Abstract
The prognosis of antineutrophil cytoplasmic antibody (ANCA)-associated glomerulonephritis (ANCA-GN) is unfavorable despite immunosuppressive therapy. It has been suggested that the loss of podocytes is a hallmark of progressive kidney disease. However, it is unclear about podocyte injuries and their predictive values on the prognosis in ANCA-GN. Therefore, the current study aimed to investigate the podocyte injury in renal histopathology and its association with renal prognosis of patients with ANCA-GN.
A total of 170 patients with ANCA-GN were recruited in this study. Morphometric investigation of podocytes by electron microscopy including foot process width (FPW), podocyte density per glomerulus (Nv), and glomerular basement membrane (GBM) width were measured and calculated in ANCA-GN patients. Cox regression analysis was used to analyze the association between podocyte injuries and prognosis of patients with ANCA-GN.
Foot processes broadening, podocyte detachment, and GBM thickening could be observed in electron micrographs in the specimens of 158/170 (92.9%), 142/170 (83.5%), and 150/170 (88.2%) patients, respectively. Compared with normal controls, FPW and GBM width in ANCA-GN patients was significantly higher (1269.39 ± 680.19 vs 585.81 ± 77.16, P = 0.004; 668.23 ± 208.73 vs 354.23 ± 52.70, P = 0.000, respectively), while the podocyte density was significantly lower (55.90 ± 36.32 vs 255.23 ± 47.29, P = 0.000). The podocyte density was independently associated with the recovery of renal function in logistic regression analysis (OR, 1.083; 95% CI, 1.025–1.440; P = 0.005). Furthermore, multivariate analysis revealed that podocyte density was an independent predictor of end-stage renal disease (ESRD) (model A: HR, 0.950; 95% CI, 0.919–1.982; P = 0.002; model B: HR, 0.953; 95% CI, 0.922–0.985; P = 0.004).
Podocyte structural damage and detachment occurred frequently in patients with ANCA-GN. Moreover, podocyte detachment was an independent predictor of renal outcomes.
INTRODUCTION
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a group of systemic vasculitis characterized by inflammation and necrosis of small vessel walls, which comprises microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA), and eosinophilic granulomatosis with polyangiits.1,2 The kidney is one of the most vulnerable organs, typically presenting with rapidly progressive glomerulonephritis. Despite that immunosuppressive therapy dramatically improves patient outcomes,3 ANCA-associated glomerulonephritis (ANCA-GN) still results in end-stage renal disease (ESRD) in about a quarter of patients over 3–4 years.4–6
The histopathological hallmark of ANCA-associated glomerulonephritis is pauci-immune necrotizing crescentic glomerulonephritis.2 In the experimental crescentic glomerulonephritis model, it has been observed that podocyte lesions, including foot processes broadening and microvillous transformation, occur in the early stage of crescent formation.7 It has been proved that various injury factors induce podocyte dysfunction and subsequently result in podocyte detachment from glomerular basement membrane (GBM).8 More importantly, the loss of podocytes is a hallmark of progressive renal diseases.9,10 In classical podocytopathies, including idiopathic focal segmental glomerulosclerosis, membranous nephropathy and HIV associated nephropathy, podocytes undergo characteristic and irreversible ultrastructural changes, and finally result in detachment from GBM.8 Previous studies suggested that the podocyte detachment is a predictor of progression in both immunological and nonimmunological glomerular injuries.11–14 It has been reported that podocyte loss contributes to increasing albuminuria and the progression of diabetic nephropathy.11,12 It has also been suggested that the podocyte number predicts progression of albuminuria and disease severity in IgA nephropathy.13,14 However, it is still unclear whether podocyte injury has predictive value on the outcomes of patients with ANCA-GN. The current study aimed to investigate the morphologic alterations of the podocytes and their clinical association in patients with ANCA-GN.
METHODS
Patients
A total of 255 consecutive ANCA-GN patients who received renal biopsy at the Renal Division of Peking University First Hospital between 1997 and 2014 were retrospectively investigated in this study. All these patients met the 2012 Chapel Hill Consensus Conference nomenclature for AAV.15 The details of the recruitment process and exclusion criteria were shown in Figure 1. Finally, there are 170 patients were enrolled in this study.
FIGURE 1.
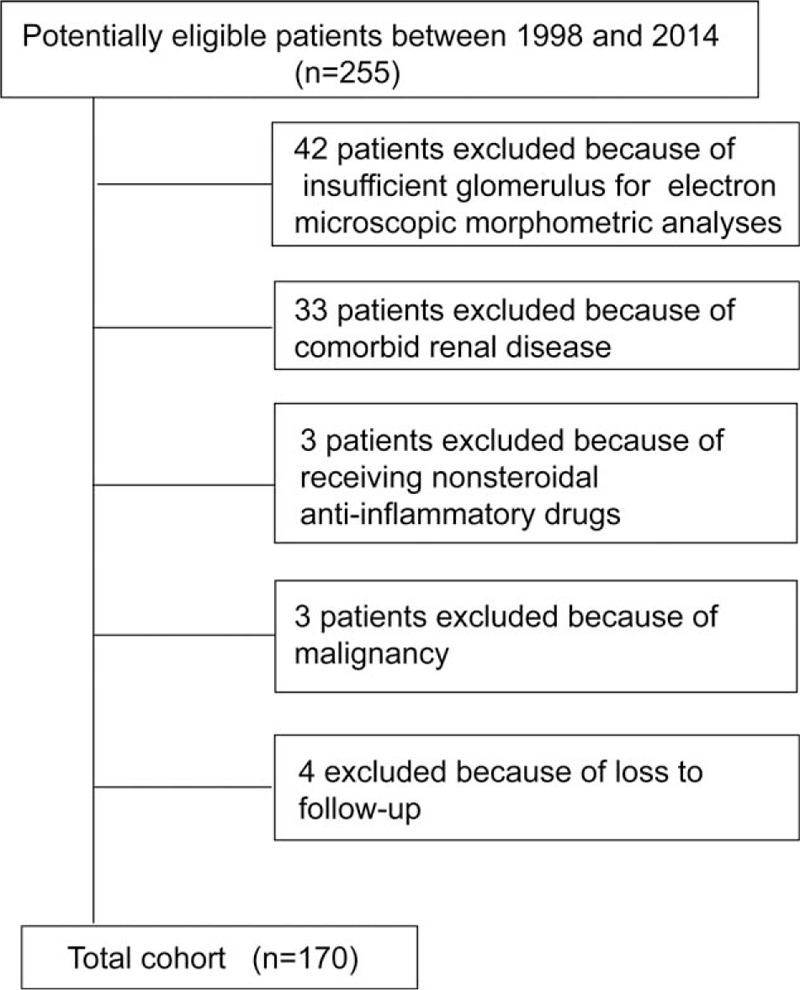
Study recruitment/inclusion process.
