Abstract
The advantage of hemodiafiltration (HDF) is well known. One of the disadvantages of HDF is loss of serum albumin, but this issue is still obscure. Some risk factors associated with mortality were age dependent. Studies on serum albumin/hypoalbuminemia and HDF in different age stratification were limited. The aim of this cross-sectional study was to assess the role of HDF and other clinical variables on serum albumin values in maintenance hemodialysis (MHD) patients of different age groups.
We recruited a total of 1216 patients on MHD. Patients were divided into 4 groups by age stratification of youth (<30 years old), young-middle age (30–44 years old), middle age (45–64 years old), and old age (≥65 years old). Biochemical, hematological, nutritional, inflammatory parameters, and receiving HDF or not were recorded. The associations between age groups, HDF, and variables mentioned above were analyzed.
Only in middle-age group, patients with HDF was significantly (P = 0.013) associated with serum albumin <4 g/dL. In middle-age group, a multivariate-forward logistic regression analysis showed that male sex (2.169 [1.029, 4.574], P = 0.042), inflammation (4.167 [2.043, 8.498], P < 0.001), cardiovascular disease (2.92 [1.019, 8.402], P = 0.046), serum creatinine level (0.639 [0.538, 0.758], P < 0.001), and cholesterol level (0.984 [0.975, 0.993], P = 0.001) were associated with serum albumin level <3.6 g/dL. Hepatitis C virus infection (1.911 [1.186, 3.077], P = 0.008), HDF (2.143 [1.298, 3.540], P = 0.003), inflammation (2.309 [1.549, 3.440], P < 0.001), use of arterio-venous fistula (0.518 [0.327, 0.820], P = 0.005), Kt/V (0.395 [0.193, 0.809], P = 0.011), nonanuria (0.542 [0.337, 0.870], P = 0.011), serum creatinine level (0.744 [0.669, 0.828], P < 0.001), and cholesterol level (0.993 [0.987, 0.998], P = 0.013) were associated with serum albumin level <4 g/dL.
HDF can predict serum albumin level <4 g/dL in middle-age HD patients. The effect of age needs to be taken into consideration when interpreting the correlation between hypoalbuminemia and HDF.
INTRODUCTION
The advantage of hemodiafiltration (HDF) is well known and well discussed.1,2 Clearance of middle-to-large molecules can be improved by convective transport through HDF. Studies have shown that HDF improves hemodynamic stability and response to erythropoietin (EPO) and reduces the incidence of hemodialysis-associated amyloidosis and chronic inflammation.3,4 HDF can also reduce all-cause mortality in hemodialysis patients.2 One of the disadvantages of HDF is loss of serum albumin.5,6 The possible mechanism of albumin loss in dialysate was high convective volume in HDF and the albumin concentration in the effluent differs according to the type of filter used and the convective volume.7 However, this issue is still obscure.8–10 den Hoedt et al showed that after 6 years follow up, there were statistically significant reduced levels of serum albumin in HDF and HD patients including all ages. But there was no difference of serum albumin level between HDF and HD patients.10 Orasan et al11 also showed that decreasing serum albumin levels were noted both in HD and HDF patients. However, Jean et al12 demonstrated recently that serum albumin (34.4 ± 3.6, 35.9 ± 3.4, and 34.1 ± 4.0 g/L, P < 0.0001, values shown for HDF1, HD, and HDF2, respectively) were significantly lower during the HDF periods in a cross over study of 51 patients. Protein-energy malnutrition is a major risk factor for mortality. Serum albumin is a surrogate of nutrition and inflammation and can predict mortality in HD patients.13 In the Dialysis Outcomes and Practice Patterns Study (DOPPS),14 older age, catheter vascular access, albumin concentration <3.5 g/dL, phosphorus concentration <3.5 g/dL, cancer, and congestive heart failure were all positively associated with 1-year mortality. Age is a most discussed risk factor for maintenance hemodialysis (MHD) patients no matter in mortality or other comorbidities. However, in different age stratification and race, the result is different. In a seminal study comparing African-American versus White dialysis patients, Kucirka et al15 reported that African-Americans have increased mortality risk in younger (≤50 years old) age groups and decreased mortality risk in older (>50 years) age groups, but did not separately consider Hispanic ethnicity. In different age categories, Lertdumrongluk et al16 pointed out that serum alkaline phosphatase (ALP) levels were positively correlated with mortality in all age groups, and the positive correlation between ALP and mortality was much stronger in patients with ages <45 years as compared with patients ≥45 years. The positive correlation between serum parathyroid hormone (PTH) levels and mortality was stronger in older patients as compared with the younger patients. There were only incremental correlation between serum PTH and mortality in middle-age and older patients (≥45 years). No significantly positive correlation was noted between serum PTH levels and mortality in patients <45 years. The effect of age needs to be considered when interpreting the prognostic implications of serum ALP and PTH levels. To our knowledge, study on serum albumin/hypoalbuminemia and HDF in different age stratification is limited. The aim of this cross-sectional study was to assess the role of HDF and other clinical variables on serum albumin values in MHD patients. We hypothesized that serum albumin levels would show differential associations with HDF across varying age groups in MHD patients.
