Abstract
Wnt/β-catenin signaling pathway is thought to be implicated in the development of arterial stiffness and vascular calcification. As a Wnt signaling pathway inhibitor, it is interesting to investigate whether sclerostin or dickkopf-1 (DKK1) level is correlated with arterial stiffness in renal transplant (RT) recipients. Fasting blood samples were obtained for biochemical data, sclerostin, DKK1, and osteoprotegerin (OPG) determinations. In this study, we applied automatic pulse wave analyzer (VaSera VS-1000) to measure brachial-ankle pulse wave velocity and either sides of brachial-ankle pulse wave velocity value, which greater than 14.0 m/s was determined as high arterial stiffness. Among 68 RT recipients, 30 patients (44.1%) were in the high arterial stiffness group. Compared with patients in the low arterial stiffness group, patients in the high arterial stiffness group had higher prevalence of hypertension (P = 0.002), diabetes (P < 0.001), metabolic syndrome (P = 0.025), longer posttransplant duration (P = 0.005), higher systolic blood pressure (P < 0.001) and diastolic blood pressure (P = 0.018), and higher fasting glucose (P = 0.004), total cholesterol (P = 0.042), blood urea nitrogen (P = 0.020), phosphorus (P = 0.042), and sclerostin levels (P = 0.001). According to our multivariable forward stepwise linear regression analysis, age (β = 0.272, P = 0.014), phosphorus (β = 0.308, P = 0.007), and logarithmically-transformed OPG (log-OPG; β = 0.222, P = 0.046) were positively associated with sclerostin levels, and multivariate logistic regression analysis, sclerostin (odds ratio 1.052, 95% confidence interval 1.007–1.099, P = 0.024), and posttransplant duration (odds ratio 1.024, 95% confidence interval 1.004–1.045, P = 0.019) were the independent predictors of peripheral arterial stiffness in RT recipients. In this study, serum sclerostin level, but not DKK1, was proved to be involved in the pathogenetic process of peripheral arterial stiffness in RT recipients.
INTRODUCTION
Cardiovascular disease is recognized as the leading cause of death in renal transplant (RT) recipients.1 Stiffness of arterial bed may augment end-systolic pressure, increase left ventricular afterload, and lead to decrease of coronary perfusion.2 To assess arterial stiffness in clinical practice, pulse wave velocity (PWV) is usually applied as a noninvasive measurement to reflect segmental arterial elasticity.3 Peripheral arterial elasticity is calculated by the difference of PWV measured from brachial to ankle regions (brachial-ankle PWV [baPWV]). Arterial stiffness can not only cause vascular damage, but can also be regarded as an independent risk factor of cardiovascular morbidity and mortality in the Framingham Heart Study.4 In fact, high arterial stiffness is also recognized to be independently related to the occurrence of cardiovascular events in RT recipients.5
Low-density lipoprotein (LDL) receptor-related protein 5 and 6 (LRP5/6) and Frizzled coreceptors, bound by a Wnt ligand, can decrease intracellular degradation of β-catenin in osteoblast. Furthermore, this increased β-catenin can trigger target gene transcription, and can regulate the bone turnover.6 In addition, Wnt/β-catenin signaling pathway is also involved in the development of vascular calcification and atherosclerosis.7 Sclerostin, a SOST gene product expressed by osteocytes,6,8 is a canonical Wnt/β-catenin signaling pathway inhibitor, through which the intracellular degradation of β-catenin can be enhanced and osteoblast differentiation and proliferation can be inhibited.8,9Sclerostin was depicted to up-regulate the expression of receptor activator of nuclear factor kappa B ligand (RANKL) mRNA and to down-regulate the expression of osteoprotegerin (OPG) mRNA in human primary preosteocytes culture.10 Moreover, sclerostin also had an impact on serum fibroblast growth factor 23 (FGF23) level and vitamin D synthesis in SOST−/− mice.11 Sclerostin had been reported to be associated with high bone turnover in hemodialysis patients,12 and high arterial stiffness in stage 2 to 5D chronic kidney disease (CKD) patients.13 As a decoy receptor for RANKL, OPG that belong to tumor necrosis factor (TNF) receptor superfamily, inhibited osteoclastogenesis, and interlinked osteoporosis with vascular stiffness. In a recent study, Morena et al14 demonstrated that either high serum sclerostin or OPG level, but not dickkopf-1 (DKK1), was correlated with coronary arterial calcification in 241 predialysis CKD patients. To the best of our knowledge, the impacts of serum Wnt pathway inhibitors (sclerostin and DKK1) on arterial stiffness in RT recipients are currently unavailable. Therefore, we sought to evaluate the relationship between serum Wnt pathway inhibitors and peripheral arterial stiffness in RT recipients.