Sixteen patients with excision of renal hematoma or carcinoma without proteinuria or hematuria, hypertension, or renal dysfunction served as controls for the morphometric studies. The research was in compliance with the Declaration of Helsinki and approved by the ethics committee of our hospital. Written informed consent was obtained from each participant.
Modified equation of modification of diet in renal disease, adjusted in Chinese population, was used to estimate the glomerular filtration rate (estimated glomerular filtration rate [eGFR]) at entry:16 eGFR (mL/min per 1.73 m2) = 175 × (plasma creatinine)−1.234 × age−0.179 × 0.79 (if female).
Regular follow-up examinations were continued for AAV patients. Either ESRD or death was defined as the end point; the combined end point was defined as a composite outcome of death or ESRD.
Detection of Serum ANCA
Both ELISA and indirect immunofluorescence technique were performed for ANCA tests as described previously.17
Renal Histology
Briefly, the renal lesions in AAV patients were scored according to a standardized protocol, as previously described.18 Both pathologists scored the biopsies separately, blinded to patients’ data and the scores of the other observer. IF was performed for the detection of IgG, IgM, IgA, C3, C1q, kappa, and lambda light chains (Dakopatts, Copenhagen, Denmark) by fluorescence photomicroscope (Zeiss Axiophot, Oberkochen, Germany). IF staining intensity was graded 0 to 3+ on a semiquantitative scaling system.
Electron Microscopy
Transmission electron microscopy and podocyte definition were performed by standard protocol described previously.11 Briefly, the arithmetic mean of foot process width (FPW) was estimated as follow:
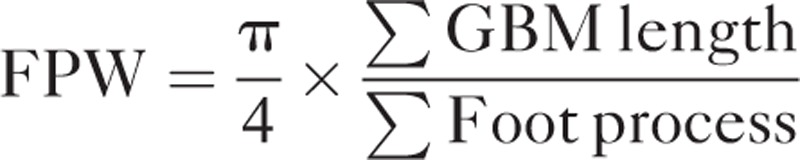 |
∑GBM length is the measurement of total GBM length in electron microscope photographs of each AAV patient, where ∑foot process is the calculation of total number of foot processes. The constant π is served for the correction of presumed random variation, which exists inevitably in the angle of section relative to the long axis of the podocyte.19,20 The intraobserver variability of the FPW was assessed by the coefficient of variation (the ratio of the SD to the mean) between 2 measurements of the same object by 1 observer.15 The interobserver variability in this study was assessed by the intraclass correlation coefficient.21
Podocyte density per glomerulus [Nv(epi/glom)] was used to estimate podocyte detachment (detailed in the Supplementary Material). In brief, the numerical density of podocytes in each glomerulus was calculated as Nv(epi/glom)=(1/1.55) × [Na(epi-nuclei/glom)3/Vv (nuclei/glom)]1/2, where 1.55 is the shape factor for the podocyte nuclei. The calculation of both Na(epi-nuclei/glom) and Vv(epi-nuclei/glom) was performed according to the standard protocol described previously.22
The GBM width was estimated by the orthogonal intercept method in 10,000 × electron microscope micrographs.23
Renal Function Recovery
The treatment protocols have been described previously24–26 (detailed in the Supplementary Material). The renal function was estimated after immunosuppressive therapy in 6 months. The renal function recovery was defined as complete recovery, partial recovery, and treatment failure, which has been detailed in the Supplementary Material.3,27,28
Statistical Analysis
Results were expressed as mean ± SD (for data that were normally distributed), or median and interquartile range (IQR) (for data that were not normally distributed). Predictors of treatment response were evaluated in all treated patients, using logistic regression; the results are expressed as odds ratios (ORs) with 95% confidence intervals (95% CIs) and P values. Cox proportional hazards models were used to evaluate time to ESRD or death among the patients given immunosuppressive treatment, and results are expressed as hazard ratios (HRs) with 95% CIs and P values. Podocyte morphometric parameters (including the FPW, podocyte density, and GBM width) were evaluated using univariate analysis. If the P value was less than 0.05, this parameter was allowed to be entered into multivariable models. Meanwhile, stepwise regression was carried out for the choice of predictive variables.29 The factors age, gender, normal glomerulus proportion, tubular atrophy, and interstitial fibrosis were forced into multivariable models, because they were potential confounding factors according to previous studies.28,30 Multicollinearity was checked using the tolerance, variance inflation factor (VIF), and condition index. The tolerance value less than 0.1 or VIF greater than 10 roughly indicates significant multicollinearity. The condition indexes were used to identify which variables were involved. The condition index for the principal component must be large (>30) to at least 2 regression coefficients.31P values less than 0.05 (2-sided) were considered significant. Statistical analysis was performed using the SPSS statistical software package (version 13.0, Chicago, IL).
