Abstract
Few studies, each limited to a single major city, have investigated the prevalence and seasonal patterns of different viruses among children with low respiratory tract infections (LRTI) in Northeastern Brazil. The aim of this study was to determine the frequency of respiratory syncytial virus (RSV) and of 7 other viruses in children for LRTI in 4 capitals from this region, and investigate their association with several risk factors, including meteorological data.
From April 2012 to March 2013, 507 children, aged up to 24 months and hospitalized with LRTI in one of the participating centers at Aracajú, Salvador, Recife, and Maceió, had a sample of nasopharyngeal aspirate collected and analyzed for the following viruses by reverse-transcription polymerase chain reaction followed by hybridization on low-density microarrays: RSV, influenza, parainfluenza, adenovirus, rhinovirus, metapneumovirus, bocavirus, and coronavirus.
The result was positive in 66.5% of cases, RSV was the most common virus (40.2%). Except for rhinovirus (17%), all other virus had frequency rates lower than 6%. Viral coinfections were detected in 13.8% of samples. Possible related risk factors for RSV infection were low age upon entry, attendance of daycare, low gestational age, and low educational level of the father. The relative frequency of viral infections was associated with increasing temperature and decreasing humidity separately, but the results also suggested both associated with increased frequency of RSV. Some of these findings differ from those reported for other regions in Brazil and may be used to guide policies that address LRTI.
INTRODUCTION
Lower respiratory tract infections (LRTIs) are an important cause of mortality among children worldwide.1 Viruses are the causative agent in a sizable proportion of pediatric LRTI cases, especially among children less than 1 year of age.2 In affluent societies, the burden of such infections may be even higher, but deaths from respiratory syncytial virus (RSV) and other associated LRTIs are several times more frequent in developing than in developed countries.3 The frequency of LRTI from different respiratory viruses is clearly influenced by a seasonal pattern that may vary according to geographic location and climate conditions.4,5 However, the relative importance of RSV as the causative agent in LRTI varies widely in different geographic locations, and there is limited information on RSV seasonal patterns in tropical areas.4,6
In Brazil, RSV is the most common viral pathogen found in episodes of LRTI; other frequent viruses include influenza, rhinovirus, parainfluenza, adenovirus, metapneumovirus, and bocavirus.7–16 Some studies conducted in Brazil have suggested a clear seasonal pattern for RSV infections, with predominance from March to July, and a smaller number of cases in the summer months, especially November to February.14–19 Other viruses have been investigated to a lesser extent, but a seasonal pattern was found for different respiratory viruses in Southern and Southeastern Brazil, where the winter is cold and dry.10,13,20 Only a few studies were conducted to investigate the prevalence and seasonal patterns of different viral infections among children with an episode of LRTI seen at hospitals from Northeastern Brazil, where the winter is warm and wet.14,16,21–24 Such studies were typically conducted in only 1 major city in the region and have not allowed for concurrent assessments within different areas of the Northeastern region of Brazil. Moreover, they have used different eligibility criteria and methods for viral identification. In the current study, we adopted standardized criteria and methods to assess the frequency of infection with RSV and 7 other viruses in children aged less than 2 years old hospitalized due to an LRTI in 4 capitals (Aracajú, Salvador, Recife, and Maceió) from Northeastern Brazil over a period of 1 year. We also seek to evaluate the association between the frequency of LRTI in general, of RSV, and of the other viruses and meteorological data (temperature, precipitation, humidity, and solar radiation).
METHODS
Study Design, Selection Criteria, and Objectives
This was a cross-sectional study of children from 0 to 24 months of age, regardless of gestational age, who were hospitalized (in the ward or intensive care unit [ICU] settings) during the 1-year (April, 2012 to March, 2013) enrollment period because of LRTI. Additional inclusion criteria were the presence of respiratory symptoms that had begun no longer than 7 days prior to the study visit, and the ability of the adult legal representative of the child to understand and comply with procedures as outlined in the protocol, providing written consent for the procedures. The only exclusion criteria were the previous use of passive immunization with palivizumab or other antibodies against RSV. All study procedures were done during a single initial visit for every enrolled patient; such procedures included the collection of demographic, social, and medical data, as well as the collection of nasopharyngeal aspirates (NPAs). There was no follow-up data after the 1st visit on patients enrolled. Medical diagnosis (bronchiolitis, pneumonia, or bronchopneumonia) was copied from the assistant physician according to their clinical evaluation or defined per protocol by the presence of symptoms of respiratory disease (for instance, cough, coryza, rhinorrhea, fever, or retractions) associated with at least 1 of the following: visible increased anteroposterior diameter, intercostal retractions, tachypnea, apnea, wheezing, or crackles, with no interference from our personal. The study was approved by the Institutional Ethics Committees of each of the 4 participating institutions.
In addition to the preplanned analyses, exploratory analyses were conducted in order to assess the prevalence of viral infections according to age upon study entry and gestational age at birth. Moreover, analyses were conducted to assess the distributions of hypoxemia during admission (defined as oxygen saturation in air <92% and <90% on pulse oximetry), duration of breastfeeding stratified by age, the relationship between age, weight and height, and the frequency of codetection of different viruses, as well as the univariate and multivariate impact of these variables on the prevalence of viral infections.
