Abstract
To investigate the clinical outcome of the Trabectome in Chinese open-angle glaucoma (OAG).
This prospective case series recruited pseudophakic glaucoma subjects with open-angle configuration. Trabeculectomy ab interno was performed using the Trabectome to 120° of the trabecular meshwork. Intraocular pressure (IOP) and medications were recorded preoperatively and every 3 months postoperatively. Visual acuity was measured preoperatively and at 1 and 6 months postoperatively. One-way ANOVA with Tukey Multiple Comparison Test were used to measure the pre and postoperative parameters.
In 19 eyes of 19 Chinese subjects, 26.3% were uveitic, 68.4% were primary open-angle glaucoma, and 5.3% had a history of chronic angle-closure glaucoma with open-angles after cataract extraction. The subjects’ mean age was 67.5 ± 14.4 years, with 4 females and 15 males. Two patients required secondary filtration procedure. At 6 months, the IOP reduced by 34.8% (24.4 ± 4.4 mm Hg to 15.9 ± 5.1 mm Hg, P < 0.0001). The number of types of antiglaucoma medications was reduced by 28.2% (3.9 ± 0.8–2.8 ± 1.6, P < 0.0001). The visual acuity was static at 1 and 6 months postoperatively (P = 0.4). There were no intraoperative complications. 26.3% of subjects had a transient IOP spike > 21 mm Hg, 1 had hyphema requiring washout, and 1 had reactivation of herpetic keratitis. The success rate at 6 months was 89.5%.
Trabectome achieved a modest reduction in IOP and medications in the majority of pseudophakic Chinese OAG eyes.
INTRODUCTION
In the past decade or so, there have been many developments in minimally invasive glaucoma surgeries in the hope to minimize complications from traditional filtration surgeries while achieving a reasonable amount of intraocular pressure (IOP) lowering. The Trabectome is a surgical device approved for use by the Food and Drug Administration in 2004. It uses an electrical probe placed through a 1.8 mm clear corneal incision to reestablish the outflow of aqueous by stripping the trabecular meshwork and the inner wall of the Schlemm canal.1,2
The Trabectome can be done as a stand-alone procedure or in combination with cataract extraction or other glaucoma surgeries. In a retrospective case series involving 1127 Trabectome cases, the mean IOP reduction was approximately 39% and antiglaucoma medication reduction was around 57% at 24 months. However, about 34.5% of the reported Trabectome cases were combined with other procedures and not just the Trabectome procedure alone. The patient demographics were also predominantly Caucasian or Hispanics, with only 3.5% of the patients being of Asian ethnicity.3 The aim of this study was to investigate the safety and efficacy of using the Trabectome as a stand-alone procedure in the treatment of pseudophakic eyes in Chinese open-angle glaucoma (OAG) patients.
PATIENTS AND METHODS
This was a prospective study conducted at a district hospital in Hong Kong Special Administrative Region, China. Subjects were recruited from a glaucoma subspecialty clinic. OAG patients requiring filtration surgery for IOP control despite maximally tolerated antiglaucoma medications were recruited. The inclusion criteria included: consenting adults >18 years of age; open-angle configuration of Grade 2 or above in ≥90° on gonioscopy (Shaffer grading), pseudophakia, and evidence of glaucomatous optic neuropathy on optical coherence tomography or Humphrey Visual Field. The exclusion criteria included subjects with only one functional eye and those with preexisting corneal pathologies or scars. Publication funding was provided by Neomedix Corporation (Tustin, CA). The authors had full autonomy over the data analysis and write-up with no involvement by the funding source.
The Trabectome procedure:
Topical anesthesia with xylocaine gel 2% and intracameral lignocaine 2%.
The operating microscope was tilted to 30° away from the surgeon and the patient's head was turned in the opposite direction of the operating eye to maximize the visualization of the nasal angle.
A 1.8 mm clear cornea incision was made temporally.
Placement of the Trabectome tip into the Schlemm canal under gonioscopic guidance.
120° ablation was made with the Trabectome to strip the inner wall of Schlemm and trabecular meshwork.
