Abstract
Several recent studies have explored efficacy and safety of different endoscopic treatments for gastrointestinal neuroendocrine tumors (GI-NETs). However, there is no definitive consensus regarding the best endoscopic approach for GI-NETs treatment. Therefore, the present study was conducted to investigate the application of various endoscopic techniques for the treatment of GI-NETs according to the previous conclusions and to summarize the optimal endoscopic modalities for GI-NETs.
Ninety-eight patients with 100 GI-NETs removed by endoscopic therapies were reviewed. The pathological complete resection rate (PCRR), complication, local recurrence, and factors possibly associated with the pathological complete resection were analyzed.
Twenty-two patients were treated by conventional polypectomy (including 6 cold biopsy forceps polypectomy and 16 snare polypectomy with electrocauterization), 41 by endoscopic mucosal resection (EMR), and 35 by endoscopic submucosal dissection (ESD). The PCRRs of conventional polypectomy, EMR, and ESD were 86.4%, 75.6%, and 85.7%, respectively. Sixteen GI-NETs that had a polypoid appearance, with a mean tumor size of 5.2 mm, were removed by snare polypectomy (PCRR 93.8%). The complication rates of conventional polypectomy, EMR, and ESD were 0.0% (0/22), 2.4% (1/41), and 2.9% (1/35), respectively. There were 2 local recurrences after cold biopsy forceps polypectomy treatment and no local recurrences in the EMR and ESD groups (P = 0.049). The results showed that PCRR was only associated with the depth of invasion (P = 0.038).
Endoscopic resection of GI-NETs is safe and effective in properly selected patients. For submucosal GI-NETs, ESD was a feasible modality, with a higher PCRR compared with EMR. For ≤5 mm polypoid-like NETs, snare polypectomy with electrocauterization was a simple procedure with a high PCRR.
INTRODUCTION
Neuroendocrine tumors are relatively rare low-grade malignant tumor that are thought to originate from amine precursor uptake and the decarboxylation cells of the neuroectoderm.1 Various organs and systems with neuroendocrine cells, such as digestive, respiratory, urinary, and reproductive systems, can be affected by neuroendocrine tumors. However, more than 75% of these tumors arise in the gastrointestinal (GI) tract, with an annual incidence of 2.5 to 5.0 per 100,000 people.1–4 Unfortunately, the incidence and prevalence of GI neuroendocrine tumors (GI-NETs) are increasing rapidly worldwide, possibly as a result of the technical improvements in endoscopy and the increased endoscopic screening for GI carcinomas.5,6
Most of early-stage GI-NETs, which rarely cause symptoms, are found incidentally during endoscopic screening and can be treated by endoscopic therapies.1 General indications for endoscopic resection are the NETs that are less than 10 mm in diameter without muscular layer invasion and peripheral lymph node metastasis.7 Various endoscopic therapies for GI-NETs have been reported.8–11 Snare polypectomy and endoscopic mucosal resection (EMR) are low cost, technically easy, and relatively safe. However, their complete resection rates are relatively low because even some ≤10 mm GI-NETs may extend to the submucosal layer.12–14 Therefore, endoscopic submucosal dissection (ESD), which can remove deep regions of the submucosa reliably, is a good option for the GI-NETs.8,15 However, this technique has a complex operating procedure, a high risk of perforation, and is time consuming.16
Recently, several studies have explored the efficacy and safety of different endoscopic treatments for GI-NETs.14,17,18 However, no consensus was reached considering the best endoscopic approach. Therefore, the present study was conducted to investigate the application of various endoscopic techniques for the treatment of GI-NETs, according to the previous conclusions, and to summarize the optimal endoscopic modality.
MATERIALS AND METHODS
Patients
The ethical committee of Ren Ji Hospital approved this study. From January 2006 to August 2014, 102 consecutive patients with GI-NETs who underwent endoscopic therapies at Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University were reviewed retrospectively. The diagnosis of GI-NETs was based on pathology, combining clinical, imaging, and endoscopic findings. The Ki67 index was used to grade the tumors. The inclusion criteria for endoscopic treatment were defined as GI-NETs up to 14 mm without lymphovascular or muscular layer invasion, or ulcers. The size of each lesion, depth of invasion, and complete resection were determined using pathological evaluation of resection specimens. Clinical data regarding age, gender, location of the lesion, tumor size, clinical manifestations, endoscopic appearance, depth of invasion, use of endoscopic ultrasound (EUS), complete resection, complication, additional treatment, and recurrence were extracted and collected from the medical records or from follow-up. The pathological complete resection rate (PCRR), which was affected by the possible factors of gender, endoscopic modalities, tumor size, endoscopic appearance, depth of invasion, and use of EUS, was analyzed. The patients were divided into 3 groups according to the different endoscopic resection modalities: the conventional polypectomy group (including cold biopsy forceps polypectomy and snare polypectomy with electrocauterization), the EMR group, and the ESD group.