Research Settings and Meteorology
Meteorological data for each city were obtained from the National Institute of Meteorology database and included the average monthly temperature (°C), relative humidity (%), solar radiation (kJ/m2), and precipitation (mm).
Viral Assessments
After collection, NPAs were frozen at −70 to −80 °C for local storage. The maximum time from NPA collection to storage in freezers was 2 hours. Periodically, NPA was sent to a central laboratory for detection of the following viruses by reverse-transcription polymerase chain reaction (RT-PCR) followed by PCR product hybridization on low-density microarrays, using CLART PneumoVir (Genomica, Madrid, Spain): RSV (A and B), influenza virus (A, A subtype H1N1, and B), parainfluenza (I and III), adenovirus, rhinovirus, metapneumovirus (A and B), bocavirus, and coronavirus (229E). Results were not known immediately by investigators, so patient care was based on clinical presentation, and was thus only available at the time of statistical analysis.
Statistical Analysis
A sample size of 504 subjects was estimated in order to allow for a 95% confidence interval (CI) of 4% around an estimated point prevalence of RSV infection of 30%, which was considered a plausible average of the rates found in previous studies.14,18,19,25 Such sample was stratified among the 4 participating sites according to the mean of annual hospitalizations due to acute respiratory infections in each corresponding city for the previous 3 years, there was not an intention to perform a comparative analysis between cities. The analysis population comprised all children included in the study in accordance with selection criteria. The frequency of each virus was computed as the number of corresponding positive samples divided by the number of tested samples, with its accompanying 95% CI. Seasonal trends were explored by assessing the frequency (the monthly frequency) of proven viral infections throughout the year. Spearman's correlation coefficients were used to explore the association between frequency of viral infections and the meteorological variables of interest. Moreover, generalized linear models were used to explore the association between the number of cases of infections from each virus and meteorological data, assuming Poisson or negative binomial distributions, as appropriate. Stepwise logistic regression models were used to explore risk factors for viral infections among variables with statistically significant results (P < 0.20) in univariate fashion. For the multivariate analysis, 2-tailed P values < 0.05 were considered statistically significant, and the analysis was done using SAS Software, version 9.3.
RESULTS
Baseline Patient Characteristics and Disposition
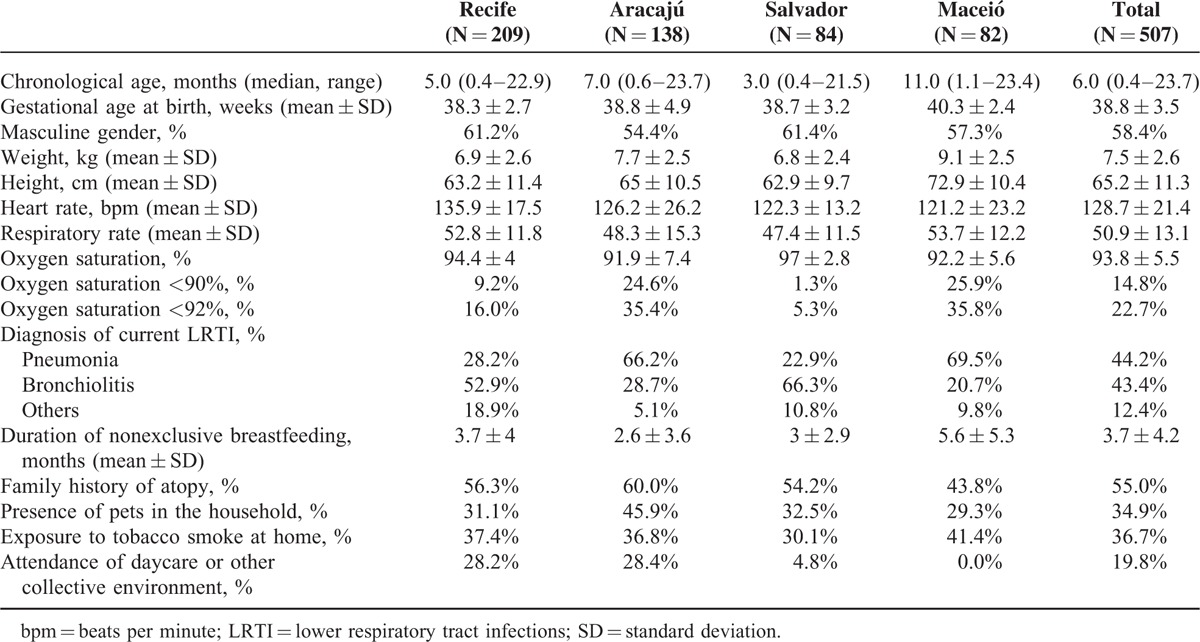
Between April 2012 and March 2013, legal representatives of 513 children signed an informed consent form and underwent the study visit; however, 5 patients were not eligible because their respiratory symptoms started longer than 7 days before study participation, and in 1 patient monitoring and source documents were missing. There was no exclusion due to use of Palivizumab in any participant. Thus, 507 children were analyzed. Overall across centers, the median time since the onset of symptoms and the study visit was 4 days (range, 0–7 days), and the median time from admission to inclusion in the study was 1 day (range, 0–5 days). Eighty-eight percent of the patients came in through the emergency room and admitted to a general ward (56.6%) or to an emergency department ward (43.2%), with only 1 directly admitted to the ICU. The chief demographic, clinical, and social features of patients are shown by center and overall in Table 1. The frequency of hypoxemia differed markedly across centers, what could reflect the different profile of the centers related to emergency/general wards and ICU hospitalizations. With regard to previous diagnoses of interest, 18.3% of children had been premature, 5.5% had congenital heart disease, and 1.0% had bronchopulmonary dysplasia. Overall, the mean duration of nonexclusive breastfeeding was 3.7 months; however, a normalized analysis describing this duration as a percentage of the ideal duration of 6 months (or child age for those aged less than 6 months) showed that the overall average was 98.8%.
