Abstract
The aim of the study was to assess the effects of bevacizumab in augmenting trabeculectomy for glaucoma.
We searched the databases of Cochrane Library, PubMed, Embase, CNKI, and VIP. All the databases were retrieved from the time databases established to September, 2015. The keywords we used were as follows: “bevacizumab,” “anti-VEGF,” “avastin,” “trabeculectomy,” “glaucoma,” and so on. We used a method of the freedom word search and the MeSH search combined, which was recommended by Cochrane Systematic Review Manual 5.1.2. Randomized controlled trails (RCTs) of frequently used bevacizumab in trabeculectomy for glaucoma were included. Study selection, data extraction, quality assessment, and data analysis were performed according to the Cochrane standards.
Eight randomized controlled trails involving 212 eyes in the experimental (bevacizumab or bevacizumab + mitomycin C) groups and 214 eyes in the control (mitomycin C or placebo) groups were selected. Compared with placebo, bevacizumab significantly increased the complete success rate [OR = 2.79, 95%CI, (1.47, 5.29), P = 0.002], what else, bevacizumab also significantly decreased the intraocular pressure (IOP) [MD = 3.07, 95% CI, (0.87, 5.27), P = 0.006] at the 6-month after trabeculectomy and the number of antiglaucoma medications [MD = 1.23, 95% CI, (0.66, 1.80), P < 0.0001]. Additionally, it also increased the risk of bleb leak [OR = 5.24, 95% CI, (1.30, 21.10), P = 0.02]. When compared with mitomycin C (MMC), bevacizumab significantly increased the rate of encysted blebs [OR = 4.62, 95% CI, (1.02, 20.91), P = 0.05]. However, there was no significantly difference between the bevacizumab + MMC groups and MMC groups whatever the items were.
Bevacizumab was an effective way in trabeculectomy concerning the complete success rate, IOP, and anti-glaucoma medications reduction when compared with placebo; however, it increased the risk of bleb leakage. And it significantly increased the rate of encysted blebs compared with MMC.
INTRODUCTION
Glaucoma as the second reason of blindness is a serious threat to human vision health in the world.1 Most commonly, the clinical course of open angle glaucoma is so insidious that the problem is found only when the visual function suffers serious damage. Thus, for open angle glaucoma, early diagnosis and treatment are very important. Usually, the operation indications of open angle glaucoma are uncontrolled cases with drugs, cases cannot tolerate medications. However, some researchers thought that once the diagnosis was clear, with significant disc and vision changes, filtration operations should be used as the preferred treatment.2,3 Trabeculectomy is the main technique of open angle glaucoma.4 There are, however, some limitations of the surgery. Scar formation and fibrosis in the process of wound healing may result in obstruction of filtration tract, leading to the operation failure.5,6 In recent 3 decades, due to the use of antimetabolites, such as MMC and 5-fluorouracil (5-FU), the rate of operation success has been higher than before.7–10 However, antimetabolites may bring some serious complications, such as low intraocular pressure, filtering bleb leakage, filtering bleb-associated endophthalmitis, epithelial toxicity, and so on.11 Hence, researchers have been searching for more effective and safer ways to inhibit scar formation and fibrosis. Recently, some researchers have found that bevacizumab may work in some ways.12–14
However, studies about this aspect were few, and high-quality researches were also seldom seen. Whether glaucoma patients after trabeculectomy could benefit more from bevacizumab than MMC or placebo, it has not been reviewed yet. The purpose of this study is to systemic review the efficacy and safety of bevacizumab in the trabeculectomy, providing more reliable evidences for clinical workers.
METHODS
Search Strategy
We searched the databases of Cochrane Library, PubMed, Embase, CNKI, and VIP. All the databases were retrieved from the time databases established to September, 2015. The keywords we used were as follows: “bevacizumab,” “anti-VEGF,” “avastin,” “trabeculectomy,” “glaucoma,” and so on. We used a method of the freedom word search and the MeSH search combined, which was recommended by Cochrane Systematic Review Manual 5.1.2. For a more comprehensive search, a manual search of cited references in published studies was done. Two researchers selected and assessed all included studies independently, and then cross-checked. Due to the fact that all analyses were based on previously published studies, the ethical approval was not necessary for our study.
Data Extraction
Two researchers extracted study characteristics and outcome data independently. If there were some discrepancies, they would be resolved through discussion or a third researcher. Data that we collected were as follows: baseline characteristics, IOP, best-corrected visual acuity (BCVA), complete success rate (CS), quality success rate (QS), failure rate, the number of glaucoma medications, and adverse events.
Statistical Analysis
Revman 5.0 (the Cochrane collaboration; http://www.cochrane.org/) was used for statistical analysis of the data. For continuous outcomes, mean difference (MD) or standard mean difference (SMD) with 95% confidence intervals (CI) was used to calculate the results; however, odds ratio (OR) with 95% confidence intervals (CI) was used for dichotomous outcomes. We used the chi-square test to assess heterogeneity between trials and the I2 statistic to assess the extent of inconsistency. If there was a significant heterogeneity, a random-effect statistical model would be used to confirm the case results. A fixed-effect model for calculations of summary estimates was applied, unless there was a significant heterogeneity. Subgroup analysis was intended to explore clinical differences among trials.
RESULTS
Search Results
We obtained 101 publications through searching literature databases and cited references. According to the inclusion criteria, only the RCTs for patients using bevacizumab during trabeculectomy were included. We eliminated the 74 articles by reading the title and abstract. Through further reading the full text, we ruled out 19 published papers, including 2 nonrandomized controlled trials, 7 retrospective case series, 6 retrospective controlled trials, and 4 prospective case series. Finally, we included 8 RCTs15–22 about use of bevacizumab in augmenting trabeculectomy for glaucoma in the meta-analysis. The process of literature screening was shown in Figure 1.
FIGURE 1.

Flowchart showing systematic review search results.
