Abstract
The aim of this study is to explore whether there is a relationship between chronic pancreatitis and cerebrovascular disease in Taiwan.
Using the claims data of the Taiwan National Health Insurance Program, we identified 16,672 subjects aged 20 to 84 years with a new diagnosis of chronic pancreatitis from 2000 to 2010 as the chronic pancreatitis group. We randomly selected 65,877 subjects aged 20 to 84 years without chronic pancreatitis as the nonchronic pancreatitis group. Both groups were matched by sex, age, comorbidities, and the index year of diagnosing chronic pancreatitis. The incidence of cerebrovascular disease at the end of 2011 was measured. The multivariable Cox proportional hazards regression model was used to measure the hazard ratio (HR) and 95% confidence interval (CI) for cerebrovascular disease risk associated with chronic pancreatitis and other comorbidities.
The overall incidence of cerebrovascular disease was 1.24-fold greater in the chronic pancreatitis group than that in the nonchronic pancreatitis group (14.2 vs. 11.5 per 1000 person-years, 95% CI = 1.19–1.30). After controlling for confounding factors, the adjusted HR of cerebrovascular disease was 1.27 (95% CI = 1.19–1.36) for the chronic pancreatitis group as compared with the nonchronic pancreatitis group. Woman (adjusted HR = 1.41, 95% CI = 1.31–1.51), age (every 1 year, HR = 1.04, 95% CI = 1.04–1.05), atrial fibrillation (adjusted HR = 1.23, 95% CI = 1.02–1.48), chronic kidney disease (adjusted HR = 1.48, 95% CI = 1.31–1.67), chronic obstructive pulmonary disease (adjusted HR = 1.27, 95% CI = 1.16–1.40), diabetes mellitus (adjusted HR = 1.82, 95% CI = 1.72–1.92), hypertension (adjusted HR = 1.66, 95% CI = 1.56–1.76), and peripheral atherosclerosis (adjusted HR = 1.26, 95% CI = 1.06–1.51) were other factors significantly associated with cerebrovascular disease.
Chronic pancreatitis is associated with increased hazard of subsequent cerebrovascular disease.
INTRODUCTION
Chronic pancreatitis can be a serious disease with severe morbidity and mortality. In the French study of Levy et al, the prevalence of chronic pancreatitis is ∼265/1,000,000 with a crude annual incidence of 7.7/100,000.1 The incidence varies worldwide depending on the populations studied, but there is an increased incidence in the past decades.2 Apart from the progressive inflammation and fibrotic destruction of the pancreatic secretory parenchyma that would cause complications and mortality, chronic pancreatitis has also been reported to be highly associated with pancreatic malignancies.3 Other studies have shown that patients with chronic pancreatitis would also possess a higher risk of developing diabetes mellitus.4 Because pancreas serves both endocrine and exocrine function, chronic pancreatitis would cause both endocrine and exocrine pancreatic insufficiency.5 The consequences of chronic pancreatitis may not only be localized to the pancreas or restricted to the gastrointestinal tract but would also be systemic. However, its correlations with cerebrovascular disease has not yet been mentioned.
Cerebrovascular disease is a worldwide public health problem associated with severe morbidity and mortality. In fact, cerebrovascular disease is the second most common cause of mortality and the third most common cause of disability worldwide.6 In recent decades, the overall rate of cerebrovascular disease-related mortality has decreased, but the absolute number of people with stroke, stroke survivors, cerebrovascular disease-related deaths, and the global burden of cerebrovascular disease-related disability is increasing.7 Previous studies have identified some nonmodifiable and modifiable risk factors of cerebrovascular disease. Diabetes mellitus is one of these.8 Atherosclerosis is one of the known direct causes of cerebrovascular disease.9
Previous studies have documented that chronic inflammation of the pancreas would cause irreversible parenchymal damage and functional impairment by destruction of the islet alpha, beta, and gamma cells combined with pre-existing risk factors for type 2 diabetes mellitus. This forms over 85% of pancreatogenic diabetes or type 3c diabetes, which often presents as a significantly larger swing in blood glucose that is more difficult to control.10 Sugar consumption is a controllable risk factor for both diabetes and cerebrovascular disease. Besides, there were previous studies which proposed that chronic inflammation is associated with accelerated atherosclerosis.11 Thus, we hypothesize that chronic pancreatitis can be linked to subsequent cerebrovascular disease based on the above-mentioned epidemiological evidence, sugar control, and chronic inflammation theory. If chronic pancreatitis substantially correlated with an increased risk of developing cerebrovascular disease, interventions could be performed to reduce the risk of cerebrovascular disease for patients with chronic pancreatitis including more intense sugar control, controlling intensity of inflammation, and treating other related comorbidities. Knowledge of the association between chronic pancreatitis and subsequent cerebrovascular disease would be helpful in developing more comprehensive ways to treat patients with chronic pancreatitis. Therefore, we conducted a retrospective cohort study to explore whether patients with chronic pancreatitis have an increased risk of developing subsequent cerebrovascular disease and whether the risk associated with chronic pancreatitis is enhanced by other comorbidities.