TABLE 1.
Patient Demographic, Clinical, and Social Features at Baseline, by Center and Overall
Frequency of LRTI and Different Viruses
Table 2 displays the numbers of cases of LRTI per centers and overall during the 1-year period of interest. Overall, the relative contribution of cases of LRTI over the whole study period was above 10.0% for the months of April to June 2012 and in March 2013, but there was wider temporal variation when each center was analyzed separately. The overall frequency of any proven viral infection is shown in Table 3. Nearly two-thirds of patients had at least 1 proven viral infection. Overall, 204 subjects tested positive for RSV, for a relative frequency of 40.2% (95% CI, 35.9%–44.7%). RSV A was nearly 3 times as frequent than RSV B. The overall prevalence of each of the other viruses was 17% for rhinovirus, 5.9% for metapneumovirus, 5.5% for parainfluenza and bocavirus, 3.7% for adenovirus, and 3.2% for influenza. Of note, there were no cases of coronavirus 229E. Any codetection (not including subtypes of the same virus) was found in 13.8% of patients. The 2 most frequent codetections were RSV and rhinovirus (3.7% of tested samples), as well as RSV and bocavirus (2.0%). Of note, 9 children had codetection with RSV A and RSV B.
TABLE 2.
Number (%) of Cases of Lower Respiratory Tract Infections Along 1 year, by Center and Overall
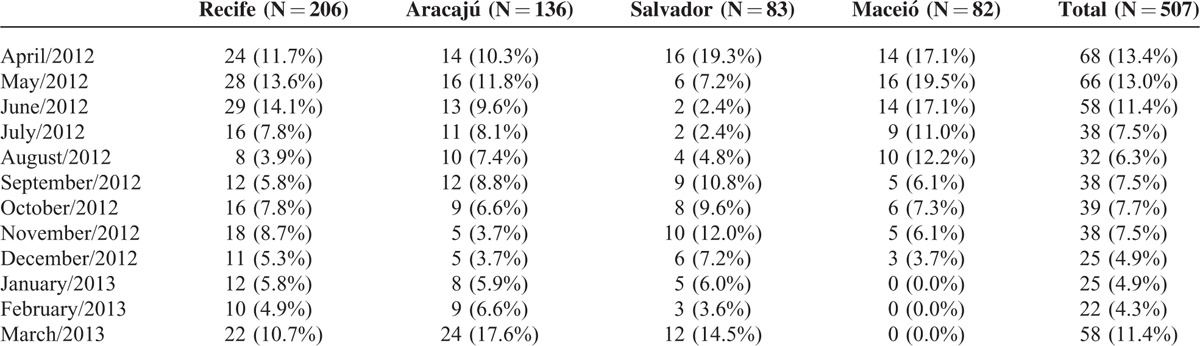
TABLE 3.
