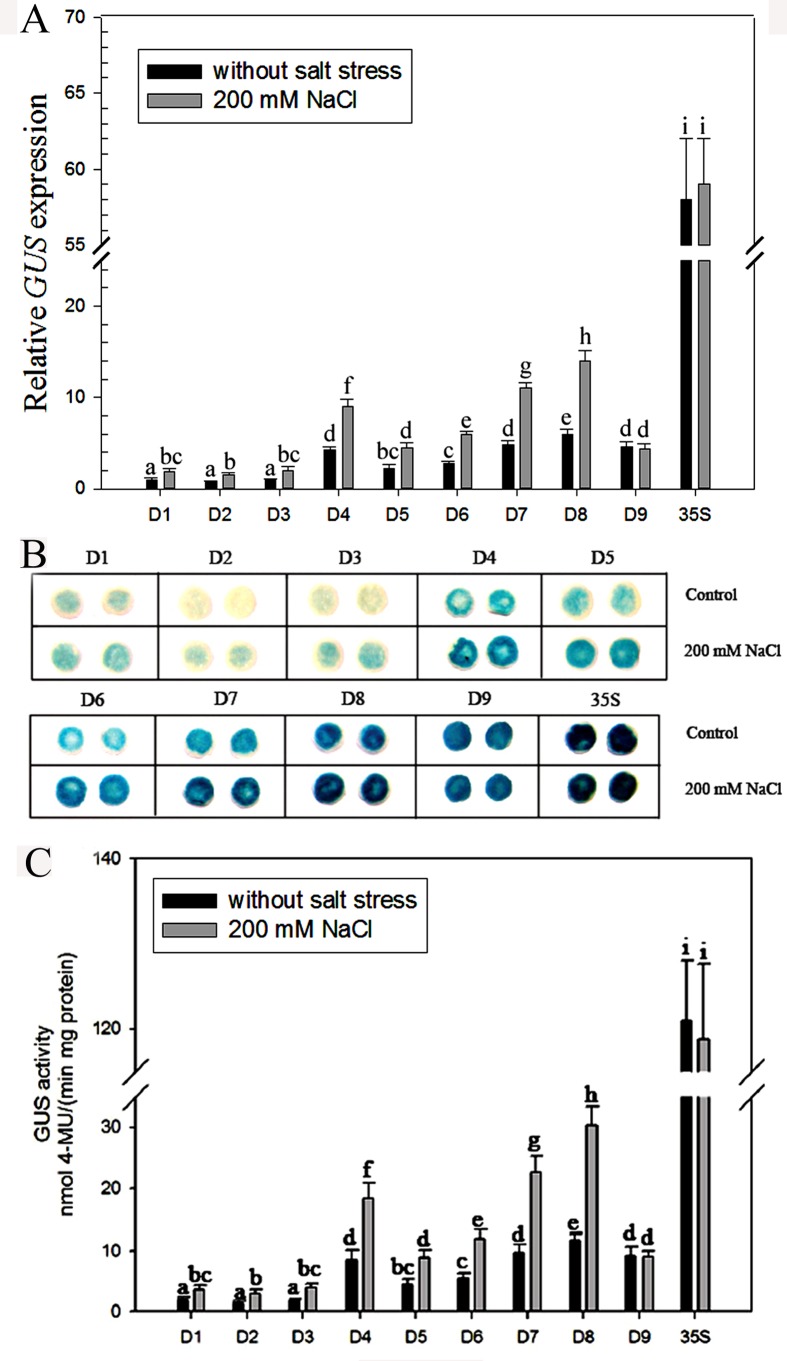
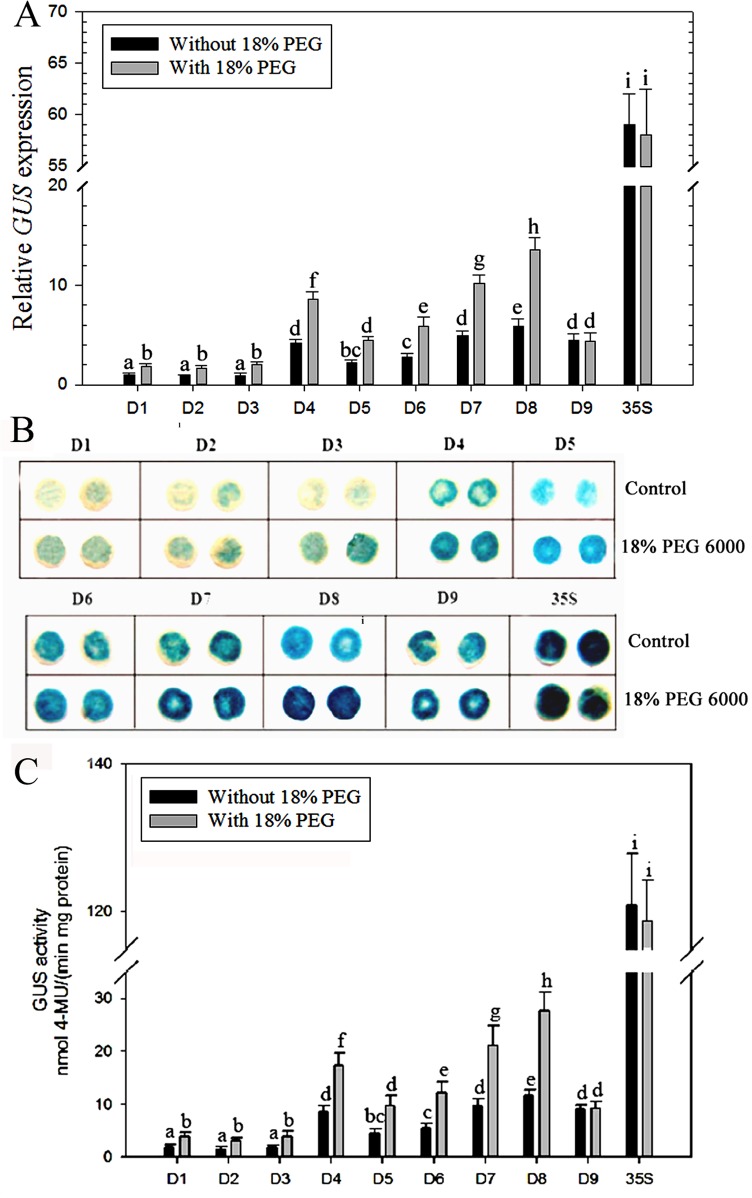
To understand the molecular basis of NaCl- or PEG-inducible expression of ZmGAPP, the promoter activities of D1–D9 were tested in leaves by incubating the detached leaves or whole plants in liquid 1/2 MS medium supplemented with 200 mM NaCl (salt stress treatment) or 18% PEG 6000 (osmotic stress treatment). CaMV 35S promoter transgenic tobacco (positive control) and wild type (negative control) plants were also treated in parallel.