Equipment
A single-channel gastroscope (GIF-H260, Olympus, Tokyo, Japan) and a single-channel colonoscope (CF-H260AL, Olympus, Tokyo, Japan) were used for the procedures of routine examination; a high-frequency generator (ICC-200, ERBE), snare (SD-230U-20, Olympus), and argon plasma coagulation unit (APC300, ERBE) were used for endoscopic resection.
Endoscopic Procedures
ESD was performed with a conventional single-channel endoscope as follows: marking dots were placed surrounding the lesion; a submucosal saline solution mixed with epinephrine and indigo carmine injection was performed beneath the lesion to elevate it; the incision was started outside the marking dots using a Flex-knife; the lesion was gradually dissected from the surrounding non-neoplastic mucosa and submucosal connective tissue from the margin to the center and then the resected specimen was retrieved with grasping forceps; and argon plasma coagulation (APC) cauterization was performed to protect the wound after resection.
The EMR procedure was carried out as follows: saline solution was injected beneath the tumor to elevate it away from the muscular layer and the lifting sign was observed; the lifted lesion was snared and resected using a blended electrosurgical current, and the resected specimen was retrieved with grasping forceps; and APC cauterization was performed to protect the wound when oozing of blood was observed from the wound surface after a snare resection.
Snare polypectomy with electrocauterization was carried out similarly to EMR, except that submucosal saline solution injection was implemented before a snare resection.
For cold biopsy forceps polypectomy, gastric biopsy forceps (FB-25KR-1, Olympus, Tokyo, Japan) or colonic biopsy forceps (FB-24UR-1, Olympus, Tokyo, Japan) were used to repeatedly clamp the polypoid tissue until no polyp was visible.