Study Quality
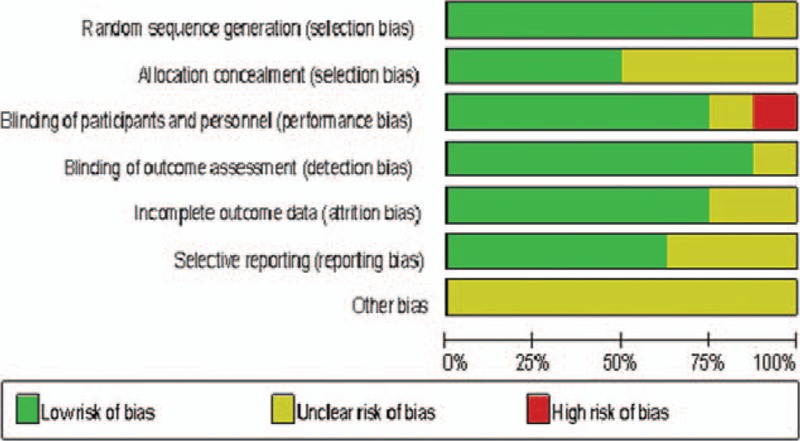
Table 1 described the specific information of the RCTs. A total of 426 eyes with 212 eyes in the experimental (bevacizumab or bevacizumab + MMC) groups and 214 eyes in the control groups separately were included in them. Figure 2 showed the methodological quality of the included RCTs, which was assessed by using the Cochrane Handbook 5.0.2. Seven studies16–22 of the included studies offered adequate descriptions of the randomization process. Five studies16,17,19,21,22 reported that masking was done either for the patients or for the practitioners; only 4 studies16,18,21,22 adequately stated allocation concealment. Six of included studies15–18,21,22 had stated incomplete outcome data. Furthermore, none of the papers adequately described other bias.
TABLE 1.
Baseline Characteristics of Included Studies†
FIGURE 2.
Quality evaluation of studies in the meta-analysis.
Studies and Baseline Characteristics
Characteristics of the 8 trials15–22 were shown in Table 1. Trabeculectomy were performed under local anaesthesia by experienced surgeons. All patients received bevacizumab, MMC, normal saline, and bevacizumab + MMC after trabeculectomy in the groups of bevacizumab, MMC, placebo, and bevacizumab + MMC respectively. Six trials reported antiglaucoma medications before trabeculectomy, and 3 studies reported the type of glaucoma including primary open-angle glaucoma (POAG) and pseudoexfoliation glaucoma (PEXG). The baseline characteristics of participants were displayed in Table 1. All studies were published between 2011 and 2015. Follow-up ranged from 3 to 24 months. There were comparable throughout age, IOP, BCVA, glaucoma medications, and glaucoma type in the papers.
IOP
All the studies15–22 reported IOP at last month. All study used the same scales to report IOP; thus the MD was used. Compared with bevacizumab groups, control groups including placebo groups (MD = 0.05, 95%CI, [−2.10, 2.20] P = 0.96) and MMC groups (MD = −1.40, 95%CI, [−4.98, 2.18] P = 0.44) were not associated with decreased IOP (Figure 3A). Additionally, bevacizumab+ MMC groups might have no advantage in decreasing IOP when compared with MMC groups (MD = −0.08, 95%CI, [−2.14, 1.98] P = 0.94) (Figure 3B). However, 5 studies15,17,18,20,21 reported IOP at the 6-month. The change of IOP in the bevacizumab groups was significantly higher than the placebo groups (MD = 3.07, 95%CI, [0.87, 5.27], P = 0.006). But there was no statistically significant difference between the bevacizumab groups and MMC groups (MD = −1.06, 95%CI, [−4.18, 2.07], P = 0.51) (Figure 4A), nor between the bevacizumab+ MMC groups and MMC groups (MD = 2.54, 95%CI, [−0.89, 5.97], P = 0.15) (Figure 4B).
FIGURE 3.
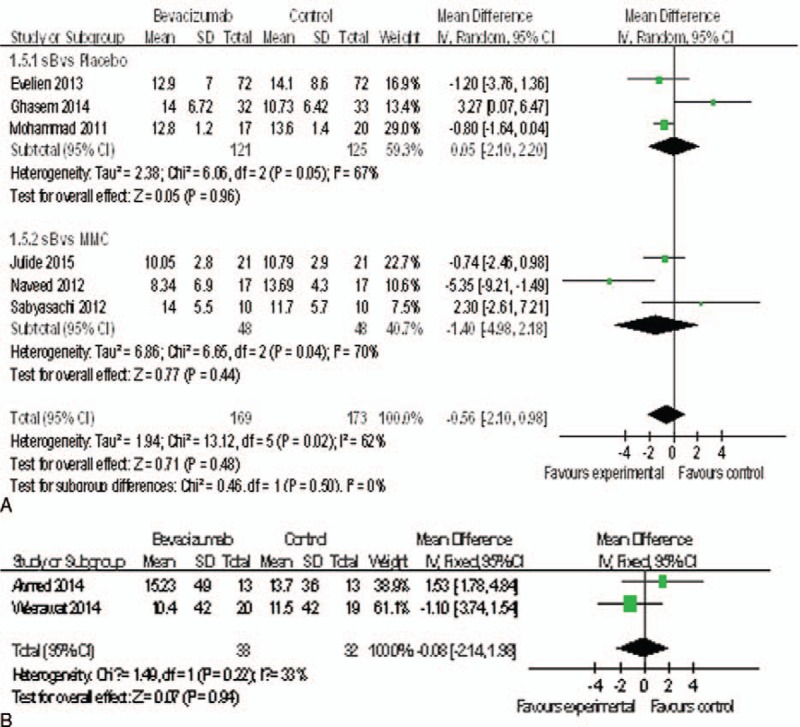
(A) Change of IOP at last month. (B) Change of IOP at last month. IOP = intraocular pressure.
FIGURE 4.
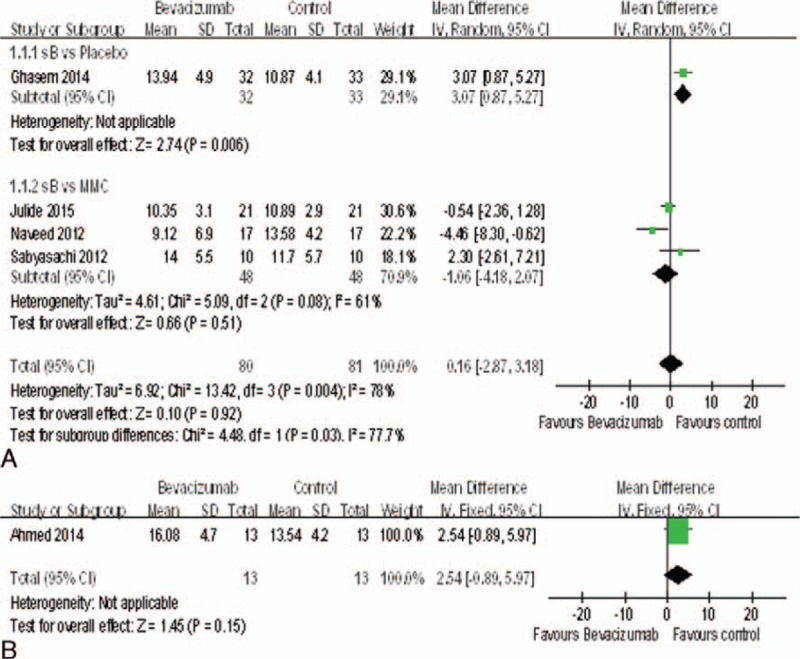
(A) Change of IOP at month 6. (B) Change of IOP at month 6. IOP = intraocular pressure.