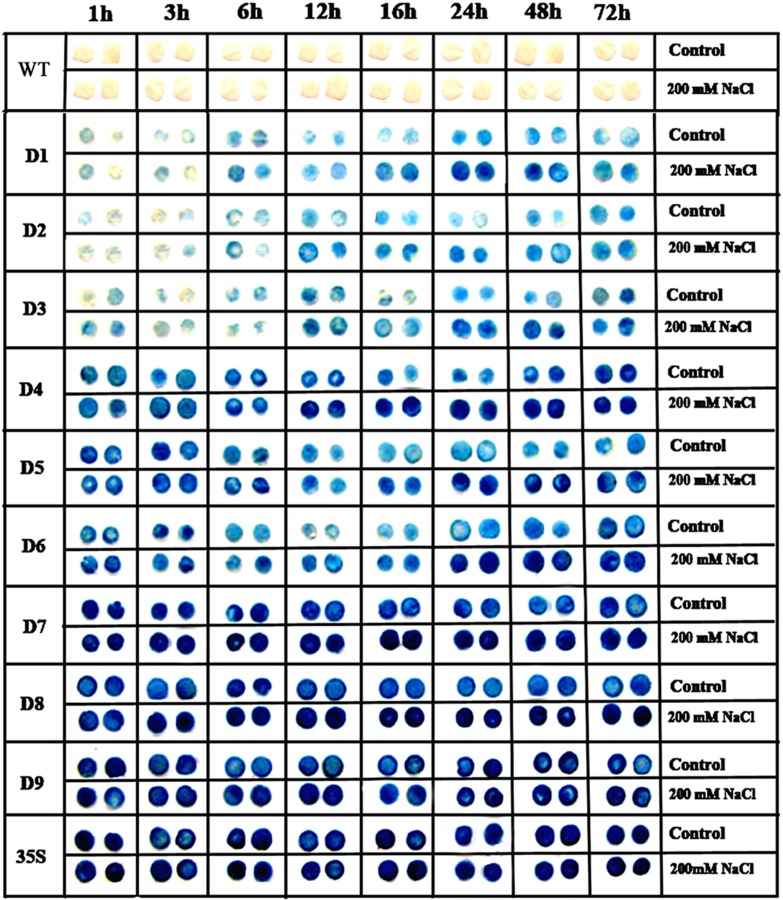
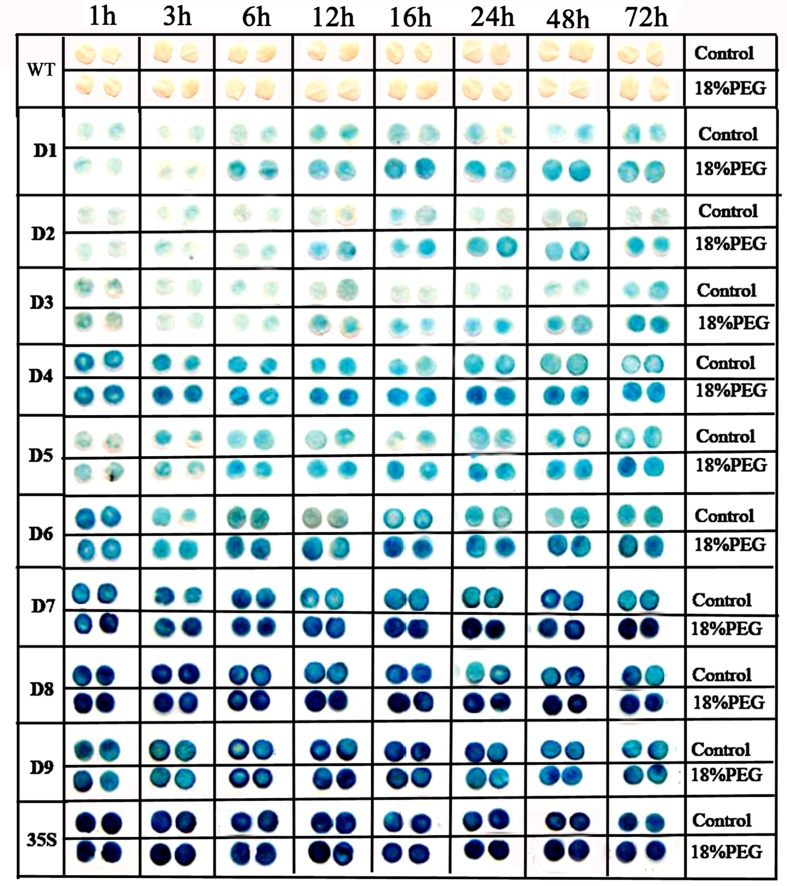
The detached leaves from 60-day-old mature tobacco plants were subjected to either 200 mM NaCl or 18% PEG 6000 treatment in a time-course experiment (Figs 4 and 5). GUS staining intensity of the leaf discs revealed no obvious differences between the stress-treated groups and control groups for 1-, 3-, or 6-h NaCl or PEG treatment. The leaf discs of D1–D8 plants displayed a stress-induced tendency following 12-h treatment, and the GUS expression level of D1–D8 leaf discs were clearly higher than those of the untreated control groups after 16-, 24-, 48-, and 72-h NaCl or PEG treatments. However, the levels of GUS expression in the leaf discs from CaMV 35S and D9 transgenic tobacco were stable during the NaCl and PEG treatments.
Fig 4. GUS staining of detached leaves of transgenic tobacco under normal and salt-stress conditions.
Ninety leaf discs (diameter 0.5 cm) from 15 individual plants (5 individual plants/ line, 3 lines for each construct) of D1–D9 and CaMV 35S transgenic tobacco plants were incubated in liquid 1/2 MS medium supplemented with 200 mM NaCl for 1, 3, 6, 12, 16, 24, 48, and 72 h; leaf discs floated in liquid 1/2 MS medium were used as control. The leaf discs of D1–D3 plants were then incubated in staining solution at 37°C for 24 h, whereas the leaf discs of D4–D9 and CaMV 35S transgenic plants were stained for 6 h. Finally, the samples were observed and photographed after decolorization.
Fig 5. GUS staining of detached leaves of transgenic tobacco under normal and PEG treatment conditions.
Ninety leaf discs (diameter 0.5 cm) from 15 individual plants (5 individual plants/ line, 3 lines for each construct) of D1–D9 and CaMV 35S transgenic tobacco plants were incubated in liquid 1/2 MS medium supplemented with 18% PEG 6000 (w/v) for 1, 3, 6, 12, 16, 24, 48, and 72 h; leaf discs floated in liquid 1/2 MS medium were used as control. The leaf discs of D1–D3 plants were then incubated in staining solution at 37°C for 24 h. The leaf discs of D4–D9 and CaMV 35S transgenic plants were stained for 6 h. Finally, the samples were observed and photographed after decolorization.
