Abstract
There are several interventional therapies that improve the prognosis and increase the survival rate of early-stage hepatocellular carcinoma (early-stage HCC), but it is uncertain about whether one is superior to others, and available researches investigating the comparative effects of different treatments are limited. The main objective of this Bayesian network meta-analysis was to compare the efficacy of these different treatment strategies for early-stage HCC and rank these interventions for practical consideration.
We performed an electronic search of PubMed, Embase, and Cochrane Library, and extracted data from randomized controlled trials that compared different interventional therapies for early-stage HCC. Direct comparison and network meta-analyses were conducted with Aggregate Data Drug Information System software. Consistency models were created to determine whether there was a significant difference between any 2 therapies, and cumulative probability was used to rank different treatments.
Twenty-one randomized controlled trials involving 2691 patients were included. In our network meta-analysis, the combination therapy of transcatheter arterial chemoembolization (TACE) and radiofrequency ablation (RFA) was associated with better 1-year survival rate, as compared with hepatic resection alone (P < 0.05, odds ratio [OR] 0.25, 95% confidence interval [CI] 0.06–0.83), percutaneous ethanol injection (PEI) alone (P < 0.05, OR 0.13, 95% CI 0.03–0.45), and RFA alone (P < 0.05, OR 0.23, 95% CI 0.07–0.70). TACE + RFA had a higher 3-year survival rate than PEI alone (P < 0.05, OR 0.32, 95% CI 0.15–0.72) and RFA alone (P < 0.05, OR 0.45, 95% CI 0.24–0.87). And there was a statistical difference between RFA + PEI and PEI alone (P < 0.05, OR 0.33, 95% CI 0.12–0.93) for 3-year survival rate. The results of rank test and cumulative probability showed that TACE + RFA ranked highest on the evaluation of 1-year, 3-year, and 5-year survival rate.
Based on Bayesian network meta-analysis combining direct and indirect comparisons, the combination therapy of TACE and RFA seemed to be the most effective strategy for early-stage HCC.
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide, ranking fifth among all malignant tumors. According to the recent reports, it is estimated that about 500,000 to 1,000,000 new cases are diagnosed as HCC annually, which has the third mortality rate among cancers.1 Based on Milan criteria, the early-stage HCC is defined as a single HCC ≤ 5 cm in the maximum diameter or up to 3 nodules <3 cm.2 Currently, for the treatment measures of HCC, in addition to the conventional hepatic resection (HR) and liver transplantation, other interventional therapies consisting of transcatheter arterial chemoembolization (TACE), radiofrequency ablation (RFA), percutaneous ethanol injection (PEI), and some combination therapies.3–5 In theory, the best treatment for HCC is liver transplantation,6–8 and its role remains fundamental for cirrhotic patients with model for end-stage liver disease (MELD) ≥ 15, and also for patients with early-stage HCC, low-MELD cirrhosis, and technical impossibility to perform surgical resection or interventional therapies.9 Although the match range of donor to recipient is becoming greater and even the match between low-risk patients (patients with HCC) and high-risk donors (older donors and/or steatosic grafts) could be successfully realized,10 the serious shortage of donor organs cannot be effectively relieved and the high costs extremely limit the application of this treatment as well, which has raised the demand for novel treatment strategies of early-stage HCC.
According to the latest studies and the National Comprehensive Cancer Network (NCCN) guidelines, the treatment strategies containing HR, TACE, RFA, and PEI were effective and recommended for the early-stage HCC.11,12 However, the problem about the best choice is always conflicting and controversial. Based on the Practice guidelines issued by the American Association for the Study of Liver Diseases (AASLD), RFA was recommended as the primary treatment option for patients with early-stage HCC.13 In fact, HR is widely accepted as curative treatment for most of the patients with early-stage HCC, who are unwilling to receive liver transplantations.4 Furthermore, a number of related studies containing the original clinical trials and meta-analysis did not achieve a general consensus, or even the opposite conclusions.14–17
With the introduction of these therapeutic options, and the lack of randomized trials that directly compare all available treatments, we conducted a systematic review and Bayesian network meta-analysis comparing the efficacy of these different treatment strategies for early-stage HCC based on existing randomized controlled trials (RCTs) and ranking these interventions for practical consideration.
METHODS
Eligibility Criteria
All the studies met the following eligibility criteria: patients: adults (age >18 years) with established early-stage HCC conforming to the Milan criteria, with no history of treatments for HCC, with removal of macroscopically visible disease; interventional measures: established treatment strategies for early-stage HCC including TACE, RFA, PEI, HR, and their different combinations; comparators: any of the above mentioned treatment strategy; outcome: survival rate with at least 24 months of follow-up after the treatments.
Information Sources and Search
An electronic search was performed using PubMed, Embase, and Cochrane Library until February 2015, of which the search strings were based on MeSH terms, including: “transcatheter arterial chemoembolization,” “radiofrequency ablation,” “percutaneous ethanol injection,” “hepatic resection,” “early-stage HCC,” and “early-stage hepatocellular carcinoma.” These terms were used in different combinations. No limitation was placed on publication status or language.
Study Selection
We included randomized clinical trials (RCTs) that compared different interventional therapies for early-stage HCC. The duration follow-up of all studies was more than 24 months, and the outcomes we evaluated were survival rate at 1, 3, and 5 years after the treatment. The study with multiple arms was preferred as much as possible so as to build comparative loops in network meta-analysis.
In addition, we excluded: repeated publications; observational studies; patients with HCC that did not conform to the Milan criteria; treatment with an adjuvant chemotherapy after the intervention; studies were not measured by the aim outcomes of survival rate, or the results were reported incompletely.
Data Collection and Quality Assessment
Two reviewers independently extracted the data from each original publication including first author's name, year of publication, number of patients, patients’ characteristics, follow-up duration, and outcomes. Missing information was estimated according to the Cochrane Handbook and was requested from the authors of original studies if necessary. Discrepancies between the 2 reviewers were resolved by discussion and consensus. The Cochrane risk of bias assessment tool was used to assess the methodological quality of individual studies, based on the following aspects: random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome and assessment; incomplete outcome data; selective reporting; and other bias. Each item was answered with high, low, or unclear risk of bias, and disagreements were resolved through open discussion or a third reviewer.