METHODS
Patients
Eighty-two RT recipients were enrolled in a prospective, cross-section study, which was conducted at Buddhist Tzu Chi General Hospital Medical Center, from April 2013 to June 2013. Patients were excluded if they had any acute infection, malignancy, acute rejection, acute myocardial infarction, pulmonary edema, heart failure (HF) at the time of blood sampling, had an arterial-venous shunt or graft in either hand, or refused to provide an informed consent. This study was approved by the Protection of Human Subjects Institutional Review Board of Tzu-Chi University and Hospital, and is consistent with the Declaration of Helsinki. Coronary artery disease (CAD) was defined as had a history of >50% stenosis in any segment by coronary angiography. HF was diagnosed according to the American College of Cardiology Foundation and the American Heart Association 2013 Guidelines.15
Anthropometric Analysis
Anthropometric parameters including body weight, body height, waist circumference, and body mass index (BMI) were checked and recorded. All the patients were examined under light clothing and naked feet. The nearest half-kilogram was recorded for body weight measurement, and body height was measured to the nearest half-centimeter. The waist circumference was determined by measuring the shortest point below the lowermost rib cage margin and iliac crest, and the data were recorded to the nearest half-centimeter. BMI was determined as body weight (kilograms) divided by the square of the body height (meters).16–19
Biochemical Determinations
All fasting blood samples were centrifuged at 3000g for 10 minutes and stored at 4°C in refrigerator before their examination. The biochemical analyses were all finished within 1 hour after collection of blood samples. The measurement including total cholesterol (TCH), triglyceride (TG), high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), fasting glucose, blood urea nitrogen (BUN), creatinine (Cre), total calcium, and phosphorus were examined by using an autoanalyzer (COBAS Integra 800, Roche Diagnostics, Basel, Switzerland). Other measurement such as serum sclerostin and DKK1 levels (Biomedica immunoassays, Vienna, Austria), OPG (eBioscience Inc., San Diego, CA, USA), and intact parathyroid hormone (iPTH; Diagnostic Systems Laboratories, TX) concentrations were quantified by using commercially enzyme-linked immunosorbent assays (ELISAs).16–19 We also calculated the estimated glomerular filtration rate (eGFR) using Modification of Diet in Renal Disease (MDRD) formula.
Metabolic Syndrome and Its Components
The definition of metabolic syndrome from the International Diabetes Federation was adopted in our study.20 Our participants who had waist circumference ≥90 cm (men) or ≥80 cm (women), and matched 2 or more of the following criteria—fasting serum glucose ≥110 mg/dL, TG ≥150 mg/dL, HDL-C level ≤40 mg/dL in men or ≤50 mg/dL in women, or blood pressure of ≥130/85 mm Hg or use of antihypertensive medication were regarded to have metabolic syndrome, and whose fasting plasma glucose level was ≥126 mg/dL, 2-hour glucose during an oral glucose tolerance test was ≥200 mg/dL, or who were using hypoglycemic medications—were diabetic patients.21
Measurements of Blood Pressure and Brachial-Ankle Pulse Wave Velocity
After blood sampling, patients were lying in the supine position and took rest for 10 minutes. Blood pressure was determined by an automatic upper-arm oscillometric device, and systolic blood pressure (SBP) and diastolic blood pressure (DBP) were checked 3 times at right brachial artery. Then, 4 pneumatic cuffs connected to both plethysmographic and oscillometric sensors were wrapped around both upper arms and ankles for assessing baPWV by a volume-plethysmographic apparatus (VaSera VS-1000, Fukuda Denshi Co. Ltd., Tokyo, Japan).17,18 For monitoring electrocardiogram and heart sound, a microphone was placed on the left edge of the sternum and electrocardiogram electrodes were placed on both wrists. Phonocardioraphy, electrograhy, and arterial pressure waveforms of brachial and ankle arteries were measured, and left-baPWV and right-baPWV were automatically calculated by the analyzer. The baPWV was calculated as the length of an arterial segment between the brachium and ankle divided by the time interval between the wavefront of the brachial waveform and that of the ankle waveform. Based on the recursive partitioning analysis in Kyushu Prevention Study of Atherosclerosis, baPWV of 14 m/s was considered to be a statistically adequate cut-off point for coronary artery events and cerebrovascular events in diabetic populations.22 Therefore, high arterial stiffness group was defined when the values of left or right baPWV were greater than 14.0 m/s in the present study.17,18,23
Statistical Analysis
Data were tested for normal distribution using the Kolmogorov–Smirnov test. Data were expressed as means ± standard deviation (SD) for normally distributed data, and comparisons between patients were performed using the Student independent t test (2-tailed). Data were expressed as medians and interquartile ranges for non-normal distributed data (TG, fasting glucose, BUN, Cre, iPTH, OPG, DKK1, sclerostin/DKK1 ratio) and comparisons between patients were performed using the Mann–Whitney U test. Data expressed as the number of patients were analyzed by the chi-square test. Variables that were significantly associated with arterial stiffness in the RT recipients were tested for independence by multivariate logistic regression analysis. Because the TG, fasting glucose, BUN, Cre, iPTH, OPG, DKK-1, and sclerostin/DKK1 ratio were not normally distributed and underwent base 10 logarithmic transformations to achieve normality. Clinical variables that correlated with serum sclerostin levels, log-DKK1 levels, or log-sclerostin/DKK1 ratio in RT recipients were evaluated by univariate linear regression analyses. Variables that were significantly associated with sclerostin levels, log-DKK1 levels, or log-sclerostin/DKK1 ratio in RT recipients were tested for independency in multivariate forward stepwise regression analyses. Data were analyzed using SPSS for Windows (version 19.0; SPSS Inc., Chicago, IL). A P value less than 0.05 was considered statistically significant.
RESULTS
Among 82 RT recipients enrolled, 14 of them were excluded on account of acute infection (n = 1), malignancy (n = 3), vascular access established for dialysis on either upper extremity (n = 3), or refused to provide informed consent (n = 7). Finally, a total of 68 RT recipients, 36 males and 32 females, aged 27 to 75 years, were included in the study (Figure 1).
FIGURE 1.