Irrigation and aspiration was performed to washout any blood reflux.
Stromal hydration to close the corneal wound.
Intracameral cefuroxime 1 mg in 0.1 mL.
After the procedure, all preexisting topical antiglaucoma medications were continued. Postoperative medications include pilocarpine 1% 4 times daily for 4 weeks, topical antibiotic 4 times daily, and topical steroids 4 times daily for 1 to 2 months postoperatively. Patients were seen the day 1 postoperatively and at 1 week, 1 month, and every 3 months thereafter. At about 1 month postoperatively, antiglaucoma medications were stepped down in the following order: pilocarpine, prostaglandin analogs, topical carbonic anhydrase inhibitors, alpha-agonists, and then beta-blockers. Fixed combination eye drops were used where available. Medications were titrated according to the attending ophthalmologist's discretion taking into consideration the severity of each patient's disease and individual target IOP's, using a 25% IOP reduction from initial IOP as a minimum reference as per the findings from the Early Manifest Glaucoma Trial.4
The main outcome measure was the Goldmann applanation-measured IOP at the preoperative baseline and after the Trabectome procedure (day 1, 1 week as well as 1, 3, and 6 months). Secondary outcome measures included: complications from the Trabectome procedure (intraoperative and postoperative) as well as the number of antiglaucoma medications at baseline as well as 3 and 6 months postoperatively. Fixed combination eye drops were counted as 2 types of medications. Success was defined as IOP ≤21 mm Hg with or without topical antiglaucoma medications at 6 months. Failure was defined as IOP >21 mm Hg at 6 months or requiring secondary filtration surgery within 6 months postoperatively.
Statistics
Normality testing of the data was performed using the D’Agostino & Pearson omnibus normality test to confirm Gaussian distribution. The following were compared using the repeated measures ANOVA with Tukey posttest:
IOP at baseline and day 1, 1 week, 1 month, 3 months, and 6 months postoperatively.
Number of antiglaucoma medications at baseline and 3 and 6 months postoperatively.
For IOP and medication analyses, subjects that had secondary filtration procedure within 6 months of the Trabectome were excluded from the analyses, as their results would not be solely contributed to the effects of the Trabectome. Their failure from the procedure was accounted for in the survival analysis.
Snellen visual acuity (VA) was converted to LogMAR units for statistical analysis. For non-numerical VA, the following denotations were used: finger count (FC) = 1.7 LogMAR, hand movement (HM) = 2.0 LogMAR, light perception (LP) = 2.3 LogMAR, and no light perception (NLP) = 3.0 LogMAR.5,6
RESULTS
In 19 eyes of 19 Chinese OAG subjects, 26.3% were uveitic, 68.4% were primary open angle glaucoma, and 5.3% had a history of chronic angle-closure glaucoma with open-angle configuration after cataract extraction. The subject mean age was 67.5 ± 14.4 years, with 4 females and 15 males. Two subjects received secondary filtration surgery at 1 and 3 months after the Trabectome surgery, respectively. Both cases had a history of uveitic glaucoma. These 2 cases were excluded from the analyses of IOP and medications changes, as per study protocol but were included in the survival analysis.
