Abstract
Histiocytoid Sweet syndrome (H-SS) is a histological variant of Sweet syndrome (SS) differing from classical neutrophilic SS (N-SS) by a dermal infiltrate mainly composed of lymphocytes and histiocytoid myeloperoxidase-positive cells. We aimed to report a large series of H-SS and compare the frequency and type of hematological malignancies associated to H-SS and N-SS. We included 62 patients with a coding histopathologic diagnosis of SS prospectively registered between 2005 and 2014 in the database of our Department of Pathology. Overall, 22 (35.5%) and 40 (64.5%) patients had a histological diagnosis of H-SS and N-SS, respectively. Median age, sex ratio, and cutaneous lesions were similar in the 2 groups. The frequency of extra-cutaneous manifestations was similar (50% vs 37.5%, P = 0.42). Recurrent forms were significantly more frequent in H-SS than in N-SS patients (21% vs 2.5%, P = 0.01). A hematological malignancy was diagnosed in 22 patients, 12 (55.5%) with H-SS and 10 (25%) with N-SS (P = 0.019). Hematological malignancy was of myeloid origin in 8/22 (36.3%) H-SS and 5/40 (12.5%) N-SS patients (P = 0.02), and of lymphoid origin without myeloid component in 4/22 (18.1%) H-SS and 4/40 (10%) N-SS patients (P = 0.35), respectively. One N-SS patient had a hematological malignancy of mixed (myeloid and lymphoid) phenotype. A myelodysplastic syndrome (MDS) was diagnosed in 7/22 (31.8%) H-SS and 1/40 (2.5%) N-SS patients (P < 0.001). Hematological disease was diagnosed before (in 8 H-SS and 3 N-SS patients) or at the time of the occurrence of the cutaneous lesions (in 1 H-SS and 7 N-SS patients). However, in 3 H-SS patients, all with MDS, cutaneous lesions preceded the hematological disease by ≤6 months. In conclusion, H-SS was associated with MDS in one third of patients but also with lymphoid malignancies, and cutaneous lesions could precede the hematological diagnosis in patients with MDS. A complete hematological assessment is mandatory at diagnosis, and monitoring blood cell counts should be recommended for at least 6 months after the diagnosis of H-SS.
INTRODUCTION
Sweet syndrome (SS) or acute febrile neutrophilic dermatosis was first described by Sweet, in 1964.1 SS has 3 main presentations: classical SS (idiopathic or associated with various inflammatory disorders), malignancy-associated SS, and drug-induced SS (mainly described with granulocyte or granulocyte-macrophage growth factors).2,3 In 20% to 30% of cases, patients show associated malignancies, mainly hematological disorders of myeloid lineage (i.e., acute myeloid leukemia [AML] and myelodysplastic syndrome [MDS]). Less frequently, associated lymphoid neoplasms such as chronic lymphocytic leukemia (CLL) and multiple myeloma were reported.4–6
Dermal neutrophilic infiltrates without vasculitis are considered the salient histological feature of SS. However, several studies described some cases with associated cellular components, such as lymphocytes and mononuclear cells of uncertain lineage but resembling histiocytes and showing myeloperoxidase expression.7–10 This histological variant was thus called “lymphocytic SS”10 or “histiocytoid SS”9 because of no consensus on the name of this SS variant to date.11–14 In a series of 9 patients, Vignon-Pennamen et al10 suggested that this histological variant could be more specifically related to MDS and AML, but only a few data regarding this association are available.
The aims of our study were to describe the epidemiological and clinical features of a series of patients with the “histiocytoid” histological variant of SS (histiocytoid Sweet syndrome, [H-SS]) and compare the frequency and type of hematological malignancies associated with H-SS and with the classical neutrophilic variant (neutrophilic Sweet syndrome [N-SS] ) in a monocentric series of SS patients.
PATIENTS AND METHODS
We retrospectively included all patients with a coding histopathologic diagnosis of SS prospectively registered between January 2005 and December 2014 in the database of the Department of Pathology of Henri Mondor Hospital, Creteil, France. Two groups of patients were defined according to the histopathologic aspect of skin biopsies in terms of previous available descriptions,7–11 the H-SS group and N-SS group. Skin biopsy specimens of H-SS and N-SS were systematically reviewed by the same pathologist (NO): H-SS was diagnosed according to Requena et al9 and Vignon-Pennamen et al10 criteria. We included patients with skin biopsies showing at histological evaluation dermal inflammatory infiltrates with inconspicuous epidermal inflammatory changes mainly composed of lymphocytes and mononuclear cells comprising CD68+ histiocytoid cells with twisted or kidney-shaped, basophilic or vesicular nuclei, with single inconspicuous nucleoli and scant, slightly eosinophilic cytoplasm expressing myeloperoxidase, and showing only few scattered neutrophils and nuclear debris (Figure 1). N-SS diagnosis was retained when we observed dermal inflammatory infiltrates mainly composed of polymorphonuclear neutrophils infiltrates with nuclear debris as previously described.1
FIGURE 1.
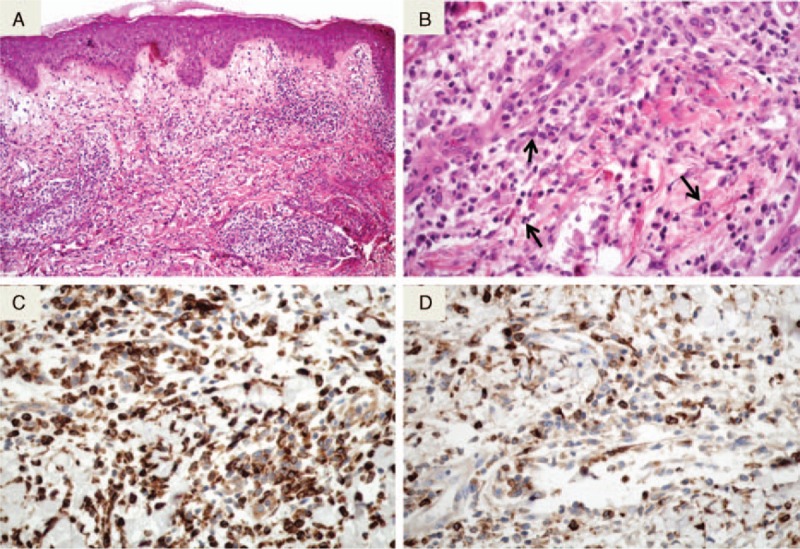
Histological aspect of H-SS. A: As in the classical form of SS (N-SS), H-SS features edema of the papillary dermis and diffuse and interstitial dermal infiltrates (HES original magnification ×100). B: The infiltrate consists of lymphocytes and histiocytoid cells. Some cells are suggestive of myeloid progenitors (HES original magnification ×400). C: The infiltrate strongly express the CD68 monocyte marker (immunohistochemistry revealed by diaminobenzidine). D: A proportion of mononuclear cells express the myeloperoxidase (immunohistochemistry revealed by diaminobenzidine). SS = Sweet syndrome, HES = hematoxylin, eosin ans saffron, H-SS = histiocytoid Sweet syndrome, N-SS = neutrophilic Sweet syndrome.