Complete Success Rate
Seven studies15–18,20,21 reported the complete success rate. The complete success rate of the bevacizumab groups was significantly higher than the placebo groups (OR = 2.79, 95%CI, [1.47, 5.29], P = 0.002). But there was no statistically significant difference between the bevacizumab groups and MMC groups (OR = 0.60, 95%CI, [0.08, 4.51], P = 0.62) (Figure 5A), nor between the bevacizumab+ MMC groups and MMC groups (OR = 1.25, 95%CI, [0.42, 3.69], P = 0.69) (Figure 5B).
FIGURE 5.
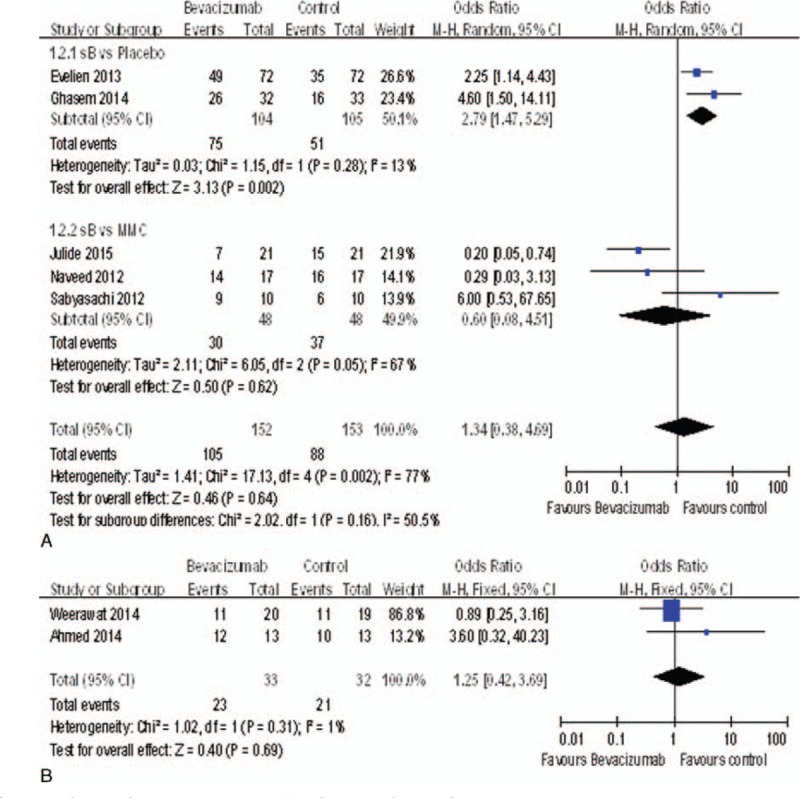
(A) Change of complete success rate. (B) Change of complete success rate.
Failure Rate
Seven studies15–18,20,21 reported the failure rate. The failure rate of the bevacizumab groups was not significantly different with control groups including the placebo groups [OR = 0.42, 95%CI, (0.08, 2.31), P = 0.32] and MMC groups [OR = 0.53, 95%CI, (0.08, 3.43), P = 0.51] (Figure 6A). Otherwise, there was no significant difference between the bevacizumab + MMC groups and MMC groups [OR = 0.73, 95%CI, (0.17, 3.19), P = 0.67] (Figure 6B).
FIGURE 6.
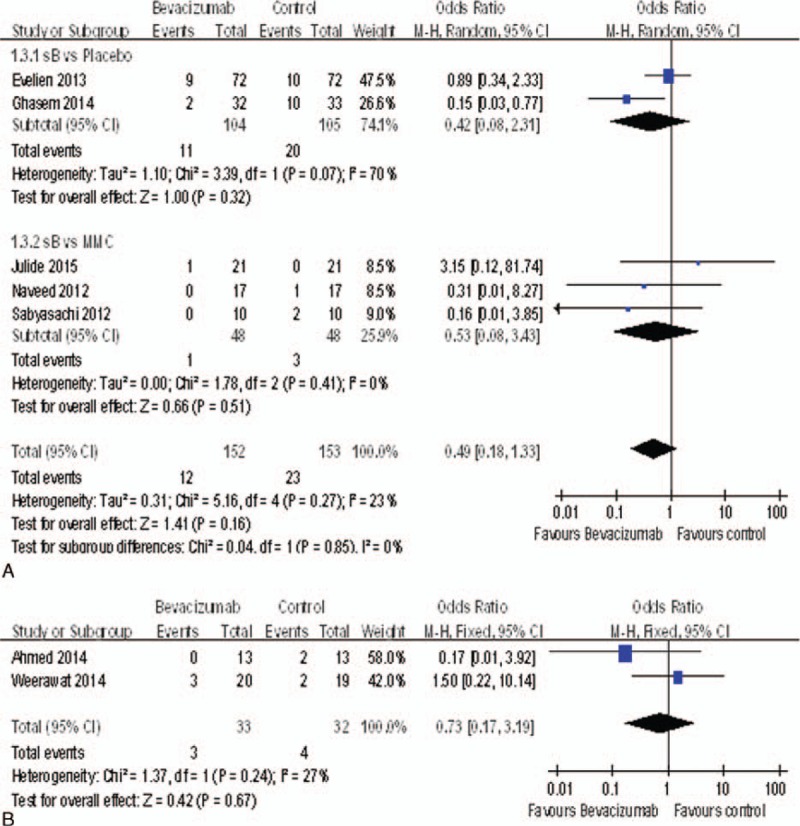
(A) Change of the failure rate. (B) Change of the failure rate.
BCVA
Only 4 studies15,18,20,22 reported the BCVA. There was no statistically significant difference between bevacizumab and MMC groups (MD = −0.01, 95%CI, [−0.11, 0.08], P = 0.77) (Figure 7A), nor between the bevacizumab + MMC groups and MMC groups (MD = −0.03, 95%CI, [−0.18, 0.11], P = 0.64) (Figure 7B).
FIGURE 7.