Outcome Measures and Statistical Analysis
The primary outcome was overall survival rate, calculated as the difference value between the date of postintervention and the date of death. The endpoints were presented as 1-year, 3-year, and 5-year survival rate. We conducted the traditional pair-wise meta-analysis, and summarized the available data for survival rate from the results of all studies. Summary measures were calculated as odd ratios (ORs) for dichotomous variable, together with 95% confidence intervals (CIs), which was pooled using RevMan 5.3 software. The chi-square statistic was used to assess the heterogeneity between trials, and I2 values over 50% indicated substantial heterogeneity. Meanwhile, subgroup analysis was used to explore the important clinical difference among studies that might be expected to affect the magnitude of the interventional effect. A Bayesian network meta-analysis was performed to simultaneously compare all interventions in the network. The network meta-analysis can be considered to be an extension of the traditional pair-wise meta-analysis, as it incorporates both direct and indirect information through a common comparator to obtain estimates of the relative interventional effects on the multiple interventions comparisons,18–20 which was performed by using the automated software Aggregate Data Drug Information System (ADDIS).21 Based on Bayesian evidence network, we evaluated consistency by combining the estimates from direct and indirect comparisons. The rank accumulate probability plot produced by the network meta-analysis was to find out which administered intervention is the best. Node-splitting models were conducted to assess whether direct and indirect effect is in agreement. It was deemed significant when P < 0.05.
RESULTS
Study Selection
According to the electronic search strategy, a total of 1110 relevant studies were identified. After removal of duplicates and title/abstract screening, 128 trials were eligible for full text. Subsequently, we excluded 46 nonoriginal clinical trials and 61 studies that were noneligible in any one of the 4 parts: patients, interventions, comparisons, and outcomes. As a result, 21 RCTs were involved in this network meta-analysis.22–42Figure 1 shows a flow chat of literatures from the initial results of the publication searches to the final inclusion or exclusion.
FIGURE 1.
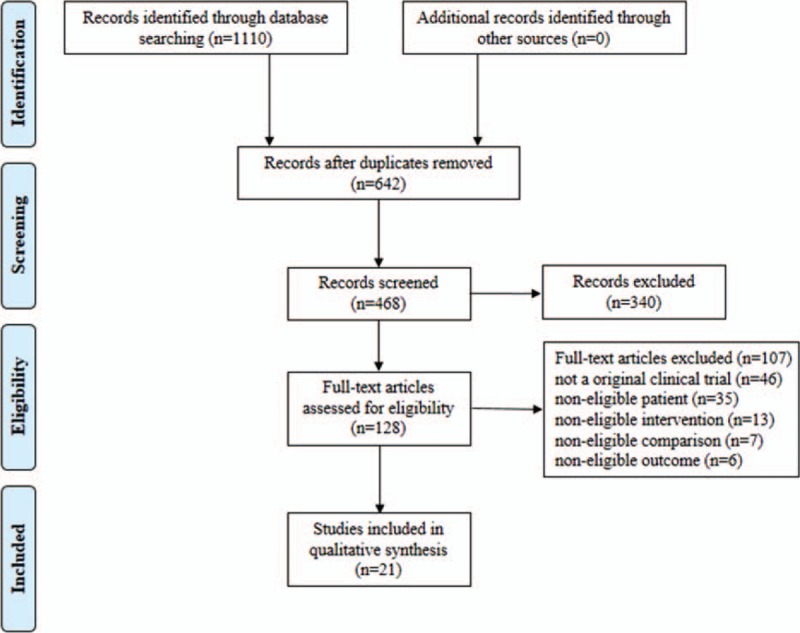
Flow chart of RCTs evaluating interventional therapies for early-stage HCC through selection process.
Study Characteristics and Quality Assessment
We included 21 RCTs containing 6 different treatments: TACE, RFA, PEI, HR, TACE + RFA and RFA + PEI, of which the sample size ranged from 37 to 285, and the duration of follow-up ranged from 24 to 60 months. Table 1 provides the characteristics of included studies. Twenty studies reported random sequence generation, and most of them used the random numbers generated from computer. Thirteen studies reported allocation concealment. However, the blinding method was not performed in most trials, leading to a high risk of bias. For the assessment of incomplete outcome data, almost all of the studies provided the information on withdrawal of patients. Similarly, we also did not find obvious risk in the evaluation of selective reporting and other bias. In summary, the studies seemed to be at low to moderate risk of bias. Overall and study-level quality assessments were summarized in Figure 2.
TABLE 1.