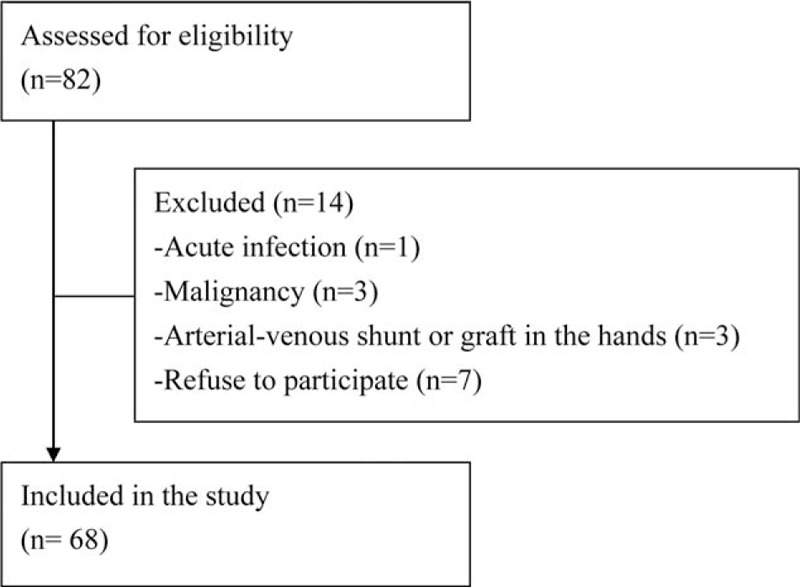
Flow chart indicates patient enrollment.
Relationships Between Baseline Demographics, Clinical Characteristics, Comorbidities, and Sclerostin Level With Peripheral Arterial Stiffness
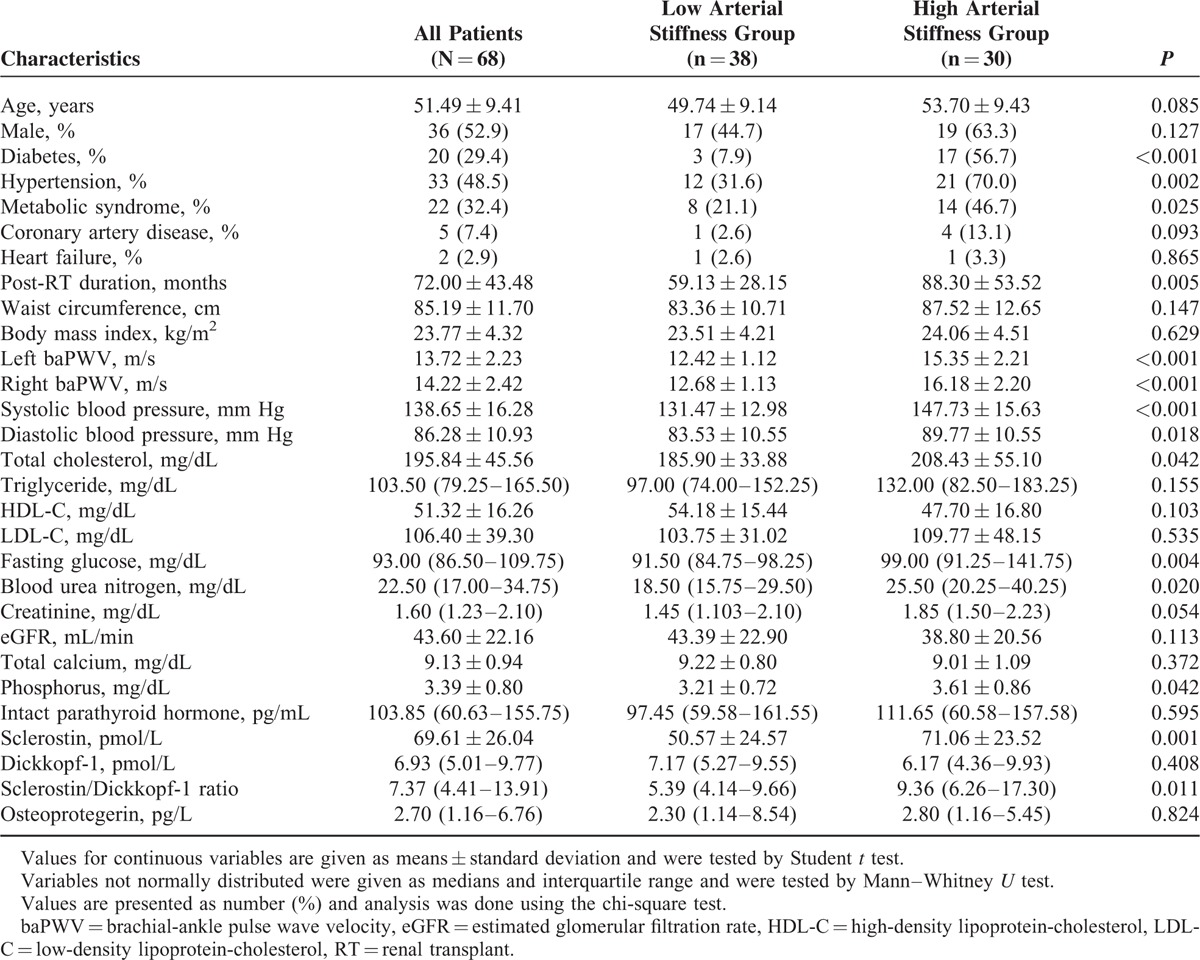
The demographics, clinical characteristics, biochemical data, and comrbidities of our 68 RT recipients are presented in Table 1. Among these patients, 20 were diabetic (29.4%), 33 hypertensive (48.5%), 5 of them had CAD (7.4%), 2 had HF (2.9%), and 22 were with metabolic syndrome (32.4%), and none had stroke. Thirty patients (44.1%) were defined to be in the high arterial stiffness group when their left or right baPWV values were greater than 14.0 m/s, and the rest of them were classified in the low arterial stiffness group. RT recipients who had diabetes (P < 0.001), hypertension (P = 0.002), or metabolic syndrome (P = 0.025) were prone to develop high arterial stiffness. Compared with the low arterial stiffness group, patients in the high arterial stiffness group had longer posttransplant duration (P = 0.005), higher SBP (P < 0.001) and DBP (P = 0.018), higher fasting glucose (P = 0.004), TCH (P = 0.042), BUN (P = 0.020), phosphorus (P = 0.042), and sclerostin (P = 0.001) levels, and sclerostin/DKK1 ratio (P = 0.011). However, serum DKK1 and OPG levels revealed no significant difference between high groups. No significant difference in arterial stiffness was observed in subgroup comparisons based on sex, HF history, transplantation model, or use of tacrolimus, mycophenolate mofetil, or mycophenolic acid, steroids, rapamycin, and cyclosporine (data not shown).