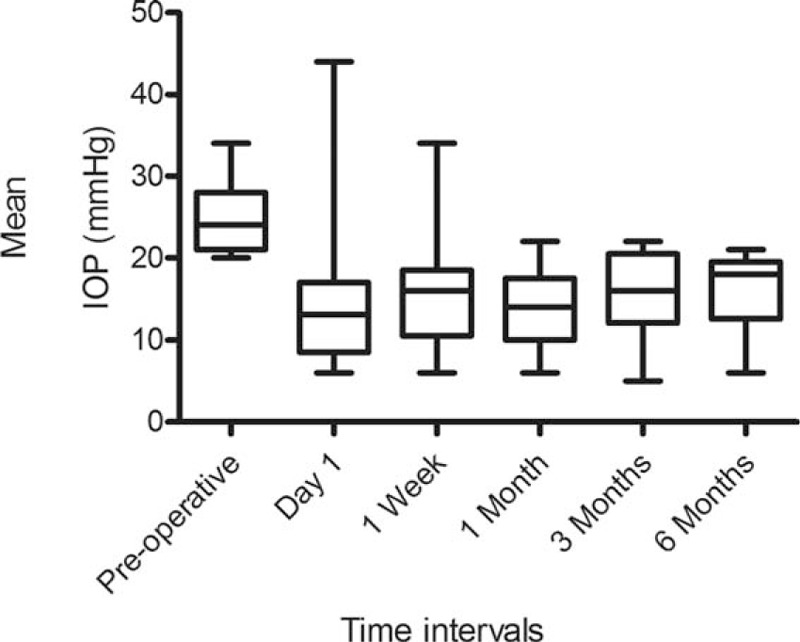
In 17 eyes of 17 remaining subjects, the mean IOP before Trabectome was 24.4 ± 4.4 mm Hg while on 3.9 ± 0.8 to 2.8 types of antiglaucoma eye drops. Table 1 summarizes the IOP at the various time intervals before and after Trabectome. Both the IOP and number of medications were significantly reduced at all time intervals following the Trabectome procedure as compared to the preoperative baseline (all P < 0.0001 and <0.0003, respectively) (Figures 1 and 2). At 6 months, the IOP was reduced by 34.8% (24.4 ± 4.4 mm Hg to 15.9 ± 5.1 mm Hg, P < 0.0001) after Trabectome. The number of types of antiglaucoma medications was also reduced by 28.2% (3.9 ± 0.8–2.8 ± 1.6, P < 0.0001) at 6 months compared to baseline (Figure 1). There was no significant difference in VA among the pretreatment (0.8 ± 0.6 LogMAR) and post-Trabectome VA 1 month (0.9 ± 0.6 LogMAR) and 6 months (0.8 ± 0.6 LogMAR) (P = 0.4).
TABLE 1.
IOP and Medications Before and After Trabectome
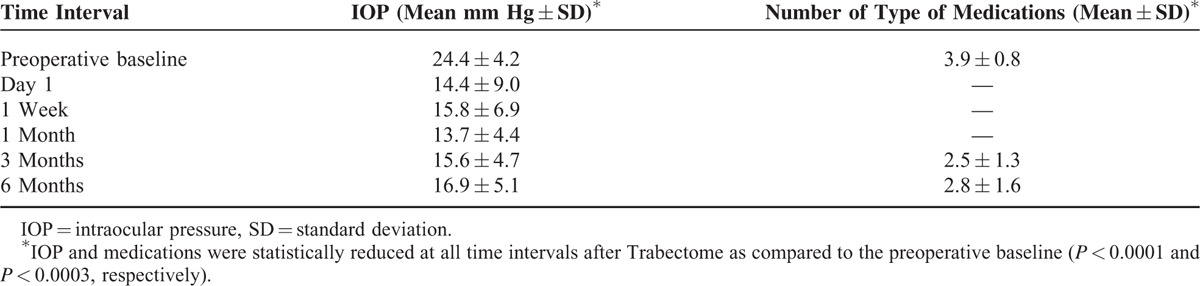
FIGURE 1.
Changes in intraocular pressure following Trabectome. Whiskers = 5–95th percentile.
FIGURE 2.
Change in antiglaucoma medications following Trabectome. Whiskers = 5–95th percentile.