Characteristics of Included Studies
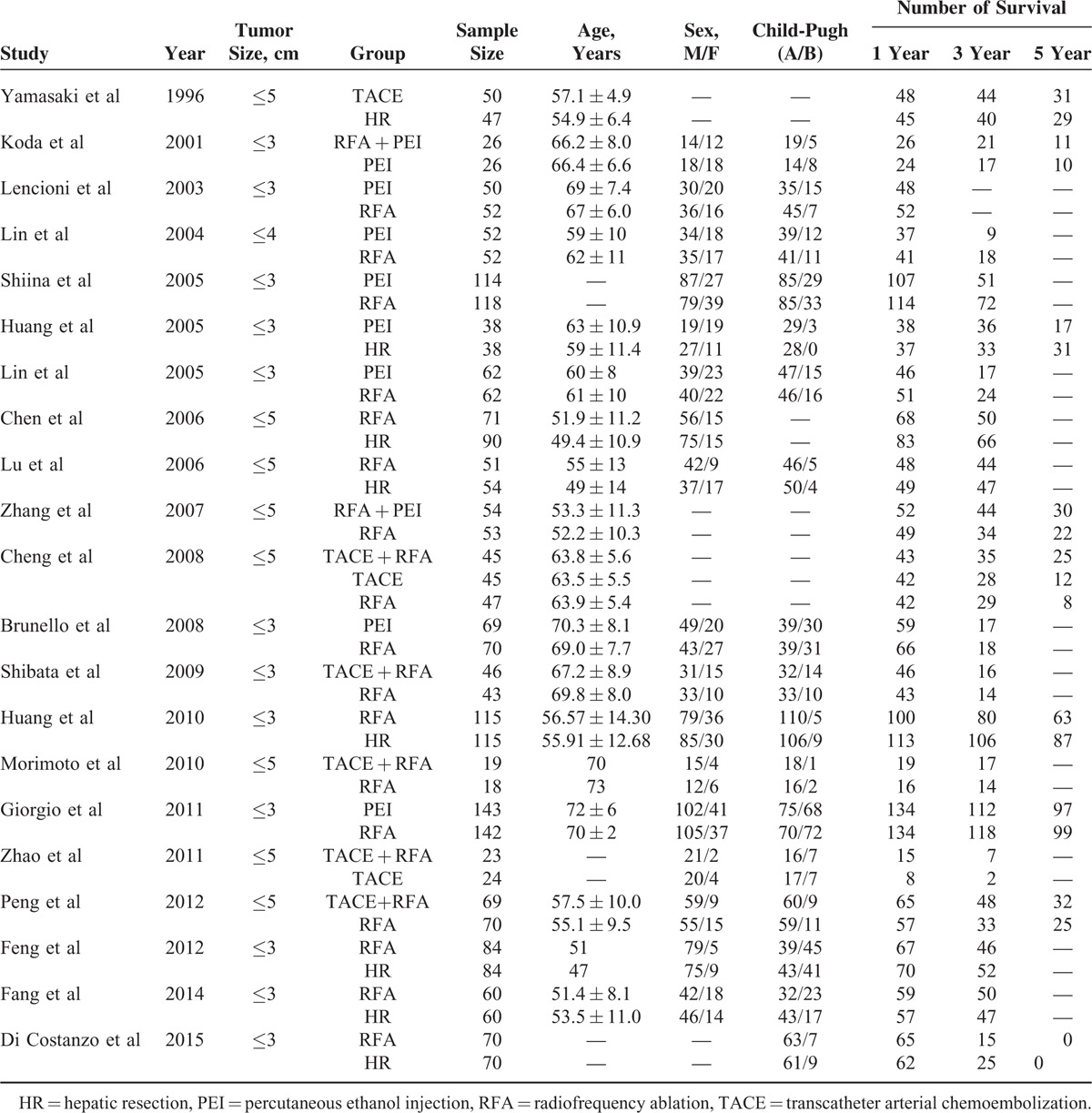
FIGURE 2.
Quality assessment of included studies: (A) overall and (B) study-level risk of bias.
Direct Meta-analysis
Results from direct pair-wise meta-analysis of interventional therapies for early-stage HCC are shown in Figure 3. Compared with the combination of TACE and RFA, RFA alone (P = 0.008, OR 3.496, 95% CI 1.395–8.759) and TACE alone (P = 0.041, OR 2.850, 95% CI 1.044–7.776) did not benefit 1-year survival rate; there was no significant difference between RFA and HR. PEI was associated with lower 1-year survival rate as compared with RFA (P = 0.016, OR 0.582, 95% CI 0.375–0.902). Similarly, there was significant reduced 3-year survival rate in patients who received RFA (P = 0.006, OR 1.877, 95% CI 1.193–2.954) and TACE (P = 0.019, OR 2.608, 95% CI 1.167–5.825) as compared with TACE + RFA; PEI was also associated with lower 3-year survival rate than RFA (P = 0.001, OR 0.616, 95% CI 0.457–0.830), which did not benefit 3-year survival rate as compared with HR (P = 0.001, OR 0.609, 95% CI 0.449–0.826). The therapy of TACE + RFA still presented higher 5-year survival rate than RFA alone (P = 0.001, OR 2.497, 95% CI 1.455–4.285). As the limit of the quantity of trials, we did not conduct the direct meta-analysis for the other comparisons of different interventional therapies.
FIGURE 3.
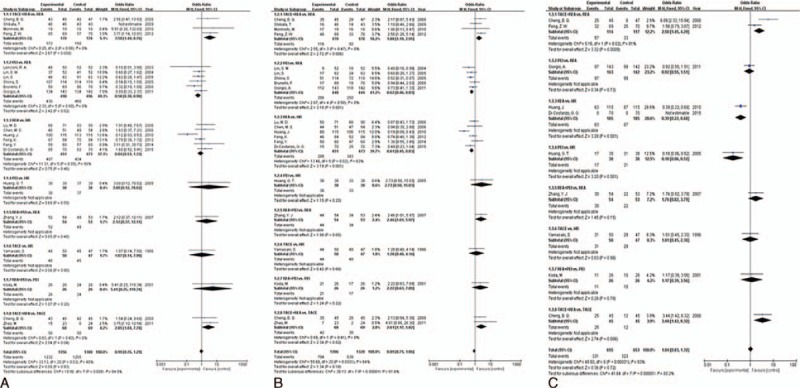
All direct meta-analyses: (A) 1-year survival rate; (B) 3-year survival rate; (C) 5-year survival rate.