METHODS
The Institutional Review Board Committee of Chang Gung Memorial Hospital approved the study protocol. All medical records during the study period, including medical history, laboratory data, and inclusion and exclusion factors were reviewed by senior nephrologists. In addition, all individual information was securely protected and was only available to the investigators.
Patients
Study patients were recruited from the 3 hemodialysis centers of Chang Gung Memorial Hospital, Lin-Kou Medical Center, Taipei and Taoyuan branches. Only MHD patients who were 18 years of age or older and had received hemodialysis for at least 6 months were enrolled in this study. The inclusion criterion of HDF was patients receiving thrice weekly HDF for ≥3 months (Figure 1). Postdilution HDF was performed for HDF patients. Synthetic high-flux dialyzers were used for HDF. Both HDF and HD were performed with ultrapure dialysate. A minimum of 300 mL/min of blood flow was required for HDF. A minimum of 20 L/session of replacement volume was requested for HDF. Patients with malignancies or obvious infectious diseases, as well as those who had been hospitalized or had undergone surgery within 3 months of the investigation, were excluded. Diabetes mellitus (DM) was defined by either a physician's diagnosis, antidiabetic drug treatment, or if 2 subsequent analyses demonstrated fasting blood glucose levels of >126 mg/dL. Most patients underwent 4 h of hemodialysis 3 times a week. Hemodialysis was performed using single-use hollow-fiber dialyzers equipped with modified cellulose, polyamide, or polysulfone membranes. The dialysate used in all cases had a standard ionic composition with a bicarbonate-based buffer. We noted the incidence of cardiovascular diseases (CVDs) including cerebrovascular disease, coronary artery disease, congestive heart failure, and peripheral vascular disease in these patients. Hypertension was defined as the regular use of antihypertensive drugs to control blood pressure or at least 2 blood pressure measurements of >140/90 mm Hg. Smoking behavior was also noted in this study. Smoking status was defined as that patients had smoked every day for more than 3 months at the time of clinical data measurements.17
FIGURE 1.
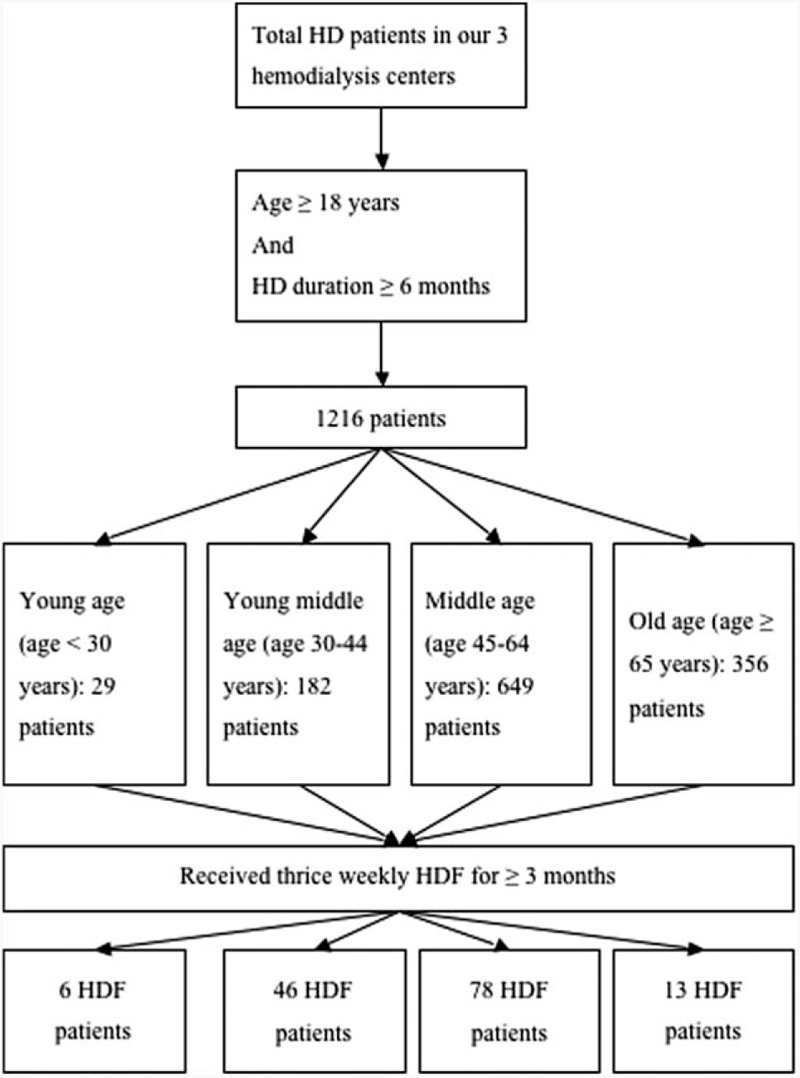
Flow chart of inclusion and exclusion criteria of HD patients in this study. HD = hemodialysis, HDF = hemodiafiltration.
Age Stratification
Because the definition of age stratification differ in countries, we divided the patients into 4 groups by age stratification of youth (<30 years old), young-middle age (30–44 years old), middle age (45–64 years old), and old age (≥65 years old) according to the study by National Health Research Institutes of Taiwan.18
Laboratory, Nutritional, and Inflammatory Parameters