MATERIALS AND METHODS
Data Sources
This retrospective nationwide cohort study analyzed the reimbursement claim data of the Taiwan National Health Insurance Program. This program was implemented in Taiwan in March 1995 and represents almost 99% of the 23 million Taiwan residents.12 The details of the program can be found in previous high-quality studies.13–19 The study was approved by the Institutional Review Board of China Medical University and Hospital in Taiwan (CMUH-104-REC2–115).
Study Participants
Using the inpatient claim dataset of the Taiwan National Health Insurance Program, we selected all of the hospitalized subjects aged 20 to 84 years with new discharge diagnosis of chronic pancreatitis (ICD-9 code 577.1) from 2000 to 2010 as the chronic pancreatitis group, based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9 code). The date of diagnosing chronic pancreatitis was defined as the index date. We randomly selected subjects aged 20 to 84 years without chronic pancreatitis from the same dataset as the nonchronic pancreatitis group. Both groups were matched by sex, age (every 5-year span), comorbidities, and the index year of diagnosing chronic pancreatitis. In both groups, subjects who had the diagnosis of cerebrovascular disease (ICD-9 codes 430–438) or pancreatic cancer (ICD-9 code 157) at baseline were excluded.
Main Outcome Measurement
All of the study subjects were followed from the index date to the date of discharge diagnosis with cerebrovascular disease or to the end of 2011. Cerebrovascular disease was divided into three categories, including ischemic type, hemorrhagic type, and other types.
Comorbidities Potentially Related to Cerebrovascular Disease
Some risk factors of cerebrovascular disease, such as alcohol consumption, tobacco use, and obesity, were not recorded in this database owing to the inherent limitation. We included alcohol-related disease instead of alcohol consumption, tobacco use-related disease such as chronic obstructive pulmonary disease instead of tobacco use, and obesity-related diseases such as hypertension, diabetes mellitus, and peripheral atherosclerosis instead of obesity. These points have been addressed in previous studies.20,21 Comorbidities before the index date which could be potentially related to cerebrovascular disease were included as follows: alcohol-related disease (ICD-9 codes 291, 303, 305.00, 305.01, 305.02, 305.03, 571.0–571.3, 790.3 and V11.3), atrial fibrillation (ICD-9 code 427.31), cancer (ICD-9 codes 140–208), chronic kidney disease (ICD-9 codes 585–586 and 588.8–588.9), chronic obstructive pulmonary disease (ICD-9 codes 491, 492, 493, and 496), coronary artery disease (ICD-9 codes 410–414), diabetes mellitus (ICD-9 code 250), heart failure (ICD-9 code 428), hyperlipidemia (ICD-9 codes 272.0, 272.1, 272.2, 272.3, and 272.4), hypertension (ICD-9 codes 401–405), and peripheral atherosclerosis (ICD-9 codes 440–448).