In both H-SS and N-SS groups, epidemiological and clinical data were collected from medical charts: gender, age at diagnosis of SS, clinical presentation, treatment of cutaneous lesions, and follow-up after SS diagnosis. Available clinical pictures were reviewed by trained dermatologists (LG, SO, and OC). A history of hematological disease was systematically investigated in the medical file of each patient. For patients with short follow-up after the diagnosis of SS (<2 months), data on outcome (recurrence of SS, occurrence or relapse of hematological disease, and survival) were updated by phone in June 2015.
The type of hematological malignancies were systematically reviewed according to the 2008 World Health Organization (WHO) classification of lymphoid and myeloid neoplasms15,16 by the same hematologist (AT).
The study was approved by the local Review Board (Comité de Protection des Personnes Ile-de-France X no: 2014-12-01).
Data are reported as median [range] for continuous variables and number (percentages) otherwise. Chi-square test was used to compare patient characteristics and the occurrence of hematological malignancies in the 2 groups of patients (StatView 5.0, StatView – SAS Institute Inc. Software Informer; www.statview.software.informer.com: STATVIEW.EXE), with P < 0.05 considered as significant.
RESULTS
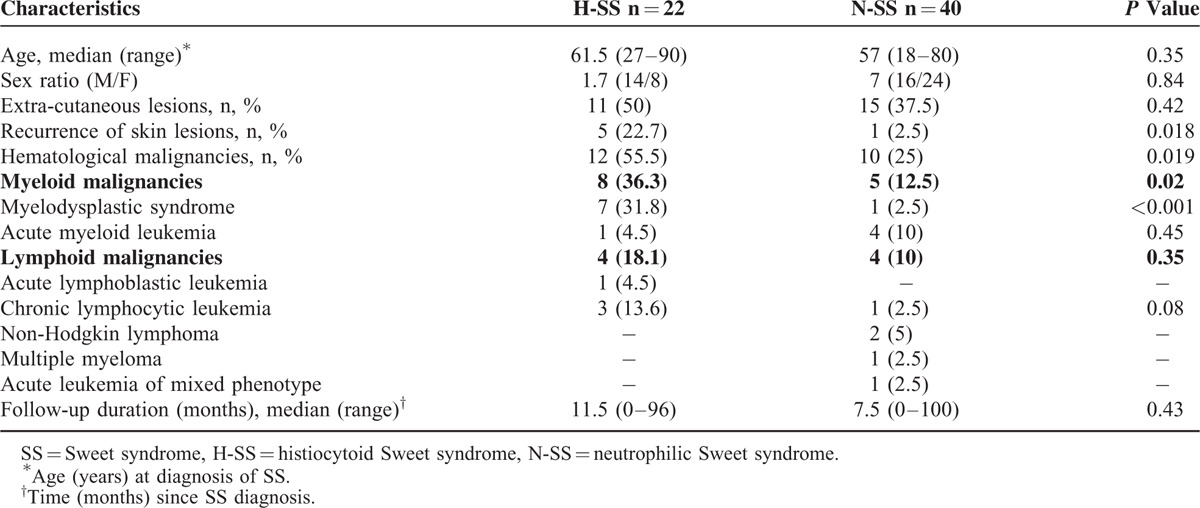
Between January 2005 and December 2014, among 62 patients with a diagnosis of SS, 22 (35.5%) and 40 (64.5%) had a histological diagnosis of H-SS and N-SS, respectively. The 2 groups did not differ in age or sex ratio (Table 1).
TABLE 1.
Patient Characteristics and Associated Hematological Malignancies in 62 Patients With H-SS and N-SS
Clinical Presentation of H-SS
Cutaneous lesions of H-SS patients were mostly similar to those with classical N-SS: they were multiple in all but 1 case, located on arms, hands, face, and trunk, and made of edematous, erythematous, infiltrative plaques or nodules, some extensive or confluent, with atypical annular disposition (Figure 2A–C). Extracutaneous manifestations (i.e., fever [n = 10], arthritis [n = 4], and episcleritis [n = 3], Figure 2D) and diarrhea (n = 1) were as frequent in H-SS as N-SS (Table 1).
FIGURE 2.
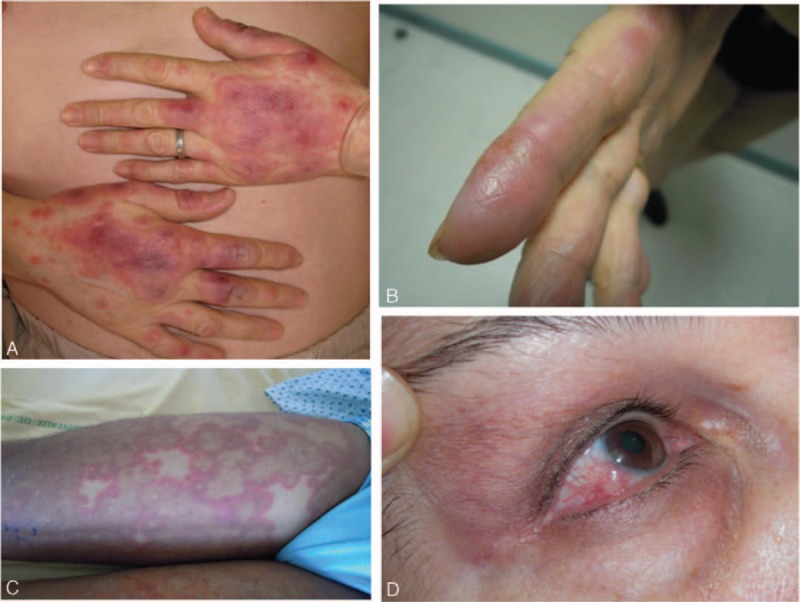
Clinical lesions in patients with histiocytoid Sweet syndrome (H-SS): (A) typical widespread erythematous and tender nodules; (B) frequent involvement of hands and fingers; (C) some patients had more atypical widespread annular lesions; and (D) eye involvement (episcleritis).