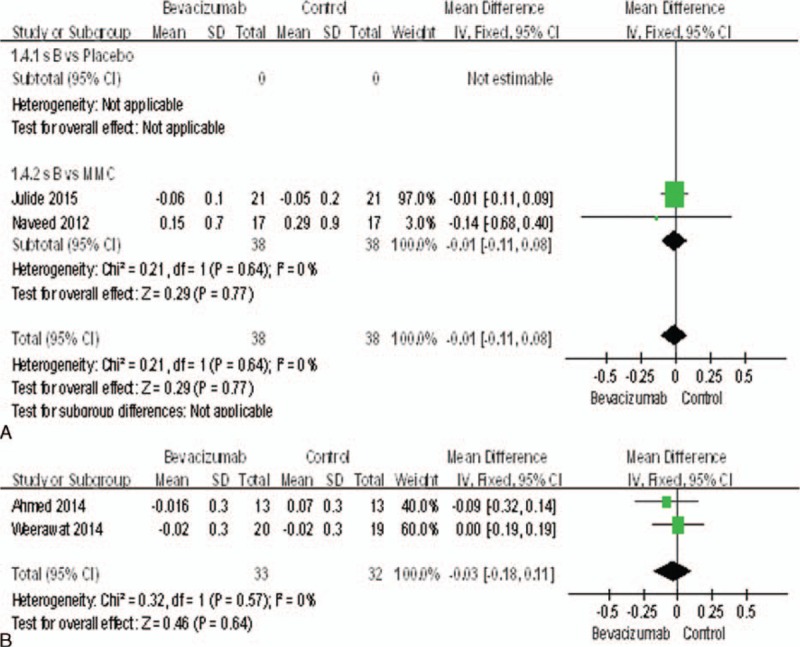
(A) Change of the BCVA. (B) Change of the BCVA. BCVA = best-corrected visual acuity.
Anti-Glaucoma Medications
Only 4 studies17,18,20,22 reported the change of antiglaucoma medications. There was statistically significant difference between bevacizumab and placebo groups (MD = 1.23, 95%CI, [0.66,1.80], P < 0.0001), but there was no statistically significant difference when compared with MMC groups (MD = −0.32, 95%CI, [−0.69,0.06], P = 0.10) (Figure 8A), nor between the bevacizumab+ MMC groups and MMC groups (MD = 0.00, 95%CI, [−0.50, 0.50], P = 1.00) (Figure 8B).
FIGURE 8.
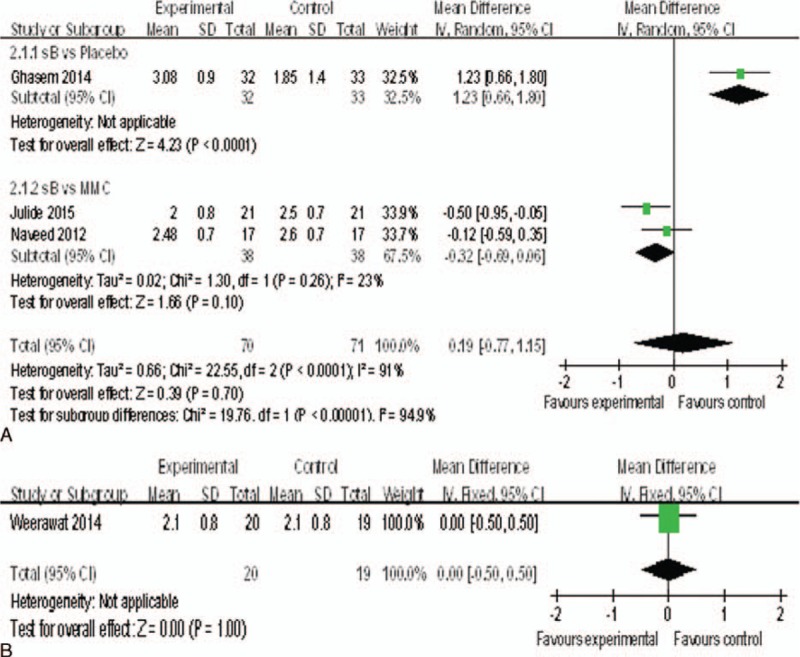
(A) Change of the antiglaucoma medications. (B) Change of the antiglaucoma medications.
Adverse Events
We could analyze 7 studies15–18,20–22 for adverse events including bleb leak, hyphema, encysted blebs, anterior chamber shallowing, and so on. Fortunately, there was no statistically significant difference between bevacizumab and control groups, including the placebo groups (OR = 1.11, 95%CI, [0.64, 1.95], P = 0.70) and MMC groups (OR = 1.12, 95%CI, [0.12, 10.87] P = 0.92) (Figure 9A), nor between the bevacizumab + MMC groups and MMC groups (OR = 1.40, 95%CI, [0.39, 5.06], P = 0.61) (Figure 9B).
FIGURE 9.
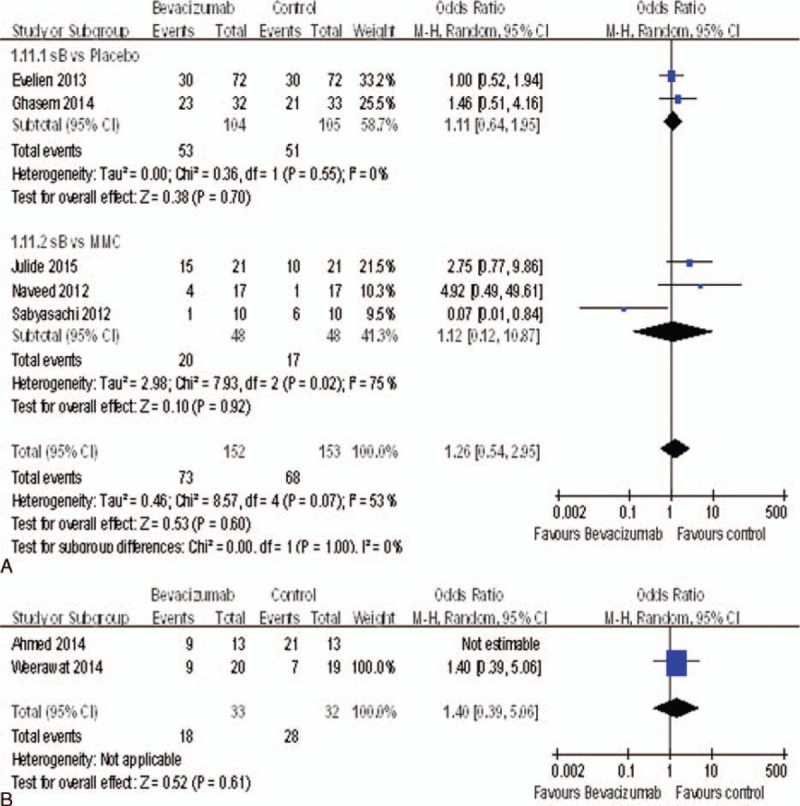
(A) Change of adverse events. (B) Change of adverse events.