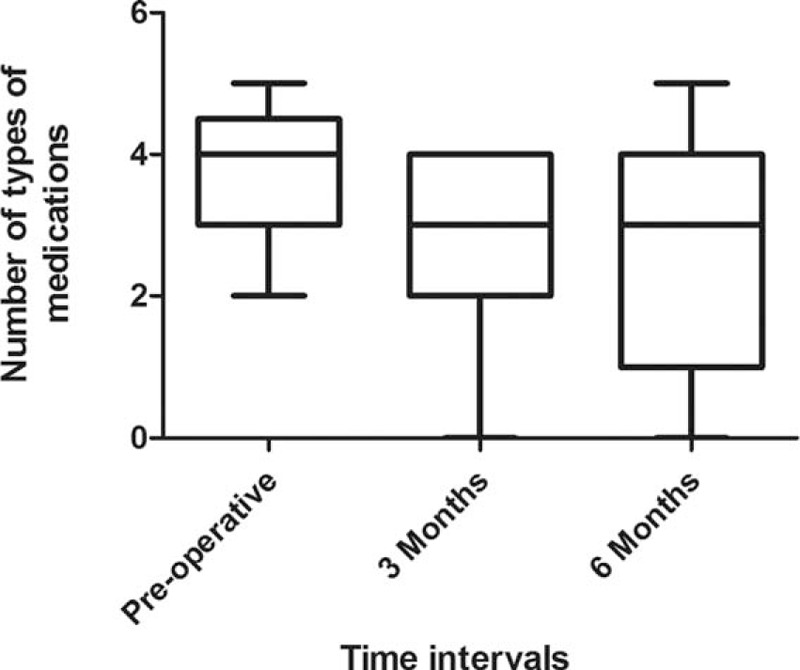
There were no intraoperative complications. One subject developed herpetic keratitis reactivation, likely from the postoperative topical steroid use, which subsided with topical antiviral medication, and 1 subject had persistent hyphema requiring anterior chamber washout. Five out of the initial 19 subjects (26.3%) had an IOP spike >21 mm Hg at 1 week after Trabectome. Three of which subsided with antiglaucoma medications and 2 required secondary filtration surgery as described above. The success rate at 6 months was 89.5% (Figure 3).
FIGURE 3.
Survival following Trabectome. Mortality = IOP > 21 mm Hg or requiring secondary glaucoma filtration surgery.
DISCUSSION
The Trabectome has been a minimally invasive procedure that has been popularized in North America and Europe for the past decade. Its main advantages are that it only requires a small clear corneal incision that is smaller than that of a phacoemulsification wound, it does not disturb or rely on the conjunctiva, and it reestablishes aqueous outflow through the physiological channel with very little risk of postoperative hypotony due to the presence of the episcleral venous pressure. It is a relatively safe procedure with the most common postoperative complications being the need of a secondary trabeculectomy or other glaucoma surgeries and its failure does not affect the success of future trabeculectomies.3,7
In our study, we selectively performed the Trabectome in pseudophakic eyes for 2 main reasons: to evaluate the stand-alone effect of the Trabectome and because in Chinese phakic eyes, even with open-angle configuration on gonsioscopy, there is often not enough space for adequate exposure of the trabecular meshwork to allow a safe access for angle surgery without damaging the surrounding structure like the iris. This was gathered from the authors’ previous experiences with similar angle surgeries like goniosynechialysis and also from the understanding that there may be higher risk of peripheral anterior synechiae formation, iridodialysis, or postoperative fibrosis leading to failure.8
Our results of an IOP reduction of 34.8% was comparable to the 25% to 38% reductions reported in the literature for Trabectome as a stand-alone procedure in primary open-angle glaucoma (POAG).9–12 The IOP reduction can vary among studies based on whether or not the Trabectome was combined with other procedures like phacoemulsification or other glaucoma filtration procedures, as these are surely to reduce the IOP more; thus, we have only quoted studies using the Trabectome as a single procedure. Furthermore, the duration of follow-up may also affect the level of IOP reduction as the Trabectome, just like another other glaucoma surgery, is not ever lasting. Our follow-up duration was comparable to studies by Pantcheva and Kahook11 while in one of the longest follow-up studies by Jordan et al,12 the IOP reduction dropped to 25% at 40 months postoperatively.
Our medication reduction of 28.2% after a single session of Trabectome also falls within the range as reported from the literature (21–67%).9–12 The more variable range of medication reduction is related to both patient and surgeon factors including patient's disease severity, individual target IOP, protocol of individual institutions, and the discretion of the attending ophthalmologist.