Statistical Analysis
The distributions of sex, age, and comorbidities were compared between the chronic pancreatitis group and nonchronic pancreatitis group using the chi-square test for categorized variables and the t-test for continuous variables. The incidence of cerebrovascular disease was measured as the number of cerebrovascular disease events identified during the follow-up period divided by the total follow-up person-years for each group. The sex-, age-, and follow-up period-specific incidence rates of cerebrovascular disease were measured. The incidence rates stratified by subtypes of cerebrovascular disease were also measured. Initially, we included all variables in the univariable Cox proportional hazards regression model. Those found to be significant in the univariable model were further included in the multivariable Cox proportional hazards regression model to estimate the hazard ratio (HR) and 95% confidence interval (CI) of cerebrovascular disease risk associated with chronic pancreatitis and other comorbidities. The statistical significance level was defined at a two-sided probability value <0.05. All analyses were performed with SAS software version 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
Baseline Characteristics of the Study Population
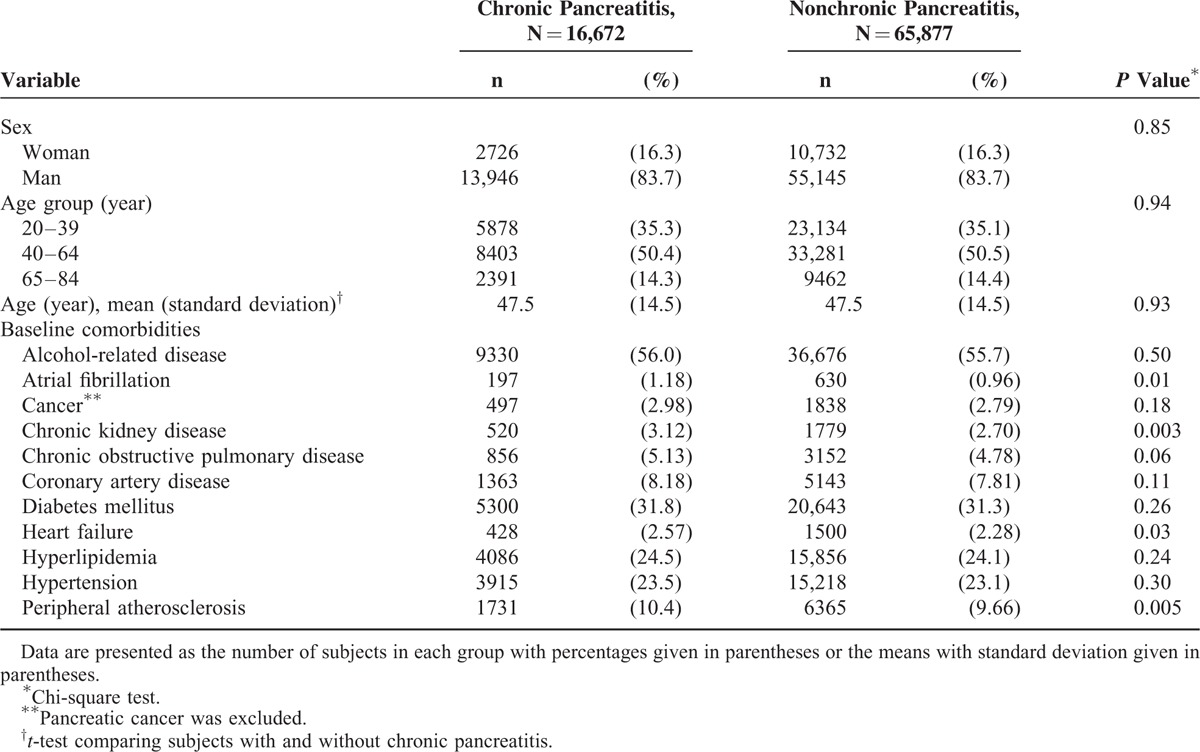
Table 1 discloses the distributions of sex, age, and comorbidities between the chronic pancreatitis group and nonchronic pancreatitis group. There were 16,672 subjects in the chronic pancreatitis group, and 65,877 subjects in the nonchronic pancreatitis group. Both groups had similar distributions of sex and age. The mean ages (standard deviation) of the study subjects were 47.5 ± 14.5 years for the chronic pancreatitis group and nonchronic pancreatitis group. The chronic pancreatitis group had a higher proportion of atrial fibrillation, chronic kidney disease, heart failure, and peripheral atherosclerosis than the nonchronic pancreatitis group (chi-square test, P < 0.05 for all).
TABLE 1.