Bleb Leak
We could analyze 5 studies15,17,18,20,22 for the bleb leak, and there was statistically significant difference between bevacizumab and placebo groups (OR = 5.24, 95%CI, [1.30, 21.10], P = 0.02). However, there was no statistically significant difference between bevacizumab and MMC groups (OR = 1.92, 95%CI, [0.38, 9.77], P = 0.43) (Figure 10A), nor between the bevacizumab + MMC groups and MMC groups (OR = 0.31, 95%CI, [0.01, 8.30], P = 0.48) (Figure 10B). Therefore, bevacizumab was associated with significantly increased the rate of bleb leak compared with placebo groups.
FIGURE 10.
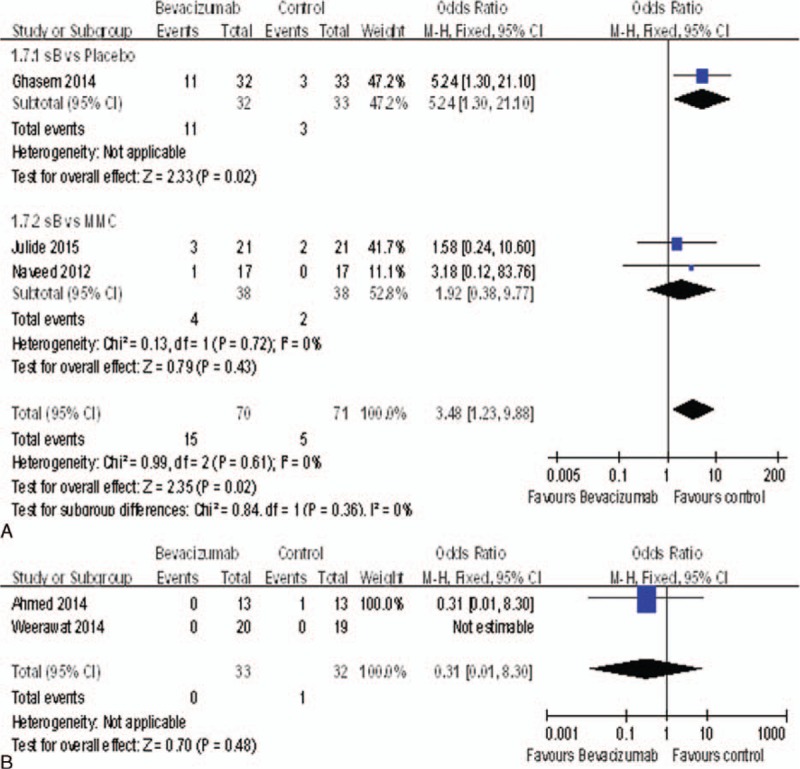
(A) The rate of bleb leak. (B) The rate of bleb leak.
Hyphema
There were 5 studies15,16,18,20,22 reported the rate of hyphema. There was no statistically significant difference between bevacizumab and control groups, including the placebo groups (OR = 0.50, 95%CI, [0.09, 2.76], P = 0.43) and MMC groups (OR = 0.18, 95%CI, [0.01, 4.02], P = 0.28) (Figure 11A), nor between the bevacizumab + MMC groups and MMC groups (OR = 0.17, 95%CI, [0.01, 3.92], P = 0.27) (Figure 11B).
FIGURE 11.
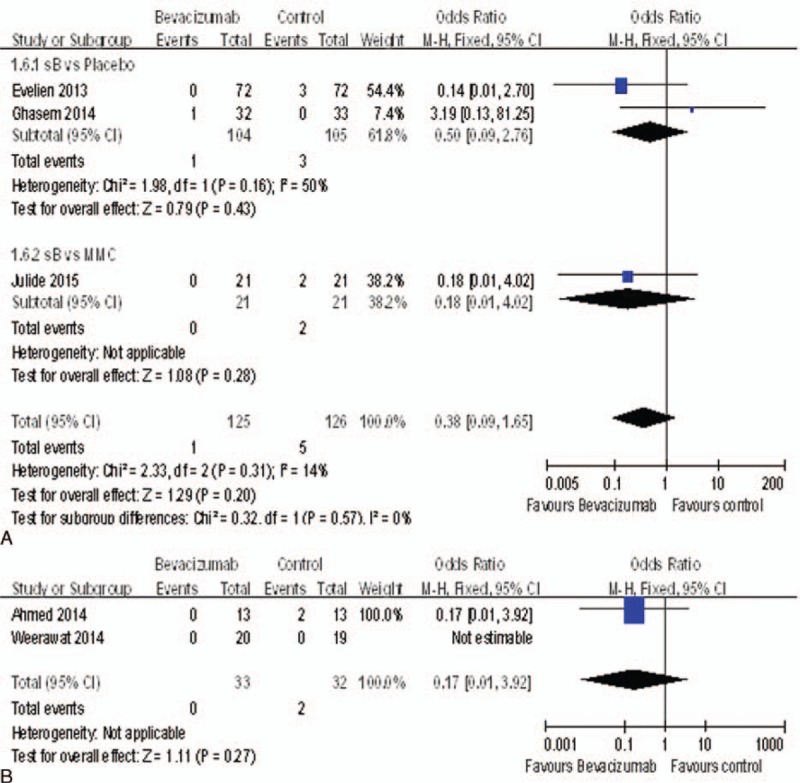
(A) The rate of hyphema. (B) The rate of hyphema.
Encysted Blebs
There were 5 studies15,17,18,20,22 reported the rate of encysted blebs. The encysted blebs rate of the bevacizumab groups was significantly higher than the MMC groups (OR = 4.62, 95%CI, [1.02, 20.91], P = 0.05). But it was no statistically significant difference between the bevacizumab groups and the placebo groups (OR = 0.45, 95%CI, [0.16, 1.30], P = 0.14) (Figure 12A), nor between the bevacizumab + MMC groups and the MMC groups (OR = 1.17, 95%CI, [0.35, 3.97], P = 0.80) (Figure 12B). Therefore, bevacizumab was associated with significantly increased the rate of encysted blebs compared with MMC.
FIGURE 12.