RESULTS
Demographic and General Data
Clinical and histological characteristics of the patients are listed in Table 1. In brief, among the 170 patients with ANCA-GN, 86 were male and 84 were female, with a median age of 57.3 (range 14–82) years at diagnosis. A total of 128 out of 170 (75.3%) patients were classified as MPA, 38/170 (22.4%) as GPA, and 4/174 (2.4%) as RLV. A total of 163 (95.9%) of the 170 patients were positive for myeloperoxidase (MPO)-ANCA and 7 (4.1%) were positive for proteinase 3 (PR3)-ANCA. In our cohort, of the patients with GPA, 31 were positive for MPO-ANCA and 7 for PR3-ANCA. This was consistent with our previous finding that a large proportion of GPA patients in China were MPO-ANCA positive.32 All patients with MPA were MPO-ANCA positive. The level of proteinuria and initial eGFR were 2.6 ± 1.7 g/24 hour and 31.1 ± 28.1 mL/min/1.73 m2, respectively.
TABLE 1.
Clinical Characteristics of the ANCA-GN Patients (n = 170)
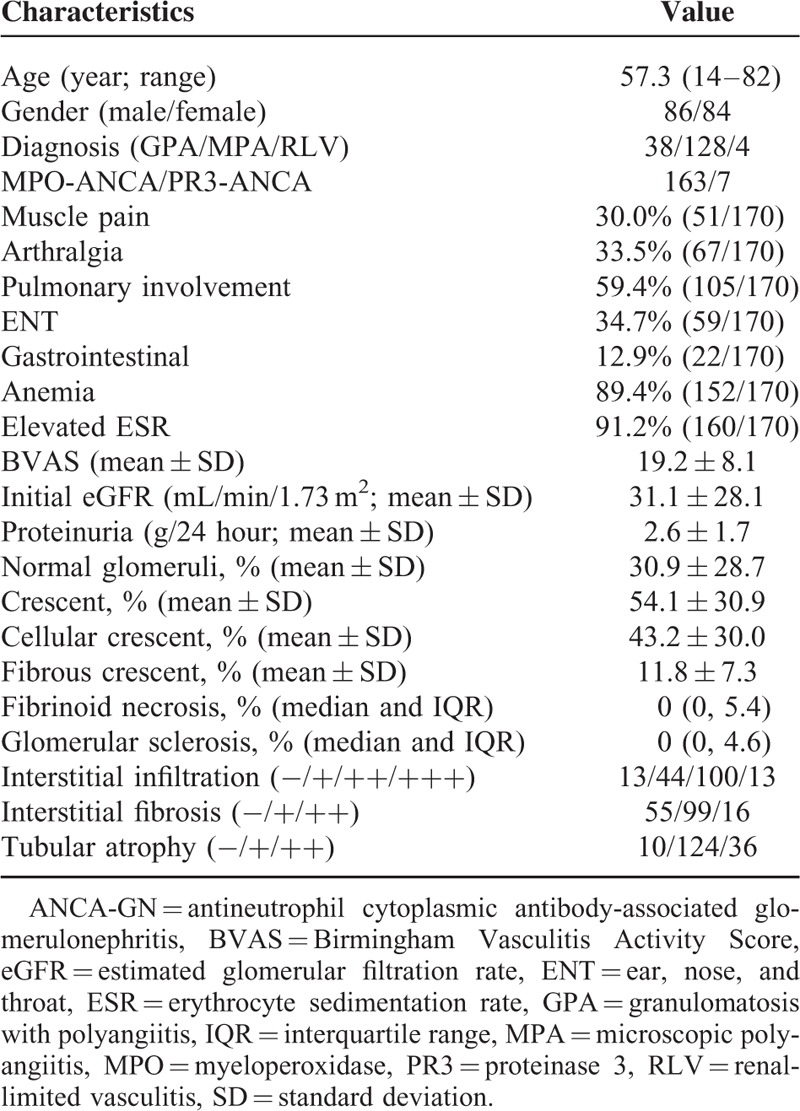
In each renal biopsy specimen, a median of 28 glomeruli (range 10–63) could be visualized. Further analysis revealed that 30.9% ± 28.7% of the glomeruli were normal and 54.1% ± 30.9% of the glomeruli had crescents. The data of interstitial infiltration, interstitial fibrosis, tubular atrophy, and extra renal manifestation were presented in Table 1.
Podocyte Morphometric Analyses
Electron microscopic measurements revealed typical podocyte injuries, including foot processes broadening, microvilli degeneration, podocyte detachment, and GBM thickening (Figure 2). Two observers independently measured the FPW, Nv, and GBM width of 10 patients with ANCA-GN and the intraclass correlation coefficient between the 2 observers were 0.92, 0.88, and 0.91, respectively, which indicated good interobserver agreement. Foot processes broadening, podocyte detachment, and GBM thickening could be observed in electron micrographs in the specimens of 158/170(92.9%), 142/170(83.5%), 150/170(88.2%) patients, respectively. FPW and GBM width in ANCA-GN patients was significantly greater than normal controls (1269.39 ± 680.19 vs 585.81 ± 77.16, P = 0.004; 668.23 ± 208.73 vs 354.23 ± 52.70, P = 0.000, respectively). Compared with the normal control, the podocyte density was significantly lower in ANCA-GN patients (55.90 ± 36.32 vs 255.23 ± 47.29, P = 0.000).
FIGURE 2.