Baseline Information Between Chronic Pancreatitis Group and Nonchronic Pancreatitis Group
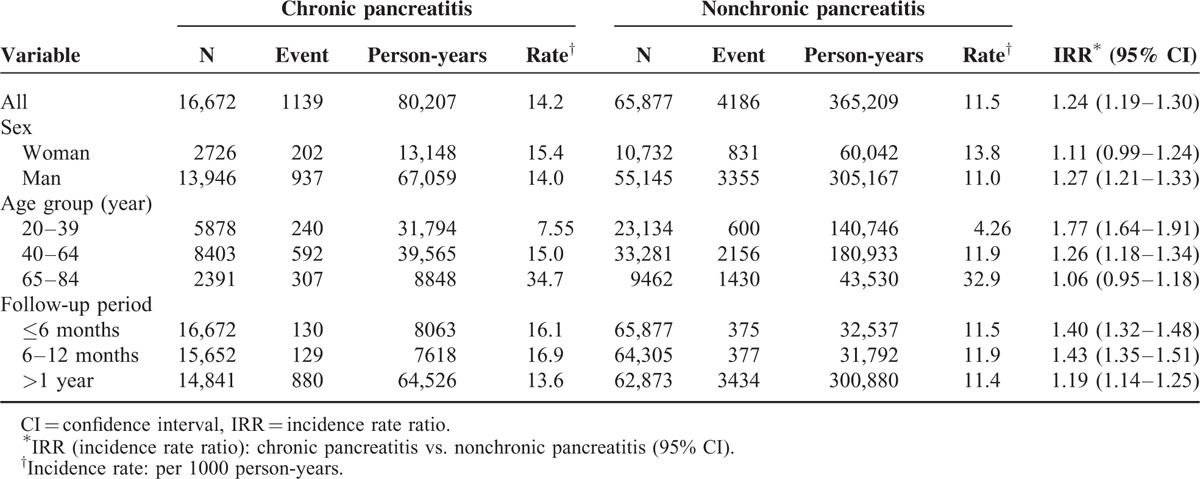
Incidence of Cerebrovascular Disease of the Study Population
The follow-up results disclosed that the overall incidence of cerebrovascular disease was 1.24-fold greater in the chronic pancreatitis group than that in the nonchronic pancreatitis group (14.2 vs. 11.5 per 1000 person-years, 95% CI = 1.19–1.30). The incidence rates of cerebrovascular disease as stratified by sexuality, age, and follow-up period were all higher in the chronic pancreatitis than in the nonchronic pancreatitis group. The chronic pancreatitis group aged 65 to 84 had the highest incidence of cerebrovascular disease (34.7 per 1000 person-years). The analysis stratified by follow-up period disclosed that the risk of cerebrovascular disease persisted over time even after 1 year from diagnosis with chronic pancreatitis. However, the incidence rate ratio was much higher during the first 1 year of follow-up (Table 2).
TABLE 2.
Incidence of Cerebrovascular Disease Stratified by Sex, Age and Follow-Up Period Between Chronic Pancreatitis Group and Nonchronic Pancreatitis Group
Cerebrovascular Disease Potentially Associated With Chronic Pancreatitis and Other Comorbidities
After controlling for potentially confounding factors, the multivariable Cox proportional hazard regression model disclosed that the adjusted HR of cerebrovascular disease was 1.27 (95% CI = 1.19–1.36) for the chronic pancreatitis group versus the nonchronic pancreatitis group. Woman (adjusted HR = 1.41, 95% CI = 1.31–1.51), age (every 1 year, adjusted HR = 1.04, 95% CI = 1.04–1.05), atrial fibrillation (adjusted HR = 1.23, 95% CI = 1.02–1.48), chronic kidney disease (adjusted HR = 1.48, 95% CI = 1.31–1.67), chronic obstructive pulmonary disease (adjusted HR = 1.27, 95% CI = 1.16–1.40), diabetes mellitus (adjusted HR = 1.82, 95% CI = 1.72–1.92), hypertension (adjusted HR = 1.66, 95% CI = 1.56–1.76), and peripheral atherosclerosis (adjusted HR = 1.26, 95% CI = 1.06–1.51) were other factors significantly associated with cerebrovascular disease (Table 3).
TABLE 3.