To further evaluate the results, stress treatments involving whole plants were also conducted. Based on the results of the detached-leaves experiment, we chose 200 mM NaCl or 18% PEG 6000 treatments for 24 h. Analysis of GUS expression, GUS staining intensity and enzyme activity demonstrated that the promoter activities of D1–D8 were induced by up to more than two-fold in the leaves for 24 h of 200 mM NaCl or 18% PEG 6000 treatment (Figs 6 and 7), whereas no significant differences were detected in D9 and CaMV 35S transgenic tobacco leaves before and after stress treatments. In other words, the D1–D8 promoter sequence appears to respond well to salinity and osmotic stress, whereas D9 and CaMV 35S did not, indicating that the 71-bp (–219 to –148 bp) fragment of the ZmGAPP promoter between D8 and D9 may contain cis-acting elements responsive to salinity and osmotic stress. Notably, the D8 fragment still exhibited the highest level of promoter activity among the D1–D9 under NaCl or PEG stress (Figs 6 and 7), and was fully six times more active than was the full-length promoter (i.e., D1). Under normal conditions, the promoter activity of D8 was about 1.4-fold that of D9 and 10% of CaMV 35S, but three-fold more so than D9 and about 25% of the CaMV 35S promoter after 200 mM NaCl or 18% PEG 6000 treatment for 24 h. Therefore, the D8 segment (219 bp; –219 to –1 bp) may confer high levels of gene expression and contain elements of an NaCl- or PEG-inducible nature.
Fig 6. Analysis of different ZmGAPP promoter deletion constructs in transgenic tobacco plants under normal and NaCl treatment conditions.
The D1–D9 and CaMV 35S transgenic tobacco plants were incubated in liquid 1/2 MS medium supplemented with 200 mM NaCl for 24 h; plants grown in liquid 1/2 MS medium were treated as control. (A) qRT-PCR analysis. The tobacco α-tubulin (AJ421411) was used as an internal control. (B) GUS histochemical staining. The leaves of D1–D3 plants were incubated in staining solution at 37°C for 24 h; leaves of D4–D9 and CaMV 35S transgenic plants were stained for 6 h. Samples were then observed and photographed after decolorization. (C) GUS activity assays. Values represent the means ± SD from 15 independent transgenic plants (5 individual plants/ line, 3 lines for each construct). Different lowercase letters above the bars indicate significant differences at P < 0.05.
Fig 7. Analysis of different ZmGAPP promoter deletion constructs in transgenic tobacco plants under normal and PEG treatment conditions.
The D1–D9 and CaMV 35S transgenic tobacco plants were incubated in liquid 1/2 MS medium supplemented with 18% PEG 6000 (w/v) for 24 h; plants grown in liquid 1/2 MS medium were treated as control. (A) qRT-PCR analysis. The tobacco α-tubulin (AJ421411) was used as an internal control. (B) GUS histochemical staining. The leaves of D1–D3 plants were incubated in staining solution at 37°C for 24 h; leaves of D4–D9 and CaMV 35S transgenic plants were stained for 6 h. Samples were then observed and photographed after decolorization. (C) GUS activity assays. Values represent the means ± SD from 15 independent transgenic plants (5 individual plants/ line, 3 lines for each construct). Different lowercase letters above the bars indicate significant differences at P < 0.05.
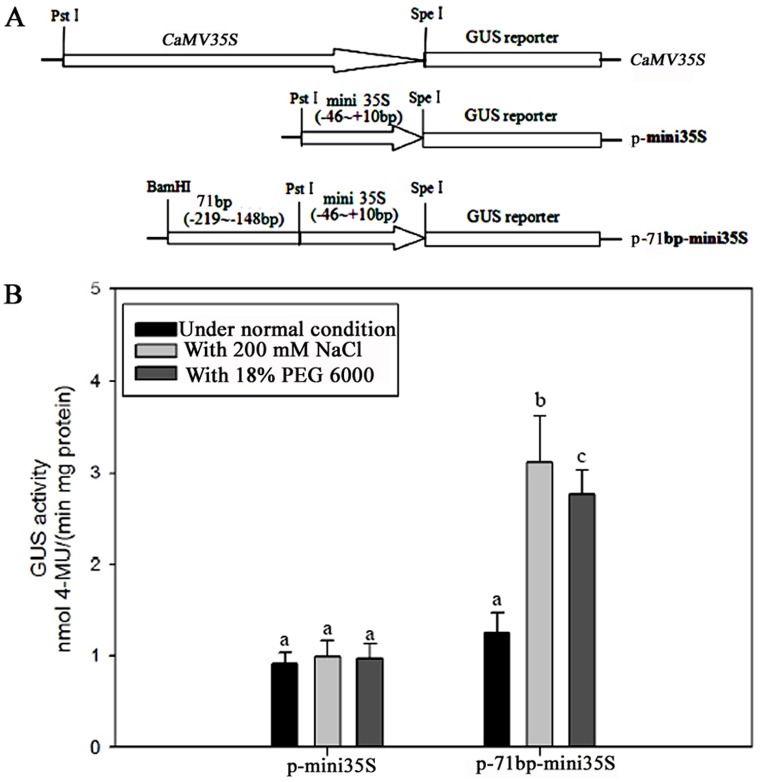
The 71-bp fragment (–219 to –148 bp) is the key region of the ZmGAPP promoter in terms of response to salinity and osmotic stress
The 71-bp (–219 to –148 bp) segment between D8 and D9 was isolated and inserted into the p-mini35S vector, as described in the Materials and Methods section. The vector was given the name p-71bp-mini35S and used for Agrobacterium-mediated GUS transient assay in tobacco leaves in order to test its NaCl- or PEG-inducible activity (Fig 8 and S3 Fig). The GUS-expression intensity of the p-71bp-mini35S vector in transiently transfected tobacco leaves had a significant increment after 200 mM NaCl or 18% PEG 6000 treatment for 24 h, whereas the level of GUS expression in the p-mini35S promoter-transformed tobacco leaves remained largely unchanged following NaCl or PEG stress treatments (Fig 8 and S3 Fig). These results suggest that the 71-bp (–219 to –148 bp) segment contains elements of salinity and osmotic stress responsiveness.
Fig 8. GUS transient assays in tobacco leaves.
(A) The plasmids used in the transient assay. The CaMV 35S represents the full-length 35S promoter; p-mini35S represents the truncated 35S (–46 to +10 bp) promoter. The test construct consisted of the p-71bp-mini35S, in which the 71-bp region (–219 to –148 bp) identified in the ZmGAPP promoter was fused to the p-mini35S promoter to drive the GUS expression. (B) GUS activity in the transiently transformed tobacco leaves with constructs p-mini35S and p-71bp-mini35S under both normal and 200 mM NaCl or 18% (w/v) PEG 6000 treatment for 24 h. Results are mean ± SD from three experiments (n = 15). Different lowercase letters above the bars indicate significant differences at P < 0.05.
The WT and CaMV 35S promoter-infiltrated tobacco leaves were also used as negative and positive controls; the CaMV 35S promoter-infiltrated tobacco leaves displayed strong GUS expression, whereas no GUS activity was detected in the WT tobacco leaves (S3 Fig).