Network Meta-analysis
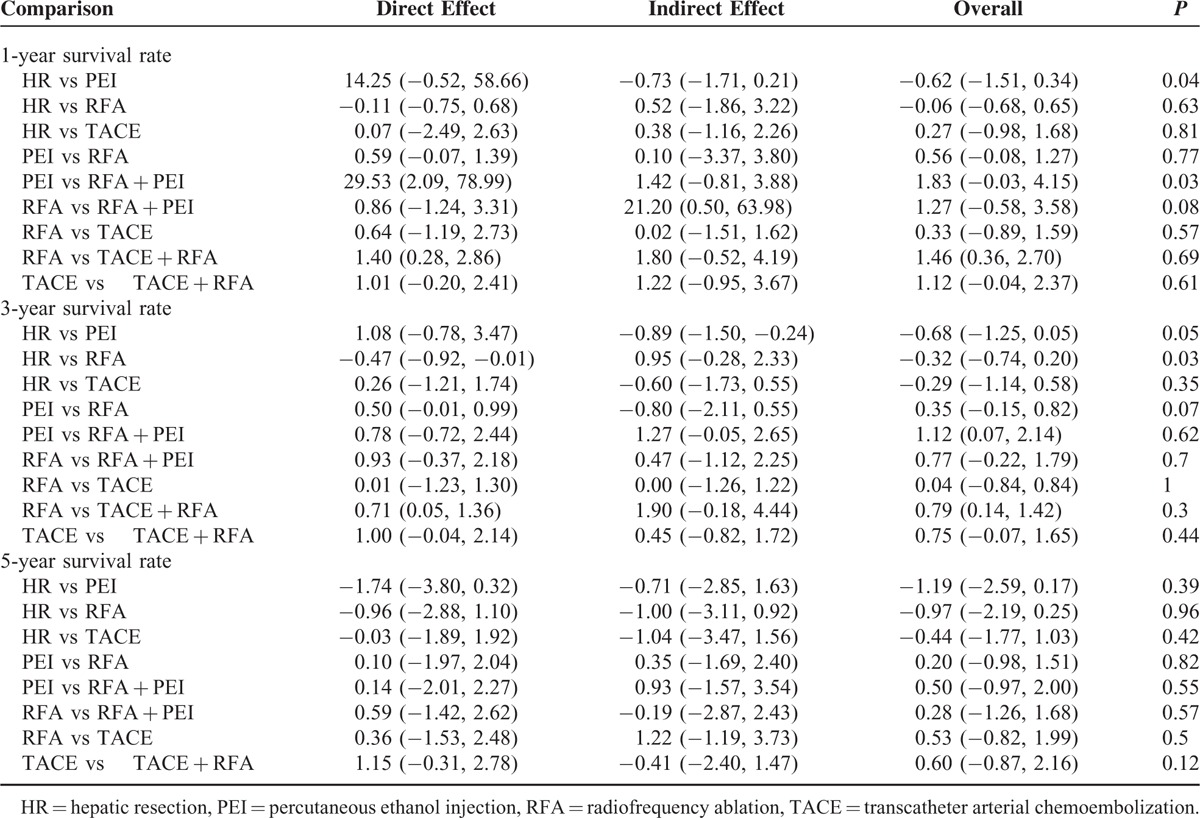
The 21 RCTs covered the presently used 6 categories of interventional therapies including TACE, RFA, PEI, HR, TACE + RFA, and RFA + PEI with a total of 2691 participants. We established a network to compare the above mentioned 6 treatments, and Figure 4 shows the overall comparison network. Node-splitting models were conducted to assess the inconsistency by testing the difference between the direct and indirect effect. If the P value is more than 0.05, it indicates that the difference between the direct and indirect effect was not significant (Table 2).
FIGURE 4.
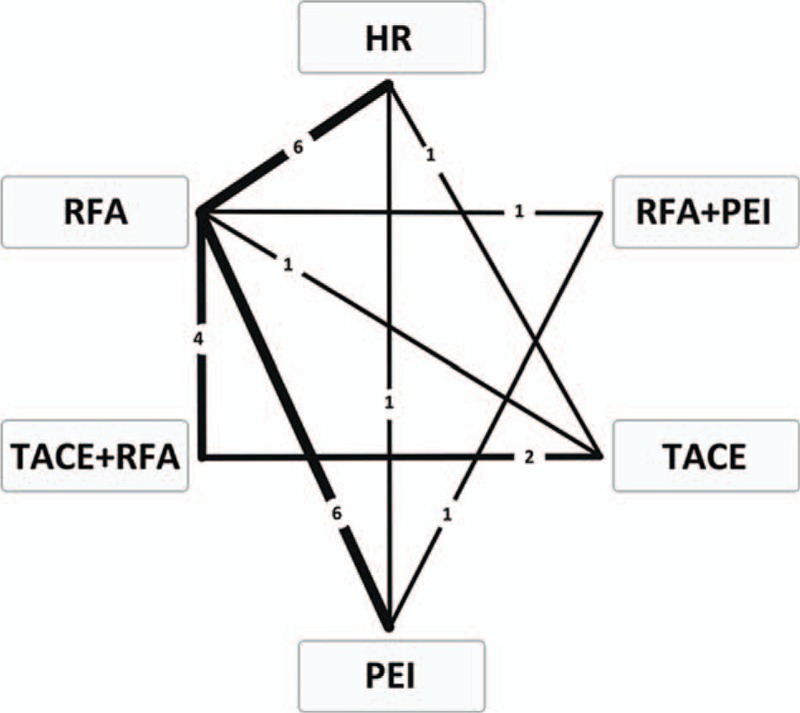
Comparison network of the included RCTs. The line linked between 2 interventional therapies means there are direct comparisons from original studies. The number on the line means the quality of studies comparing every pair of treatments, which were also represented by the width of the lines. RCTs = randomized controlled trials.
TABLE 2.