Hazard Ratio and 95% Confidence Interval of Cerebrovascular Disease Associated With Chronic Pancreatitis and Other Comorbidities

Incidence Density and Hazard Ratio of Ischemic, Hemorrhagic, and Other Types of Cerebrovascular Disease Potentially Associated With Chronic Pancreatitis
In further analysis, the incidence rates of ischemic, hemorrhagic, and other types of cerebrovascular disease were all higher in the chronic pancreatitis than those in the nonchronic pancreatitis group (Table 4). After controlling for potentially confounding factors, the adjusted HRs were 1.20 (95% CI = 1.08–1.33) for ischemic cerebrovascular disease, 1.33 (95% CI = 1.15–1.52) for hemorrhagic cerebrovascular disease, and 1.32 (95% CI = 1.19–1.47) for other types of cerebrovascular disease, when comparing the chronic pancreatitis group with the nonchronic pancreatitis group.
TABLE 4.
Incidence Density and Hazard Ratio of Ischemic, Hemorrhagic, and Other Types of Cerebrovascular Disease Between Chronic Pancreatitis Group and Nonchronic Pancreatitis Group

Figure 1A–D disclose that the chronic pancreatitis group had a significantly higher cumulative incidences of overall cerebrovascular disease (P < 0.001) (Figure 1A), ischemic (P = 0.006) (Figure 1B), hemorrhagic (P < 0.001) (Figure 1C), and other types of cerebrovascular disease (P < 0.001) (Figure 1D), when compared with the nonchronic pancreatitis group.
FIGURE 1.

A–1D disclose that the chronic pancreatitis group had a significantly higher cumulative incidences of overall cerebrovascular disease (P < 0.001) (A); ischemic type (P = 0.006) (B); hemorrhagic type (P < 0.001), (C); and other types of cerebrovascular disease (P < 0.001) (D), when compared with the nonchronic pancreatitis group.
DISCUSSION
This is the first population-based cohort study to study the risk of subsequent cerebrovascular disease in patients with chronic pancreatitis and the effects associated with comorbidities in Taiwan. No other study has reported this association. We used a well-preserved database in Taiwan that has been analyzed to explore numerous epidemiological problems with an excellent established role in the literature. The accuracy and reliability of the diagnosis codes in the present study have been well examined in previous high-quality journals.22–26
We found that the incidence of cerebrovascular disease in the chronic pancreatitis group was ∼1.24-fold greater than that in the nonchronic pancreatitis group. We noted that the risk of cerebrovascular disease persisted over time even after 1 year from diagnosis with chronic pancreatitis (Table 2). The multivariable regression analysis disclosed that the adjusted HR of cerebrovascular disease was 1.27 (95% CI = 1.19–1.36) for the chronic pancreatitis group when compared with the nonchronic pancreatitis group (Table 3). In further analysis, we found the adjusted HRs were 1.20 for ischemic cerebrovascular disease, 1.33 for hemorrhagic cerebrovascular disease, and 1.32 for other types of cerebrovascular disease when comparing the chronic pancreatitis group with the nonchronic pancreatitis group (Table 4). These findings indicate that chronic pancreatitis is associated with increased hazard of cerebrovascular disease, no matter ischemic, hemorrhagic, or other types.
Although the cause of the association between chronic pancreatitis and cerebrovascular disease cannot be clarified in this observational study, we reviewed some relevant studies to explain the mechanism between chronic pancreatitis and the risk of cerebrovascular disease. First, chronic pancreatic inflammation because of chronic pancreatitis causes irreversible parenchymal damage and functional impairment of the pancreatic islets that contribute to the development of 85% of pancreatogenic diabetes. This accounts for 5% to 10% of all diabetes mellitus.10 Diabetes mellitus is a known risk factor for cerebrovascular disease, although most of the previous studies about diabetes related to cerebrovascular disease are type 2 diabetes mellitus. After diabetes developed—regardless of the diabetes type—they shared at least a common pathway in diabetes-related macrovascular diseases, causing cerebrovascular disease. Second, chronic inflammation of the pancreas aggregate macrophage, neutrophils, and injured acinar cell, releasing cytokines such as interleukin-1β, interleukin-6, tumor necrosis factor-α, transforming growth factor-β, platelet-derived growth factor, and reactive oxygen species.27,28 Reactive oxygen species were shown to be increased significantly in patient with chronic pancreatitis.29 There are multiple animal models pointing that excessive level of reactive oxygen species is related to cerebrovascular disease, including both ischemic and hemorrhagic cerebrovascular disease.30 Some researchers even proposed nicotinamide adenine dinucleotide phosphate oxidase as a therapeutic target for neuroprotection against ischemic stroke, based on animal studies.31
We found that women had a rather large HR besides the comorbidities listed above which carried HR >1. Traditionally, men were known to have a higher incidence of cerebrovascular disease before the incidence reversed—women were found to have higher incidence when aged >75 years.32 This was probably because of the differences in the production of reactive oxygen species, and the antioxidant capacity between the two sexes.33
Endothelial dysfunction was often noted in chronic inflammation and was associated with atherosclerosis, hypertension, and diabetes mellitus.11 There were complicated interactions among inflammation, reactive oxygen species, hyperglycemia, hypertension, atherosclerosis, and thromboembolism. Each may aggravate the others, and the definite mechanism cannot be clarified here.34 However, since appropriate treatment of modifiable risk factor would reduce the incidence of cerebrovascular disease, we think that by treating chronic pancreatitis intensively—especially when the extent of the blood sugar swing can be lessened—cerebrovascular disease may be avoided in some individuals, whereas usefulness of antioxidant therapy to treat chronic pancreatitis, diabetic complications, or to prevent cerebrovascular disease is yet to be concluded.