Discussion
Plants have two phylogenetically distinct subclasses of H+-PPase. The genes encoding Type I H+-PPase have been characterized in several plant species. Its transcriptional expression was up-regulated by salinity and drought [43–47]. The TsVP1 (Type I H+-PPase gene) promoter from Thellungiella halophila displayed strong activity and NaCl stress inducibility [34]. Genetic manipulation of Type I H+-PPase in plants results in enhanced salinity or drought tolerance [43–56]. However, the genes encoding Type II H+-PPase have thus far been rarely examined. In previous studies, the ZmGAPP (Type II H+-PPase; GenBank accession no. EF051578) gene was cloned by our laboratory from maize, which shares only 39% of its amino acid sequence identity with that of maize VPP1 (Type I H+-PPase; GenBank accession no. AJ715528). The qRT-PCR analysis of ZmGAPP showed that its expression is up-regulated in multiple stress conditions, such as 200 mM NaCl and 18% PEG 6000 [59]. Bioinformatic analysis demonstrated that the upstream regulatory region of ZmGAPP contains potential cis-acting elements related to abiotic stresses, including salinity (GT1 motif) and drought (MBS), which may be involved in the induced expression of ZmGAPP (Fig 1 and Table 2). To understand the molecular basis of the stress response and identify ideal candidate promoters for the transgenic breeding of crop drought or salinity resistance, the ZmGAPP promoter was cloned, characterized, and functionally validated in this study.
The analysis of 5′ deleted mutants of the ZmGAPP promoter (D1–D9) under NaCl and PEG 6000 treatments revealed that a 71-bp sequence (–219 to –148 bp, the upstream of the translation initiation codon ATG) is the key region for ZmGAPP response to NaCl or PEG stress. GUS transient assay of leaves of 60-day-old tobacco plants displayed that this 71-bp sequence was sufficient for the response of NaCl or PEG stress. Bioinformatics analysis determined that the 71-bp (–219 to –148 bp) region contains a CAAT box, a TGACG motif and a GT1 motif (GAAAAA). The TGACG motif is a cis-acting element involved in MeJA-responsiveness, whereas the CAAT box is a common cis-acting element in promoter and enhancer regions that typically exhibits a putative effect in enhancing gene expression, resulting in the increment of p-71bp-mini35S promoter activity compared with p-mini35S under normal conditions (Fig 8B). Park et al. (2004) found that the transcription of SCaM-4 is dramatically induced by NaCl or pathogen treatment [67]. A GT-1 motif (GAAAAA) was ultimately identified as a core element responsible for the NaCl- or pathogen-induced expression of SCaM-4 in part by GT-1 interaction with AtGT-3b (an Arabidopsis GT-1-like transcription factor) in both soybean and Arabidopsis [67]. In our present study, a GT-1 motif (GAAAAA) also was identified within the ZmGAPP promoter between -219 and -148 bp. And the 71-bp (–219 to –148 bp) sequence has been shown to respond well to salt and osmotic stresses. Further detection whether GT-1 or other motif that have not been reported involved in stress inducible response of the 71-bp fragment and identification its interacting protein will provide a better understanding on the inducible gene expression of ZmGAPP during salt or osmotic stress.
Moreover, the deletion of the 189-bp (–1091 to –903 bp) sequence between D3 and D4 resulted in significant increase of GUS activity in leaves of transgenic tobacco plants (Fig 3). The results showed that the 189-bp sequence mediates transcriptional repression and contributes to relatively weak promoter activity of D1-D3 fragments in tobacco leaves. However, the 189-bp fragment does not appear to contain known cis-acting elements that inhibit gene expression by sequence analysis. Indeed, the silencers are generally varying in size and showing sequence degeneracy that make it difficult to recognize them in comparative analysis [8]. The activities of some well-studied plant silencer are associated with tissue specific expression, regulation by light, etc. Castresana et al. (1988) reported a A/T-rich DNA sequence that reduced the expression of photoregulated gene CAB in light in Nicotiana plumbaginifolia [68]. Delaney et al. (2007) identified an 84-bp A/T-rich sequence in the cotton FSltp4 promoter that suppressed the expression of FSltp4 in non-fiber tissues [69]. Lai et al. (2009) identified a 43-bp A/T-rich element in the AtKP1 promoter that mediated the transcriptional repression in both roots and leaves [70]. These A/T-rich DNA sequences usually mediate gene expression in plants [71], while no A/T-rich similar sequence is present in the 189-bp fragment. Further discovering the negatively regulatory element that located within the 189-bp (–1091 to –903 bp) sequence by deletion analysis and site-specific sequence mutation will promote our understanding on the transcriptional regulation of ZmGAPP.
Transgenic technology offers a powerful tool for gene function characterization and crop improvement [7], and appropriate promoter selection has become increasingly important for the successful application of transgenic technology [8]. Each additional transgene requires its own promoter, making it necessary to identify different promoters that achieve the same expression profile [6]. Cloning and identification of inducible or tissue-specific promoters would be of great practical value, as doing so would eliminate unnecessary burdens by restricting genetic expression to specific tissues or in response to specific environmental conditions [8, 72–74]. As such, the cloning and functional validation of salinity or drought-stress inducible promoters are therefore, of great importance to the effective management of salinity tolerance or drought resistance in commercial crops. In the current study, a 219-bp (D8) NaCl- and PEG-stress inducible core fragment extracted from the ZmGAPP regulatory region was identified by 5′ deleted mutant analysis. The GUS expression of D8 was highest in all tissues, with the exception of petals, among D1–D9 transgenic tobacco plants, which corresponds to about 10% and 25% of the CaMV 35S promoter under normal and NaCl- or PEG-stress conditions, respectively. The D8 fragment exhibited high promoter activity, especially under salt or osmotic stress, but was lower than that of the CaMV 35S promoter. It has been shown that excessive expression of transgenes in host plants may inhibit their growth and development, often resulting in host-plant morphological and physiological dysfunction [7, 8, 75]. Thus, the D8 fragment may be useful for moderating expression of transgenes and, more importantly, facilitates the expression of transgenes at desired levels under conditions of salt and osmotic stress.
Abiotic stress adaptability of crops is complex, and single transgene introductions may not be sufficient to improve crop stress resistance under natural conditions. Multiple-gene transformation is becoming routine in the genetic engineering of plants, as researchers strive for transgenic plants that present more complex and ambitious phenotypes [6]. However, expression of the multiple transgenes that are introduced into the host plants are regulated by the same promoter in the vector, which often results in homology dependent gene silencing [7, 76–78]. The D8 fragment is only 219 bp; such a small inducible promoter would be very useful in avoiding the repetitive usage of the same constitutive promoter and would reduce the vector size for plant genetic transformation [7], but also expresses target transgenes in an inducible manner, which will help to improve the adaptability of crops to adverse environmental conditions. It is also known that utilizing heterologous promoters to drive the expression of transgenes in host plants can help to prevent homology dependent gene silencing [79, 80]. Thus, this truncated 219-bp fragment (D8) of the maize promoter ZmGAPP could be used to confer high levels of gene expression and salinity or osmotic stress inducibility to transgenic tobacco plants, and as such, this monocot promoter fragment may be an ideal candidate for improving salinity or drought resistance in dicot crops. The transcriptional behave of promoters may have obvious difference in monocot and dicot. For example, CaMV 35S promoter displays strong transcriptional activity in dicot, while ubiquitin promoters are generally more capable of driving transgene expression in monocot [9, 81]. However, some promoters, such as 0.3 kb AtTCTP promoter [7], have strong transcriptional activity in both monocot and dicot. The D8 fragment derived from maize (monocot) displays high transcriptional activity in salinity and osmotic stresses inducible manner in tobacco (dicot). Further characterization of D8 promoter in monocot and evaluation of its application prospect in transgenic breeding of monocot crops will also be meaningful.