Results of Node-splitting Models for the Test of Difference Between Direct and Indirect Effect in the Analysis of Primary Outcomes of 1-Year, 3-Year, and 5-Year Survival Rate
One-year Survival Rate
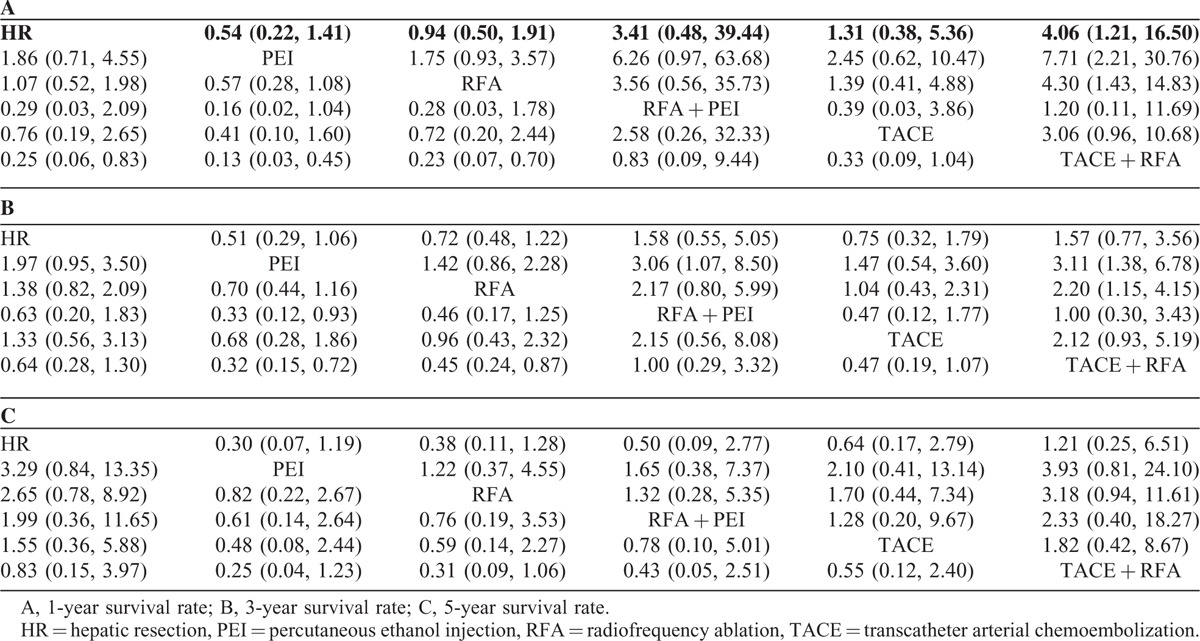
A total of 21 studies involving all 6 kinds of interventional therapies analyzed 1-year survival rate. Table 3A presented the comparison of different interventional therapies on 1-year survival rate. The network meta-analysis showed that compared with the combination of TACE and RFA, HR alone (P < 0.05, OR 0.25, 95% CI 0.06–0.83), PEI alone (P < 0.05, OR 0.13, 95% CI 0.03–0.45), and RFA alone (P < 0.05, OR 0.23, 95% CI 0.07–0.70) were associated with lower 1-year survival rate. No significant difference was found in the other comparisons. The node-splitting models (Table 2) indicated that there were significant differences between direct and indirect effect in the comparisons of HR versus PEI and PEI versus RFA + PEI.
TABLE 3.
The Network Meta-analysis Results For 6 Kinds of Interventional Therapies
Three-year Survival Rate
A total of 20 studies containing all 6 categories of interventional therapies described the analysis of 3-year survival rate. The network meta-analysis demonstrated that 2 interventional therapies had a significant effect on the reduction of 3-year survival rate as compared with TACE + RFA, which were PEI alone (P < 0.05, OR 0.32, 95% CI 0.15–0.72) and RFA alone (P < 0.05, OR 0.45, 95% CI 0.24–0.87). In addition, there was a statistical difference between RFA + PEI and PEI alone (P < 0.05, OR 0.33, 95% CI 0.12–0.93), but not in the other comparisons (Table 3B). Simultaneously, the results of node-splitting models (Table 2) illuminated that there was statistical difference between direct and indirect effect in the comparisons of HR versus RFA.
Five-year Survival Rate
A total of 9 studies including all 6 different kinds of interventional therapies provided the analysis of 5-year survival rate. The results of network meta-analysis revealed that there was no significant difference in any comparison (Table 3C). Likewise, the node-splitting models detect no statistical differences between direct and indirect effect (Table 2).
Rank Test
The Bayesian network meta-analysis could apply the rank probabilities of each interventional therapy and the cumulative probability sum of the rank probabilities to give an overall probability. And larger cumulative probability means the better effect on the improvement of 1-year, 3-year, and 5-year survival rate. Table 4 and Figure 5 show the results of rank test and cumulative probability of all the 6 interventional therapies on each outcome, which demonstrated that among all the interventional therapies, the combination of TACE and RFA ranked highest on the evaluation of 1-year, 3-year, and 5-year survival rate. RFA combined with PEI ranked the second highest for 1-year and 3-year survival rate. However, the rank of HR moved to second place for 5-year survival rate.
TABLE 4.
Results of Rank Test for Different Interventional Therapies
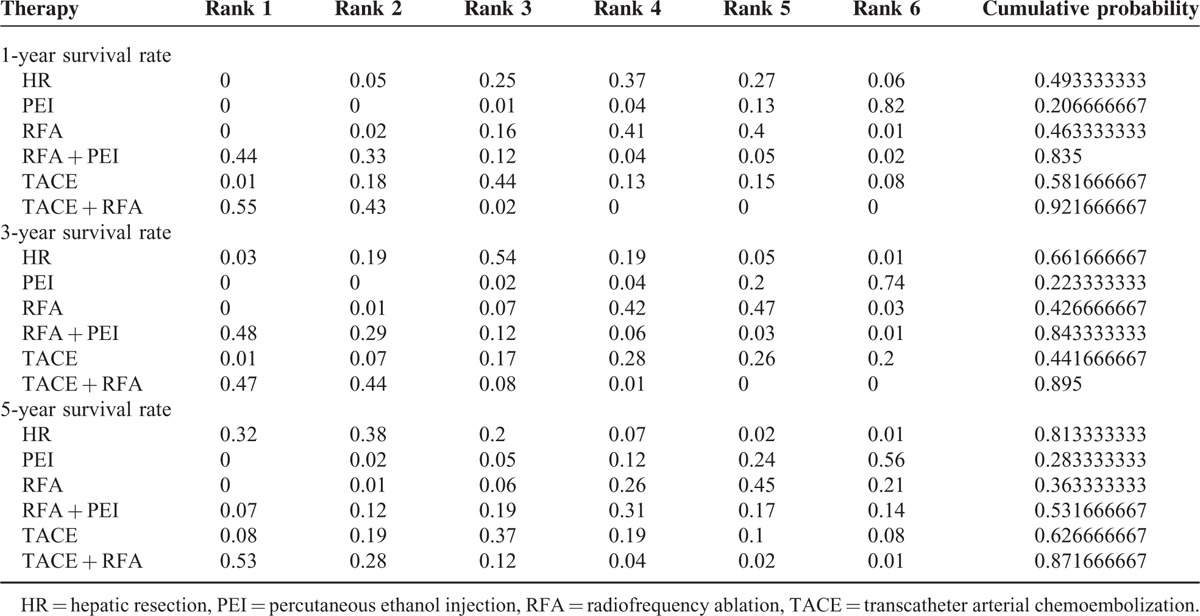
FIGURE 5.