We also found that although the increased risk of cerebrovascular disease persisted over long periods, the risk was particularly increased during the first 12 months (Table 2). This may indicate a golden period after chronic pancreatitis is diagnosed that certain intervention may be useful to effectively lower the risk of cerebrovascular disease. Before some further larger or prospective investigation can be done, we suggest that clinicians should be particularly cautious about cerebrovascular disease risk after diagnosing chronic pancreatitis and treating the sugar problems more intensively.
Limitations of this present study are as follow. First, this database does not record how chronic pancreatitis and cerebrovascular disease were diagnosed. Only discharge diagnosis based on ICD-9 codes were recorded in the hospitalization dataset. From a view of high quality of the Taiwan National Health Insurance Program, the accuracy of discharge diagnosis can be sure. Therefore, chronic pancreatitis, cerebrovascular disease, and other comorbidities were included based on ICD-9 codes. Second, there was no information concerning the severity of chronic pancreatitis, cerebrovascular disease, or the subsequent type of diabetes mellitus—this plays an important role in the development of cerebrovascular disease and the potentially most practical management in our hypothesis. Owing to the same reason, we were unable to detect the difference based on pancreatitis severity or whether the existence of chronic pancreatitis complications, such as pseudoaneurysms, biliary/duodenal obstruction, pseudocysts, or splenic vein thrombosis, has different impacts on risk of cerebrovascular disease. Third, owing to the same limitations, the exact etiologies of chronic pancreatitis were not recorded. Alcohol abuse, which is an important cause of chronic pancreatitis, is also a known modifiable risk factor for cerebrovascular disease. Ideally, this should be analyzed independently—this might bring a more statistically and clinically useful result that may block the still unspecified pathway from chronic pancreatitis to cerebrovascular disease. Fourth, although our results showed that chronic pancreatitis is associated with increased risk of subsequent cerebrovascular disease, we still cannot precisely state how intense the treatment should be nor how to suggest treatment that prevents patients with chronic pancreatitis from getting cerebrovascular disease. Further prospective investigation such as randomized controlled trials should be done to determine the actual benefits of different levels of intensive treatment for chronic pancreatitis to prevent the development of cerebrovascular disease. Although we proposed a hypothesis that poor sugar control may be one of the reasons underlying cerebrovascular disease, we could not definitively conclude that chronic pancreatitis is the reason behind cerebrovascular disease.
Some strengths of this study should be mentioned. This study provides a different interpretation of the association between chronic pancreatitis and cerebrovascular disease. We also suggest a likely probable biological mechanism that links the two diseases. Further investigation may be conducted to develop a risk of subsequent cerebrovascular disease assessment calculator for patients with chronic pancreatitis or other comorbidities. In addition, none of our investigators faced any of the subjects directly while collecting the data, yet the diagnostic codes are clear and reliable in the database. This makes the study free from selection bias and misclassification bias while also minimizing information bias.