SS-Associated Hematologic Malignancies
In total, 12/22 (55.5%) H-SS patients and 10/40 (25%) N-SS patients had an associated diagnosis of hematological malignancy (P = 0.019) (Table 1). H-SS was associated with MDS (n = 7), AML (n = 1), acute lymphoblastic leukemia (n = 1), and CLL (n = 3). N-SS was associated with MDS (n = 1), AML (n = 4), CLL (n = 1), non-Hodgkin lymphoma (n = 2), multiple myeloma (n = 1), and acute leukemia of mixed phenotype (n = 1) (Table 1, Figure 3).
FIGURE 3.
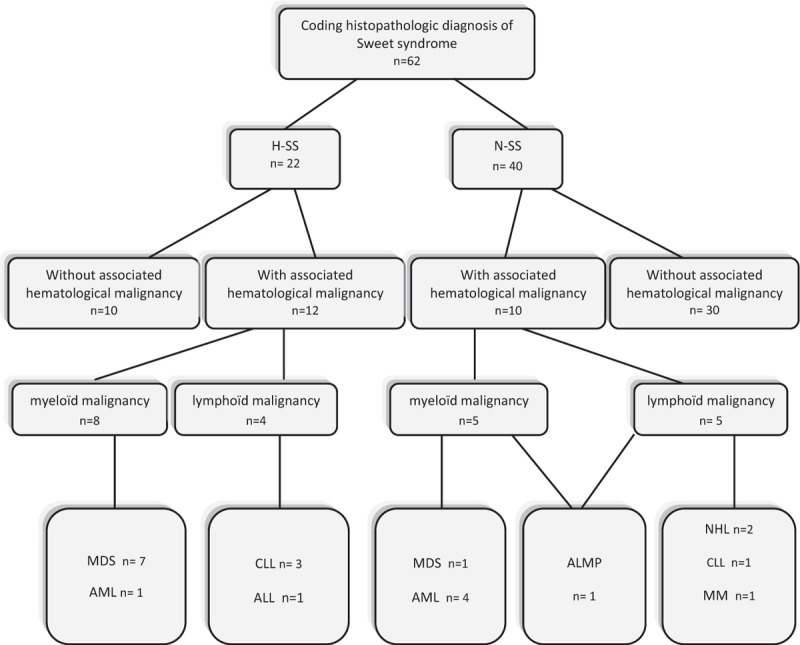
Flow chart of the study and type of hematological malignancies associated with Sweet syndrome in 62 patients.
Diagnosis and status of hematological disease at SS occurrence time are described in Table 2. For the 22 patients with SS associated to a hematological malignancy (12 H-SS and 10 N-SS), the median age was 60 years (range 27–89) and sex ratio (M/F) was 14/8 (1.75), with no difference between H-SS and N-SS groups (data not shown). Overall, 8/22 (36.3%) and 5/40 (12.5%) of H-SS and N-SS patients, respectively, presented a myeloid malignancy (P = 0.02), whereas 4/22 (18.1%) and 4/40 (10%) H-SS and N-SS patients, respectively, presented a lymphoid malignancy without myeloid component (P = 0.35, Table 1).
TABLE 2.
Hematological Diagnosis and Disease Status in Patients With H-SS and N-SS
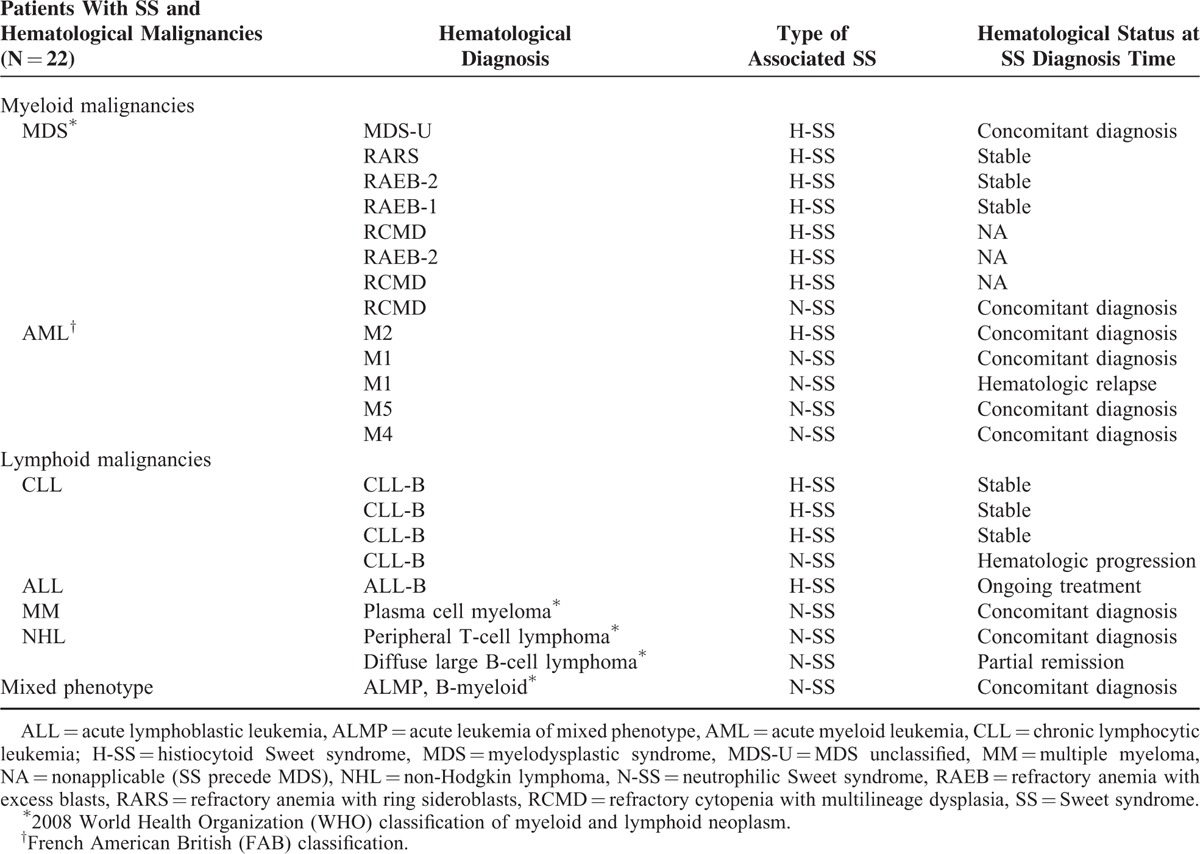
In all, 31.8% (7/22) and 2% (1/40) of H-SS and N-SS patients, respectively, had an associated diagnosis of MDS (P < 0.001), with no other significant difference between the 2 groups regarding associated hematological malignancies (Table 1).