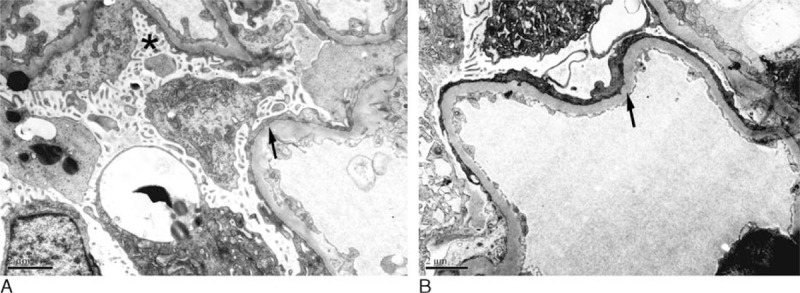
 |
 |
Among the 170 patients, 67 patients manifested massive proteinuria (≥3 g/24 hour). Compared with patients with massive proteinuria, the GBM width calculated in patients without massive proteinuria (<3 g/24 hour) was significantly thinner (0.62 ± 0.18 vs 0.75 ± 0.34, P = 0.000). No significant difference of FPW and podocyte density was observed between these 2 groups of patients (935.17 [IQR: 621.84–1610.83] vs 1242.93 [IQR: 658.04–2014.67], P = 0.172; 30.75 ± 15.20 vs 28.03 ± 20.33, P = 0.293, respectively). A total of 85 of 170 patients were detected with sparse immune deposits confined to the glomerular mesangium in renal histopathology. No significant difference of FPW, podocyte density, or GBM width was observed between ANCA-GN patients with and without immune complex deposition. A total of 33 of 170 ANCA-GN patients had normal serum creatinine (≤133 μmol/L) and 137 ANCA-GN patients had elevated serum creatinine (>133 μmol/L) at the time of renal biopsy. Compared with ANCA-GN patients with normal serum creatinine, FPW was significantly greater in patients with elevated serum creatinine (561.22 [IQR: 396.97, 852.22] vs 1056.62 [IQR: 721.62, 1303.82], P = 0.000). Podocyte density in ANCA-GN patients with elevated serum creatinine was significantly lower than patients with normal serum creatinine (26.39 ± 11.29 vs 43.22 ± 30.96, P = 0.000).
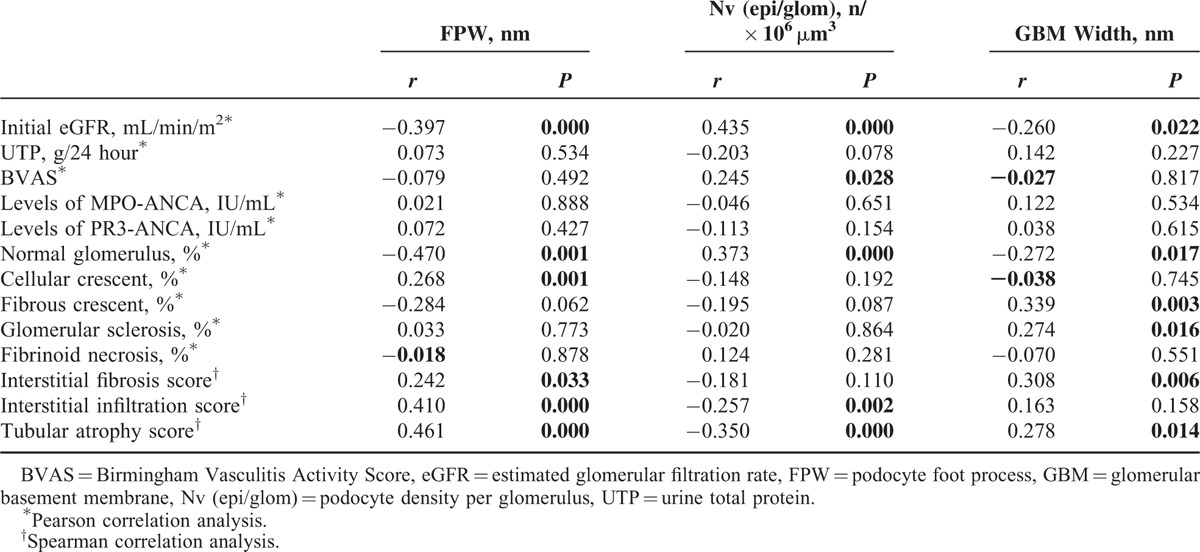
Pearson correlation analysis and Spearman rank correlation analysis was performed to investigate the associations between the podocyte morphometric parameters and the clinicopathological parameters in ANCA-GN patients (Table 2). It was found that FPW correlated with initial eGFR, proportion of normal glomerulus, fibrous crescent, total crescents, and the extent of interstitial fibrosis, interstitial infiltration, and tubular atrophy (r = −0.397, P = 0.000; r = −0.470, P = 0.001; r = 0.284, P = 0.062; r = 0.242, P = 0.033; r = 0.410, P = 0.000; and r = 0.461, P = 0.000, respectively). Podocyte density correlated with initial eGFR, proportion of normal glomerulus, and the extent of interstitial infiltration and tubular atrophy. Furthermore, podocyte density correlated with Birmingham Vasculitis Activity Score (r = 0.284, P = 0.001). GBM width negatively correlated with initial eGFR and the proportion of normal glomerulus (r = −0.260, P = 0.022; r = −0.272, P = 0.017, respectively), and positively correlated with the proportion of fibrous crescent, glomerular sclerosis, and the extent of interstitial fibrosis and tubular atrophy (r = 0.339, P = 0.003; r = 0.274, P = 0.016; r = 0.308, P = 0.006; r = 0.278, P = 0.014, respectively). The magnitude of podocyte injury was not associated with quantification of MPO-ANCA or PR3-ANCA.
TABLE 2.