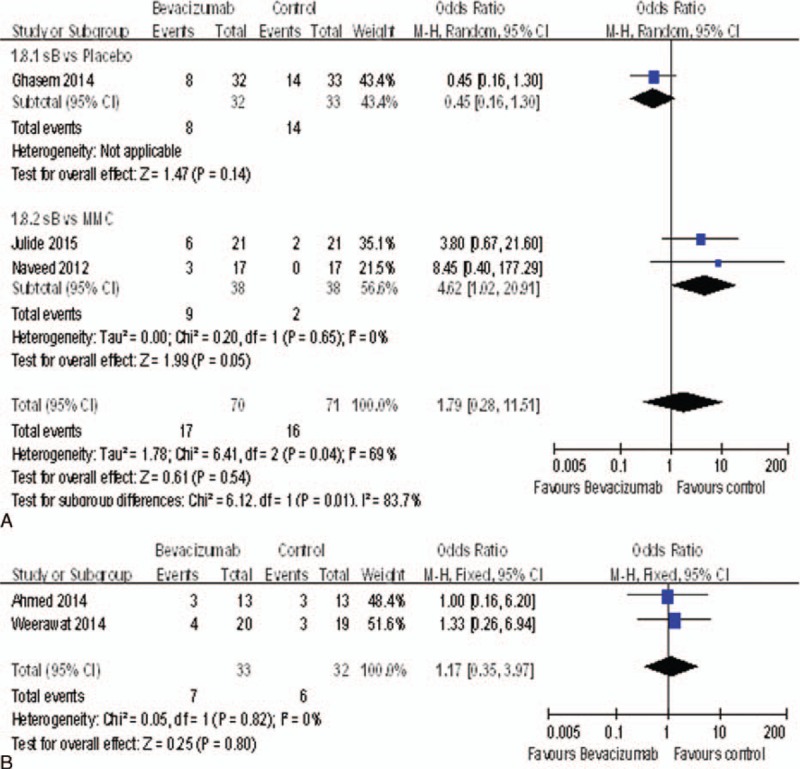
(A) The rate of encysted blebs. (B) The rate of encysted blebs.
Anterior Chamber Shallowing
Only 2 studies17,18 reported the anterior chamber shallowing. There was no statistically significant difference between bevacizumab and control groups (OR = 1.02, 95%CI, [0.14, 7.44], P = 0.99) (Figure 13).
FIGURE 13.
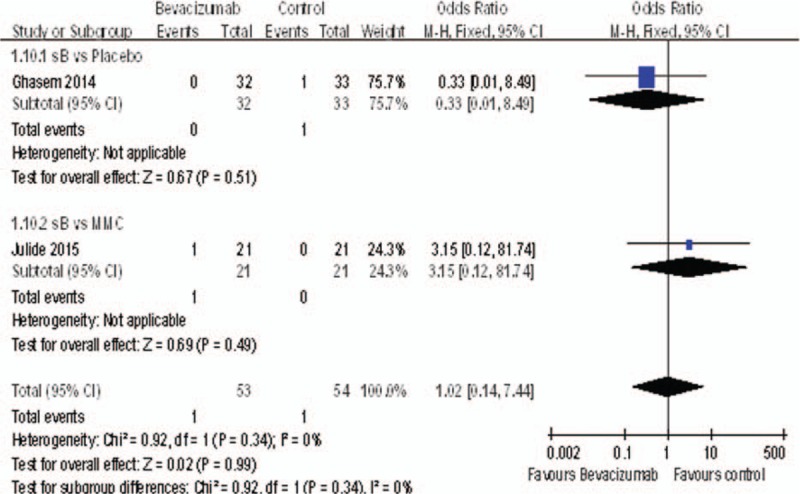
The rate of anterior chamber shallowing.
Bleb Morphology
There were 5 studies15,18,20–22 reported bleb characteristics. Two15,20 showed no significantly difference between the experimental (bevacizumab or bevacizumab + MMC) groups and control groups. Two21,22 found that the vascularity scores of the experimental (bevacizumab or bevacizumab + MMC) groups were significantly lower when compared with the control groups at the 1-month follow-up. But these were not retained for longer time. One18 showed a statistically significant difference between 2 groups in regard to maximal bleb area, with the control group exhibiting more diffuse bleb area.
DISCUSSION
The failure of trabeculectomy is mainly due to fibrosis and scar formation of subconjunctival tissue around the scleral flap and bleb during the wound-healing process.23,24 Bevacizumab, a humanized nonselective monoclonal antibody against vascular endothelial growth factor (VEGF), has been successfully used for diabetic retinopathy (DR),25 neovascular glaucoma,26,27 may work in some ways. As is known to all, tissue growth requires nutrients which provided by blood. Thus, bevacizumab are expected to act a role of inhibiting scar formation and fibrosis through the inhibition of angiogenesis information.28 On the other hand, the vascularization of conjunctiva is an important reason of bleb filtration failure. What is more, there were also evidences showed that VEGF had a direct effect on fibroblasts, which if inhibited by bevacizumab, scar formation, and fibrosis would be modulated.28–32 Previous studies found that the VEGF levels were elevated in patients who had a trabeculectomy.33,34 And the concentration of VEGF was significantly reduced after application of bevacizumab.32,35 Thus, bevacizumab may have the potential to work in trabeculectomy.
In the present study, 8 RCT studies were reviewed, consisting of 3 studies about bevacizumab vs placebo, 3 about bevacizumab vs MMC, and 2 about bevacizumab + MMC vs MMC. We found similar efficacy of reduction in the IOP and BCVA in the experimental (bevacizumab or bevacizumab + MMC) groups and control groups at last visit. Because of the lack of data reported in all phases of follow-up and trials with different durations, we chose the data from the end-point. The operative failure rate was also similar between the 2 groups. There were 5 studies, including 1 in the bevacizumab vs placebo groups, 3 in the bevacizumab vs MMC groups, and 1 in the bevacizumab + MMC vs MMC groups, reported IOP at the 6-month; we found the change of IOP was more remarkable in bevacizumab groups when compared with placebo groups (MD = 3.07, 95%CI, [0.87, 5.27], P = 0.006). However, there was no statistically difference when compared with MMC (MD = -1.06, 95%CI, [-4.18, 2.07], P = 0.51), nor between the bevacizumab + MMC groups and MMC groups (MD = 2.54, 95%CI, [-0.89, 5.97], P = 0.15). With respect to the complete success rate, bevacizumab was more likely to achieve complete success than placebo (OR = 2.79, 95%CI, [1.47, 5.29], P = 0.002), but there was no statistically significant difference between the bevacizumab groups and the MMC groups (OR = 0.60, 95%CI, [0.08, 4.51], P = 0.62), nor between the bevacizumab + MMC groups and MMC groups (OR = 1.25, 95%CI, [0.42, 3.69], P = 0.69). What is more, bevacizumab was associated with the reduction of antiglaucoma medications compared with placebo (MD = 1.23, 95%CI, [0.66,1.80], P < 0.0001).