Data Analysis
Continuous variables were expressed as the mean ± standard deviations (SD) and percentage. The comparisons for continuous variables of the 2 or 3 treatment groups were performed using Student t test and one-way ANOVA. Chi-squared or Fisher exact test was used to compare categorical variables. The SPSS 15.0.1 statistical software package for Windows was used to perform the statistical analyses. A P-value <0.05 was considered statistically significant.
RESULTS
Characteristics of Patients and Tumors
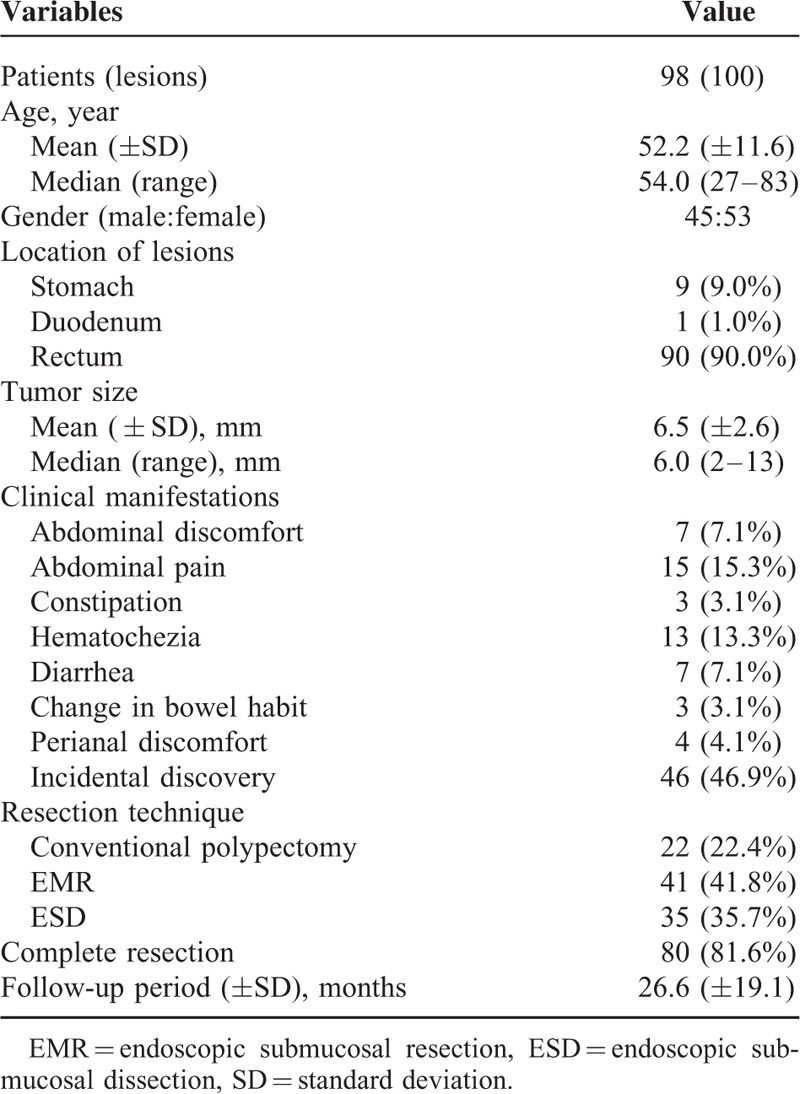
Among 102 patients, 4 were excluded because 1 had a lesion greater than the upper limit; 1 underwent additional ESD for remnants after primary endoscopic treatment in another hospital; 1 had lymphovascular invasion in the resection specimen; and 1 had no pathological report after treatment. Of the remaining 98 patients, 22 were treated by conventional polypectomy (including 6 cold biopsy forceps polypectomy and 16 snare polypectomy with electrocauterization), 41 by EMR, and 35 by ESD. One patient in the ESD group had 3 rectal lesions. All the ki67 indices were <5% according to the WHO 2010 classification.19 No tumors involved the lateral resection margins. The average age of the patients was 52.2 ± 11.6 years and the male:female ratio was 45:53. The mean size of the tumors was 6.5 ± 2.6 mm. The majority of tumors were located in the rectum (90.0%), and the remaining were gastric tumors (9.0%) and duodenal tumors (1.0%). Almost half of the cases (46.9%) were incidental findings during screening or examinations indicated for other diseases. Table 1 shows the baseline characteristics of the tumors and patients.
TABLE 1.
Characteristics of Patients and Tumors
Characteristics and Clinical Outcomes According to Endoscopic Modalities
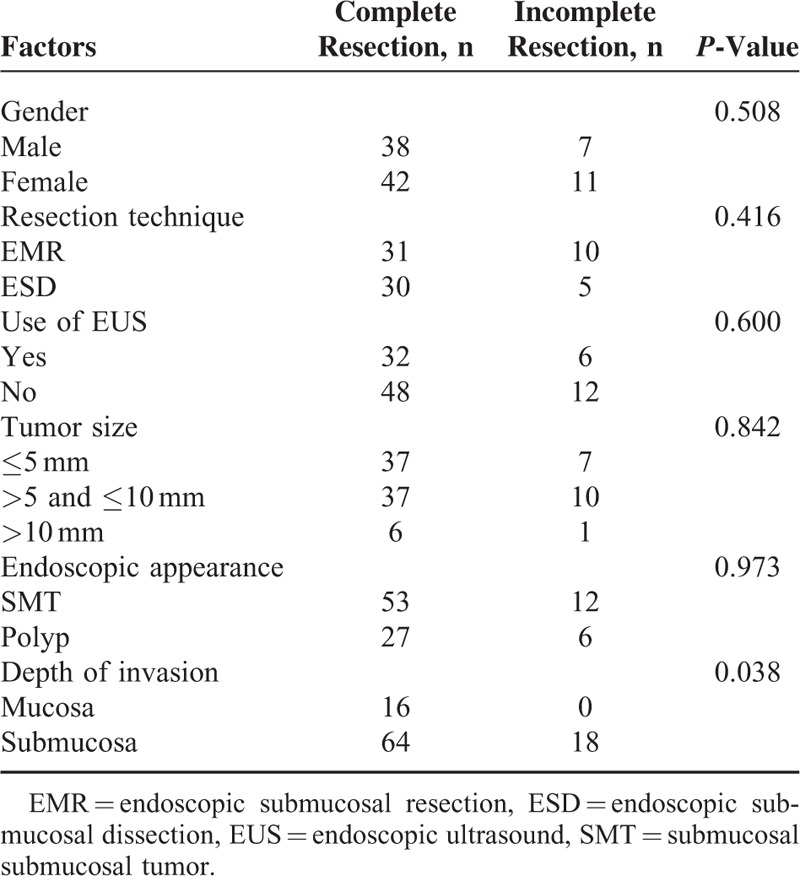