TABLE 1.
Clinical Variables of the 68 Renal Transplant Recipients With High or Low Arterial stiffness
Subgroup Comparisons of Serum Sclerostin and DKK1 Levels in RT Recipients
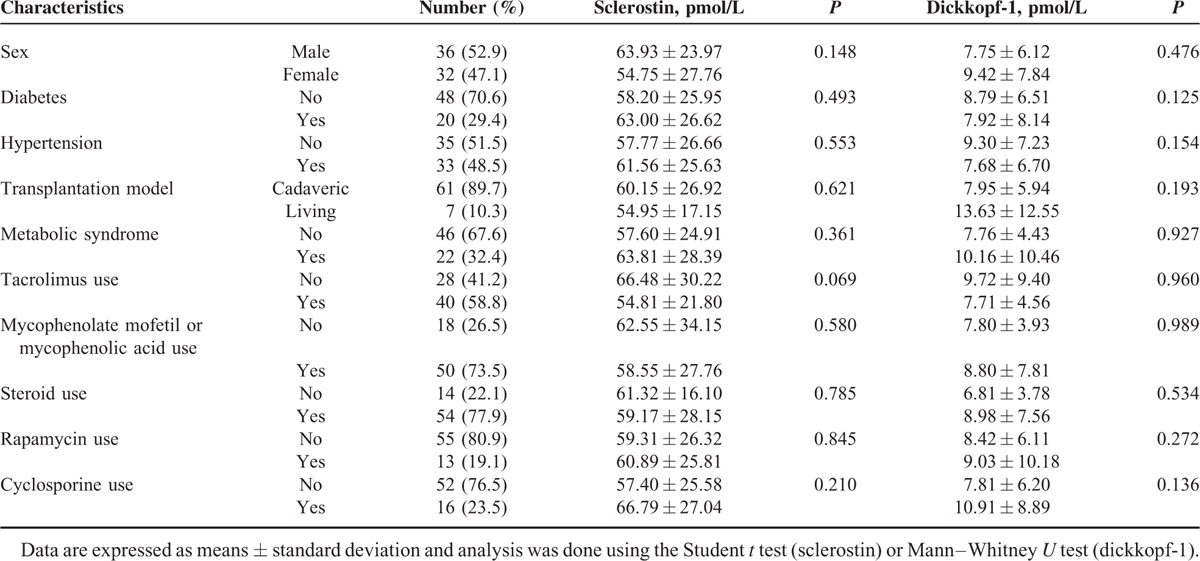
Pairwise comparisons of serum sclerostin and DKK1 levels in subgroups of RT recipients, according to sex, diabetes, hypertension, metabolic syndrome, or transplantation model, and use of tacrolimus, mycophenolate mofetil, or mycophenolic acid, steroids, rapamycin, or cyclosporine are shown in Table 2. However, either serum sclerostin or DKK1 level failed to reach any significance in subgroup analysis.
TABLE 2.
Subgroup Analysis of Serum Sclerostin and Dickkopf-1 Levels in 68 Renal Transplant Recipients with High or Low Arterial Stiffness
Correlation Between Sclerostin or DKK1 Levels and Selected Clinical and Biochemical Parameters in RT Recipients
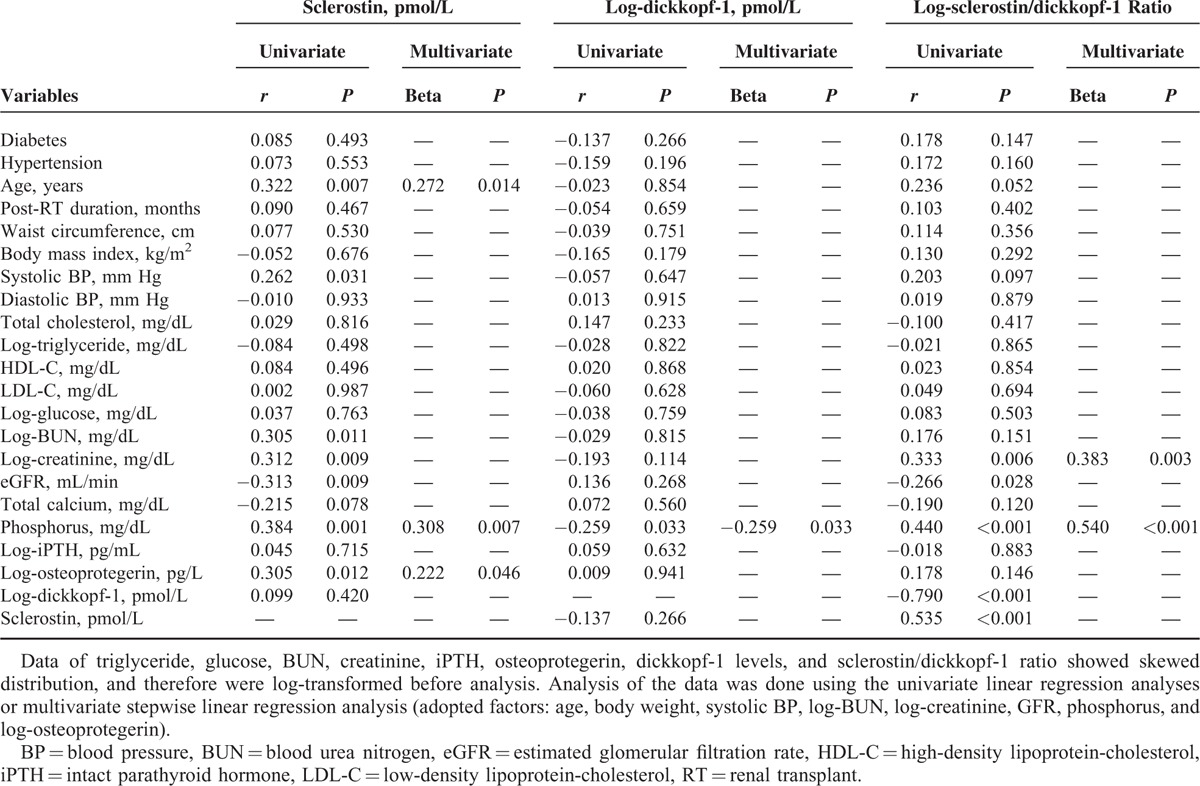
We applied univariable linear analysis and multivariable forward stepwise linear regression analysis to delineate the association of either serum sclerostin levels, log-DKK1 levels, or log-sclerostin/DKK1 ratio with demographic data, comorbidities, and biochemical data in RT recipients, and the results are presented in Table 3. We found serum sclerostin level was positively correlated with age (r = 0.322, P = 0.007), SBP (r = 0.262, P = 0.031), log-BUN (r = 0.305, P = 0.011), log-Cre (r = 0.312, P = 0.009), phosphorus (r = 0.384, P = 0.001), log-OPG level (r = 0.305, P = 0.012), and was negatively correlated with GFR (r = −0.313, P = 0.009) in RT recipients. In multivariable forward stepwise linear regression analysis, only age (β = 0.272, P = 0.014), phosphorus (β = 0.308, P = 0.007), and log-OPG (β = 0.222, P = 0.046) were positively associated with serum sclerostin level. While, serum DKK1 level was negatively correlated with phosphorus (β = −0.259, P = 0.033) only in univariable linear analysis and multivariable forward stepwise linear regression analysis. Moreover, log-sclerostin/DKK1 level was positively correlated with body weight (r = 0.247, P = 0.043), log-Cre (r = 0.333, P = 0.006), phosphorus (r = 0.440, P < 0.001), and was negatively correlated with GFR (r = −0.266, P = 0.028) in RT recipients. In multivariable forward stepwise linear regression analysis, only log-Cre (β = 0.383, P = 0.003) and phosphorus (β = 0.540, P < 0.001) were positively associated with serum sclerostin/DKK1 ratio.
TABLE 3.
Correlation Between Sclerostin and Log-Dickkopf-1 Levels and Variables Among 68 Renal Transplant Recipients
Sclerostin is Independently Associated With Peripheral Arterial Stiffness
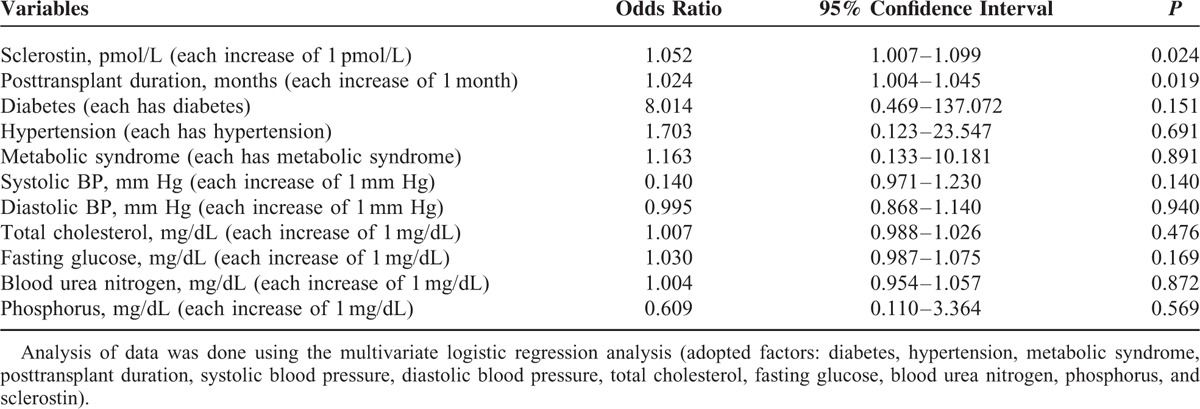
After adjusted diabetes, hypertension, metabolic syndrome, posttransplant duration, SBP, DBP, TCH, fasting glucose, BUN, phosphorus, and sclerostin, our multivariate logistic regression analysis showed that sclerostin (odds ratio [OR] 1.052, 95% confidence interval [CI] 1.007–1.099, P = 0.024) and posttransplant duration (OR 1.024, 95% CI 1.004–1.045, P = 0.019) were the 2 independent predictors of peripheral arterial stiffness in RT recipients (Table 4). We also found sclerostin/DKK1 ratio was significantly elevated only in our high arterial stiffness group and was unable to predict the occurrence of arterial stiffness according to multivariate logistic regression analysis (P = 0.074).