Relative Frequency (95% CI) of Proven Viral Infections
Related Risk Factors for Different Viruses
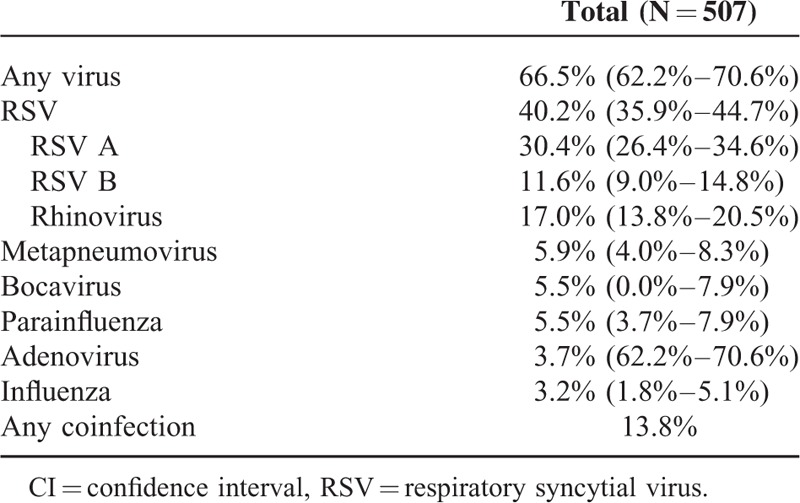
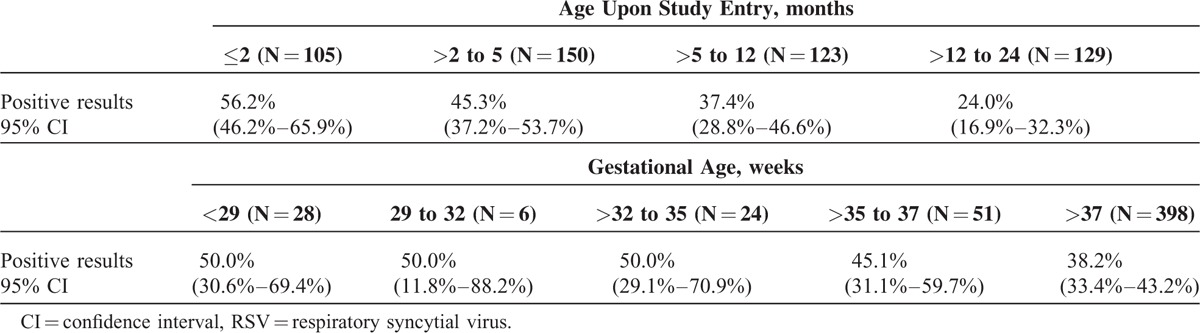
Statistically significant possible related risk factors for viral infections in multivariate analysis were decreasing age upon study entry, increasing weight, and lower educational level of the father (Table 4). The frequency of RSV decreased with increasing age upon study entry and gestational age at birth (Table 5), as we can observe that nearly half of all children hospitalized with RSV LRTI (49%, n = 127) are in the less than 6 months age group. Proportionally, RSV positivity was higher among children born with gestational age <35 weeks (50%, n = 29) compared to children born with a gestational age of >35 weeks (39%, n = 175). For RSV infection, statistically significant risk factors in multivariate analysis were decreasing age upon study entry, decreasing gestational age, attendance of daycare or other collective environment, and lower educational level of the father (Table 4). No statistically significant risk factors were found for other viruses in multivariate analysis, except for increasing age upon study entry in the case of influenza and the following factors in the case of metapneumovirus: increasing gestational age, the presence of bronchopulmonary dysplasia, higher educational level of the mother, and lower educational level of the father (data not shown).
TABLE 4.
Significant Risk Factors for Viral Infections and for RSV Infections in Multivariate Analysis
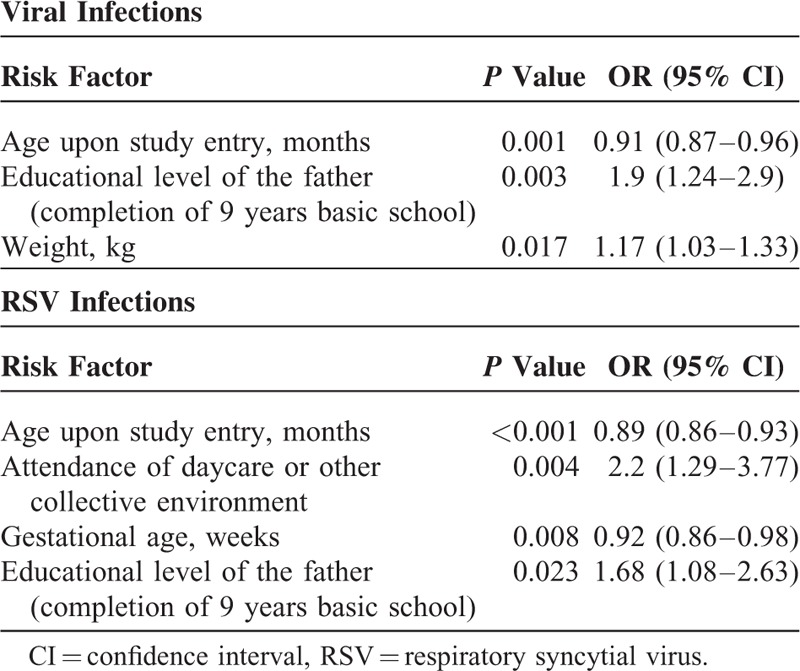
TABLE 5.
Association Between the Prevalence of RSV and Both Age Upon Study Entry and Gestational Age at Birth
Seasonality of Viral Infections and Influence of Meteorological Factors
The monthly frequency of each viral infection along 1 year is shown in Figure 1; as expected from its predominance in this age group, RSV frequency is the major driver of the frequency of viral infections in general.
FIGURE 1.