The blood samples were drawn and measured as described in our previous study.19 The procedures were summarized briefly as follows. Normalized protein catabolism rate (nPCR) and serum albumin levels were measured and used as nutritional markers. Serum albumin was detected by colorimetric method in our 3 HD centers. High-sensitivity C-reactive protein (hsCRP) levels were assayed as the index of inflammation. Dialyzer clearance of urea was measured using a Daugirdas method and was expressed as Kt/V urea.20 The nPCR of HD patients was calculated by approved equations and normalized to their body weight.21
Definition of Malnutrition and Inflammation
Till now, the accurate level of albumin for nutrition evaluation is not clear. Serum albumin level of <3.6 g/dL to be malnourished upon the lower limit of the normal range of serum albumin levels in our hospital, that is, 3.5 g/dL, and represented the 10th percentile of the Third National Health and Nutrition Examination Survey of Americans.22,23 However, in K/DOQI Clinical Practice Guidelines for Chronic Kidney Disease, an acceptable goal level of serum albumin is ≥4.0 g/dL in patients with MHD.24 From above cited studies and description, we tried to analyze the association between variables and status of malnutrition as serum albumin <3.6 and <4 g/dL, by multivariate logistic regression analysis, respectively. The presence of inflammation in the MHD patients was defined as an hsCRP level of >3 mg/L, a level that was correlated with elevated cardiovascular risk in the general population.25,26
Statistical Analysis
Data were analyzed using SPSS, version 12.0 for Windows 95 (SPSS Inc., Chicago, IL). The Kolmogorov–Smirnov test was used to test if variables were normally distributed. A P value of >0.05 was required to assume a normal distribution. Unless otherwise stated, continuous variables are expressed as mean ± standard deviation, and categorical variables are expressed as numbers or percentages. Chi-squared test or Fisher exact test was used to analyze the correlation between categorical variables. Comparisons among the 4 study groups were analyzed using a trend test. Comparisons between 2 groups were performed using the Mann–Whitney U test and Student t test. The data of intact parathyroid hormone (iPTH) and hsCRP levels were log-transformed for analysis. To evaluate the variables related to malnutrition (serum albumin, <3.6 and <4 g/dL), multivariate logistic regression analyses in forward method were performed to assess the odds ratio and 95% confidence interval for the baseline variables including age, male sex, body mass index (BMI), smoking status, DM, hypertension, previous CVD, hepatitis B virus (HBV) infection, hepatitis C virus (HCV) infection, hemodialysis duration, fistula as blood access, HDF, Kt/V Daugirdes, nPCR, residual daily urine of >100 mL, hemoglobin, serum creatinine levels, inorganic phosphate levels, hsCRP >3.0 mg/dL, cholesterol levels, and triglyceride levels. All the nominal variables in logistic regression were transformed into dummy coding. Missing data were approached with listwise deletion. One-way ANOVA and a post hoc test (Bonferroni) were used to analyze the difference of continuous variables between 4 age groups. The level of significance was set at P < 0.05.