TABLE 4.
Multivariate Logistic Regression Analysis of the Factors Correlated to Arterial Stiffness Among 68 Renal Transplant Recipients
DISCUSSION
Our study showed that RT recipients with longer posttransplant duration, hypertension, diabetes, and higher serum phosphorus and sclerostin levels had high arterial stiffness. Serum sclerostin level is an independent predictor of peripheral arterial stiffness in RT recipients, whereas the serum DKK1 level and sclerostin/DKK1 ratio are not.
In a meta-analysis study, Vlachopoulos et al24 elucidated a 12%, 13%, and 6% increase of total cardiovascular events, cardiovascular mortality, and all-cause mortality rate, respectively, by per 1 m/s increase of baPWV. Besides, endothelial dysfunction, inflammation, oxidative stress, atherosclerosis, and vascular calcification were claimed to link patients with hyperlipidemia, dyslipidaemia, hypertension, obesity, insulin resistance, or type 2 diabetes mellitus to the higher prevalence of arterial stiffness25 and higher risk of cardiovascular insults.26 In this study, diabetes, hypertension, and metabolic syndrome were more frequent in RT patients with high arterial stiffness. Furthermore, fasting glucose, TCH, SBP, and DBP were also higher in high arterial stiffness group. However, after multivariate logistic regression analysis of confounding variables, diabetes, hypertension, metabolic syndrome, serum glucose, TCH, SBP, and DBP were not as independent predictors of peripheral arterial stiffness in our RT recipients. The impacts of diabetes on baPWV values could be multifactorial, which included hyperglycemia, insulin resistance, oxidative stress, chronic inflammatory status, malnutrition, low turnover bone disease, vascular calcification, and the coexistence of hypertension, dyslipidemia, and cardiovascular events. Moreover, drug therapy for hypertension, dyslipidemia, diabetes mellitus, and lifestyle modifications may also affect the baPWV values.26 Further studies are therefore required to elucidate the relationship between peripheral arterial stiffness and drugs in RT recipients.
The RT recipients in our study, who had high arterial stiffness, were found to have a higher serum phosphorus level. High phosphorus load had been demonstrated to provoke a transdifferentiation process, thus leading to osteochondrogenesis of vascular smooth muscle cells,7,27 vascular calcification, and arterial stiffness in RT recipients.28–30
Recently, Wnt/β-catenin signaling pathway was also introduced to be one of the inducers in this vascular osteochondrogenesis process.7 Inhibition of sclerostin by using its monoclonal antibody enhances Wnt/β-catenin signaling and stimulates osteoblastic bone formation,6,8 which provide a cornerstone for clinical application of sclerostin monoclonal antibodies in the population with osteoporosis.31,32 Compared with sclerostin that was found as a specific canonical Wnt/β-catenin signaling pathway inhibitor, DKK1 was another Wnt inhibitor that involved more extensive Wnt pathways.9 Sclerostin level was observed to be positively correlated with carotid-femoral PWV in CKD patients, according to EUTox data.13 However, only serum sclerostin level, but not DKK1, was positively correlated with carotid-femoral PWV in postmenopausal women33 and with coronary artery calcification in nondialysis CKD patients.14 On the contrary, Claes et al34 failed to find such correlation in CKD patients. In addition, plasma DKK1 level was inversely associated with coronary artery and aortoiliac arterial calcified plaque in African-Americans with type 2 diabetes.35 In our study, serum sclerostin level was significantly higher in the high arterial stiffness group compared with that in the low arterial stiffness group, whereas the serum DKK1 level was not. After adjusting a variety of factors in multivariate logistic regression analysis, only serum sclerostin level, but not DKK1, was regarded as an independent predictor of peripheral arterial stiffness in our RT recipients. Even sclerostin/DKK1 ratio was significantly elevated in our high arterial stiffness group, but there was loss of its predictability on the occurrence of arterial stiffness.
Positive correlations between age, serum phosphorus, FGF23, 1,25(OH)2 D, OPG, and sclerostin levels, and negative correlation between eGFR and sclerostin had been reported in CKD patients.13,14,36 We also noted that age, serum phosphorus, log-BUN, log-Cre, and log-OPG levels were positively and eGFR was negatively correlated with sclerostin levels in our RT recipients. After adjustment for a variety of confounders in multivariable forward stepwise linear regression analysis, age, serum phosphorus, and log-OPG levels remained to be positively associated with sclerostin level.
Sclerostin level had been found to be negatively correlated with iPTH level in CKD-5D patients,12 in maintenance hemodialysis patients,37,38 and in patients who underwent peritoneal dialysis.39 However, several studies failed to observe such correlation in predialysis CKD patients.14,36,40 In fact, we also failed to find a significant correlation between sclerostin and iPTH levels in our RT recipients. We hypothesized lower level of iPTH in our RT recipients, who, with either normal or early stages of CKD, may partially be responsible for this observation. However, further studies are required to explore the precise mechanisms for sclerostin regulation.
Furthermore, a positive correlation between posttransplant duration and increase of PWV value in RT recipients was reported by Strozecki et al.41 According to our previous reports, posttransplant vintage was proved to be an independent predictor of peripheral arterial stiffness in RT recipients.17,18 Again, in this study, RT vintage was also independently correlated with high peripheral arterial stiffness.
There are several limitations in this study. First, this is an observational, single-center study with a limited number of RT recipients enrolled, and the possibility of bias cannot be excluded. Therefore, this observational design prevents us from exploring the mechanisms underlying our observation between serum sclerostin and peripheral arterial stiffness in RT recipients. Several studies had reported that serum sclerostin level was positively correlated with FGF23, and negatively correlated with 1,25 (OH)2 D levels in CKD patients.13,14 In this study, we did not evaluate the relationship among serum sclerostin, FGF23, and 1,25(OH)2 D levels.