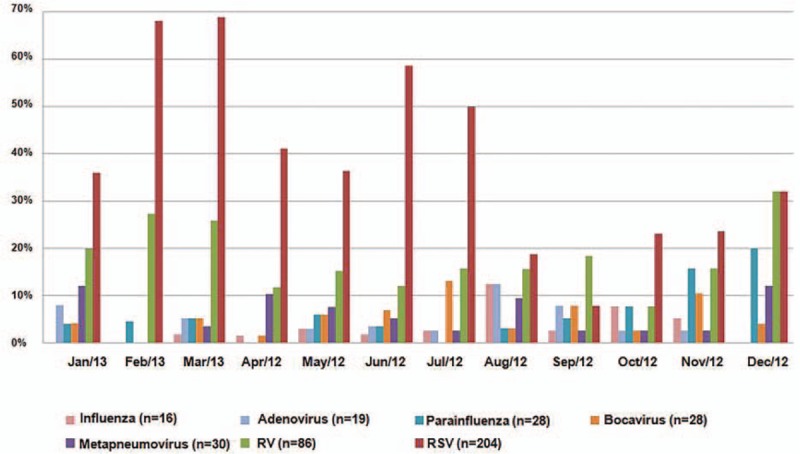
Monthly relative frequency of different viral infections along 1 year.
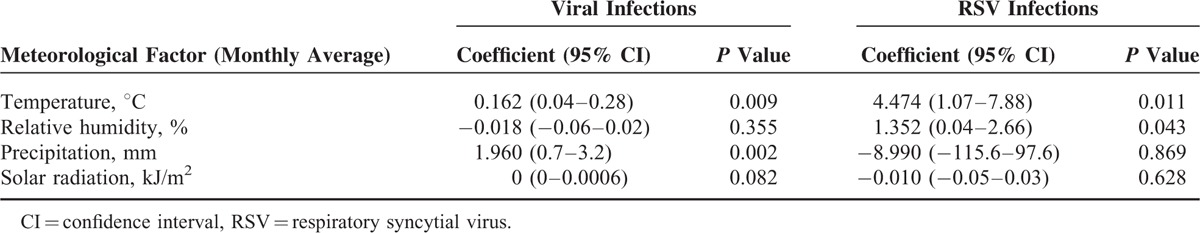
Statistically significant associations were found between the frequency of viral infections in general and both temperature and humidity. For temperature, such correlation was moderate and positive (Spearman correlation coefficient, 0.401), whereas it was moderate and negative for humidity (Spearman correlation coefficient, −0.416). On the other hand, generalized linear models disclosed statistically significant correlations between the number of cases of viral infections in general and both temperature and precipitation (Figure 2; Table 6). There was statistically significant association between the relative frequency of RSV infection and both temperature and humidity (Table 6). There were no statistically significant correlations between the frequency of other viral infections and any of the meteorological variables investigated, except for parainfluenza, whose relative frequency was moderately and negatively correlated with precipitation (Spearman correlation coefficient, −0.383), and moderately and positively correlated with solar radiation (Spearman correlation coefficient, 0.354). With regard to the numbers of other viral infections investigated in the study, some statistically significant correlations were found between the frequency of such infections and some of the meteorological variables investigated (data not shown). There were negative correlations between temperature and the number of cases of both influenza and adenovirus infections, as well as a positive correlation between precipitation and the number of cases of bocavirus infections.
FIGURE 2.
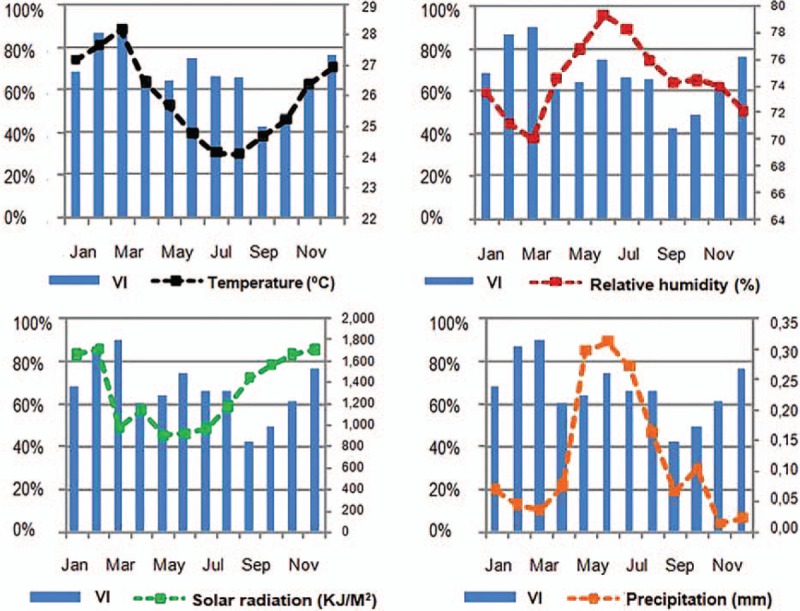
Monthly frequency of viral infections (VI) along 1 year and variation of meteorological factors in the same period.
TABLE 6.