The characteristics and clinical outcomes of the 3 groups are presented in Table 2. According to the pathological reports, 17 tumors were limited to the mucosa. There were significantly more NETs that were confined to the mucosa in the conventional polypectomy group than in the EMR and ESD groups (54.5% vs 2.4% and 10.8%, P < 0.001 and P = 0.001). Of the 18 patients with suspected remnants, 10 were treated by EMR, 3 by conventional polypectomy, and 5 by ESD. The PCRRs of the conventional polypectomy, EMR, and ESD groups were 86.4%, 75.6%, and 85.7%, respectively. In the conventional polypectomy group, 16 polypoid-like GI-NETs, with a mean tumor size of 5.2 mm, were removed by snare polypectomy (PCRR 93.8%). The tumor size was significantly smaller in the conventional polypectomy group than in the EMR and ESD groups (4.8 ± 2.3 vs 6.7 ± 2.4 and 7.4 ± 2.6, P = 0.004 and P < 0.001). Submucosal tumors (SMT) were encountered in 33 and 34 patients in the EMR and ESD groups, respectively, but none in the conventional polypectomy group (80.5% and 91.9% vs 0.0%, P < 0.001 and P < 0.001). The use of EUS before resection in the EMR and ESD groups was significantly higher than that in the conventional polypectomy group (56.1% and 42.9% vs 0.0%, P < 0.001 and P < 0.001). There was 1 bleeding event in the EMR group and 1 in the ESD group. The 2 cases were successfully managed by endoscopic treatment. No perforation was found in the 3 groups. There were no significantly statistical differences in age, gender, tumor location, and complication rate among the 3 groups. Univariate analysis suggested that PCRR was only associated with depth of invasion, but not modality, use of EUS, tumor size, and endoscopic appearance (Table 3).
TABLE 2.
Baseline Characteristics and Clinical Outcomes of Different Endoscopic Treatment Groups
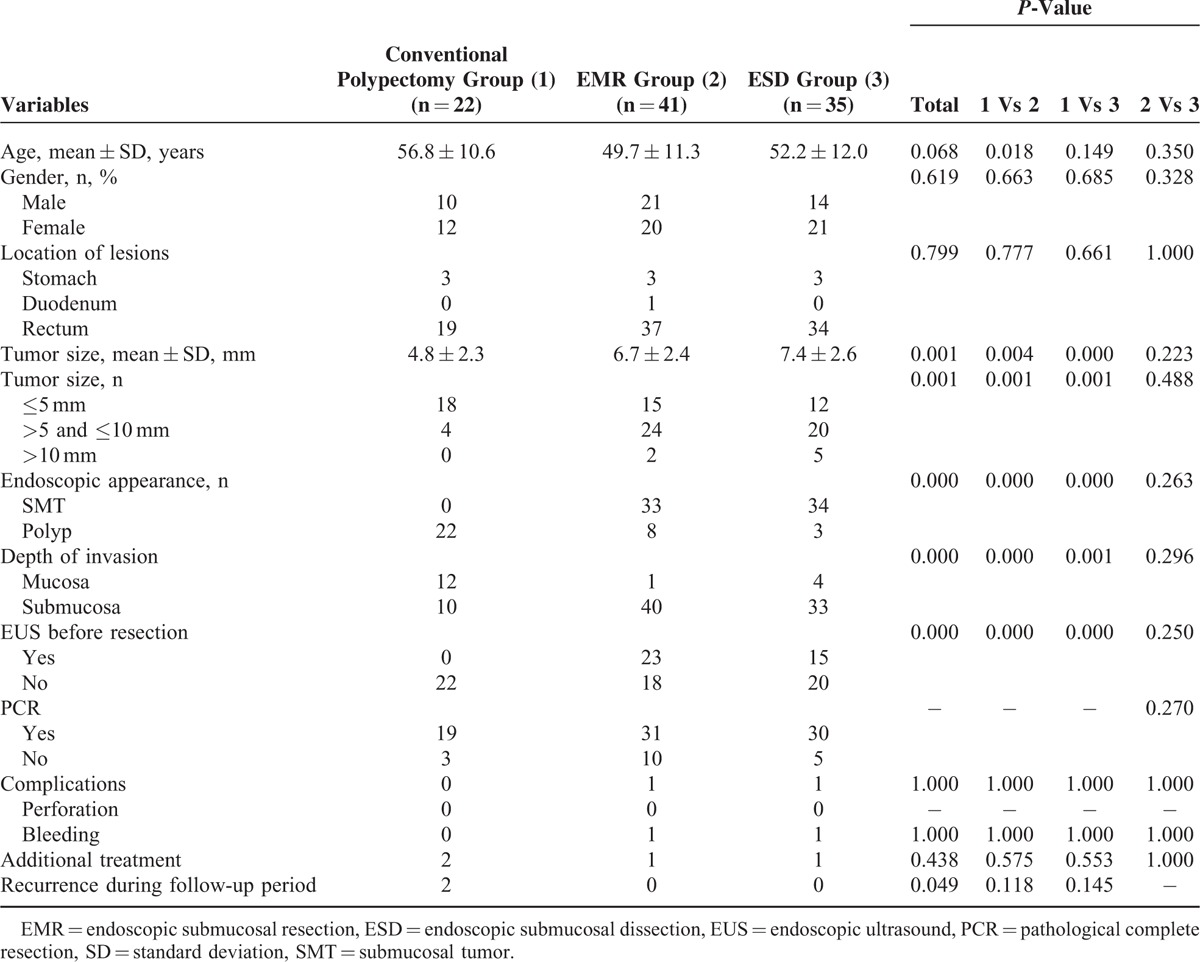
TABLE 3.