In conclusion, the present study showed that serum sclerostin level is independently associated with peripheral arterial stiffness, whereas serum DKK1 is not, in our RT recipients. In addition, age, serum phosphorus, and log-OPG are positively correlated with sclerostin level, whereas serum phosphorus is negatively correlated with DKK1 level. Further long-term prospective studies are needed to elucidate the causal relationship between serum sclerostin and peripheral arterial stiffness in RT recipients.
Acknowledgment
We thank Ling-Yi Wang (Department of Medical Research, Hualien Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Hualien, Taiwan) for statistical consultation.
Footnotes
Abbreviations: baPWV = brachial-ankle pulse wave velocity, BMI = body mass index, BUN = blood urea nitrogen, CAD = coronary artery disease, CI = confidence interval, CKD = chronic kidney disease, Cre = creatinine, DBP = diastolic blood pressure, DKK1 = dickkopf-1, ELISA = enzyme-linked immunosorbent assay, FGF23 = fibroblast growth factor 23, GFR = glomerular filtration rate, HDL-C = high-density lipoprotein-cholesterol, HF = heart failure, iPTH = intact parathyroid hormone, LDL-C = low-density lipoprotein-cholesterol, LRP5/6 = lipoprotein receptor-related protein 5 and 6, MDRD = Modification of Diet in Renal Disease, OPG = osteoprotegerin, OR = odds ratio, PWV = pulse wave velocity, RANKL = receptor activator of nuclear factor kappa B ligand, RT = renal transplant, SBP = systolic blood pressure, TCH = total cholesterol, TG = triglyceride, TNF = tumor necrosis factor.
B-GH and H-HL contributed equally to this study.
This study was presented at the 14th Congress of the Asian Society of Transplantation (Singapore, August 23–26, 2015).
Funding: This study was supported by a grant from Buddhist Tzu Chi General Hospital, Hualien, Taiwan (TCRD102–26).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Cianciolo G, Capelli I, Angelini ML, et al. Importance of vascular calcification in kidney transplant recipients. Am J Nephrol 2014; 39:418–426. [DOI] [PubMed] [Google Scholar]
- 2.Jia G, Aroor AR, Sowers JR. Arterial Stiffness: a nexus between cardiac and renal disease. Cardiorenal Med 2014; 4:60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomiyama H, Yamashina A. Non-invasive vascular function tests: their pathophysiological background and clinical application. Circ J 2010; 74:24–33. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell GF, Hwang SJ, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 2010; 121:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khoshdel AR, Carney SL. Arterial stiffness in kidney transplant recipients: an overview of methodology and applications. Urol J 2008; 5:3–14. [PubMed] [Google Scholar]
- 6.Lewiecki EM. Role of sclerostin in bone and cartilage and its potential as a therapeutic target in bone diseases. Ther Adv Musculoskelet Dis 2014; 6:48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evrard S, Delanaye P, Kamel S, et al. SFBC/SN joined working group on vascular calcifications. Vascular calcification: from pathophysiology to biomarkers. Clin Chim Acta 2015; 438:401–414. [DOI] [PubMed] [Google Scholar]
- 8.Ke HZ, Richards WG, Li X, et al. Sclerostin and Dickkopf-1 as therapeutic targets in bone diseases. Endocr Rev 2012; 33:747–783. [DOI] [PubMed] [Google Scholar]
- 9.Evenepoel P, D’Haese P, Brandenburg V. Sclerostin and DKK1: new players in renal bone and vascular disease. Kidney Int 2015; 88:235–240. [DOI] [PubMed] [Google Scholar]
- 10.Wijenayaka AR, Kogawa M, Lim HP, et al. Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway. PLoS One 2011; 6:e25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryan ZC, Ketha H, McNulty MS, et al. Sclerostin alters serum vitamin D metabolite and fibroblast growth factor 23 concentrations and the urinary excretion of calcium. Proc Natl Acad Sci U S A 2013; 110:6199–6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cejka D, Herberth J, Branscum AJ, et al. Sclerostin and Dickkopf-1 in renal osteodystrophy. Clin J Am Soc Nephrol 2011; 6:877–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desjardins L, Liabeuf S, Oliveira RB, et al. Uremic toxicity and sclerostin in chronic kidney disease patients. Nephrol Ther 2014; 10:463–470. [DOI] [PubMed] [Google Scholar]
- 14.Morena M, Jaussent I, Dupuy AM, et al. Osteoprotegerin and sclerostin in chronic kidney disease prior to dialysis: potential partners in vascular calcifications. Nephrol Dial Transplant 2015; 30:1345–1356. [DOI] [PubMed] [Google Scholar]
- 15.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62:e147–239. [DOI] [PubMed] [Google Scholar]
- 16.Hsu BG, Shih MH, Chen YC, et al. High serum osteoprotegerin is associated with arterial stiffness in Renal transplant recipients. Tohoku J Exp Med 2015; 236:247–253. [DOI] [PubMed] [Google Scholar]
- 17.Lee MC, Chen YC, Ho GJ, et al. Serum leptin levels positively correlate with peripheral arterial stiffness in kidney transplantation patients. Transplant Proc 2014; 46:353–358. [DOI] [PubMed] [Google Scholar]