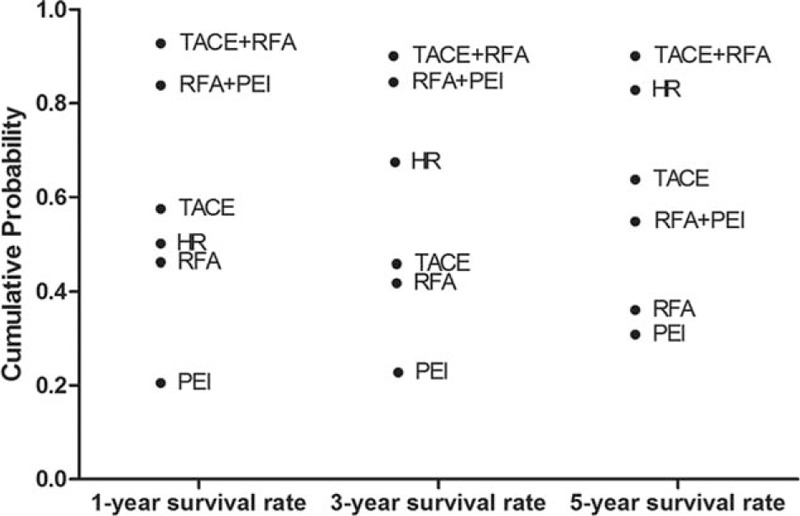
Cumulative probability of different interventional therapies as measured by the included outcomes.
DISCUSSION
Currently, several interventional therapies have been applied in early-stage HCC, TACE, RFA, PEI, and HR, which has always been regarded as the traditional first-line treatment for early-stage HCC. However, as the idea of minimally invasive treatment gained more and more popularity, the other nonsurgical therapies have been developed. Among them, PEI—injecting absolute ethanol directly into lesions through a fine needle—can achieve complete necrosis of lesions and has been widely used as a standard therapy for early-stage HCC.43 It has been proved that the long-term survival rates in patients treated with PEI are similar to those in matched patients who underwent HR, but the spread of injected ethanol is largely affected by the capsule or septa of lesion, which could lead to a long treatment time and a local recurrence rate of 10% to 30%.44 RFA is a medical procedure in which part of the tumor is ablated using the heat generated from a high-frequency alternating current under image guidance. It has been identified that RFA has been regarded as a suitable treatment for HCC because of its low trauma, low number of complications, and strong repeatability.45 TACE, which concentrates chemotherapeutic agents at the tumor site while blocking the primary artery feeding the tumor, was considered to be the most frequent loco-regional treatment for unresectable HCC.13 But now, it is reported that TACE is starting to be used in early-stage HCC and shows a prolonged survival rate.42 In addition, the combination therapies such as TACE + RFA and RFA + PEI have raised researchers’ concerns. Though numerous studies including the original RCTs and meta-analyses have examined whether a certain therapy was more effective than another in the treatment of patients with early-stage HCC, the conclusions were controversial, and we cannot get a best advice from these several measures.
Summary of Evidence
This is the first systematic review and Bayesian network meta-analysis comparing the effectiveness of different interventional therapies for early-stage HCC through direct and indirect statistical comparisons based on all available information from the included RCTs. Our study found that in comparison to HR alone, PEI alone, and RFA alone, the combination therapy of TACE and RFA was associated with better 1-year survival rate. For 3-year survival rate, there was no significant difference between the HR alone and TACE + RFA, but the latter still benefited the survival as compared with the other 2 therapies. Meanwhile, the combination therapy of RFA and PEI had a higher 3-year survival rate than PEI alone. For 5-year survival rate, we observed no statistical difference in any comparison, indicating that the long-term survival rate of the patients with early-stage HCC was invariable, though they received 6 different interventional therapies. Nevertheless, the results of direct comparison and network meta-analysis were not consistent, which suggested that these results were unstable. Anyway, in such a condition, we would rather believe the results from direct comparison meta-analysis, because the network meta-analysis incorporates indirect comparisons which could increase the risk of statistical errors. So its confidence needs to be confirmed by future multicenter large-sample high-quality RCTs.
The data of rank test demonstrated that among all the 6 interventional therapies, TACE + RFA ranked highest in efficacy for increasing of 1-year, 3-year, and 5-year survival rate. Furthermore, the other combination therapy of RFA and PEI ranked the second highest for 1-year and 3-year survival rate. Therefore, the results suggested that the combination therapies, especially TACE + RFA, had the greatest efficacy for prognosis of early-HCC. In addition, the rank of HR also interested us. As time went on, it showed a gradual increase in survival rate, and its rank moved to second place for 5-year survival rate. So we deduced that the trauma caused by HR was the most difficult to tolerate for patients with early-stage HCC, coupled with the influence of postoperative complications, leading to a relatively low short-term survival rate. However, through an intuitive way, the surgical approach could completely eliminate the tumor as far as possible, which was associated with a low local recurrence rate. This is consistent with a recent meta-analysis,14 which revealed that whether for 1-year disease-free survival, for 3-year disease-free survival, or for 5-year disease-free survival, HR was significantly higher than RFA.
The aim of our network meta-analysis was to evaluate the effect of different interventional therapies for early-stage HCC. Results from this network meta-analysis may guide clinicians in the recommendations of different treatments in the absence of head-to-head comparisons.