Conclusions
In the study, we identified and characterized a salinity or osmotic stress inducible promoter from maize Type-II H+-pyrophosphatase gene (ZmGAPP) in transgenic tobacco. By analyzing nine 5′ deleted mutants under normal and NaCl or PEG stress conditions, a 219-bp fragment (D8) of the ZmGAPP promoter was identified and functionally validated. This fragment may provide an efficient means of conferring high levels of inducible transgene expression (Fig 9). The use of alternative plant promoters suitable to the plant′s background and the type of transgenes utilized is essential for the stacking of multiple genes to avoid the homology dependent gene silencing that often occurs in transgenic plants. The novel D8 fragment isolated from monocot maize described in this study could therefore, be of great use in regulating gene expression in salinity or drought tolerance transgenic breeding of dicot crops based on its heterogeneous promoter activity and inducibility.
Fig 9. Diagrams of the D8 fragment of the ZmGAPP promoter and the 71-bp region for NaCl/PEG stress response.
Putative cis-regulatory elements in the 71-bp (–219 to –148 bp) sequence of the ZmGAPP promoter predicted by PlantCARE and PLACE are shown in the border. CAAT box: common cis-acting element in promoter and enhancer regions; TGACG motif: cis-acting element involved in MeJA-responsiveness; GT-1 motif: cis-acting element involved in pathogen- and NaCl-induced gene expression of SCaM-4 in soybean and Arabidopsis.
Furthermore, a 71-bp segment (–219 to –148 bp) of the ZmGAPP promoter was identified as the key region for the plant′s salinity and osmotic stress responsiveness (Fig 8), with analysis of GUS expression in transient transformed tobacco leaves revealing that the 71-bp segment was sufficient for the salinity or osmotic stress response.
Supporting Information
A series of 5′ deleted fragments of the ZmGAPP promoter were ligated into the upstream of the GUSA gene of the pCAMBIA1391Z vector (A). The numbers indicate the nucleotide position from the translational initiate codon ATG (A as +1). The fused plasmids were confirmed by restriction digestion analysis with HindШ/EcoR1 (B).
(TIF)
(A) Genomic PCR analysis of transformed plants using primers HPTFR (Table 1) designed for the hygromycin gene. DL2000 was the marker; + = the PCR result of pCAMBIA1391Z plasmid; CK = non-transformed plants; 1–11 = transformed tobacco plants. (B) GUS histochemical staining of transgenic plants. 35S = transgenic tobacco of the CaMV 35S promoter; D1–D9 = transgenic tobacco containing one of nine truncated promoter fragments.
(TIF)
The CaMV 35S represents full-length 35S promoter-driven GUS expression; p-mini35S represents the mini35S (–46 to +10 bp) promoter-driven GUS expression. The test construct p-71bp-mini35S, in which the 71-bp region (–219 to –148 bp) identified in the ZmGAPP promoter was fused to the p-mini35S promoter to drive the GUS expression. (A) GUS staining resulting from non-transformed tobacco leaves (WT) and the transient transformed tobacco leaves with constructs CaMV 35S, p-mini35S and p-71bp-mini35S under both normal and 200 mM NaCl-stress conditions for 24 h. (B) Histochemical GUS staining resulting from non-transformed tobacco leaves (WT) and the transient transformed tobacco leaves with constructs CaMV 35S, p-mini35S and p-71bp-mini35S under both normal and 18% (w/v) PEG 6000-stress conditions for 24 h.
(TIF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants from the National Program of Transgenic Variety Development of China (2014ZX0800922B), State Key Laboratory of Crop Biology (2015KF03) and Shandong Province Agricultural Seed Project of China (SDLZGC2014960302)
References
- 1.Agarwal PK, Shukla PS, Gupta K, Jha B. Bioengineering for salinity tolerance in plants: state of the art. Mol Biotechnol. 2013; 54: 102–123. 10.1007/s12033-012-9538-3 [DOI] [PubMed] [Google Scholar]
- 2.Golldack D, Li C, Mohan H, Probst N. Tolerance to drought and salt stress in plants: unraveling the signaling networks. Front Plant Sci. 2014; 5: 151 10.3389/fpls.2014.00151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaur C, Kumar G, Kaur S, Ansari MW, Pareek A, Sopory SK, et al. Molecular cloning and characterization of salt overly sensitive gene promoter from Brassica juncea (BjSOS2). Mol Biol Rep. 2015; 42: 1139–1148. 10.1007/s11033-015-3851-4 [DOI] [PubMed] [Google Scholar]
- 4.Tester M, Langridge P. Breeding technologies to increase crop production in a changing world. Science. 2010; 327: 818–822. 10.1126/science.1183700 [DOI] [PubMed] [Google Scholar]
- 5.Halpin C. Gene stacking in transgenic plants–the challenge for 21st century plant biotechnology. Plant Biotechnol J. 2005; 3: 141–155. [DOI] [PubMed] [Google Scholar]
- 6.Peremarti A, Twyman RM, Go´mez-Galera S, Naqvi S, Farre´ G, Sabalza M, et al. Promoter diversity in multigene transformation. Plant Mol Biol. 2010; 73: 363–378. 10.1007/s11103-010-9628-1 [DOI] [PubMed] [Google Scholar]
- 7.Han YJ, Kim YM, Hwang OJ, Kim JI. Characterization of a small constitutive promoter from Arabidopsis translationally controlled tumor protein (AtTCTP) gene for plant transformation. Plant Cell Rep. 2015; 34: 265–275. 10.1007/s00299-014-1705-5 [DOI] [PubMed] [Google Scholar]
- 8.Potenza C, Aleman L, Sengupta-Gopalan C. Targeting transgene expression in research, agricultural, and environmental applications: promoters used in plant transformation. In Vitro Cell Dev-Pl. 2004; 40: 1–22. [Google Scholar]
- 9.Tao YB, He LL, Niu LJ, Xu ZF. Isolation and characterization of an ubiquitin extension protein gene (JcUEP) promoter from Jatropha curcas. Planta. 2015; 241: 823–836. 10.1007/s00425-014-2222-z [DOI] [PubMed] [Google Scholar]
- 10.Cornejo MJ, Luth D, Blankenship KM, Anderson OD, Blechl AE. Activity of a maize ubiquitin promoter in transgenic rice. Plant Mol Biol. 1993; 23: 567–581. [DOI] [PubMed] [Google Scholar]