There are very few publications available in the literature reporting the effects of the Trabectome for the Chinese population. Huang et al published an article in Chinese in February 2015 reporting an IOP reduction of 21.7%, medication reduction of 40.0% and a success rate of 85.0% at 12 months, which is comparable to our results.13 Mizoguchi et al14 reported the results of the Trabectome in a Japanese population, demonstrating IOP reductions of 23.0% and success rates of 51.2% at 2 years. In many of the patients, the surgical groove was no longer clearly visible at 1 month after surgery as it was covered by normal iris tissue (not peripheral anterior synechiae) although this finding did not correlate with IOP rise. This is because in the Chinese population, there tends to be a smaller anterior chamber area/volume so even in those with open angles, visibility of the angle structures may be limited by gonioscopy alone but during angle surgery, the influx of balanced salt solution helps to deepen the angle improving accessibility.15
None of our subjects had any intraoperative complications. One subject developed herpetic keratitis reactivation, which was unrelated to the procedure itself but arising from the postoperative steroid use. Another subject had persistent hyphema requiring anterior chamber washout and on retrospect, keeping the initial IOP higher at the end of the operation by reforming the anterior chamber or injecting an air bubble temponade may reduce the chance of bleeding in those high-risk subjects. Hyphema is often due to the persistent intraoperative reflux of blood from the episcleral venous system after the sudden pressure lowering; reflux has been reported to be as frequent as 78%.3 About 26.3% of our subjects had an IOP spike >21 mm Hg at 1 week after Trabectome which may be related to postoperative inflammation or an hyphened sensitivity to steroid response after removal of the inner wall of the Schelmm canal16; most pressures went down after the temporary addition of antiglaucoma medications. The percentage of IOP spike is much higher than the previously reported 5.8% in other studies and this may be related to the release of iris pigments due to the relatively shallower angle and more pigmented iris in our population.3 Of the 2 subjects that required additional glaucoma filtration surgery for IOP control, both had a history of uveitic glaucoma. While the Trabectome has the theoretical advantage of being minimally invasive without the need of a peripheral iridotomy and has minimal risk of postoperative hypotony, making it a seemingly ideal surgery in uveitic glaucoma, our experience with uveitic patients is that the IOP control from Trabectome alone may be short-lived or inadequate. On the whole, the success rate at 6 months was 89.5%. Another point of interest from our study is that 5% of our study subjects had a history of angle-closure configuration before phacoemulsification but at the time of Trabectome, all had open-angle configuration suggesting that the procedure can still be useful in those with angle-closure as long as the angles are reopened with other modalities before Trabectome. In a study by Bussel et al,17 there was no significant difference in IOP or medication reduction between those with Shaffer angle grading ≤2 versus those with grading ≥3.
Our study was limited by the relative small sample size although there are very few studies in the literature reporting the effects of Trabectome for the Chinese glaucoma population. Ideally, a larger sample size would also follow us to conduct subclasses analyses on the different subtypes of OAG. Furthermore, a longer follow-up duration will enable us to have a clearer picture on the long-term effects and sustainability of the Trabectome. Future studies involving randomization of treatment and comparison to other glaucoma surgeries can provide a head-to-head comparison to conventional treatments. Lastly, the results of this study may not be generalizable to other populations that are significantly different to the one studied.
In conclusion, the Trabectome's performance in the Chinese OAG population was comparable to the data reported in Caucasian populations. The mean IOP and medication requirement was reduced by about a third from the pretreatment baseline. The procedure was more effective in POAG or even angle-closure glaucoma after angle reopening but less so in uveitic glaucoma; the overall success rate was around 90% at 6 months.