Histological Features of H-SS (Figure 1)
Papillary dermal edema was present as well in idiopathic H-SS (5/10, 50%) than in H-SS associated to hematological malignancies (7/12, 58%). However, this inflammatory feature seemed less often encountered in H-SS with MDS than in H-SS associated to other hematological malignancies (3/7, 43% and 4/5, 80%, respectively).
Dermal infiltrate was organized in confluent clusters in 7/10 (70%) and/or dense infiltrate in 3/10 (30%) cases of idiopathic H-SS, compared to 4/12 (33%) and 7/12 (58%) cases, respectively, in samples of H-SS with underlying hematological malignancies. However, 1/7 (14%) of H-SS associated to MDS samples showed dispersed dermal histiocytoid cells which were not evidenced in H-SS with non-MDS malignancies or in idiopathic H-SS samples (Table 3).
TABLE 3.
Histopathological and Immunohistochemical Features of H-SS Cases Associated With MDS, Another Hematological Disorder or No Underlying Disease
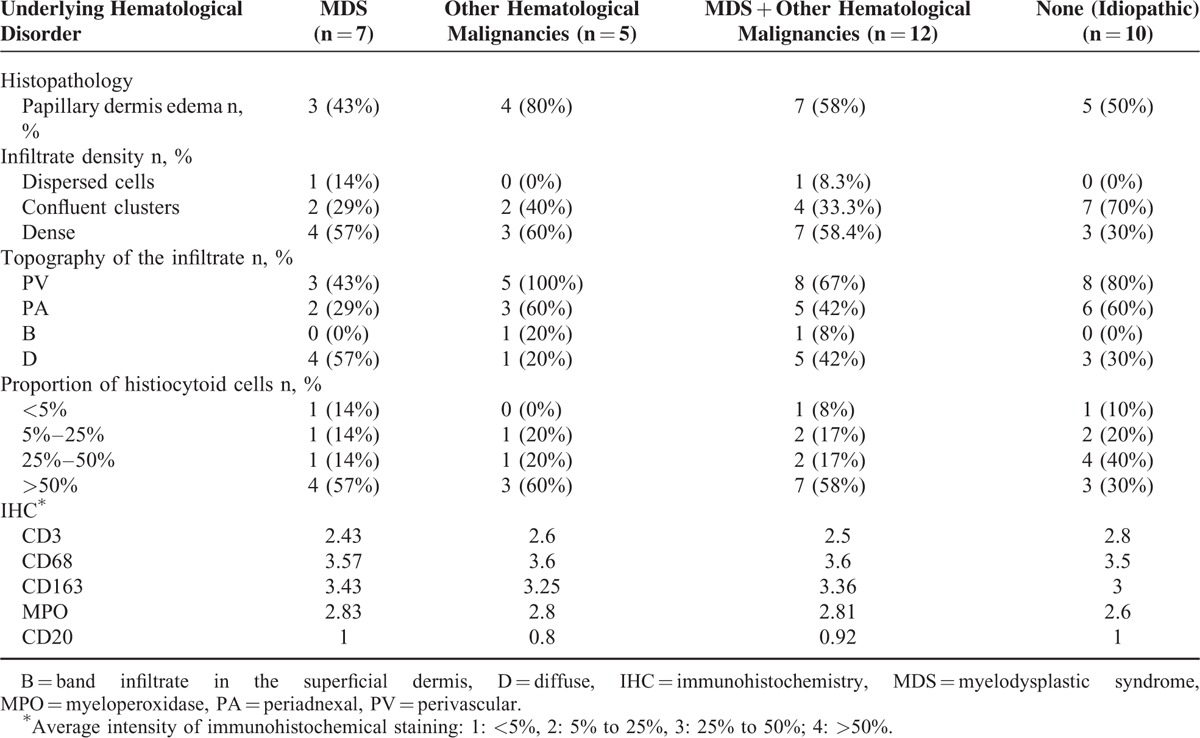
A perivascular topography of the infiltrate was observed in all H-SS with non-MDS malignancies, 8/10 (80%) cases of idiopathic H-SS, and 3/7 (43%) cases of H-SS associated to MDS. Histiocytoid cells were the predominant cell component in 7/12 (58%) cases of H-SS associated to a hematological disease. The immunohistochemical staining of CD3, CD68, CD163, myeloperoxidase, and CD20 was comparable in H-SS associated to hematological diseases and idiopathic H-SS (Table 3). A statistical comparison between the different sub-groups was not addressed because of the small size of the samples.