- 11.Odell JT, Nagy F, Chua NH. Identification of DNA sequences required for activity of the cauliflower mosaic virus 35S promoter. Nature. 1985; 313: 810–812. [DOI] [PubMed] [Google Scholar]
- 12.Cheon BY, Kim HJ, Oh KH, Bahn SC, Ahn JH, Choi JW, et al. Overexpression of human erythropoietin (EPO) affects plant morphologies: retarded vegetative growth in tobacco and male sterility in tobacco and Arabidopsis. Transgenic Res. 2004; 13: 541–549. [DOI] [PubMed] [Google Scholar]
- 13.Zhao J, Ren W, Zhi D, Wang L, Xia G. Arabidopsis DREB1A/CBF3 bestowed transgenic tall fescue increased tolerance to drought stress. Plant Cell Rep. 2007; 26: 1521–1528. [DOI] [PubMed] [Google Scholar]
- 14.Kasuga M, Miura S, Shinozaki K, Yamaguchi-Shinozaki K. A Combination of the Arabidopsis DREB1A Gene and Stress-Inducible rd29A Promoter Improved Drought- and Low-Temperature Stress Tolerance in Tobacco by Gene Transfer. Plant Cell Physiol. 2004; 45: 346–350. [DOI] [PubMed] [Google Scholar]
- 15.Kasuga M, Liu Q, Miura S, Yamaguchi-Shinozaki K, Shinozaki K. Improving plant drought, salt and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat Biotechnol. 1999; 17: 287–291. [DOI] [PubMed] [Google Scholar]
- 16.Furtado A, Henry RJ. The wheat Em promoter drives reporter gene expression in embryo and aleurone tissue of transgenic barley and rice. Plant Biotechnol J. 2005; 3: 421–434. [DOI] [PubMed] [Google Scholar]
- 17.Opsahl-Sorteberg HG, Divon HH, Nielsen PS, Kalla R, Hammon-Kosach M, Shimamoto K, et al. Identification of a 49-bp fragment of the HvLTP2 promoter directing aleurone cell specific expression. Gene. 2004; 341: 49–58. [DOI] [PubMed] [Google Scholar]
- 18.Pan Y, Ma X, Liang H, Zhao Q, Zhu D, Yu J. Spatial and temporal activity of the foxtail millet (Setaria italica) seed-specific promoter pF128. Planta. 2015; 241: 57–67. 10.1007/s00425-014-2164-5 [DOI] [PubMed] [Google Scholar]
- 19.Qu LQ, Takaiwa F. Evaluation of tissue specificity and expression strength of rice seed component gene promoters in transgenic rice. Plant Biotechnol J. 2004; 2: 113–125. [DOI] [PubMed] [Google Scholar]
- 20.Jeon JS, Chung YY, Lee S, Yi GH, Oh BG, An G. Isolation and characterization of an anther-specific gene, RA8, from rice (Oryza sativa, L). Plant Mol Biol. 1999; 39: 35–44. [DOI] [PubMed] [Google Scholar]
- 21.Koltunow AM, Truettner J, Cox KH, Walroth M, Goldberg RB. Different temporal and spatial gene expression patterns occur during anther development. Plant Cell. 1990; 2: 1201–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paul W, Hodge R, Smartt S, Draper J, Scott R. The isolation and characterization of the tapetum specific Arabidopsis thaliana A9 gene. Plant Mol Biol. 1992; 19: 611–622. [DOI] [PubMed] [Google Scholar]
- 23.Chen A, Zhong N, Qu Z, Wang F, Liu N, Xia G. Root and vascular tissue-specific expression of glycine-rich protein AtGRP9 and its interaction with AtCAD5, a cinnamyl alcohol dehydrogenase, in Arabidopsis thaliana. J Plant Res. 2007; 120: 337–343. [DOI] [PubMed] [Google Scholar]
- 24.Chen L, Jiang B, Wu C, Sun S, Hou W, Han T. The characterization of GmTIP, a root-specific gene from soybean, and the expression analysis of its promoter. Plant Cell Tiss Organ Cult. 2015; 121: 259–274. [Google Scholar]
- 25.Huda KM, Banu MS, Pathi KM, Tuteja N. Reproductive organ and vascular specific promoter of the rice plasma membrane Ca2+ ATPase mediates environmental stress responses in plants. PLoS One. 2013; 8: e57803 10.1371/journal.pone.0057803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamamoto YT, Taylor CG, Acedo GN, Cheng CL, Conkling MA. Characterization of cis-acting sequences regulating root-specific gene expression in tobacco. Plant Cell. 1991; 3: 371–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carre IA, Kay SA. Multiple DNA–protein complexes at a circadian regulated promoter element. Plant Cell. 1995; 7: 2039–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marraccini P, Courjault C, Caillet V, Lausanne F, Lepage B, Rogers W J, et al. Rubisco small subunit of Coffea Aabica: cDNA sequence, gene cloning and promoter analysis in transgenic tobacco plants. Plant Physiol Biochem. 2003; 41: 17–25. [Google Scholar]
- 29.Thilmony R, Guttman M, Thomson JG, Blechl AE. The LP2 leucine-rich repeat receptor kinase gene promoter directs organ-specific, light-responsive expression in transgenic rice. Plant Biotechnol J. 2009; 7: 867–882. 10.1111/j.1467-7652.2009.00449.x [DOI] [PubMed] [Google Scholar]
- 30.Bang SW, Park SH, Jeong JS, Kim YS, Jung H, Ha SH, et al. Characterization of the stress-inducible OsNCED3 promoter in different transgenic rice organs and over three homozygous generations. Planta. 2013; 237: 211–224. 10.1007/s00425-012-1764-1 [DOI] [PubMed] [Google Scholar]
- 31.Shinozaki Y, Shinozaki K. Characterization of the expression of a desiccation-responsive rd29 gene of Arabidopsis thaliana and analysis of its promoter in transgenic plants. Mol Gen Genet. 1993; 236: 331–340. [DOI] [PubMed] [Google Scholar]
- 32.Tavakol E, Sardaro ML, Shariati JV, Rossini L, Porceddu E. Promoter analysis and expression profile of in response to drought stress in wheat ancestors. Gene. 2014; 549: 24–32. 10.1016/j.gene.2014.07.020 [DOI] [PubMed] [Google Scholar]
- 33.Rai M, He C, Wu R. Comparative functional analysis of three abiotic stress-inducible promoters in transgenic rice. Transgenic Res. 2009; 18: 787–799. 10.1007/s11248-009-9263-2 [DOI] [PubMed] [Google Scholar]
- 34.Sun QH, Gao F, Zhao L, Li KP, Zhang JR. Identification of a new 130 bp cis-acting element in the TsVP1 promoter involved in the salt stress response from Thellungiella halophila. BMC Plant Biol. 2010; 10: 90 10.1186/1471-2229-10-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Yin H, Li D, Zhu W, Li Q. Functional analysis of BADH gene promoter from Suaeda liaotungensis K. Plant Cell Rep. 2008; 27: 585–592. [DOI] [PubMed] [Google Scholar]