Correlation Between the Number of Cases of Viral Infections or of RSV Infections and Meteorological Factors Using Generalized Linear Models
DISCUSSION
This cross-sectional study has shown that 7 of the 8 tested viruses were found using RT-PCR in 66.5% of children aged 0 to 24 months hospitalized because of an LRTI in 4 centers located in major cities of Northeastern Brazil, a tropical region. The most commonly detected virus was RSV, which was found in 40.2% (95% CI, 35.9%–44.7%) of children. With the exception of rhinovirus (17%), a rising viral agent has been identified worldwide, all other individual virus had prevalence rates lower than 6%. Coinfection with at least 2 different viruses was found in 13.8% of tested samples, the most common being RSV with rhinovirus (3.7%). CLART PneumoVir could have some limitation on picornaviruses sensitivity;26 rhinovirus could be in somehow undetected, even though was the 2nd most frequent virus identified as noted.
Of the available studies that were conducted to investigate the prevalence and seasonal patterns of various viral infections among children from Northeastern Brazil, designs, methods, and objectives have varied considerably. In the study by Moura et al,14 RSV was detected during a 43-month period in 21% of 1950 children. However, around half of the children in this study had upper respiratory tract infection and 26% were more than 2 years old. On the other hand, a study by Bezerra et al,7 which investigated the same viruses as in this current study tested by us plus Mycoplasma pneumoniae in 407 children with a median age of 8 months whose main diagnostic was an LTRI, pathogens were detected in 85.5% of them, with RSV being the most prevalent (37.3%). In that work, coinfection was detected in 39.6% of the cases, with the most frequent combination being RSV with bocavirus (6.4%). Finally, another large study by Moura group based on patients seen over a period of 8 years reported a rate of 59.7% of RSV among children up to 2 years old with an acute respiratory infection. Considering all children, from 0 to 16 years old, coinfections were found in less than 1% of them.16 This same group also reported their attempt to investigate parainfluenza infections among children from Fortaleza,22 as well as adenovirus infections in Salvador.23 Silva et al24 assessed the prevalence of influenza viruses among children in Salvador.
RSV infects virtually every child in the 1st few years of life and is the most important cause of hospitalization for respiratory infection in young children worldwide. Statistically significant risk factors for RSV infection in this study were low age upon entry, attendance of daycare or other collective environment, low gestational age, and low educational level of the father. We have to take in consideration the limitation of this study design to infer causality. Although the latter variable has questionable biological role, the others are established risk factors for RSV infection in the literature.27,28 On the other hand, other risk factors previously indentified in the literature, such as exposure to tobacco smoke at home, smoking during pregnancy, family history of atopy, the presence of siblings in school age, the duration of breast-feeding, the presence of pets in the home, and the number of residents and visitors at home, could not be confirmed as independent predictors of RSV infection in this study. Fortunately, severe RSV infection is largely preventable by using palivizumab in high-risk children.29–31
Although there was general standardization on selection criteria and study methods across centers, the study did not interfere with the centers’ criteria for the diagnosis of bronchiolitis and pneumonia (or other LRTI) and time of oximetry, and the center profile regarding emergency/general ward and ICU admissions. This is likely the cause for the differences observed, such as the marked variation in the frequency of hypoxemia. The higher proportion of children with lower oxygen saturation (Maceió 25.9% and Aracaju 24.6% <90%) may be due to the service characteristic in the 2 cities, with more children at the emergency ward being enrolled. Actually, oxygen saturation, which was not measured in 2.8% of cases, was assessed upon admission in 34.7% of children, and during the study visit in the remaining 62.8% of cases, this may represent a limitation of our study.
In the current study, statistically significant associations were found between the frequency of viral infections and increasing temperature and decreasing humidity. Moreover, there was a statistically significant correlation between the absolute number of cases of RSV infection and an interaction term for these 2 meteorological variables, thus suggesting that higher temperatures associated with decreased humidity increase the number of cases of RSV infection. In a study in Southern Brazil, viral respiratory infections were found to increase as both temperature and the relative air humidity decreased.32 In that study, 54% of hospitalized children aged less than 6 years had any viral infection by RT-PCR, with 29% of cases overall being due to RSV and 23.1% to rhinovirus. Thus, besides some limitation of the assay used different associations between viral infections and temperature may represent the play of chance or else indicate biological phenomena modulated by different viral prevalence and climate conditions between these 2 regions, being another possible cause. With regard to seasonality, viral infections in our study peaked in June, July 2012, February, and March 2013, mostly as a result of the higher frequency of RSV in those months. In a similar study conducted in Fortaleza, Moura et al14 found a higher frequency of RSV infections during the rainy season, especially during the 8 consecutive months starting in January, in their study of 1950 children seen as outpatients or inpatients. In the study by Bezerra et al,7 conducted in Recife, RSV also peaked in the rainy season, from April to July. Cuevas et al21 assessed a smaller number of outpatient and hospitalized children (N = 111) in Aracajú; in this study that lasted only 2 months, seasonal trends could not be assessed, but there was a time relation between number of cases and precipitation. When RSV seasonality was assessed in Southeastern Brazil, 88.5% of cases occurred from late summer to mid-autumn in 1 study,33 and in another the frequency was higher in autumn and near-negligible in winter.25 Recently, data from the Brazilian surveillance system of influenza-like illness in sentinel sites showed that RSV cases peaked from March to June, but there were several regional differences.9 Likewise, results from a study on RSV circulation in 7 countries suggest that RSV prevalence is higher during wet months in climates with high annual precipitation, while cold temperatures may be the main factor in other areas; furthermore, no association with meteorological factors or weather trends was observed in one of the countries.4 Therefore, interventions that target the prevalence of RSV have to take into account regional differences in the seasonality of this infection.