Limitations
However, there were some limitations in this study. To begin with, all information was extracted from published data rather than individual patient data, which may have resulted in publication bias. Second, due to the limited number of direct comparative studies, the estimates purely based on indirect studies warranted lower confidence compared with those based on combined direct and indirect comparisons, and the strength of the inference was not high enough for these estimates. Third, we performed the node-splitting models to assess the inconsistency between direct and indirect effect, and some inconsistency was detected in the comparison of HR versus PEI and PEI versus RFA + PEI on 1-year survival rate, and HR versus RFA on 3-year survival rate that the overall effect was not in line with the direct effect, which suggested the conclusions comparing these interventional therapies should be carefully considered. Next, due to the limitations of original researches, we could not determine whether the patients were diagnosed as having liver cirrhosis that largely affected the prognosis. Actually, in the clinical practice, patients with liver cirrhosis and early-stage HCC who received HR are often associated with very poor survival rate, indicating that when formulating the therapeutic plan of early HCC, we should consider whether the patients are suffering from liver cirrhosis. Lastly, we cannot assume that the choice between the treatment modalities is only related to results in terms of outcome. On the contrary, the location of the lesion in relation to the liver anatomy; the visibility of lesion using ultrasounds; the degree of portal hypertension; the liver function represent major biases in the choice of the treatment. This fact should be recognized. In other words, lesions located in the anterior segments can be easily approached by all treatment modalities (including laparoscopic resection), whereas lesions close to supra-hepatic veins or close to portal bifurcation cannot be treated by RFA, and small sub-Glissonian lesions of the VIII or II segments close to the diaphragm are very difficult to approach with loco-regional therapies. Furthermore, HCC close to the vena cava remains a challenging issue, particularly in the cirrhotic patient. In these cases, liver transplantation is the sole option. Therefore, the results of this network meta-analysis may not completely conform to the clinical practice to some extent.
CONCLUSIONS
In conclusion, by using a Bayesian network meta-analysis involving 21 RCTs comparing 6 different interventional therapies, our research demonstrated that the combination therapy of TACE and RFA was the best therapeutic option for early-stage HCC in terms of improving outcomes of 1-year, 3-year, and 5-year survival rate. However, taking above mentioned shortcomings into consideration, multicenter large-sample high-quality RCTs are warranted to establish the comparative efficacy of different interventional therapies for early-stage HCC. Nonetheless, the application of this Bayesian network meta-analysis in this setting can help inform current therapeutic decision-making and guide the design of future studies.
Ethical Review
Ethical approval was not necessary, because this article is a meta-analysis and it does not involve the participation of ethics committee.
Footnotes
Abbreviations: AASLD = American Association for the Study of Liver Diseases, ADDIS = Aggregate Data Drug Information System, CI = confidence interval, HCC = hepatocellular carcinoma, HR = hepatic resection, NCCN = National Comprehensive Cancer Network, OR = odds ratio, PEI = percutaneous ethanol injection, RCTs = randomized controlled trials, RFA = radiofrequency ablation, TACE = transcatheter arterial chemoembolization.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007; 132:2557–2576. [DOI] [PubMed] [Google Scholar]
- 2.Simonetti RG, Camma C, Fiorello F, et al. Hepatocellular carcinoma. A worldwide problem and the major risk factors. Digest Dis Sci 1991; 36:962–972. [DOI] [PubMed] [Google Scholar]
- 3.Benson AB3rd, Abrams TA, Ben-Josef E, et al. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. J Natl Comprehensive Cancer Netw 2009; 7:350–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol 2008; 48 Suppl 1:S20–37. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003; 362:1907–1917. [DOI] [PubMed] [Google Scholar]
- 6.Seshadri RM, Besur S, Niemeyer DJ, et al. Survival analysis of patients with stage I and II hepatocellular carcinoma after a liver transplantation or liver resection. HPB 2014; 16:1102–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee KK, Kim DG, Moon IS, et al. Liver transplantation versus liver resection for the treatment of hepatocellular carcinoma. J Surg Oncol 2010; 101:47–53. [DOI] [PubMed] [Google Scholar]
- 8.Hasegawa K, Kokudo N, Makuuchi M. Surgical management of hepatocellular carcinoma. Liver resection and liver transplantation. Saudi Med J 2007; 28:1171–1179. [PubMed] [Google Scholar]
- 9.Biggins SW, Gralla J, Dodge JL, et al. Survival benefit of repeat liver transplantation in the United States: a serial MELD analysis by hepatitis C status and donor risk index. Am J Transplant 2014; 14:2588–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avolio AW, Halldorson JB, Burra P, et al. Balancing utility and need by means of donor-to-recipient matching: a challenging problem. Am J Transplant 2013; 13:522–523. [DOI] [PubMed] [Google Scholar]
- 11.Zhang YY, Xia HH. Novel therapeutic approaches for hepatocellulcar carcinoma: fact and fiction. World J Gastroenterol 2008; 14:1641–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramanathan R, Sharma A, Lee DD, et al. Multimodality therapy and liver transplantation for hepatocellular carcinoma: a 14-year prospective analysis of outcomes. Transplantation 2014; 98:100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology (Baltimore, Md) 2011; 53:1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duan C, Liu M, Zhang Z, et al. Radiofrequency ablation versus hepatic resection for the treatment of early-stage hepatocellular carcinoma meeting Milan criteria: a systematic review and meta-analysis. World J Surg Oncol 2013; 11:190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salhab M, Canelo R. An overview of evidence-based management of hepatocellular carcinoma: a meta-analysis. J Cancer Res Therapeut 2011; 7:463–475. [DOI] [PubMed] [Google Scholar]