- 18.Ho GJ, Lee MC, Lee CJ, et al. Hypoadiponectinemia correlates with arterial stiffness in kidney transplantation patients. Clin Exp Nephrol 2015; 19:534–541. [DOI] [PubMed] [Google Scholar]
- 19.Chen YC, Lee MC, Lee CJ, et al. N-terminal pro-B-type natriuretic peptide associated with arterial stiffness by cardio-ankle vascular index in renal transplant recipients. J Atheroscler Thromb 2013; 20:646–653. [DOI] [PubMed] [Google Scholar]
- 20.Alberti KG, Zimmet PZ, Shaw J. Metabolic syndrome: a new world-wide definition. A consensus statement from the International Diabetes Federation. Diab Med 2006; 23:469–480. [DOI] [PubMed] [Google Scholar]
- 21.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. I. Diagnosis and classification of diabetes mellitus: provisional report of a WHO consultation. Diabet Med 1998; 15:539–553. [DOI] [PubMed] [Google Scholar]
- 22.Maeda Y, Inoguchi T, Etoh E, et al. Brachial-ankle pulse wave velocity predicts all-cause mortality and cardiovascular events in patients with diabetes: the Kyushu Prevention Study of Atherosclerosis. Diabetes Care 2014; 37:2383–2390. [DOI] [PubMed] [Google Scholar]
- 23.Kato A, Ishida J, Endo Y, et al. Association of abdominal visceral adiposity and thigh sarcopenia with changes of arteriosclerosis in haemodialysis patients. Nephrol Dial Transplant 2011; 26:1967–1976. [DOI] [PubMed] [Google Scholar]
- 24.Vlachopoulos C, Aznaouridis K, Terentes-Printzios D, et al. Prediction of cardiovascular events and all-cause mortality with brachial-ankle elasticity index: a systematic review and meta-analysis. Hypertension 2012; 60:556–562. [DOI] [PubMed] [Google Scholar]
- 25.Cecelja M, Chowienczyk P. Role of arterial stiffness in cardiovascular disease. JRSM Cardiovasc Dis 2012; 1:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vlachopoulos C, Xaplanteris P, Aboyans V, et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis 2015; 241:507–532. [DOI] [PubMed] [Google Scholar]
- 27.Giachelli CM. The emerging role of phosphate in vascular calcification. Kidney Int 2009; 75:890–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cianciolo G, Capelli I, Angelini ML, et al. Importance of vascular calcification in kidney transplant recipients. Am J Nephrol 2014; 39:418–426. [DOI] [PubMed] [Google Scholar]
- 29.Connolly GM, Cunningham R, McNamee PT, et al. Elevated serum phosphate predicts mortality in renal transplant recipients. Transplantation 2009; 87:1040–1044. [DOI] [PubMed] [Google Scholar]
- 30.Stevens KK, Morgan IR, Patel RK, et al. Serum phosphate and outcome at one year after deceased donor renal transplantation. Clin Transplant 2011; 25:E199–204. [DOI] [PubMed] [Google Scholar]
- 31.Padhi D, Jang G, Stouch B, et al. Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res 2011; 26:19–26. [DOI] [PubMed] [Google Scholar]
- 32.Padhi D, Allison M, Kivitz AJ, et al. Multiple doses of sclerostin antibody romosozumab in healthy men and postmenopausal women with low bone mass: a randomized, double-blind, placebo-controlled study. J Clin Pharmacol 2014; 54:168–178. [DOI] [PubMed] [Google Scholar]
- 33.Hampson G, Edwards S, Conroy S, et al. The relationship between inhibitors of the Wnt signaling pathway (Dickkopf-1(DKK1) and sclerostin), bone mineral density, vascular calcification and arterial stiffness in post-menopausal women. Bone 2013; 56:42–47. [DOI] [PubMed] [Google Scholar]
- 34.Claes KJ, Viaene L, Heye S, et al. Sclerostin: another vascular calcification inhibitor? J Clin Endocrinol Metab 2013; 98:3221–3228. [DOI] [PubMed] [Google Scholar]
- 35.Register TC, Hruska KA, Divers J, et al. Plasma Dickkopf1 (DKK1) concentrations negatively associate with atherosclerotic calcified plaque in African-Americans with type 2 diabetes. J Clin Endocrinol Metab 2013; 98:E60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thambiah S, Roplekar R, Manghat P, et al. Circulating sclerostin and Dickkopf-1 (DKK1) in predialysis chronic kidney disease (CKD): relationship with bone density and arterial stiffness. Calcif Tissue Int 2012; 90:473–480. [DOI] [PubMed] [Google Scholar]
- 37.Ishimura E, Okuno S, Ichii M, et al. Relationship between serum sclerostin, bone metabolism markers, and bone mineral density in maintenance hemodialysis patients. J Clin Endocrinol Metab 2014; 99:4315–4320. [DOI] [PubMed] [Google Scholar]
- 38.Yang CY, Chang ZF, Chau YP, et al. Circulating Wnt/(-catenin signaling inhibitors and uremic vascular calcifications. Nephrol Dial Transplant 2015; 30:1356–1363. [DOI] [PubMed] [Google Scholar]
- 39.Yamada S, Tsuruya K, Tokumoto M, et al. Factors associated with serum soluble inhibitors of Wnt-(-catenin signaling (sclerostin and dickkopf-1) in patients undergoing peritoneal dialysis. Nephrology 2015; 20:639–645. [DOI] [PubMed] [Google Scholar]
- 40.Pelletier S, Dubourg L, Carlier MC, et al. The relation between renal function and serum sclerostin in adult patients with CKD. Clin J Am Soc Nephrol 2013; 8:819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strozecki P, Adamowicz A, Kozlowski M, et al. Progressive arterial stiffening in kidney transplant recipients. Ann Transplant 2011; 16:30–35. [DOI] [PubMed] [Google Scholar]