Analysis of Various Potential Risk Factors Associated With Pathologically Complete Resection Rates
Follow-Up and Additional Treatment
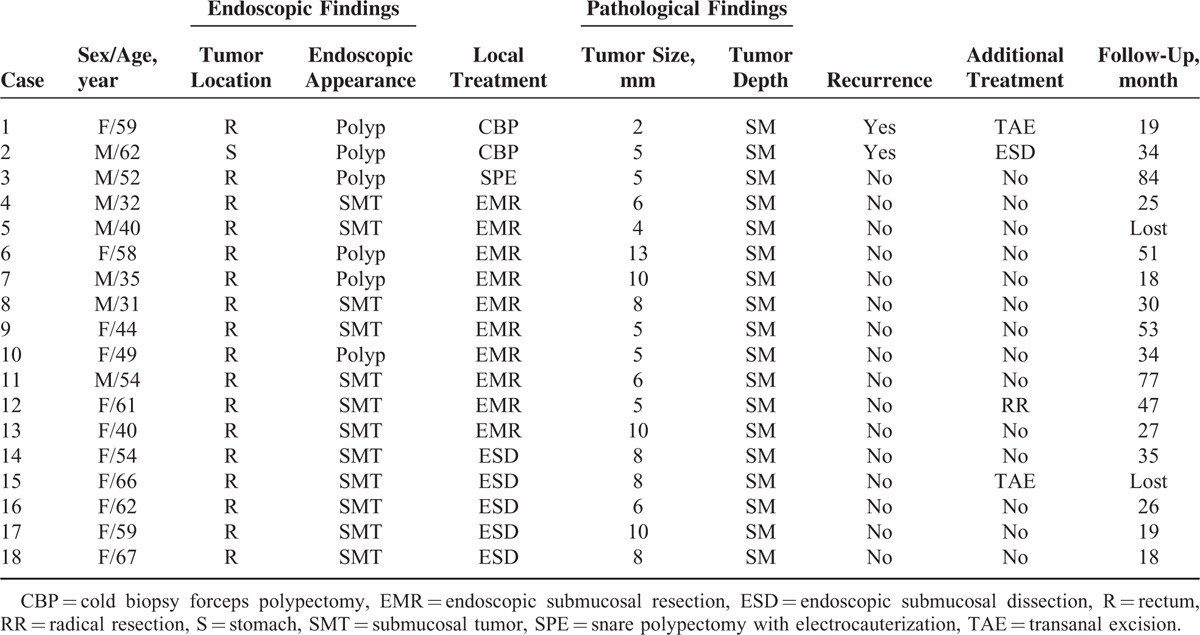
Of the 98 patients, 89 were followed up for 26.6 ± 19.1 months, 8 were lost and 1 died from heart failure. Among the 18 patients with suspected incomplete resection, 4 patients undertook additional treatments, including 1 radical operation, 1 ESD, and 2 transanal excisions. The remaining 14 did not undergo additional treatments because of old age, patients’ refusal, or other reasons. There were 2 local recurrences, which were treated by cold biopsy forceps polypectomy, after 2 and 4 months of follow-up, respectively. No local recurrence occurred in the snare polypectomy, EMR, or ESD groups (Table 4).
TABLE 4.
Detail Information of Patients With Suspected Remnant Tumor
DISCUSSION
To date, various endoscopic modalities, such as snare polypectomy, EMR, and ESD, have been reported as safe and effective modalities to treat GI-NETs. In addition, assessment of efficacy of these treatments is mainly dependent on the PCRR. Therefore, endoscopists consider ESD as the best because of its highest PCRR. However, in our medical center, not only ESD, but also EMR and snare polypectomy were used to remove GI-NETs.