Treatment of H-SS and Follow-Up
In H-SS patients, cutaneous lesions at the acute phase were treated with high potent topical steroids (n = 17) and/or systemic steroids (n = 4). Recurrent lesions were treated with hydroxychloroquine (n = 3), colchicine (n = 4), thalidomide (n = 1), dapsone (n = 1), nonsteroid antiinflammatory drugs (n = 2), and intravenous immunoglobulins (n = 1) with poor efficacy.
Evolution of cutaneous lesions in patients with H-SS was acute without further relapse in 15 cases (68.2%, 9 with associated hematological malignancy), and chronic with persistent lesions or cutaneous relapses in 4 cases (18.2%, 1 with hematological malignancy). One patient had a second biopsy 3 years after the diagnosis of SS, showing the same H-SS pattern. Three patients were lost to follow-up. Recurrence of the SS was significantly more frequent in patients with H-SS (4/19, 21%) than N-SS (1/40, 2.5%, P = 0.01). The median follow-up after diagnosis of SS was 11.5 (0–96) and 7.5 (0–100) months for H-SS and N-SS patients, respectively (P = 0.43) (Table 1).
Time of Occurrence of Hematological Disease and Follow-Up
Among the 22 patients with SS and hematological malignancies, the hematological disease was diagnosed before the cutaneous lesions in 11 (50%) patients, 8 with H-SS and 3 with N-SS, at a median interval of 7 (0–121) months and 1 (0–93) months, respectively. Hematological disease was diagnosed at the time of occurrence of cutaneous SS lesions in 8 patients (36.4%), 1 patient with H-SS and 7 patients with N-SS. In 3 patients (13.6%), all H-SS patients with MDS, cutaneous lesions preceded the diagnosis of the hematologic disease by ≤6 months (Figure 4).
FIGURE 4.
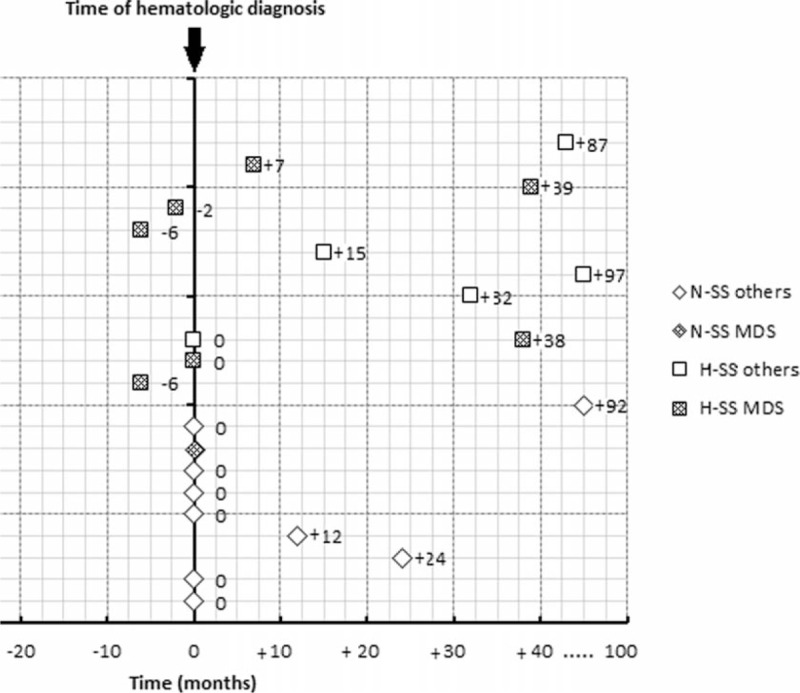
Time between Sweet syndrome and hematological diagnosis.
Finally, 10 patients with H-SS and 30 with N-SS were not associated to a hematological malignancy. Blood cell counts measured within a median follow-up of 14 (0–96) months after the diagnosis of SS were in the normal range. Bone-marrow aspiration was performed in 3 patients with H-SS, with normal medullar cytology and cytogenetics.
Two of 12 patients with H-SS and hematological malignancies died during the follow-up. Causes of death were septic shock (MDS, death 8 months after the diagnosis of H-SS and 2 years after the onset of the hematological disease) and disseminated pulmonary aspergillosis (CLL, death 11 months after the diagnosis of H-SS and 9 years after the onset of the hematological disease). In patients with N-SS and hematological malignancies, 6/10 patients died from uncontrolled AML (at diagnosis or relapse, n = 4), MDS (follow-up 32 months, n = 1), and non-Hodgkin lymphoma (bulky form, follow-up <2 months, n = 1). Thus, the mortality rate was higher in N-SS than in H-SS patients with underlying hematological malignancy (P = 0.04). All the patients with H-SS or N-SS and without an associated hematological malignancy were still alive at the end of the study.
DISCUSSION
We here report a large clinical series of patients with H-SS and compare hematological malignant diseases associated with H-SS and N-SS, respectively. H-SS was more frequent than described in previous studies, since it represented 35.5% of all SS diagnosed in our Pathology Department. Clinical presentation did not differ between H-SS and N-SS patients. Cutaneous lesions of H-SS were mostly similar to those of N-SS, although some patients may present atypical dermatological lesions such as widespread annular lesions. Most H-SS patients showed a single flare of the disease, even those with associated hematological conditions, whereas a few showed chronic disease refractory to conventional antiinflammatory or immunomodulatory drugs. Hematological malignancies were more frequently associated with H-SS than N-SS (55.5% vs 25%, respectively, P = 0.019), and MDS was more frequently associated with H-SS than with N-SS (31.8% vs 2.5%, respectively, P < 0.001). Lymphoid malignancies could be associated with H-SS, although less frequently than with N-SS.