We conclude that chronic pancreatitis is associated with increased hazard of subsequent cerebrovascular disease, no matter ischemic, hemorrhagic, or other types. Woman, age, atrial fibrillation, chronic kidney disease, chronic obstructive pulmonary disease, diabetes mellitus, hypertension, and peripheral atherosclerosis are other factors significantly associated with cerebrovascular disease. Further studies are needed to clarify the actual biological mechanism by which chronic pancreatitis is associated with risk of developing cerebrovascular disease while exploring which specific groups may benefit the most from receiving intensive management of chronic pancreatitis in order to lower the risk of cerebrovascular disease.
Footnotes
Abbreviation: ICD-9 code = International Classification of Diseases, 9th Revision, Clinical Modification.
Funding: this study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW105-TDU-B-212–133019), China Medical University Hospital, Academia Sinica Taiwan Biobank Stroke Biosignature Project (BM10501010037), National Research Program for Biopharmaceuticals (NRPB) Stroke Clinical Trial Consortium (MOST 104–2325-B-039 -005), Tseng-Lien Lin Foundation, Taichung, Taiwan, Taiwan Brain Disease Foundation, Taipei, Taiwan, and Katsuzo and Kiyo Aoshima Memorial Funds, Japan. These funding agencies did not influence the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions: T-SW, K-FL, and C-ML substantially contributed equally to the conception of the article. They initiated the draft of the article and critically revised the article.
C-LL and W-CC conducted the data analysis and critically revised the article.
S-WL planned and conducted this study. He substantially contributed to the conception of the article, initiated the draft of the article and critically revised the article.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Levy P, Barthet M, Mollard BR, et al. Estimation of the prevalence and incidence of chronic pancreatitis and its complications. Gastroenterologie Clinique Biologique 2006; 30:838–844. [DOI] [PubMed] [Google Scholar]
- 2.Yadav D, Timmons L, Benson JT, et al. Incidence, prevalence, and survival of chronic pancreatitis: a population-based study. Am J Gastroenterol 2011; 106:2192–2199. [DOI] [PubMed] [Google Scholar]
- 3.Pinho AV, Chantrill L, Rooman I. Chronic pancreatitis: a path to pancreatic cancer. Cancer Lett 2014; 345:203–209. [DOI] [PubMed] [Google Scholar]
- 4.Ewald N, Hardt PD. Diagnosis and treatment of diabetes mellitus in chronic pancreatitis. World J Gastroenterol 2013; 19:7276–7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hyun JJ, Lee HS. Experimental models of pancreatitis. Clin Endosc 2014; 47:212–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380:2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krishnamurthi RV, Feigin VL, Forouzanfar MH, et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet Global Health 2013; 1:e259–e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arboix A. Cardiovascular risk factors for acute stroke: Risk profiles in the different subtypes of ischemic stroke. World J Clin Cases 2015; 3:418–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hollander M, Hak AE, Koudstaal PJ, et al. Comparison between measures of atherosclerosis and risk of stroke: the Rotterdam Study. Stroke 2003; 34:2367–2372. [DOI] [PubMed] [Google Scholar]
- 10.Rickels MR, Bellin M, Toledo FG, et al. Detection, evaluation and treatment of diabetes mellitus in chronic pancreatitis: recommendations from Pancreas Fest 2012. Pancreatology 2013; 13:336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steyers CM, 3rd, Miller FJ., Jr Endothelial dysfunction in chronic inflammatory diseases. Int J Mol Sci 2014; 15:11324–11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Health Insurance Research Database, Taiwan. Available at: http://nhird.nhri.org.tw/en/index.html. [cited in 2016 April 1, English version]. [Google Scholar]
- 13.Lai SW, Liao KF, Liao CC, et al. Polypharmacy correlates with increased risk for hip fracture in the elderly: a population-based study. Medicine 2010; 89:295–299. [DOI] [PubMed] [Google Scholar]