Footnotes
Abbreviations: FC = finger count, HM = hand movement, IOP = intraocular pressure, LP = light perception, NLP = no light perception, OAG = open-angle glaucoma, POAG = primary open-angle glaucoma, VA = visual acuity.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Minckler D, Baerveldt G, Ramirez MA, et al. Clinical results with the Trabectome, a novel surgical device for treatment of open-angle glaucoma. Trans Am Ophthalmol Soc 2006; 104:40–50. [PMC free article] [PubMed] [Google Scholar]
- 2.Minckler DS, Baerveldt G, Alfaro MR, et al. Clinical results with the Trabectome for treatment of open-angle glaucoma. Ophthalmology 2005; 112:962–967.doi: 10.1016/j.ophtha.2004.12.043 [published online first: Epub date]. [DOI] [PubMed] [Google Scholar]
- 3.Minckler D, Mosaed S, Dustin L, et al. Trabectome (trabeculectomy-internal approach): additional experience and extended follow-up. Trans Am Ophthalmol Soc 2008; 106:149–159.discussion 59–60. [PMC free article] [PubMed] [Google Scholar]
- 4.Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol 2002; 120:1268–1279. [DOI] [PubMed] [Google Scholar]
- 5.Lee JW, Lai JS, Yick DW, et al. Retrospective case series on the long-term visual and intraocular pressure outcomes of phacomorphic glaucoma. Eye (Lond) 2010; 24:1675–1680.doi: 10.1038/eye.2010.108 [published online first: Epub date]. [DOI] [PubMed] [Google Scholar]
- 6.Lee JW, Woo TT, Yau GS, et al. Cross-sectional study of the retinal nerve fiber layer thickness at 7 years after an acute episode of unilateral primary acute angle closure. Medicine 2015; 94:e391.doi: 10.1097/MD.0000000000000391 [published online first: Epub date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jea SY, Mosaed S, Vold SD, et al. Effect of a failed trabectome on subsequent trabeculectomy. J Glaucoma 2012; 21:71–75.doi: 10.1097/IJG.0b013e31820bcfda [published online first: Epub date]. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Jung J, Francis BA. Ab interno trabeculotomy: Trabectome™ surgical treatment for open-angle glaucoma. Expert Rev Ophthalmol 2009; 4:119–128. [Google Scholar]
- 9.Ting JL, Damji KF, Stiles MC. Ab interno trabeculectomy: outcomes in exfoliation versus primary open-angle glaucoma. J Cataract Refract Surg 2012; 38:315–323.doi: 10.1016/j.jcrs.2011.08.043 [published online first: Epub date]. [DOI] [PubMed] [Google Scholar]
- 10.Maeda M, Watanabe M, Ichikawa K. Evaluation of trabectome in open-angle glaucoma. J Glaucoma 2013; 22:205–208.doi: 10.1097/IJG.0b013e3182311b92 [published online first: Epub date]. [DOI] [PubMed] [Google Scholar]
- 11.Pantcheva MB, Kahook MY. Ab interno trabeculectomy. Middle East Afr J Ophthalmol 2010; 17:287–289.doi: 10.4103/0974-9233.71585 [published online first: Epub date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jordan JF, Wecker T, van Oterendorp C, et al. Trabectome surgery for primary and secondary open angle glaucomas. Graefes Arch Clin Exp Ophthalmol 2013; 251:2753–2760.doi: 10.1007/s00417-013-2500-7 [published online first: Epub date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang P, Wang H, Wu H, et al. Preliminary investigation on the safety and efficacy of Trabectome. Zhonghua Yan Ke Za Zhi 2015; 51:115–119. [PubMed] [Google Scholar]
- 14.Mizoguchi T, Nishigaki S, Sato T, et al. Clinical results of Trabectome surgery for open-angle glaucoma. Clin Ophthalmol 2015; 9:1889–1894.doi: 10.2147/OPTH.S83958 [published online first: Epub date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang D, Qi M, He M, et al. Ethnic difference of the anterior chamber area and volume and its association with angle width. Invest Ophthalmol Vis Sci 2012; 53:3139–3144.doi: 10.1167/iovs.12-9776 [published online first: Epub date]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vold S. Improving surgical outcomes with the Trabectome. Glaucoma Today. 2009. URL: http://glaucomatoday.com/2009/08/GT0709_06.php/ (Accessed on December 2, 2015). [Google Scholar]
- 17.Bussel II, Kaplowitz K, Schuman JS, et al. Outcomes of ab interno trabeculectomy with the trabectome by degree of angle opening. Br J Ophthalmol 2015; 99:914–919.doi: 10.1136/bjophthalmol-2014-305577 [published online first: Epub date]. [DOI] [PMC free article] [PubMed] [Google Scholar]