Correlation of Clinical and Histological Parameters with Podocyte Injury
Predictors of Renal Function Recovery
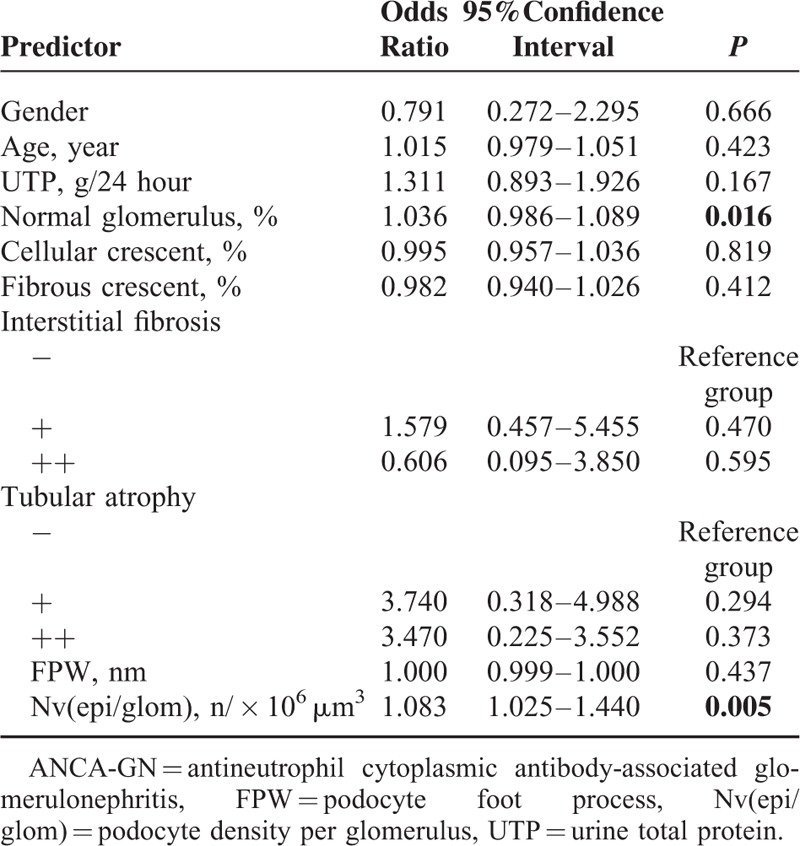
After the aforementioned induction therapy, 133 out of the 170 (78.2%) ANCA-GN patients achieved recovery of renal function, of which 63 patients achieved complete recovery and 70 patients achieved partial recovery; 37 of 170 (21.8%) patients had treatment failure. The candidate parameters were determined by stepwise regression and entered the multivariate analysis are shown in Table 3. The VIFs were less than 10 and the condition index was less than 30, respectively. The proportion of normal glomeruli and the podocyte density were independently associated with the recovery of renal function (OR, 1.036; 95% CI, 0.986−1.089; P = 0.016; OR, 1.083; 95% CI, 1.025−1.440; P = 0.005, respectively).
TABLE 3.
Multivariate Analysis of Renal Function Recovery in ANCA-GN Patients
Predictors of ESRD or Death
During the median follow-up period of 29 months (IQR, 12–63), 49 of 170 (28.8%) patients died and 48 of 170 (28.2%) patients progressed to ESRD.