- 14.Hung SC, Liao KF, Lai SW, et al. Risk factors associated with symptomatic cholelithiasis in Taiwan: a population-based study. BMC Gastroenterol 2011; 11:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen HY, Lai SW, Muo CH, et al. Ethambutol-induced optic neuropathy: a nationwide population-based study from Taiwan. Br J Ophthalmol 2012; 96:1368–1371. [DOI] [PubMed] [Google Scholar]
- 16.Lai HC, Chang SN, Lin CC, et al. Does diabetes mellitus with or without gallstones increase the risk of gallbladder cancer? Results from a population-based cohort study. J Gastroenterol 2013; 48:856–865. [DOI] [PubMed] [Google Scholar]
- 17.Lai HC, Tsai IJ, Chen PC, et al. Gallstones, a cholecystectomy, chronic pancreatitis, and the risk of subsequent pancreatic cancer in diabetic patients: a population-based cohort study. J Gastroenterol 2013; 48:721–727. [DOI] [PubMed] [Google Scholar]
- 18.Lai HC, Lin CC, Cheng KS, et al. Increased incidence of gastrointestinal cancers among patients with pyogenic liver abscess: a population-based cohort study. Gastroenterology 2014; 146:129–137. [DOI] [PubMed] [Google Scholar]
- 19.Yang SP, Muo CH, Wang IK, et al. Risk of type 2 diabetes mellitus in female breast cancer patients treated with morphine: a retrospective population-based time-dependent cohort study. Diabetes Res Clin Pract 2015; 110:285–290. [DOI] [PubMed] [Google Scholar]
- 20.Liao KF, Lin CL, Lai SW, et al. Sitagliptin use and risk of acute pancreatitis in type 2 diabetes mellitus: A population-based case-control study in Taiwan. Eur J Intern Med 2016; 27:76–79. [DOI] [PubMed] [Google Scholar]
- 21.Lai SW, Lin CL, Liao KF. Atrial fibrillation associated with acute pancreatitis: a retrospective cohort study in Taiwan. J Hepatobiliary Pancreat Sci 2016; 3:331. [DOI] [PubMed] [Google Scholar]
- 22.Lai S-W, Sung F-C, Lin C-L, et al. Use of proton pump inhibitors correlates with increased risk of pancreatic cancer: a case-control study in Taiwan. Kuwait Med J 2014; 46:44–48. [Google Scholar]
- 23.Lai SW, Lai HC, Lin CL, et al. Finasteride use and acute pancreatitis in Taiwan. J Clin Pharmacol 2015; 55:657–660. [DOI] [PubMed] [Google Scholar]
- 24.Liao K-F, Lin C-L, Lai S-W. Schizophrenia correlates with increased risk of hepatocellular carcinoma in men: a Cohort study in Taiwan. Int Med J 2015; 22:273–276. [Google Scholar]
- 25.Liao K-F, Lin C-L, Lai S-W, et al. Parkinson's disease and risk of pancreatic cancer: a population-based case-control study in Taiwan. Neurol Asia 2015; 20:251–255. [Google Scholar]
- 26.Lai SW, Lai HC, Lin CL, et al. Chronic osteomyelitis correlates with increased risk of acute pancreatitis in a case-control study in Taiwan. Eur J Intern Med 2015; 26:429–432. [DOI] [PubMed] [Google Scholar]
- 27.Zheng L, Xue J, Jaffee EM, et al. Role of immune cells and immune-based therapies in pancreatitis and pancreatic ductal adenocarcinoma. Gastroenterology 2013; 144:1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lesina M, Wormann SM, Neuhofer P, et al. Interleukin-6 in inflammatory and malignant diseases of the pancreas. Semin Immunol 2014; 26:80–87. [DOI] [PubMed] [Google Scholar]
- 29.Leung PS, Chan YC. Role of oxidative stress in pancreatic inflammation. Antioxidants Redox Signaling 2009; 11:135–165. [DOI] [PubMed] [Google Scholar]
- 30.Chrissobolis S, Faraci FM. The role of oxidative stress and NADPH oxidase in cerebrovascular disease. Trends Mol Med 2008; 14:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCann SK, Roulston CL. NADPH oxidase as a therapeutic target for neuroprotection against ischaemic stroke: future perspectives. Brain Sci 2013; 3:561–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 2014; 129:e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brunelli E, Domanico F, La Russa D, et al. Sex differences in oxidative stress biomarkers. Curr Drug Targets 2014; 15:811–815. [DOI] [PubMed] [Google Scholar]
- 34.Aksu K, Donmez A, Keser G. Inflammation-induced thrombosis: mechanisms, disease associations and management. Curr Pharm Des 2012; 18:1478–1493. [DOI] [PubMed] [Google Scholar]