This study has some limitations we want to declare: as a cross-sectional study, one only measure of clinical parameters was taken, during admission, by the physician responsible for the patient, which may have admitted patients with different criteria. As it was a diagnostic study to identify viruses’ etiology, we have not evaluated bacterial associations nor the treatment given.
CONCLUSION
In summary, this study confirms RSV as the most important viral pathogen found in episodes of LRTI in Northeastern Brazil. Moreover, it shows that other viruses are often present, sometimes in coinfection with RSV. The differences found in terms of prevalence rates and associations with climate conditions across the 4 study centers highlight the importance of local policies regarding the medical and public health interventions that may be used to address pediatric LRTI, especially among younger children hospitalized because of these infections.
Acknowledgments
The authors thank AbbVie (AbbVie contributed to the study design, research, analysis and interpretation of data, and publication writing, reviewing, and approval). The authors also thank Eloisa de Sá Moreira and Everardo D. Saad, from Dendrix for providing medical writing support; AbbVie Farmacêutica Brasil for the support; subinvestigators Ana Cláudia Dowsley (Universidade de Ciências de Saúde de Alagoas); Fernanda Rodrigues Dubourg (Universidade Federal da Bahia); Marlene Amália da Silva (Universidade Federal da Bahia); Marcela Pinheiro de Albuquerque (Instituto de Medicina Integral Professor Fernando Figueira); Marilha Will Albuquerque (Hospital Memorial Arthur Ramos); and study manager Karla Salomão (AbbVie).
Footnotes
Abbreviations: ICU = intensive care unit, LRTI = lower respiratory tract infection, NPA = nasopharyngeal aspirate, RSV = respiratory syncytial virus.
RQG, PGdMB, MdCMBD, AÁM, and ELS contributed equally to this work.
This study was funded by AbbVie, Inc.; RQG, MCMBD, AAM, LSS. PGMB received traveling support from AbbVie and Roche; ELS received traveling support from AbbVie, Roche, and Novartis; RBP and CES are employees of AbbVie Brazil and may hold stock or options.
The remaining authors have no conflicts of interest to disclose
REFERENCES
- 1.Bryce J, Boschi-Pinto C, Shibuya K, et al. WHO estimates of the causes of death in children. Lancet 2005; 365:1147–1152. [DOI] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380:2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 2010; 375:1545–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haynes AK, Manangan AP, Iwane MK, et al. Respiratory syncytial virus circulation in seven countries with Global Disease Detection Regional Centers. J Infect Dis 2013; 208 Suppl 3:S246–S254. [DOI] [PubMed] [Google Scholar]
- 5.Hervas D, Reina J, Hervas JA. Meteorologic conditions and respiratory syncytial virus activity. Pediatr Infect Dis J 2012; 31:e176–e181. [DOI] [PubMed] [Google Scholar]
- 6.Bloom-Feshbach K, Alonso WJ, Charu V, et al. Latitudinal variations in seasonal activity of influenza and respiratory syncytial virus (RSV): a global comparative review. PLoS One 2013; 8:e54445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bezerra PG, Britto MC, Correia JB, et al. Viral and atypical bacterial detection in acute respiratory infection in children under five years. PLoS One 2011; 6:e18928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferone EA, Berezin EN, Durigon GS, et al. Clinical and epidemiological aspects related to the detection of adenovirus or respiratory syncytial virus in infants hospitalized for acute lower respiratory tract infection. J Pediatr (Rio J) 2014; 90:42–49. [DOI] [PubMed] [Google Scholar]
- 9.Freitas FT. Sentinel surveillance of influenza and other respiratory viruses, Brazil, 2000-2010. Braz J Infect Dis 2013; 17:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.do Amaral de Leon C, Amantea SL, Pilger DA, et al. Clinical and epidemiologic profile of lower respiratory tract infections associated with human bocavirus. Pediatr Pulmonol 2013; 48:1112–1118. [DOI] [PubMed] [Google Scholar]
- 11.Salomao Junior JB, Gardinassi LG, Simas PV, et al. Human respiratory syncytial virus in children hospitalized for acute lower respiratory infection. J Pediatr (Rio J) 2011; 87:219–224. [DOI] [PubMed] [Google Scholar]
- 12.Pilger DA, Cantarelli VV, Amantea SL, et al. Detection of human bocavirus and human metapneumovirus by real-time PCR from patients with respiratory symptoms in Southern Brazil. Mem Inst Oswaldo Cruz 2011; 106:56–60. [DOI] [PubMed] [Google Scholar]