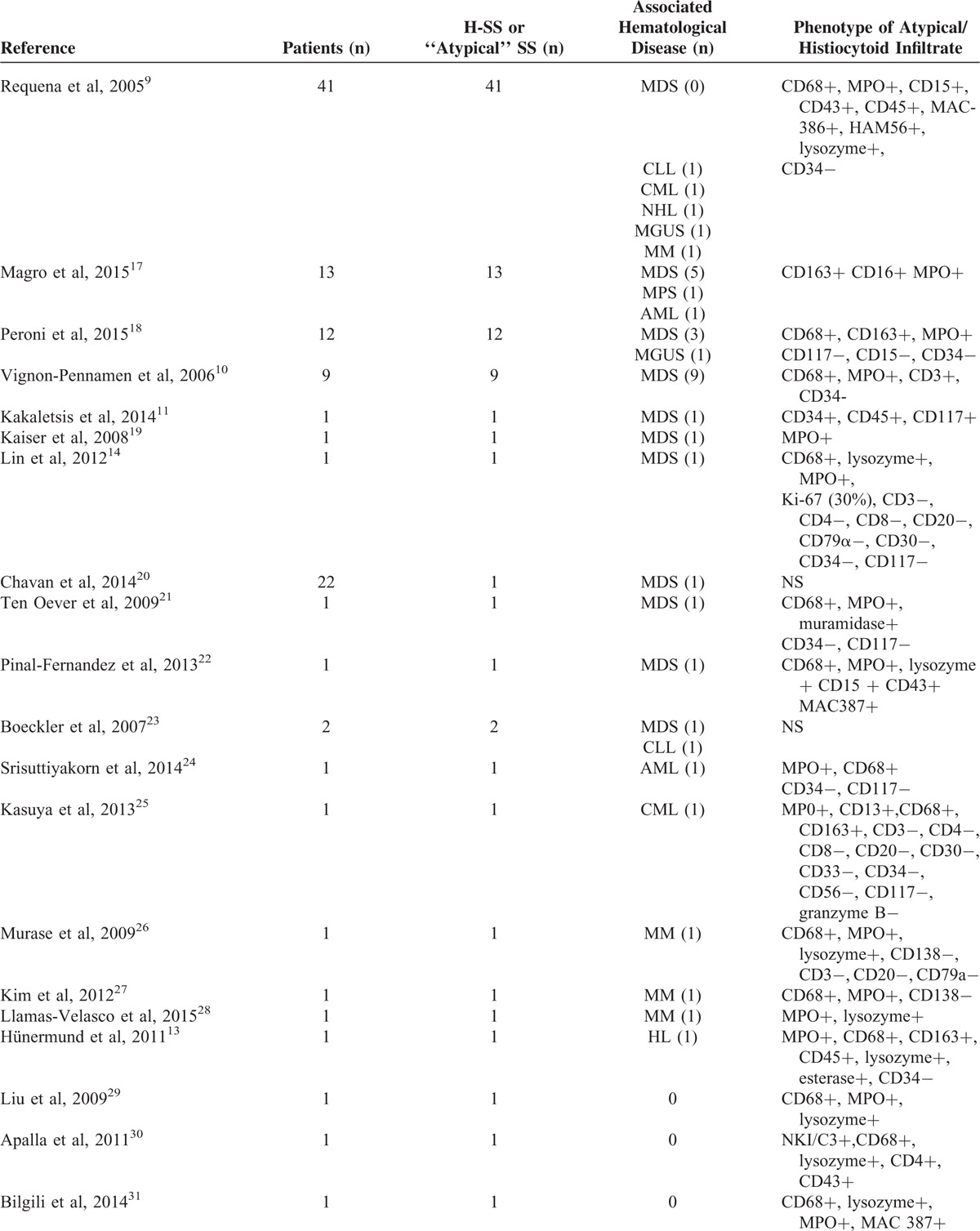
H-SS is a rare histological variant of SS, and only few previous series of patients were published. In order to compare our findings to those previously described, we reviewed published cases of H-SS in the last decade (Table 4). We retrieved 100 cases of atypical SS with histiocytoid component described between 2005 and 2015, especially in 4 series of 41, 13, 12, and 9 patients, respectively.9,10,17,18 The remaining cases were described in case reports.11,13,14,19–38 In Vignon-Pennamen et al series, the 9 H-SS cases studied were all associated to MDS. Overall, 40% (40/100) of these previously published cases of H-SS were associated to a hematological malignancy, and 24% (24/100) with a MDS, while these associations were of 55.5% (12/22) and 31.8% (7/22), respectively, in our series. This high proportion of H-SS in our series may be due to the areas of expertise of our Hematology Department, with a recognized expertise for myeloid disorders, including MDS. Immunohistochemical study, when performed, evidenced the presence of atypical cells of myeloid origin, CD68, and myeloperoxidase positive in all cases. Further comparison between our series and previous published cases is difficult because of the heterogeneity of clinical descriptions and immunohistochemical stainings used (Table 4).
TABLE 4.
Review of Previously Published H-SS Cases in the Last Decade
H-SS was previously shown to occur at the onset or preceding MDS in most cases.10,39,40 In our study, the hematological disease was mostly diagnosed before the diagnosis of H-SS. However, 3 patients presented cutaneous lesions of H-SS before the diagnosis of hematological malignancy, MDS in all these cases, within a 6-month interval. Consequently, a careful hematological assessment is recommended in H-SS at diagnosis time and for at least 6 months later.
In previous series, the occurrence of N-SS in a patient with MDS was suggested to indicate a transformation into AML, thereby indicating a poor prognosis of MDS when associated with classical SS.41 In our study, the outcome seemed better for patients with underlying hematological malignancy associated with H-SS than with N-SS, but no correlation can be made between the type of SS and the hematological disease status.
Moreover, the evolution of H-SS was acute without further relapse in most patients, including those with associated hematological malignancies.
Previous studies have suggested that H-SS may represent an early stage of classical N-SS, because cases with 1st histiocytoid mononuclear and lymphocytic infiltrates, then neutrophilic infiltrates were described in the same patients.7,10 H-SS and N-SS may belong to the same spectrum of a disease. However, in our study, only 1 H-SS patient with recurrent lesions had a 2nd biopsy 3 years after the former biopsy, without histological modification. Overall, in our series, relapsing evolution was rare but significantly more frequent in patients with H-SS than N-SS.