Conventional polypectomy is a safe and simple procedure to resect GI-NETs. Martínez-Ares et al20 reported that conventional snare polypectomy could achieve 100% (13/13) complete resection in tumors that were less than 10 mm without invasion to the muscularis propria. However, it is generally recognized that about 75% of GI-NETs infiltrate into submucosa,21 and conventional polypectomy has difficulty achieving pathological complete resection in those tumors. The PCRR of conventional polypectomy ranges from 20.0% to 100% with an average of 60.0%,10,14,20,22–24 which is lower than the 86.4% (19/22) noted in our study. Six GI-NETs were unconsciously removed by cold biopsy forceps polypectomy, and we confirmed that it was inadequate because of the high risk of remnant tumors.13 Sixteen GI-NETs were removed by snare polypectomy with electrocauterization, with a PCRR of 93.8% and no local or distant recurrences during follow-up. Several reasons might explain the high PCRR of snare polypectomy. First, snare polypectomy was applied more frequently for smaller sized tumors (5.2 mm) with a polypoid appearance that were more likely to be confined to the mucosa. The 2nd reason is that electrosurgical devices, such as APC, damage larger fields during treatment.25 As some studies have reported, it seems feasible to accept snare polypectomy as an available treatment for some specific forms of tumors, such as diminutive (≤5 mm), polypoid-like GI-NETs.20,26
Compared with conventional polypectomy, EMR theoretically enables the resection of sufficient lateral margins for GI-NETs. However, for the most of the GI-NETs that infiltrate into the deep submucosa, EMR also cannot achieve a high pathological complete resection. The complete resection rate of EMR varies from 52.2% to 84.6% in the reports,27,28 and the PCRR in our study was 75.6% (31/41) in the EMR group, which was consistent with the previous reports. To improve the PCRR, various modified EMRs have been developed, including endoscopic submucosal resection with a ligation device,17 EMR with a ligation device,29 EMR using a transparent cap,23 EMR using a band-ligation device,8 and EMR using a dual-channel endoscope.30 All of these methods were designed to provide a deeper vertical resection margin. However, modified EMR includes many different techniques and the numbers of patients in those studies were small and the follow-up periods were limited.
ESD provides a larger lateral and deeper vertical resection margin compared with EMR. In recent years, ESD has been considered as a standard treatment for early gastric carcinomas in many countries, especially in Japan.16 In a meta-analysis comparing the efficacy of ESD and EMR for the treatment of rectal NETs, ESD was more effective, with a significantly higher complete resection rate. Moreover, there was no significant difference in complications between the 2 groups.27 In our study, the baseline of patients and indications for resection of tumors were not significantly different between the EMR and ESD groups. The PCRR of the ESD group was 85.7% (30/35), which was consistent with the 77.8% to 100% reported in the previous studies.27,28 However, ESD has a higher reported risk of complications compared with EMR in the report.16 In our study, there were no significant differences in complication rates between the 2 groups. The complication rate for ESD was 2.9% (1/35), which was similar to that (3.3%) of a previous report on rectal NETs.27 ESD needs a high level of experience and expertise to provide a deep enough resection to achieve complete resection, as well as to achieve a low complication rate. In addition, this method is also associated with disadvantages of a complex operating procedure, dependence on specialized equipment, and instruments, as well as being time consuming.27 Although tumor size and PCRR were not significant different between EMR and ESD groups in our study, we preferred ESD for the resection of submucosal GI-NETs, since ESD had a higher PCRR of bigger tumors than EMR.
Our study showed that the depth of invasion was the only factor associated with the PCRR. Tumors limited to the mucosa could be easily resected by endoscopic treatments, including snare polypectomy with electrocauterization, EMR, and ESD, and snare polypectomy was reasonable as the simplest procedure. Son et al12 reported that the method was the only factor associated with the PCRR of small rectal NETs, and the PCRR of ESD (72.3%) or surgical local excision (81.8%) was much higher than that of polypectomy (18.5%) and strip biopsy (42.9%). In our study, because conventional polypectomy was applied more frequently in the smaller and polypoid-like tumors with higher possibility of mucosal involvement, the PCRR of conventional polypectomy was higher than that reported by Son et al.