- 16.Dong W, Zhang T, Wang ZG, et al. Clinical outcome of small hepatocellular carcinoma after different treatments: a meta-analysis. World J Gastroenterol 2014; 20:10174–10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marelli L, Stigliano R, Triantos C, et al. Treatment outcomes for hepatocellular carcinoma using chemoembolization in combination with other therapies. Cancer Treatment Rev 2006; 32:594–606. [DOI] [PubMed] [Google Scholar]
- 18.Salanti G, Higgins JP, Ades AE, et al. Evaluation of networks of randomized trials. Stat Methods Med Res 2008; 17:279–301. [DOI] [PubMed] [Google Scholar]
- 19.Jansen JP, Crawford B, Bergman G, et al. Bayesian meta-analysis of multiple treatment comparisons: an introduction to mixed treatment comparisons. Value Health 2008; 11:956–964. [DOI] [PubMed] [Google Scholar]
- 20.Glenny AM, Altman DG, Song F, et al. Indirect comparisons of competing interventions. Health Technol Assess (Winchester, England) 2005; 9:1–134.iii-iv. [DOI] [PubMed] [Google Scholar]
- 21.Valkenhoef G, Tervonen T, Brock B, et al. Deficiencies in the transfer and availability of clinical trials evidence: a review of existing systems and standards. BMC Med Inform Decision Making 2012; 12:95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shibata T, Isoda H, Hirokawa Y, et al. Small hepatocellular carcinoma: is radiofrequency ablation combined with transcatheter arterial chemoembolization more effective than radiofrequency ablation alone for treatment? Radiology 2009; 252:905–913. [DOI] [PubMed] [Google Scholar]
- 23.Morimoto M, Numata K, Kondou M, et al. Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: a randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Cancer 2010; 116:5452–5460. [DOI] [PubMed] [Google Scholar]
- 24.Peng ZW, Zhang YJ, Liang HH, et al. Recurrent hepatocellular carcinoma treated with sequential transcatheter arterial chemoembolization and RF ablation versus RF ablation alone: a prospective randomized trial. Radiology 2012; 262:689–700. [DOI] [PubMed] [Google Scholar]
- 25.Lencioni RA, Allgaier HP, Cioni D, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology 2003; 228:235–240. [DOI] [PubMed] [Google Scholar]
- 26.Lin SM, Lin CJ, Lin CC, et al. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or =4 cm. Gastroenterology 2004; 127:1714–1723. [DOI] [PubMed] [Google Scholar]
- 27.Lin SM, Lin CJ, Lin CC, et al. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut 2005; 54:1151–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shiina S, Teratani T, Obi S, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology 2005; 129:122–130. [DOI] [PubMed] [Google Scholar]
- 29.Giorgio A, Di Sarno A, De Stefano G, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma compared to percutaneous ethanol injection in treatment of cirrhotic patients: an Italian randomized controlled trial. Anticancer Res 2011; 31:2291–2295. [PubMed] [Google Scholar]
- 30.Brunello F, Veltri A, Carucci P, et al. Radiofrequency ablation versus ethanol injection for early hepatocellular carcinoma: a randomized controlled trial. Scand J Gastroenterol 2008; 43:727–735. [DOI] [PubMed] [Google Scholar]
- 31.Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg 2006; 243:321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fang Y, Chen W, Liang X, et al. Comparison of long-term effectiveness and complications of radiofrequency ablation with hepatectomy for small hepatocellular carcinoma. J Gastroenterol Hepatol 2014; 29:193–200. [DOI] [PubMed] [Google Scholar]
- 33.Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol 2012; 57:794–802. [DOI] [PubMed] [Google Scholar]
- 34.Huang J, Yan L, Cheng Z, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg 2010; 252:903–912. [DOI] [PubMed] [Google Scholar]
- 35.Di Costanzo GG, Tortora R, D’Adamo G, et al. Radiofrequency ablation versus laser ablation for the treatment of small hepatocellular carcinoma in cirrhosis: a randomized trial. J Gastroenterol Hepatol 2015; 30:559–565. [DOI] [PubMed] [Google Scholar]
- 36.Lu MD, Kuang M, Liang LJ, et al. [Surgical resection versus percutaneous thermal ablation for early-stage hepatocellular carcinoma: a randomized clinical trial]. Zhonghua yi xue za zhi 2006; 86:801–805. [PubMed] [Google Scholar]
- 37.Huang GT, Lee PH, Tsang YM, et al. Percutaneous ethanol injection versus surgical resection for the treatment of small hepatocellular carcinoma: a prospective study. Ann Surg 2005; 242:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang YJ, Liang HH, Chen MS, et al. Hepatocellular carcinoma treated with radiofrequency ablation with or without ethanol injection: a prospective randomized trial. Radiology 2007; 244:599–607. [DOI] [PubMed] [Google Scholar]
- 39.Yamasaki S, Hasegawa H, Kinoshita H, et al. A prospective randomized trial of the preventive effect of pre-operative transcatheter arterial embolization against recurrence of hepatocellular carcinoma. Jpn J Cancer Res 1996; 87:206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koda M, Murawaki Y, Mitsuda A, et al. Combination therapy with transcatheter arterial chemoembolization and percutaneous ethanol injection compared with percutaneous ethanol injection alone for patients with small hepatocellular carcinoma: a randomized control study. Cancer 2001; 92:1516–1524. [DOI] [PubMed] [Google Scholar]
- 41.Zhao M, Wang JP, Li W, et al. [Comparison of safety and efficacy for transcatheter arterial chemoembolization alone and plus radiofrequency ablation in the treatment of single branch portal vein tumor thrombus of hepatocellular carcinoma and their prognosis factors]. Zhonghua yi xue za zhi 2011; 91:1167–1172. [PubMed] [Google Scholar]
- 42.Cheng BQ, Jia CQ, Liu CT, et al. Chemoembolization combined with radiofrequency ablation for patients with hepatocellular carcinoma larger than 3 cm: a randomized controlled trial. JAMA 2008; 299:1669–1677. [DOI] [PubMed] [Google Scholar]
- 43.Livraghi T, Giorgio A, Marin G, et al. Hepatocellular carcinoma and cirrhosis in 746 patients: long-term results of percutaneous ethanol injection. Radiology 1995; 197:101–108. [DOI] [PubMed] [Google Scholar]
- 44.Shiina S, Tagawa K, Unuma T, et al. Percutaneous ethanol injection therapy for hepatocellular carcinoma. A histopathologic study. Cancer 1991; 68:1524–1530. [DOI] [PubMed] [Google Scholar]
- 45.Xu HX, Lu MD, Xie XY, et al. Prognostic factors for long-term outcome after percutaneous thermal ablation for hepatocellular carcinoma: a survival analysis of 137 consecutive patients. Clin Radiol 2005; 60:1018–1025. [DOI] [PubMed] [Google Scholar]