For safety, results of adverse events were reported in 7 studies.15–18,20–22 Concerned overall adverse events, there was no statistically difference between the experimental (bevacizumab or bevacizumab + MMC) groups and control groups. And no one died patient was associated with bevacizumab and MMC in including studies. The adverse events included bleb leak, hyphema, encysted blebs, anterior chamber shallowing, hypotony, and so on. This meta-analysis showed bevacizumab not only increased the rate of bleb leak compared with placebo groups, but also increased the rate of encysted blebs compared with MMC.
Concerned with bleb morphology, 2 studies21,22 found bevacizumab had some advantages in reduce the vascularity scores in 1 month, which was similar to a recent cohort study.36 This might be associated with mechanism of bevacizumab, inhibiting the angiogenesis information. Akkan and Cilsim17 reported that the bevacizumab showed less efficiency in diffuse bleb area. This was in contrast with 1 recent study,36 revealing the bevacizumab group had greater extent.
Despite bevacizumab and MMC had similar efficacy in the IOP reduction and success rate, bevacizumab was much more expensive than MMC, with approximately $450 for each bevacizumab vial.37 If we use each vial of bevacizumab for multiple injections, the per dose price will potentially much lower than $450, depending on the number of injections per vial. However, each bevacizumab vial was allowed to use for only 1 injection because of the contamination outbreaks, discarding the leftover amount. Therefore, MMC might be the preferred choice concerned cost-effectiveness.
The present study is the meta-analysis that evaluates the efficiency and safety of bevacizumab in trabeculectomy. All the studies we included were RCT studies. Seven studies16–22 of the included studies offered adequate descriptions of the randomization process. The randomization process of 6 studies16–18,20–22 was generated by computer. Five studies16,17,19,21,22 reported that masking was done either for the patients or for the practitioners; only 4 studies16,18,21,22 adequately stated allocation concealment. Six of included studies15–18,21,22 had stated incomplete outcome data. Furthermore, none of the papers adequately described other bias.
Of course, there are some limitations in our meta-analysis that should be taken into consideration when considering the results. First, the number of RCTs and the sample sizes of these studies were very small, all of the studies15–22 enrolled only 426 eyes, resulting in the possibility of false-negative statistical error. Second, the varying definitions of surgical success in the literature and absence of patient's stratification into different types of glaucoma and risk of surgical failure should be taken into consideration. Furthermore, the different operative methods and procedures were performed by different surgeons would lead to an unavoidable potential bias. Additionally, the data came from the end-point owing to the lack of data reported in all phases of follow-up and trials with different durations introduced a potential heterogeneity. Finally, publication bias was inevitable.
CONCLUSION
From the current evidences, we found bevacizumab was an effective way in trabeculectomy concerned the complete success rate, IOP, and antiglaucoma medications reduction when compare with placebo, but bevacizumab did not show any advantages when compared with MMC. However, bevacizumab not only increased the rate of bleb leak compared with placebo groups, but also increased the rate of encysted blebs compared with MMC. What is more, there was no difference between bevacizumab+ MMC and MMC whatever the items were. However, MMC might be the preferred choice concerned cost-effectiveness. Further intensive RCTs of large sample, high-quality, multiple centres, and vary phases of follow-up should be carried out to provide more clear and reliable evidence.
Acknowledgments
All authors are in agreement with the content of the manuscript. The copyright will be sent to your Journal. Thanks to Professor Shen Xi-ping gives guidance on the statistical analysis.
Footnotes
Abbreviations: CNKI = China National Knowledge Infrastructure, VIP database = China Science and Technology Journal Database, anti-VEGF = anti-vascular endothelial growth factor, MeSH = Medical Subject Headings, RCTs = Randomized controlled trails, OR = odds ratio, MD = mean difference, CI = confidence intervals, IOP = intraocular pressure, MMC = mitomycin C, 5-FU = 5-fluorouracil, BCVA = best-corrected visual acuity, CS = complete success rate, QS = quality success rate, SMD = standard mean difference, POAG = primary open-angle glaucoma, PEXG = pseudoexfoliation glaucoma, logMAR = logarithm of minimal angle of resolution, VEGF = vascular endothelial growth factor.
Funding: This work was supported by the National Major Scientific Equipment program (Grant No. 2012YQ12008005).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Foster PJ, Johnson GJ. Glaucoma in China: how big is the problem? Br J Ophthalmol 2001; 85:1277–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lavin MJ, Wormald RPL, Migdal CS, et al. The influence of prior therapy on the success of trabeculectomy. Arch Ophthalmol 1990; 108:1543–1548. [DOI] [PubMed] [Google Scholar]
- 3.Migdal C, Gregory W, Hitchings R. Long-term functional outcome after early surgery compared with laser and medicine in open-angle glaucoma. Ophthalmology 1994; 101:1651–1656.discussion 1657. [DOI] [PubMed] [Google Scholar]
- 4.Cairns JE. Trabeculectomy. Preliminary report of a new method. Am J Ophthalmol 1968; 66:673–679. [PubMed] [Google Scholar]
- 5.Nouri-Mahdavi K, Brigatti L, Weitzman M, et al. Outcomes of trabeculectomy for primary open-angle glaucoma. Ophthalmology 1995; 102:1760–1769. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen NX, Kuchle M, Martus P, et al. Quantification of blood–aqueous barrier breakdown after trabeculectomy: pseudoexfoliation versus primary open-angle glaucoma. J Glaucoma 1999; 8:18–23. [PubMed] [Google Scholar]
- 7.Chen CW, Huang HT, Bair JS, et al. Trabeculectomy with simultaneous topical application of mitomycin-C in refractory glaucoma. J Ocul Pharmacol 1990; 6:175–182. [DOI] [PubMed] [Google Scholar]
- 8.WuDunn D, Cantor LB, Palanca-Capistrano AM, et al. A prospective randomized trial comparing intraoperative 5-fluorouracil vs mitomycin C in primary trabeculectomy. Am J Ophthalmol 2002; 134:521–528. [DOI] [PubMed] [Google Scholar]