In the present study, 4 of 18 patients with suspected incomplete resection undertook additional treatments, including 2 in the conventional polypectomy group, 1 in the EMR group, and 1 in the ESD group. After additional treatments, all resection specimens showed negative resection margins. There were no relapses in the remaining 14 patients who did not undergo any additional treatment during follow-up. This may be explained by the electrocauterization effect, which generates high heat during the procedure to kill the remnant tumor cells.25 However, because of the slow-growing characteristics of NETs and limited follow-up period, we could not exclude the possibility of recurrence or metastasis during long-term follow-up. Therefore, further careful follow-up is required in these patients.
Several limitations in our study deserve discussion. The 1st limitation was that a retrospective study might be associated with selection bias. Although there were some differences on baseline characteristics among the 3 groups, the results, such as PCRRs, were consistent with previous reports, which suggested that there was a low possibility of selection bias. Second, the follow-up period was too limited, such that local relapses and metastasis could not been evaluated adequately. However, as the main indicator for the efficacy of endoscopic treatment, PCRR was fully assessed. Furthermore, the number of patients in each group was relatively small. But GI-NET was a rare disease, and the present number of patients was considered to be appropriate.
In conclusion, endoscopic resection of GI-NETs is safe and effective in properly selected patients. For submucosal GI-NETs, ESD was a feasible modality with a higher PCRR compared with EMR. For diminutive, polypoid-like NETs, snare polypectomy with electrocauterization was reasonable, being simple and providing a high PCRR.
Acknowledgments
The authors thank National Natural Science Foundation of China (81370509) (http://www.nsfc.gov.cn/publish/portal1/) for the support.
Footnotes
Abbreviations: EMR = endoscopic mucosal resection, ESD = endoscopic submucosal dissection, GI-NET = gastrointestinal neuroendocrine tumor, PCRR = pathological complete resection rate.
WS, SW, and XH were responsible for data collection and the statistical analysis; WS designed and wrote the article; CY critically revised the manuscript and contributed to conception of the study; and all authors approved the final version of the manuscript.
The study was supported by National Natural Science Foundation of China (81370509) (http://www.nsfc.gov.cn/publish/portal1/).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Woodside KJ, Townsend CM, Jr, Mark Evers B. Current management of gastrointestinal carcinoid tumors. J Gastrointest Surg 2004; 8:742–756. [DOI] [PubMed] [Google Scholar]
- 2.Modlin IM, Sandor A. An analysis of 8305 cases of carcinoid tumors. Cancer 1997; 79:813–829. [DOI] [PubMed] [Google Scholar]
- 3.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003; 97:934–959. [DOI] [PubMed] [Google Scholar]
- 4.Modlin IM, Oberg K, Chung DC, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol 2008; 9:61–72. [DOI] [PubMed] [Google Scholar]
- 5.Scherubl H. Rectal carcinoids are on the rise: early detection by screening endoscopy. Endoscopy 2009; 41:162–165. [DOI] [PubMed] [Google Scholar]
- 6.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008; 26:3063–3072. [DOI] [PubMed] [Google Scholar]
- 7.Park CH, Cheon JH, Kim JO, et al. Criteria for decision making after endoscopic resection of well-differentiated rectal carcinoids with regard to potential lymphatic spread. Endoscopy 2011; 43:790–795. [DOI] [PubMed] [Google Scholar]
- 8.Choi CW, Kang DH, Kim HW, et al. Comparison of endoscopic resection therapies for rectal carcinoid tumor: endoscopic submucosal dissection versus endoscopic mucosal resection using band ligation. J Clin Gastroenterol 2013; 47:432–436. [DOI] [PubMed] [Google Scholar]
- 9.Higaki S, Nishiaki M, Mitani N, et al. Effectiveness of local endoscopic resection of rectal carcinoid tumors. Endoscopy 1997; 29:171–175. [DOI] [PubMed] [Google Scholar]