N-SS may be triggered by an external antigen (virus, bacteria, and drug) or induced by a dysregulation of the innate immune system as in autoinflammatory diseases, which are often associated with neutrophil recruitment in lesional skin. For SS associated with hematological malignancies, whether SS reflects a paraneoplastic syndrome or a “differentiated” leukemia cutis is still unclear. The “histiocytoid” myeloperoxidase-positive cells that are dominant in H-SS may be considered immature myeloid progenitors recruited into the skin. These cells, although much less numerous, can be identified in classical N-SS infiltrates, where mature neutrophils are the main cell type.42 Whether these cells are abnormal cells clonally related to the hematological malignant clone or normal myeloid progenitors recruited into the skin remains unknown.
Interestingly, fluorescence in situ hybridization assays in patients with H-SS and N-SS and associated myeloid malignancies with identified chromosomal abnormalities (AML, chronic myelogenous leukemia, and refractory anemia with excess blasts) showed the same chromosomal abnormalities in skin-infiltrating neutrophils.20,43 However, recently, histiocytoid cells of H-SS infiltrates were found to exhibit a phenotype similar to M2 macrophages, involved in TH2-inflammatory responses.18 Regardless, the physiopathological significance of H-SS still remains poorly understood and needs further studies.
The limitations of our series are the monocentric setting, the retrospective design, although we included all consecutive patients diagnosed with SS in the study period, and the small size of study population, probably due to the rare incidence of this histological feature of SS. The short median follow-up is limiting for concluding about the potential risk of hematological disease in the long term after the skin disease.
In conclusion, we show that H-SS is associated with MDS in almost one third of patients and may precede the hematological disease. H-SS may also be associated with lymphoid diseases. Thus, a complete hematologic investigation is mandatory at diagnosis of H-SS and should be renewed later in order to early diagnose a hematological malignancy.
Footnotes
Abbreviations: AML = acute myeloid leukemia, CLL = chronic lymphocytic leukemia, H-SS = histiocytoid Sweet syndrome, MDS = myelodysplastic syndrome, N-SS = neutrophilic Sweet syndrome, SS = Sweet syndrome.
NO, SI-HO, OC, and AT contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol 1964; 76:349–356. [DOI] [PubMed] [Google Scholar]
- 2.Cohen PR. Sweet's syndrome – a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis 2007; 2:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petit T, Frances C, Marinho E, et al. Lymphoedema-area-restricted Sweet syndrome during G-CSF treatment. Lancet 1996; 347:690. [DOI] [PubMed] [Google Scholar]
- 4.Cohen PR, Talpaz M, Kurzrock R. Malignancy-associated Sweet's syndrome: review of the world literature. J Clin Oncol 1988; 6:1887–1897. [DOI] [PubMed] [Google Scholar]
- 5.Bourke JF, Keohane S, Long CC, et al. Sweet's syndrome and malignancy in the UK. Br J Dermatol 1997; 137:609–613. [DOI] [PubMed] [Google Scholar]
- 6.Bayer-Garner IB, Cottler-Fox M, Smoller BR. Sweet syndrome in multiple myeloma: a series of six cases. J Cutan Pathol 2003; 30:261–264. [DOI] [PubMed] [Google Scholar]
- 7.Jordaan HF. Acute febrile neutrophilic dermatosis. A histopathological study of 37 patients and a review of the literature. Am J Dermatopathol 1989; 11:99–111. [PubMed] [Google Scholar]
- 8.Delabie J, De Wolf-Peeters C, Mooren M, et al. Histiocytes in Sweet's syndrome. Br J Dermatol 1991; 124:348–353. [DOI] [PubMed] [Google Scholar]
- 9.Requena L, Kutzner H, Palmedo G, et al. Histiocytoid Sweet syndrome: a dermal infiltration of immature neutrophilic granulocytes. Arch Dermatol 2005; 141:834–842. [DOI] [PubMed] [Google Scholar]
- 10.Vignon-Pennamen MD, Juillard C, Rybojad M, et al. Chronic recurrent lymphocytic Sweet syndrome as a predictive marker of myelodysplasia: a report of 9 cases. Arch Dermatol 2006; 142:1170–1176. [DOI] [PubMed] [Google Scholar]
- 11.Kakaletsis N, Kaiafa G, Savopoulos C, et al. Initially lymphocytic Sweet's syndrome in male patients with myelodysplasia: a distinguished clinicopathological entity? Case report and systematic review of the literature. Acta haematol 2014; 132:220–225. [DOI] [PubMed] [Google Scholar]
- 12.Heymann WR. Histiocytoid Sweet syndrome. J Am Acad Dermatol 2009; 61:693–694. [DOI] [PubMed] [Google Scholar]
- 13.Hunermund A, Wendel AM, Geissinger E, et al. Typically atypical: histiocytoid Sweet syndrome, associated with malignancy. J Deutsch Dermatol Ges 2011; 9:666–669. [DOI] [PubMed] [Google Scholar]
- 14.Lin J, Zhang Q, Chen M. Subcutaneous histiocytoid Sweet's syndrome in a patient associated with myelodysplastic syndrome-refractory anemia. J Dermatol 2012; 39:99–101. [DOI] [PubMed] [Google Scholar]
- 15.Campo E, Swerdlow SH, Harris NL, et al. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood 2011; 117:5019–5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 2009; 114:937–951. [DOI] [PubMed] [Google Scholar]
- 17.Magro CM, Momtahen S, Nguyen GH, et al. Histiocytoid Sweet's syndrome: a localized cutaneous proliferation of macrophages frequently associated with chronic myeloproliferative disease. Eur J Dermatol 2015; 25:335–341. [DOI] [PubMed] [Google Scholar]