- 36.Vijayan J, Devanna BN, Singh NK, Sharma TR. Cloning and functional validation of early inducible Magnaporthe oryzaere sponsive CYP76M7 promoter from rice. Front Plant Sci. 2015; 6: 371 10.3389/fpls.2015.00371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ballas N, Wong L, Ke M, Theologis A. Two auxin-responsive domains interact positively to induce expression of the early indoleacetic acid-inducible gene PS-IAA4/5. PNAS. 1995; 92: 3483–3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Y, Tan BA, Distal AB. A responsive element in AtNCED3 promoter is required for positive feedback regulation of ABA biosynthesis in Arabidopsis. PLoS One. 2014; 9: e87283 10.1371/journal.pone.0087283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gurr SJ, Rushton PJ. Engineering plants with increased disease resistance: how are we going to express it? Trends Biotech. 2005; 23: 283–290. [DOI] [PubMed] [Google Scholar]
- 40.Maeshima M. Vacuolar H+-pyrophosphatase, Biochim Biophys Acta 2000; 1465: 37–51. [DOI] [PubMed] [Google Scholar]
- 41.Rea PA, Poole RJ. Vacuolar H+-translocating pyrophosphatase. Annu Rev Plant Physiol Plant Mol Biol. 1993; 44: 157–180. [Google Scholar]
- 42.Drozdowicz YM, Rea PA. Vacuolar H+-pyrophosphatases: From the evolutionary backwaters into the mainstream. Trends Plant Sci. 2001; 6: 206–211. [DOI] [PubMed] [Google Scholar]
- 43.Gao F, Gao Q, Duan X, Yue G, Yang A, Zhang J. Cloning of an H+-PPase gene from Thellungiella halophila and its heterologous expression to improve tobacco salt tolerance. J Exp Bot. 2006; 57: 3259–3270. [DOI] [PubMed] [Google Scholar]
- 44.Guo S, Yin H, Zhang X, Zhao F, Li P, Chen S, et al. Molecular cloning and characterization of a vacuolar H+-pyrophosphatase gene, SsVP, from the halophyte Suaeda salsa and its overexpression increases salt and drought tolerance of Arabidopsis, Plant Mol Biol. 2006; 60: 41–50. [DOI] [PubMed] [Google Scholar]
- 45.Li X, Guo C, Gu J, Duan W, Zhao M, Ma C, et al. Overexpression of VP, a vacuolar H+-pyrophosphatase gene in wheat (Triticum aestivum L.), improves tobacco plant growth under Pi and N deprivation, high salinity, and drought. J Exp Bot. 2014; 65: 683–696. 10.1093/jxb/ert442 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Liu L, Wang Y, Wang N, Dong YY, Fan XD, Liu XM, et al. Cloning of a vacuolar H+-pyrophosphatase gene from the Halophyte Suaeda corniculata whose heterologous overexpression improves salt, saline-alkali and drought tolerance in Arabidopsis. J Integrative Plant Biol. 2011; 53: 731–742. [DOI] [PubMed] [Google Scholar]
- 47.Lv S, Jiang P, Nie L, Chen X, Tai F, Wang D, et al. H+-pyrophosphatase from Salicornia europaea confers tolerance to simultaneously occurring salt stress and nitrogen deficiency in Arabidopsis and wheat. Plant Cell Environ. 2015; 38: 2433–2449. 10.1111/pce.12557 [DOI] [PubMed] [Google Scholar]
- 48.Gaxiola RA, Li JS, Undurraga S, Dang LM, Allen GJ, Alper SL, et al. Drought- and salt-tolerant plants result from overexpression of theAVP1 H+-pump. PNAS. 2001; 98: 11444–11449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li B, Wei A, Song C, Li N, Zhang J. Heterologous expression of the TsVP gene improves the drought resistance of maize. Plant Biotechnol J. 2008; 6: 146–159. [DOI] [PubMed] [Google Scholar]
- 50.Wu GQ, Feng RJ, Wang SM, Wang CM, Bao AK, Wei L, et al. Co-expression of xerophyte Zygophyllum xanthoxylum ZxNHX and ZxVP1-1 confers enhanced salinity tolerance in chimeric sugar beet (Betavulgaris L.). Front Plant Sci. 2015; 6: 581 10.3389/fpls.2015.00581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lv S, Zhang KW, Gao Q, Lian LJ, Song YJ, Zhang JR. Overexpression of an H+-PPase gene from Thellungiella halophila in cotton enhances salt tolerance and improves growth and photosynthetic performance. Plant Cell Physiol. 2008; 49: 1150–1164. 10.1093/pcp/pcn090 [DOI] [PubMed] [Google Scholar]
- 52.Lv SL, Lian LJ, Tao PL, Li ZX, Zhang KW, Zhang JR. Overexpression of Thellungiella halophila H+-PPase (TsVP) in cotton enhances drought stress resistance of plants. Planta. 2009; 229: 899–910. 10.1007/s00425-008-0880-4 [DOI] [PubMed] [Google Scholar]
- 53.Pasapula V, Shen G, Kuppu S, Paez-Valencia J, Mendoza M, Hou P, et al. Expression of an Arabidopsis vacuolar H+-pyrophosphatase gene (AVP1) in cotton improves drought- and salt tolerance and increases fibre yield in the field conditions. Plant Biotechnol J. 2011; 9: 88–99. 10.1111/j.1467-7652.2010.00535.x [DOI] [PubMed] [Google Scholar]
- 54.Park S, Li J, Pittman JK, Berkowitz GA, Yang H, Undurraga S, et al. Up-regulation of a H+-pyrophosphatase (H+-PPase) as a strategy to engineer drought-resistant crop plants. PNAS. 2005; 102: 18830–18835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bao AK, Wang SM, Wu GQ, Xi JJ, Zhang JL, Wang CM. Overexpression of the Arabidopsis H+-PPase enhanced resistance to salt and drought stress in transgenic alfalfa (Medicago sativa L.). Plant Sci. 2009; 176: 232–240. [Google Scholar]
- 56.Li Z., Baldwin CM, Hu Q, Liu H, Luo H. Heterologous expression of Arabidopsis H+-pyrophosphatase enhances salt tolerance in transgenic creeping bentgrass (Agrostis stolonifera L.). Plant Cell Environ. 2010; 33: 272–289. 10.1111/j.1365-3040.2009.02080.x [DOI] [PubMed] [Google Scholar]
- 57.Drozdowicz YM, Kissinger JC, Rea PA. AVP2, a sequence-divergent, K+-insensitive H+-translocating inorganic pyrophosphatase from Arabidopsis thaliana. Plant Physiol. 2000; 123: 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mitsuda N, Enami K, Nakata M, Takeyasu K, Sato MH. Novel type Arabidopsis thaliana H+-PPase is localized to the Golgi apparatus. FEBS Lett. 2001; 488: 29–33. [DOI] [PubMed] [Google Scholar]
- 59.Yue G, Sui Z, Gao Q, Zhang J. Molecular cloning and characterization of a novel H+-translocating pyrophosphatase gene in Zea mays, DNA Seq. 2008; 19: 79–86. [DOI] [PubMed] [Google Scholar]