Univariate analysis of renal survival in ANCA-GN patients showed that FPW and podocyte density were associated with ESRD (P = 0.000 and P = 0.000, respectively). Besides podocyte morphometric parameters, the predictors of ESRD in univariate analysis included age, proportion of normal glomerulus, serum creatinine at diagnosis, cellular crescent, fibrous crescent, and the extent of interstitial fibrosis. As the close correlation between the serum creatinine level and the proportion of normal glomerulus (the correlation coefficient was 0.522, P = 0.000), the 2 parameters were put in a multivariate analysis separately using model A and model B, respectively, as shown in Tables 4 and 5. The VIFs were less than 10 and the condition index were less than 30, respectively. Multivariate analysis revealed that podocyte density was an independent predictor of ESRD (model A: HR, 0.950; 95% CI, 0.919−1.982; P = 0.002; model B: HR, 0.953; 95% CI, 0.922−0.985; P = 0.004) (Table 4).
TABLE 4.
Multivariate Analysis of Patients’ Renal Survival in ANCA-GN
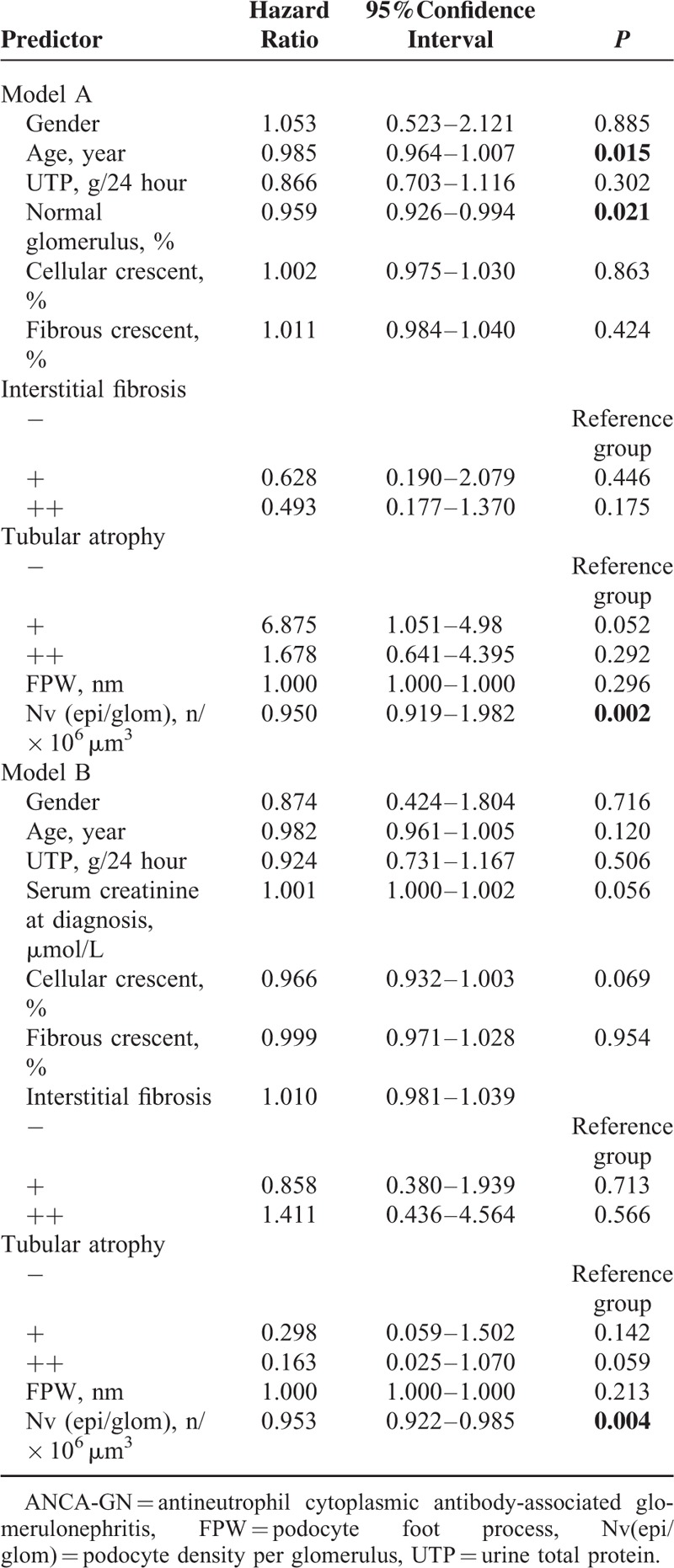
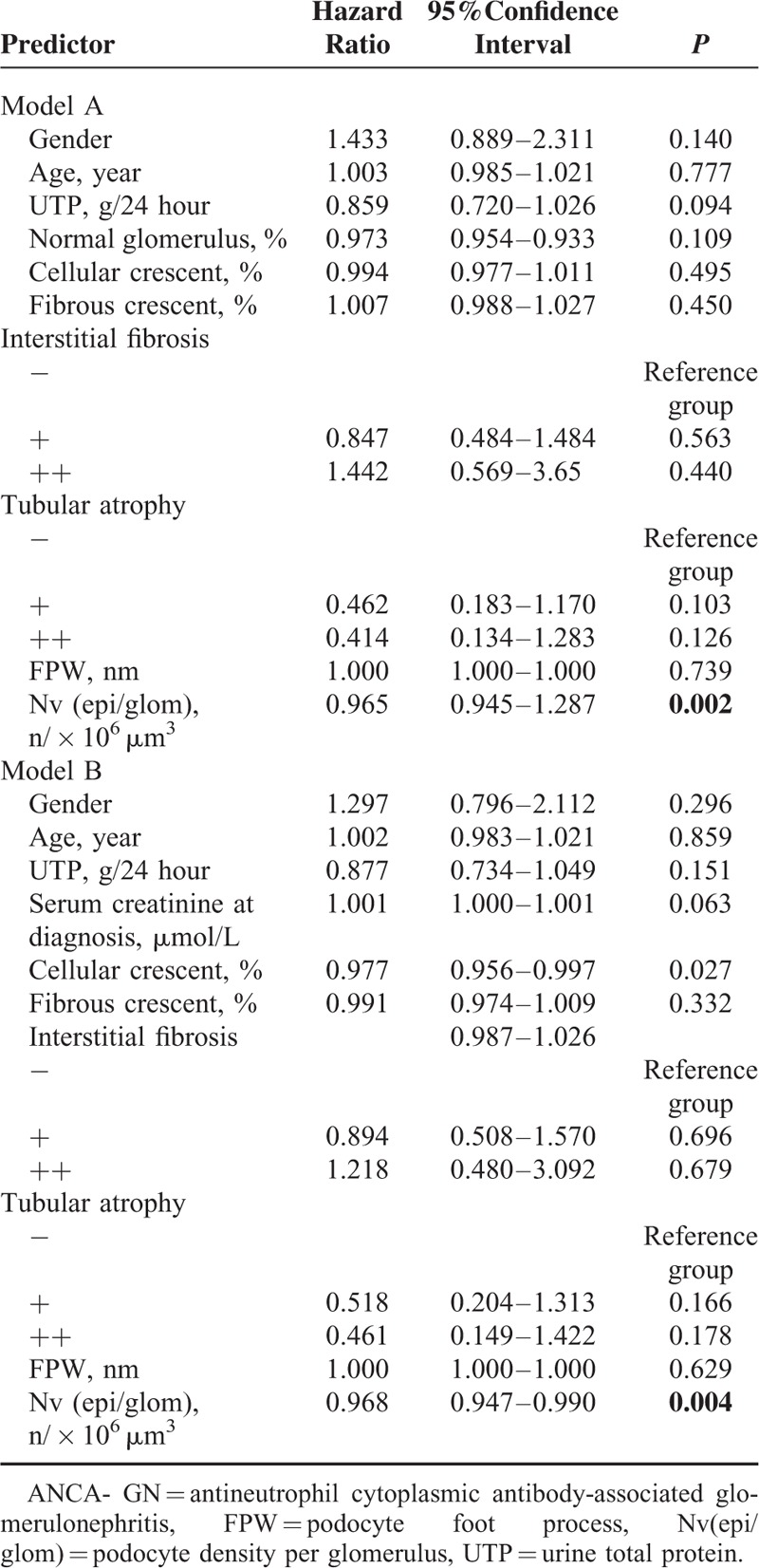
TABLE 5.
Multivariate Analysis of ANCA-GN Patients’ Combined end Points
None of the podocyte morphometric parameters correlated with all-cause mortality in multivariate analysis.