- 9.Mostafaei A. Augmenting trabeculectomy in glaucoma with subconjunctival mitomycin C versus subconjunctival 5-fluorouracil: a randomized clinical trial. Clin Ophthalmol 2011; 5:491–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palanca-Capistrano AM, Hall J, Cantor LB, et al. Long-term outcomes of intraoperative 5-fluorouracil versus intraoperative mitomycin C in primary trabeculectomy surgery. Ophthalmology 2009; 116:185–190. [DOI] [PubMed] [Google Scholar]
- 11.Anand N, Arora S, Clowes M. Mitomycin C augmented glaucoma surgery: evolution of filtering bleb avascularity, transconjunctival oozing, and leaks. Br J Ophthalmol 2006; 90:175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biteli LG, Prata TS. Subconjunctival bevacizumab as an adjuvant in first-time filtration surgery for patients with primary glaucomas. Int Ophthalmol 2013; 33:741–746. [DOI] [PubMed] [Google Scholar]
- 13.Simsek T, Cankaya AB, Elgin U. Comparison of needle revision with subconjunctival bevacizumab and 5-fluorouracil injection of failed trabeculectomy blebs. J Ocul Pharmacol Ther 2012; 28:542–546. [DOI] [PubMed] [Google Scholar]
- 14.Suh W, Kee C. The effect of bevacizumab on the outcome of trabeculectomy with 5-fluorouracil. J Ocul Pharmacol Ther 2013; 29:646–651. [DOI] [PubMed] [Google Scholar]
- 15.Saeed AM, AboulNasr TT. Subconjunctival bevacizumab to augment trabeculectomy with mitomycin C in the management of failed glaucoma surgery. Clin Ophthalmol 2014; 8:1745–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vandewalle E, Abegao Pinto L, Van Bergen T, et al. Intracameral bevacizumab as an adjunct to trabeculectomy: a 1-year prospective, randomised study. Br J Ophthalmol 2014; 98:73–78. [DOI] [PubMed] [Google Scholar]
- 17.Fakhraie G, Ghadimi H, Eslami Y, et al. Short-term results of trabeculectomy using adjunctive intracameral bevacizumab: a randomized controlled trial. J Glaucoma 2016; 25:182–188. [DOI] [PubMed] [Google Scholar]
- 18.Akkan JU, Cilsim S. Role of subconjunctival bevacizumab as an adjuvant to primary trabeculectomy: a prospective randomized comparative 1-year follow-up study. J Glaucoma 2015; 24:1–8. [DOI] [PubMed] [Google Scholar]
- 19.Sedghipour MR, Mostafaei A, Taghavi Y. Low-dose subconjunctival bevacizumab to augment trabeculectomy for glaucoma. Clin Ophthalmol 2011; 5:797–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nilforushan N, Yadgari M, Kish SK, et al. Subconjunctival bevacizumab versus mitomycin C adjunctive to trabeculectomy. Am J Ophthalmol 2012; 153:352–357. [DOI] [PubMed] [Google Scholar]
- 21.Sengupta S, Venkatesh R, Ravindran RD. Safety and efficacy of using off-label bevacizumab versus mitomycin C to prevent bleb failure in a single-site phacotrabeculectomy by a randomized controlled clinical trial. J Glaucoma 2012; 21:450–459. [DOI] [PubMed] [Google Scholar]
- 22.Kiddee W, Orapiriyakul L, Kittigoonpaisan K, et al. Efficacy of adjunctive subconjunctival bevacizumab on the outcomes of primary trabeculectomy with mitomycin C: a prospective randomized placebo-controlled trial. J Glaucoma 2015; 24:600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hitchings RA, Grierson I. Clinicopathological correlation in eyes with failed fistulizing surgery. Trans Ophthalmol Soc U K 1983; 103 (Pt1):84–88. [PubMed] [Google Scholar]
- 24.Addicks EM, Quigley HA, Green WR, et al. Histologic characteristics of filtering blebs in glaucomatous eyes. Arch Ophthalmol 1983; 101:795–798. [DOI] [PubMed] [Google Scholar]
- 25.Zhang ZH, Liu HY, Hernandez-Da Mota SE, et al. Vitrectomy with or without preoperative intravitreal bevacizumab for proliferative diabetic retinopathy: a meta-analysis of randomized controlled trials. Am J Ophthalmol 2013; 156:106–115. [DOI] [PubMed] [Google Scholar]
- 26.Zhou M, Xu X, Zhang X, et al. Clinical outcomes of Ahmed glaucoma valve implantation with or without intravitreal bevacizumab pretreatment for neovascular glaucoma: a systematic review and meta-analysis. J Glaucoma 2015; doi: 10.1097/j.0000000000000241. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 27.Hwang HB, Han JW, Yim HB, et al. Beneficial effects of adjuvant intravitreal bevacizumab injection on outcomes of Ahmed glaucoma valve implantation in patients with neovascular glaucoma: systematic literature review. J Ocul Pharmacol Ther 2015; 31:198–203. [DOI] [PubMed] [Google Scholar]
- 28.Wong J, Wang N, Miller JW, et al. Modulation of human fibroblast activity by selected angiogenesis inhibitors. Exp Eye Res 1994; 58:439–451. [DOI] [PubMed] [Google Scholar]
- 29.Cheng G, Xiang H, Yang G, et al. Direct effects of bevacizumab on rat conjunctival fibroblast. Cell Biochem Biophys 2015; 73:45–50. [DOI] [PubMed] [Google Scholar]
- 30.O’Neill EC, Qin Q, Van Bergen NJ, et al. Antifibrotic activity of bevacizumab on human Tenon's fibroblasts in vitro. Invest Ophthalmol Vis Sci 2010; 51:6524–6532. [DOI] [PubMed] [Google Scholar]
- 31.Wilgus TA, Ferreira AM, Oberyszyn TM, et al. Regulation of scar formation by vascular endothelial growth factor. Lab Invest 2008; 88:579–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Z, Van Bergen T, Van de Veire S, et al. Inhibition of vascular endothelial growth factor reduces scar formation after glaucoma filtration surgery. Invest Ophtahlmol Vis Sci 2009; 50:5217–5225. [DOI] [PubMed] [Google Scholar]
- 33.Seibold LK, Sherwood MB, Kahook MY. Wound modulation after filtration surgery. Surv Ophthalmol 2012; 57:530–550. [DOI] [PubMed] [Google Scholar]
- 34.Skuta GL, Parrish RKII. Wound healing in glaucoma filtering surgery. Surv Ophthalmol 1987; 32:149–170. [DOI] [PubMed] [Google Scholar]
- 35.Hu D-N, Ritch R, Liebmann J, et al. Vascular endothelial growth factor is increased in aqueous humor of glaucomatous eyes. J Glaucoma 2002; 11:406–410. [DOI] [PubMed] [Google Scholar]
- 36.Tai TY, Moster MR, Pro MJ, et al. Needle bleb revision with bevacizumab and mitomycin C compared with mitomycin C alone for failing filtration blebs. J Glaucoma 2015; 24:311–315. [DOI] [PubMed] [Google Scholar]
- 37.Suzann Pershing, Christine Pal Chee, Asch Steven M, et al. Treating age-related macular degeneration: comparing the use of two drugs among medicare and veterans affairs populations. Health Aff (Millwood) 2015; 34:229–238. [DOI] [PubMed] [Google Scholar]