- 10.Iishi H, Tatsuta M, Yano H, et al. More effective endoscopic resection with a two-channel colonoscope for carcinoid tumors of the rectum. Dis Colon Rectum 1996; 39:1438–1439. [DOI] [PubMed] [Google Scholar]
- 11.Imada-Shirakata Y, Sakai M, Kajiyama T, et al. Endoscopic resection of rectal carcinoid tumors using aspiration lumpectomy. Endoscopy 1997; 29:34–38. [DOI] [PubMed] [Google Scholar]
- 12.Son HJ, Sohn DK, Hong CW, et al. Factors associated with complete local excision of small rectal carcinoid tumor. Int J Colorectal Dis 2013; 28:57–61. [DOI] [PubMed] [Google Scholar]
- 13.Han X, Cui Y, Yang C, et al. Endoscopic biopsy in gastrointestinal neuroendocrine neoplasms: a retrospective study. PLoS One 2014; 9:e103210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onozato Y, Kakizaki S, Iizuka H, et al. Endoscopic treatment of rectal carcinoid tumors. Dis Colon Rectum 2010; 53:169–176. [DOI] [PubMed] [Google Scholar]
- 15.Lee DS, Jeon SW, Park SY, et al. The feasibility of endoscopic submucosal dissection for rectal carcinoid tumors: comparison with endoscopic mucosal resection. Endoscopy 2010; 42:647–651. [DOI] [PubMed] [Google Scholar]
- 16.Lian J, Chen S, Zhang Y, et al. A meta-analysis of endoscopic submucosal dissection and EMR for early gastric cancer. Gastrointest Endosc 2012; 76:763–770. [DOI] [PubMed] [Google Scholar]
- 17.Kim KM, Eo SJ, Shim SG, et al. Treatment outcomes according to endoscopic treatment modalities for rectal carcinoid tumors. Clin Res Hepatol Gastroenterol 2013; 37:275–282. [DOI] [PubMed] [Google Scholar]
- 18.Zhao ZF, Zhang N, Ma SR, et al. A comparative study on endoscopy treatment in rectal carcinoid tumors. Surg Laparosc Endosc Percutan Tech 2012; 22:260–263. [DOI] [PubMed] [Google Scholar]
- 19.Bosman TF, Carneiro F, Hruban RH, et al. WHO Classification of Tumours of the Digestive System. 2010; Lyon: IARC Press, 13–14. [Google Scholar]
- 20.Martínez-Ares D, Souto-Ruzo J, Varas Lorenzo MJ, et al. Endoscopic ultrasound-assisted endoscopic resection of carcinoid tumors of the gastrointestinal tract. Rev Esp Enferm Dig 2004; 96:847–855. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto T, Iida M, Suekane H, et al. Endoscopic ultrasonography in rectal carcinoid tumors: contribution to selection of therapy. Gastrointest Endosc 1991; 37:539–542. [DOI] [PubMed] [Google Scholar]
- 22.Kumar AS, Sidani SM, Kolli K, et al. Transanal endoscopic microsurgery for rectal carcinoids: the largest reported United States experience. Colorectal Dis 2012; 14:562–566. [DOI] [PubMed] [Google Scholar]
- 23.Kim YJ, Lee SK, Cheon JH, et al. Efficacy of endoscopic resection for small rectal carcinoid: a retrospective study. Korean J Gastroenterol 2008; 51:174–180. [PubMed] [Google Scholar]
- 24.Ono A, Fujii T, Saito Y, et al. Endoscopic submucosal resection of rectal carcinoid tumors with a ligation device. Gastrointest Endosc 2003; 57:583–587. [DOI] [PubMed] [Google Scholar]
- 25.Norton ID, Wang L, Levine SA, et al. In vivo characterization of colonic thermal injury caused by argon plasma coagulation. Gastrointest Endosc 2002; 55:631–636. [DOI] [PubMed] [Google Scholar]
- 26.Matsui K, Iwase T, Kitagawa M. Small, polypoid-appearing carcinoid tumors of the rectum: clinicopathologic study of 16 cases and effectiveness of endoscopic treatment. Am J Gastroenterol 1993; 88:1949–1953. [PubMed] [Google Scholar]
- 27.Zhong DD, Shao LM, Cai JT. Endoscopic mucosal resection vs endoscopic submucosal dissection for rectal carcinoid tumours: a systematic review and meta-analysis. Colorectal Dis 2013; 15:283–291. [DOI] [PubMed] [Google Scholar]
- 28.Zhou X, Xie H, Xie L, et al. Endoscopic resection therapies for rectal neuroendocrine tumors: a systematic review and meta-analysis. J Gastroenterol Hepatol 2014; 29:259–268. [DOI] [PubMed] [Google Scholar]
- 29.Niimi K, Goto O, Fujishiro M, et al. Endoscopic mucosal resection with a ligation device or endoscopic submucosal dissection for rectal carcinoid tumors: an analysis of 24 consecutive cases. Dig Endosc 2012; 24:443–447. [DOI] [PubMed] [Google Scholar]
- 30.Lee WH, Kim SW, Lim CH, et al. Efficacy of endoscopic mucosal resection using a dual-channel endoscope compared with endoscopic submucosal dissection in the treatment of rectal neuroendocrine tumors. Surg Endosc 2013; 27:4313–4318. [DOI] [PubMed] [Google Scholar]