Additionally, univariate survival analysis revealed that FPW and podocyte density were associated with combined end points, that is, a composite outcome of death or ESRD (P = 0.000 and P = 0.000, respectively). Multivariate analysis revealed that podocyte density was an independent risk factor for combined end points after adjusting for age, sex, the proportion of normal glomerulus, and the extent of tubular atrophy and interstitial fibrosis (model A: HR, 0.965; 95% CI, 0.945−1.287; P = 0.002; model B: HR, 0.968; 95% CI, 0.947−0.990; P = 0.004)(Table 5).
DISCUSSION
The crescent formation and pauci-immune deposits are recognized as major histopathological characteristics of ANCA-associated glomerulonephritis.2 In a murine crescentic glomerulonephritis model, it has been shown that podocyte bridging acts as a key initial event in crescent formation.7,33 Podocytes form a bridge between the tuft and Bowman's capsule triggering the proliferation of parietal epithelial cells and the formation of crescents.7,34 In this murine model, it was also observed that the podocyte lesions such as the effacement of podocyte foot processes and microvillous transformation occurred in the early stage of crescent formation.7 However, in ANCA-GN, a typical crescentic glomerulonephritis, the podocyte injury has not been investigated. Although podocyte loss has been proved to be a hallmark of progressive nephron damage, it is unclear whether podocyte detachment correlated with renal prognosis in patients with ANCA-GN.
Morphometric studies have contributed greatly to our understanding of renal diseases.11,12,14,35 In the current study, morphometric analysis confirmed that the structural damage and podocyte detachment occurred in most of ANCA-GN patients, even in those with mild proteinuria and normal level of serum creatinine.
Rapid deterioration of renal function is a major but unfavorable feature of ANCA-GN.27 Even with active immunosuppressive therapy, the renal outcome of ANCA-GN patients is heterogeneous.4,5 Previous studies found that the proportion of normal glomeruli, GFR at diagnosis, and tubulointerstitial injury were predictors of renal outcomes.36–38 In the current study, the correlation analysis revealed that podocyte density was a factor independently associated with the recovery of renal function in ANCA-GN patients. More importantly, multivariate regression analysis showed that podocyte density was an independent predictor of ESRD. The predictive value of podocyte density is even better than the proportion of normal glomeruli, which is the well-recognized predictor for ESRD in ANCA-GN.18
In conclusion, podocyte density was an independent predictor of renal outcomes. The role of podocyte played in the pathogenesis of ANCA-associated glomerulonephritis needs further investigation.
Supplementary Material
Acknowledgments
The authors thank the grant of the Chinese 973 project (No. 2012CB517702), and three grants from the National Natural Science Fund (No. 81425008, 81321064 and 81370829) for the support.
Footnotes
Abbreviations: AAV = antineutrophil cytoplasmic antibody-associated vasculitis, ANCA = antineutrophil cytoplasmic antibody, ANCA-GN = ANCA-associated glomerulonephritis, eGFR = estimated glomerular filtration rate, ESR = erythrocyte sedimentation rate, FPW = foot process width, FSGS = focal segmental glomerulosclerosis, GBM = glomerular basement membrane, GPA = granulomatosis with polyangiitis, HR = hazard ratio, ICC = intraclass correlation coefficient, MPA = microscopic polyangiitis, MPO = myeloperoxidase, Nv = podocyte density per glomerulus, OR = odds ratio, PR3 = proteinase 3, VIF = variance inflation factor.
Research idea and study design: FY, MC; data acquisition: RZ, S-xW; data analysis: RZ, MC; statistical analysis: RZ, GL; supervision: M-HZ. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. MC takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
This study was supported by a grant of the Chinese 973 project (No. 2012CB517702), and three grants from the National Natural Science Fund (No. 81425008, 81321064 and 81370829).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Jennette JC, Falk RJ, Andrassy K, et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum 1994; 37:187–192. [DOI] [PubMed] [Google Scholar]
- 2.Kallenberg CG. Pathogenesis of ANCA-associated vasculitis, an update. Clin Rev Allergy Immunol 2011; 41:224–231. [DOI] [PubMed] [Google Scholar]
- 3.Hogan SL, Falk RJ, Chin H, et al. Predictors of relapse and treatment resistance in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis. Ann Intern Med 2005; 143:621–631. [DOI] [PubMed] [Google Scholar]
- 4.Li ZY, Chang DY, Zhao MH, et al. Predictors of treatment resistance and relapse in antineutrophil cytoplasmic antibody-associated vasculitis: a study of 439 cases in a single Chinese center. Arthritis Rheumatol 2014; 66:1920–1926. [DOI] [PubMed] [Google Scholar]
- 5.Booth AD, Almond MK, Burns A, et al. Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. Am J Kidney Dis 2003; 41:776–784. [DOI] [PubMed] [Google Scholar]
- 6.Flossmann O, Berden A, de Groot K, et al. Long-term patient survival in ANCA-associated vasculitis. Ann Rheum Dis 2011; 70:488–494. [DOI] [PubMed] [Google Scholar]
- 7.Le Hir M, Keller C, Eschmann V, et al. Podocyte bridges between the tuft and Bowman's capsule: an early event in experimental crescentic glomerulonephritis. J Am Soc Nephrol 2001; 12:2060–2071. [DOI] [PubMed] [Google Scholar]
- 8.Mundel P, Shankland SJ. Podocyte biology and response to injury. J Am Soc Nephrol 2002; 13:3005–3015. [DOI] [PubMed] [Google Scholar]