- 60.Higo K, Ugawa Y, Iwamoto M, Korenaga T. Plant cis-acting regulatory DNA elements (PLACE) database. Nucleic Acids Res. 1999; 27: 297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, et al. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002; 30: 325–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Voelker T, Sturm A, Chrispeels MJ. Differences in expression between two seed lectin alleles obtained from normal and lectindeficient beans are maintained in transgenic tobacco. EMBO J. 1987; 6: 3571–3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 64.Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987; 6: 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Ann Biochem. 1976; 72: 248–254. [DOI] [PubMed] [Google Scholar]
- 66.Yang Y, Li R, Qi M. In vivo analysis of plant promoters and transcription factors by agroinfiltration of tobacco leaves. Plant J. 2000; 22, 543–551. [DOI] [PubMed] [Google Scholar]
- 67.Park HC, Kim ML, Kang YH, Jeon JM, Yoo JH, Kim MC, et al. Pathogen- and NaCl-induced expression of the SCaM-4 promoter is mediated in part by a GT-1 box that interacts with a GT-1-like transcription factor. Plant Physiol. 2004; 135: 2150–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Castresana C, Garcia-Luque I, Alonso E, Malik VS, Cashmore AR. Both positive and negative regulatory elements mediate expression of a photoregulated CAB gene from Nicotiana plumbaginifolia. EMBO J. 1988; 7: 1929–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Delaney SK, Orford SJ, Martin-Harris M, Timmis JN. The fiber specificity of the cotton FSltp4 gene promoter is regulated by an AT-rich promoter region and the AT-hook transcription factor GhAT1. Plant Cell Physiol. 2007; 48: 1426–1437. [DOI] [PubMed] [Google Scholar]
- 70.Lai C, Xiong J, Li X, Qin X. A 43-bp A/T-rich element upstream of the kinesin gene AtKP1 promoter functions as a silencer in Arabidopsis. Plant Cell Rep. 2009; 28: 851–860. 10.1007/s00299-009-0689-z [DOI] [PubMed] [Google Scholar]
- 71.Laursen NB, Larsen K, Knudsen JY, Hoffmann HJ, Poulsen C, Marcker KA, et al. A protein binding AT-rich sequence in the soybean leghemoglobin c3 promoter is a general cis element that requires proximal DNA elements to stimulate transcription. Plant Cell. 1994; 6: 659–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hsieh TH, Lee JT, Charng YY, Chan MT. Tomato plants ectopically expressing Arabidopsis CBF1 show enhanced resistance to water deficit stress. Plant Physiol. 2002; 130: 618–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee JT, Prasad V, Yang PT, Wu JF, David Ho TH, Charng YY, et al. Expression of Arabidopsis CBF1 regulated by an ABA/stress inducible promoter in transgenic tomato confers stress tolerance without affecting yield, Plant cell environ. 2003; 26: 1181–1190. [Google Scholar]
- 74.Zavallo D, Bilbao ML, Hopp HE, Heinz R. Isolation and functional characterization of two novel seed-specific promoters from sunflower (Helianthus annuus L.). Plant Cell Rep. 2010; 29: 239–248. 10.1007/s00299-010-0816-x [DOI] [PubMed] [Google Scholar]
- 75.Zhou J, Yang Y, Wang X, Yu F, Yu C, Chen J, et al. Enhanced transgene expression in rice following selection controlled by weak promoters. BMC Biotechnol. 2013; 13: 29 10.1186/1472-6750-13-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.De Wilde C, Van Houdt H, De Buck S, Angenon G, De Jaeger G, Depicker A. Plants as bioreactors for protein production: avoiding the problem of transgene silencing. Plant Mol Biol. 2000; 43: 347–359. [DOI] [PubMed] [Google Scholar]
- 77.Matzke MA, Matzke AJ. Homology-dependent gene silencing in transgenic plants: What does it really tell us? Trends Genet. 1995; 11: 1–3. [DOI] [PubMed] [Google Scholar]
- 78.Verdaguer B, de Kochko A, Beachy RN, Fauquet C. Isolation and expression in transgenic tobacco and rice plants, of the cassava vein mosaic virus (CVMV) promoter. Plant Mol Biol. 1996; 31: 1129–1139. [DOI] [PubMed] [Google Scholar]
- 79.Dong Q, Jiang H, Xu Q, Li X, Peng X, Yu H, et al. Cloning and characterization of a multifunctional promoter from Maize (Zea mays L.). Appl Biochem Biotechnol. 2015; 175: 1344–1357. 10.1007/s12010-014-1277-4 [DOI] [PubMed] [Google Scholar]
- 80.Kumpatla SP, Chandrasekharan MB, Lyer LM. Genome intruder scanning and modulation systems and transgene sileneing. Trends Plant Sci. 1998; 3: 96–104. [Google Scholar]
- 81.Schledzewski K, Mendel RR. Quantitative transient gene expression: comparison of the promoters for maize polyubiquitin1, rice actin1, maize-derived Emu and CaMV35S in cells of barley, maize and tobacco. Transgenic Res. 1994; 3: 249–255. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A series of 5′ deleted fragments of the ZmGAPP promoter were ligated into the upstream of the GUSA gene of the pCAMBIA1391Z vector (A). The numbers indicate the nucleotide position from the translational initiate codon ATG (A as +1). The fused plasmids were confirmed by restriction digestion analysis with HindШ/EcoR1 (B).
(TIF)
(A) Genomic PCR analysis of transformed plants using primers HPTFR (Table 1) designed for the hygromycin gene. DL2000 was the marker; + = the PCR result of pCAMBIA1391Z plasmid; CK = non-transformed plants; 1–11 = transformed tobacco plants. (B) GUS histochemical staining of transgenic plants. 35S = transgenic tobacco of the CaMV 35S promoter; D1–D9 = transgenic tobacco containing one of nine truncated promoter fragments.
(TIF)
The CaMV 35S represents full-length 35S promoter-driven GUS expression; p-mini35S represents the mini35S (–46 to +10 bp) promoter-driven GUS expression. The test construct p-71bp-mini35S, in which the 71-bp region (–219 to –148 bp) identified in the ZmGAPP promoter was fused to the p-mini35S promoter to drive the GUS expression. (A) GUS staining resulting from non-transformed tobacco leaves (WT) and the transient transformed tobacco leaves with constructs CaMV 35S, p-mini35S and p-71bp-mini35S under both normal and 200 mM NaCl-stress conditions for 24 h. (B) Histochemical GUS staining resulting from non-transformed tobacco leaves (WT) and the transient transformed tobacco leaves with constructs CaMV 35S, p-mini35S and p-71bp-mini35S under both normal and 18% (w/v) PEG 6000-stress conditions for 24 h.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.