- 18.Peroni A, Colato C, Schena D, et al. Histiocytoid Sweet syndrome is infiltrated predominantly by M2-like macrophages. J Am Acad Dermatol 2015; 72:131–139. [DOI] [PubMed] [Google Scholar]
- 19.Kaiser R, Connolly K, Linker C, et al. Stem cell transplant for myelodysplastic syndrome-associated histiocytoid Sweet's syndrome in a patient with arthritis and myalgias. Arthritis Rheum 2008; 59:1832–1834. [DOI] [PubMed] [Google Scholar]
- 20.Chavan RN, Cappel MA, Ketterling RP, et al. Histiocytoid Sweet syndrome may indicate leukemia cutis: a novel application of fluorescence in situ hybridization. J Am Acad Dermatol 2014; 70:1021–1027. [DOI] [PubMed] [Google Scholar]
- 21.Ten Oever J, Kuijper PH, Kuijpers AL, et al. Complete remission of MDS RAEB following immunosuppressive treatment in a patient with Sweet's syndrome. Neth J Med 2009; 67:347–350. [PubMed] [Google Scholar]
- 22.Pinal-Fernandez I, Ferrer Fabrega B, Ramentol Sintas M, et al. Histiocytoid Sweet syndrome and cutaneous polyarteritis nodosa secondary to myelodysplastic syndrome. Int J Rheum 2013; 16:777–779. [DOI] [PubMed] [Google Scholar]
- 23.Boeckler P, Noacco G, Maradeix S, et al. Lymphocytic infiltrate of the dermis preceding typical Sweet's syndrome. Ann Dermatol Venereol 2007; 134:559–563. [DOI] [PubMed] [Google Scholar]
- 24.Srisuttiyakorn C, Reeve J, Reddy S, et al. Subcutaneous histiocytoid Sweet's syndrome in a patient with myelodysplastic syndrome and acute myeloblastic leukemia. J Cutan Pathol 2014; 41:475–479. [DOI] [PubMed] [Google Scholar]
- 25.Kasuya A, Fujiyama T, Hashizume H, et al. Histiocytoid Sweet's syndrome associated with t(9;22)(q34;q11)-positive chronic myelogenous leukemia: immature granulocytic origin of histiocytic cells. Int J Dermatol 2013; 52:1577–1579. [DOI] [PubMed] [Google Scholar]
- 26.Murase JE, Wu JJ, Theate I, et al. Bortezomib-induced histiocytoid Sweet syndrome. J Am Acad Dermatol 2009; 60:496–497. [DOI] [PubMed] [Google Scholar]
- 27.Kim JS, Roh HS, Lee JW, et al. Distinct variant of Sweet's syndrome: bortezomib-induced histiocytoid Sweet's syndrome in a patient with multiple myeloma. Int J Dermatol 2012; 51:1491–1493. [DOI] [PubMed] [Google Scholar]
- 28.Llamas-Velasco M, Concha-Garzon MJ, Fraga J, et al. Histiocytoid sweet syndrome related to bortezomib: a mimicker of cutaneous infiltration by myeloma. Indian J Dermatol Venereol Leprol 2015; 81:305–306. [DOI] [PubMed] [Google Scholar]
- 29.Liu CI, Hsiao CH, Wu JT, et al. Sweet syndrome with histiocytoid infiltrate and neutropenia: a rare combination. J Am Acad Dermatol 2009; 61:882–884. [DOI] [PubMed] [Google Scholar]
- 30.Apalla Z, Kanatli L, Sotiriou E, et al. Histiocytoid Sweet syndrome. Clin Exp Dermatol 2011; 36:562–563. [DOI] [PubMed] [Google Scholar]
- 31.Bilgili SG, Karadag AS, Calka O, et al. Histiocytoid Sweet syndrome. Int J Dermatol 2014; 53:e80–e82. [DOI] [PubMed] [Google Scholar]
- 32.Wu AJ, Rodgers T, Fullen DR. Drug-associated histiocytoid Sweet's syndrome: a true neutrophilic maturation arrest variant. J Cutan Pathol 2008; 35:220–224. [PubMed] [Google Scholar]
- 33.Spencer B, Nanavati A, Greene J, et al. Dapsone-responsive histiocytoid Sweet's syndrome associated with Crohn's disease. J Am Acad Dermatol 2008; 59 (2 Suppl 1):S58–S60. [DOI] [PubMed] [Google Scholar]
- 34.Camarillo D, McCalmont TH, Frieden IJ, et al. Two pediatric cases of nonbullous histiocytoid neutrophilic dermatitis presenting as a cutaneous manifestation of lupus erythematosus. Arch Dermatol 2008; 144:1495–1498. [DOI] [PubMed] [Google Scholar]
- 35.Wilson TC, Stone MS, Swick BL. Histiocytoid Sweet syndrome with haloed myeloid cells masquerading as a cryptococcal infection. Am J Dermatopathol 2014; 36:264–269. [DOI] [PubMed] [Google Scholar]
- 36.Wang T, Liu Y, Zheng H. Histiocytoid Sweet's syndrome associated with rheumatoid arthritis and pleuritis. Chin Med J 2014; 127:1396. [PubMed] [Google Scholar]
- 37.Fernandez-Torres RM, Castro S, Moreno A, et al. Subcutaneous histiocytoid sweet syndrome associated with crohn disease in an adolescent. Case Rep Dermatol Med 2014; 2014:954254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arima Y, Namiki T, Ueno M, et al. Histiocytoid sweet syndrome: a novel association with relapsing polychondritis. Br J Dermatol 2016; 174:691–694. [DOI] [PubMed] [Google Scholar]
- 39.Evans AV, Sabroe RA, Liddell K, et al. Lymphocytic infiltrates as a presenting feature of Sweet's syndrome with myelodysplasia and response to cyclophosphamide. Br J Dermatol 2002; 146:1087–1090. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto T, Soejima K, Yokozeki H, et al. Unusual annular erythema associated with myelodysplastic syndrome. Dermatology 2001; 202:70–72. [DOI] [PubMed] [Google Scholar]
- 41.Avivi I, Rosenbaum H, Levy Y, et al. Myelodysplastic syndrome and associated skin lesions: a review of the literature. Leuk Res 1999; 23:323–330. [DOI] [PubMed] [Google Scholar]
- 42.Tomasini C, Aloi F, Osella-Abate S, et al. Immature myeloid precursors in chronic neutrophilic dermatosis associated with myelodysplastic syndrome. Am J Dermatopathol 2000; 22:429–433. [DOI] [PubMed] [Google Scholar]
- 43.Sujobert P, Cuccuini W, Vignon-Pennamen D, et al. Evidence of differentiation in myeloid malignancies associated neutrophilic dermatosis: a fluorescent in situ hybridization study of 14 patients. J Invest Dermatol 2013; 133:1111–1114. [DOI] [PubMed] [Google Scholar]