- 9.Kriz W, LeHir M. Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int 2005; 67:404–419. [DOI] [PubMed] [Google Scholar]
- 10.Kim YH, Goyal M, Kurnit D, et al. Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int 2001; 60:957–968. [DOI] [PubMed] [Google Scholar]
- 11.Dalla VM, Masiero A, Roiter AM, et al. Is podocyte injury relevant in diabetic nephropathy? Studies in patients with type 2 diabetes. Diabetes 2003; 52:1031–1035. [DOI] [PubMed] [Google Scholar]
- 12.Pagtalunan ME, Miller PL, Jumping-Eagle S, et al. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest 1997; 99:342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu L, Yang HC, Hao CM, et al. Podocyte number predicts progression of proteinuria in IgA nephropathy. Mod Pathol 2010; 23:1241–1250. [DOI] [PubMed] [Google Scholar]
- 14.Lemley KV, Lafayette RA, Safai M, et al. Podocytopenia and disease severity in IgA nephropathy. Kidney Int 2002; 61:1475–1485. [DOI] [PubMed] [Google Scholar]
- 15.Jennette JC, Falk RJ, Bacon PA, et al. 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013; 65:1–11. [DOI] [PubMed] [Google Scholar]
- 16.Ma YC, Zuo L, Chen JH, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 2006; 17:2937–2944. [DOI] [PubMed] [Google Scholar]
- 17.Zhao MH, Lockwood CM. A comprehensive method to purify three major ANCA antigens: proteinase 3, myeloperoxidase and bactericidal/permeability-increasing protein from human neutrophil granule acid extract. J Immunol Methods 1996; 197:121–130. [DOI] [PubMed] [Google Scholar]
- 18.Bajema IM, Hagen EC, Hermans J, et al. Kidney biopsy as a predictor for renal outcome in ANCA-associated necrotizing glomerulonephritis. Kidney Int 1999; 56:1751–1758. [DOI] [PubMed] [Google Scholar]
- 19.Gundersen HJ, Seefeldt T, Osterby R. Glomerular epithelial foot processes in normal man and rats. Distribution of true width and its intra- and inter-individual variation. Cell Tissue Res 1980; 205:147–155. [DOI] [PubMed] [Google Scholar]
- 20.van den Berg JG, van den Bergh WM, Assmann KJ, et al. Podocyte foot process effacement is not correlated with the level of proteinuria in human glomerulopathies. Kidney Int 2004; 66:1901–1906. [DOI] [PubMed] [Google Scholar]
- 21.Mandrekar JN. Measures of interrater agreement. J Thorac Oncol 2011; 6:6–7. [DOI] [PubMed] [Google Scholar]
- 22.White KE, Bilous RW. Estimation of podocyte number: a comparison of methods. Kidney Int 2004; 66:663–667. [DOI] [PubMed] [Google Scholar]
- 23.Jensen EB, Gundersen HJ, Osterby R. Determination of membrane thickness distribution from orthogonal intercepts. J Microsc 1979; 115:19–33. [DOI] [PubMed] [Google Scholar]
- 24.Chen M, Yu F, Zhang Y, et al. Clinical and pathological characteristics of Chinese patients with antineutrophil cytoplasmic autoantibody associated systemic vasculitides: a study of 426 patients from a single centre. Postgrad Med J 2005; 81:723–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen M, Yu F, Zhang Y, et al. Antineutrophil cytoplasmic autoantibody-associated vasculitis in older patients. Medicine (Baltimore) 2008; 87:203–209. [DOI] [PubMed] [Google Scholar]
- 26.Jennette JC. Rapidly progressive crescentic glomerulonephritis. Kidney Int 2003; 63:1164–1177. [DOI] [PubMed] [Google Scholar]
- 27.Hogan SL, Nachman PH, Wilkman AS, et al. Prognostic markers in patients with antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol 1996; 7:23–32. [DOI] [PubMed] [Google Scholar]
- 28.Nachman PH, Hogan SL, Jennette JC, et al. Treatment response and relapse in antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol 1996; 7:33–39. [DOI] [PubMed] [Google Scholar]
- 29.Roecker, Ellen B. Prediction error and its estimation for subset-selected models. Technometrics 1991; 33:459–468. [Google Scholar]
- 30.Pagnoux C, Hogan SL, Chin H, et al. Predictors of treatment resistance and relapse in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis: comparison of two independent cohorts. Arthritis Rheum 2008; 58:2908–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu RX, Kuangb J, Gong Q, et al. Principal component regression analysis with spss. Comput Methods Programs Biomed 2003; 71:141–147. [DOI] [PubMed] [Google Scholar]
- 32.Chen M, Yu F, Zhang Y, et al. Characteristics of Chinese patients with Wegener's granulomatosis with anti-myeloperoxidase autoantibodies. Kidney Int 2005; 68:2225–2229. [DOI] [PubMed] [Google Scholar]
- 33.Singh SK, Jeansson M, Quaggin SE. New insights into the pathogenesis of cellular crescents. Curr Opin Nephrol Hypertens 2011; 20:258–262. [DOI] [PubMed] [Google Scholar]
- 34.Thorner PS, Ho M, Eremina V, et al. Podocytes contribute to the formation of glomerular crescents. J Am Soc Nephrol 2008; 19:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kriz W, Gretz N, Lemley KV. Progression of glomerular diseases: is the podocyte the culprit? Kidney Int 1998; 54:687–697. [DOI] [PubMed] [Google Scholar]
- 36.Ford SL, Polkinghorne KR, Longano A, et al. Histopathologic and clinical predictors of kidney outcomes in ANCA-associated vasculitis. Am J Kidney Dis 2014; 63:227–235. [DOI] [PubMed] [Google Scholar]
- 37.Hauer HA, Bajema IM, Van Houwelingen HC, et al. Determinants of outcome in ANCA-associated glomerulonephritis: a prospective clinico-histopathological analysis of 96 patients. Kidney Int 2002; 62:1732–1742. [DOI] [PubMed] [Google Scholar]
- 38.de Lind van Wijngaarden RA, Hauer HA, Wolterbeek R, et al. Clinical and histologic determinants of renal outcome in ANCA-associated vasculitis: a prospective analysis of 100 patients with severe renal involvement. J Am Soc Nephrol 2006; 17:2264–2274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