- 13.Thomazelli LM, Vieira S, Leal AL, et al. Surveillance of eight respiratory viruses in clinical samples of pediatric patients in southeast Brazil. J Pediatr (Rio J) 2007; 83:422–428. [DOI] [PubMed] [Google Scholar]
- 14.Moura FE, Nunes IF, Silva GB, Jr, et al. Respiratory syncytial virus infections in northeastern Brazil: seasonal trends and general aspects. Am J Trop Med Hyg 2006; 74:165–167. [PubMed] [Google Scholar]
- 15.Nascimento JP, Siqueira MM, Sutmoller F, et al. Longitudinal study of acute respiratory diseases in Rio de Janeiro: occurrence of respiratory viruses during four consecutive years. Rev Inst Med Trop Sao Paulo 1991; 33:287–296. [DOI] [PubMed] [Google Scholar]
- 16.Alonso WJ, Laranjeira BJ, Pereira SA, et al. Comparative dynamics, morbidity and mortality burden of pediatric viral respiratory infections in an equatorial city. Pediatr Infect Dis J 2012; 31:e9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moura FE, Perdigao AC, Ribeiro JF, et al. Respiratory syncytial virus epidemic periods in an equatorial city of Brazil. Influenza Other Respir Viruses 2013; 7:1128–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Straliotto SM, Siqueira MM, Muller RL, et al. Viral etiology of acute respiratory infections among children in Porto Alegre, RS, Brazil. Rev Soc Bras Med Trop 2002; 35:283–291. [DOI] [PubMed] [Google Scholar]
- 19.Vieira SE, Stewien KE, Queiroz DA, et al. Clinical patterns and seasonal trends in respiratory syncytial virus hospitalizations in Sao Paulo, Brazil. Rev Inst Med Trop Sao Paulo 2001; 43:125–131. [DOI] [PubMed] [Google Scholar]
- 20.Tsuchiya LR, Costa LM, Raboni SM, et al. Viral respiratory infection in Curitiba, Southern Brazil. J Infect 2005; 51:401–407. [DOI] [PubMed] [Google Scholar]
- 21.Cuevas LE, Nasser AM, Dove W, et al. Human metapneumovirus and respiratory syncytial virus, Brazil. Emerg Infect Dis 2003; 9:1626–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fe MM, Monteiro AJ, Moura FE. Parainfluenza virus infections in a tropical city: clinical and epidemiological aspects. Braz J Infect Dis 2008; 12:192–197. [DOI] [PubMed] [Google Scholar]
- 23.Moura FE, Mesquita JR, Portes SA, et al. Antigenic and genomic characterization of adenovirus associated to respiratory infections in children living in Northeast Brazil. Mem Inst Oswaldo Cruz 2007; 102:937–941. [DOI] [PubMed] [Google Scholar]
- 24.Silva Rde C, Siqueira MA, Netto EM, et al. Epidemiological aspects of influenza A related to climatic conditions during and after a pandemic period in the city of Salvador, northeastern Brazil. Mem Inst Oswaldo Cruz 2014; 109:229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cintra OA, Owa MA, Machado AA, et al. Occurrence and severity of infections caused by subgroup A and B respiratory syncytial virus in children in southeast Brazil. J Med Virol 2001; 65:408–412. [DOI] [PubMed] [Google Scholar]
- 26.Costa E, Rodríguez-Domínguez M, Clari MA, et al. Comparison of the performance of 2 commercial multiplex PCR platforms for detection of respiratory viruses in upper and lower tract respiratory specimens. Diagn Microbiol Infect Dis 2015; 82:40–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alvarez AE, Marson FA, Bertuzzo CS, et al. Epidemiological and genetic characteristics associated with the severity of acute viral bronchiolitis by respiratory syncytial virus. J Pediatr (Rio J) 2013; 89:531–543. [DOI] [PubMed] [Google Scholar]
- 28.Figueras-Aloy J, Carbonell-Estrany X, Quero-Jimenez J, et al. FLIP-2 Study: risk factors linked to respiratory syncytial virus infection requiring hospitalization in premature infants born in Spain at a gestational age of 32 to 35 weeks. Pediatr Infect Dis J 2008; 27:788–793. [DOI] [PubMed] [Google Scholar]
- 29.The IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 1998; 102:531–537. [PubMed] [Google Scholar]
- 30.Carbonell-Estrany X, Quero J, Bustos G, et al. Rehospitalization because of respiratory syncytial virus infection in premature infants younger than 33 weeks of gestation: a prospective study. IRIS Study Group. Pediatr Infect Dis J 2000; 19:592–597. [DOI] [PubMed] [Google Scholar]
- 31.Feltes TF, Cabalka AK, Meissner HC, et al. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr 2003; 143:532–540. [DOI] [PubMed] [Google Scholar]
- 32.Gardinassi LG, Marques Simas PV, Salomao JB, et al. Seasonality of viral respiratory infections in southeast of Brazil: the influence of temperature and air humidity. Braz J Microbiol 2012; 43:98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Checon RE, Siqueira MM, Lugon AK, et al. Short report: seasonal pattern of respiratory syncytial virus in a region with a tropical climate in southeastern Brazil. Am J Trop Med Hyg 2002; 67:490–491. [DOI] [PubMed] [Google Scholar]