RESULTS
Study Population Characteristics
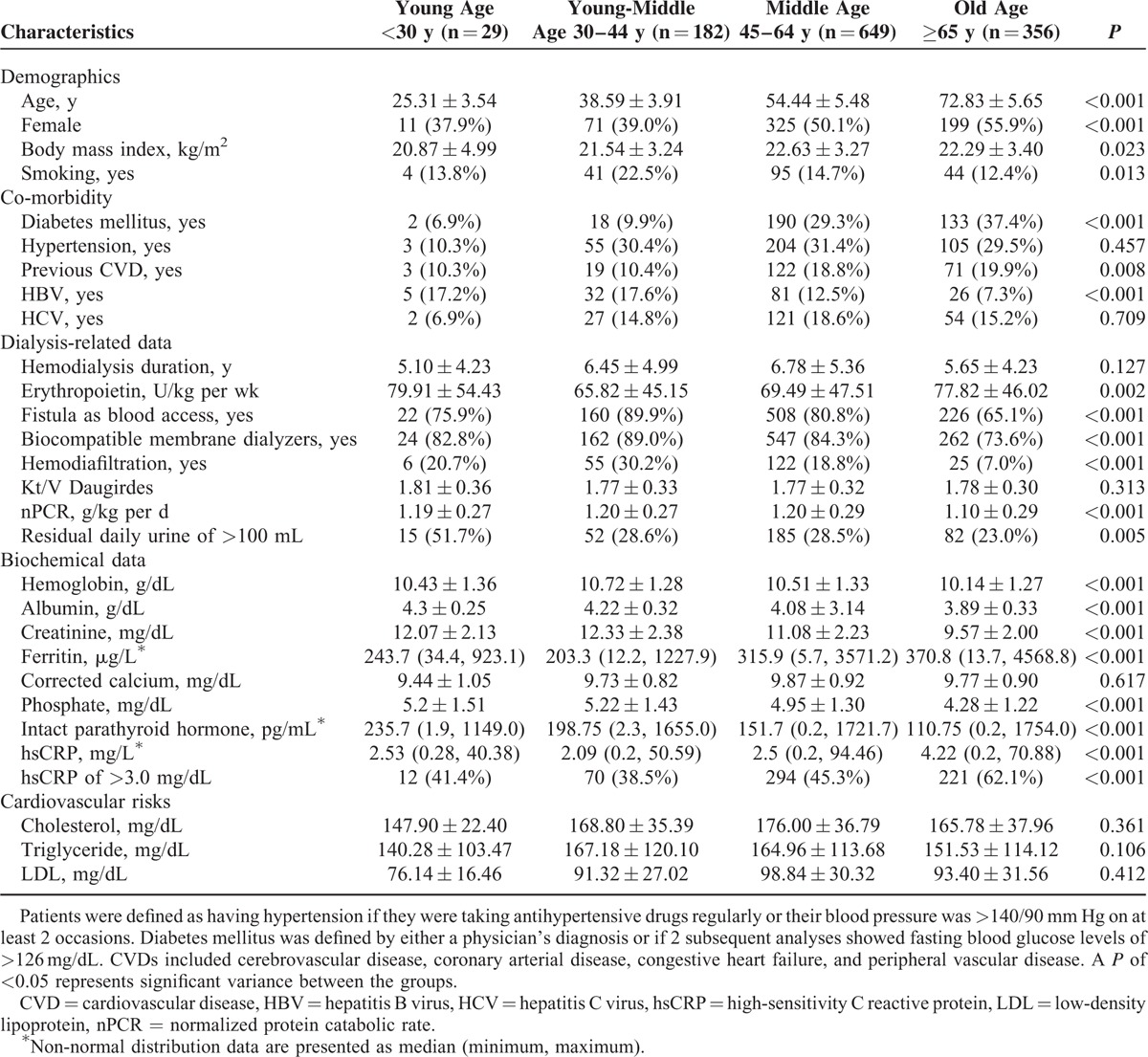
A total of 1216 MHD patients (610 men and 606 women) met the inclusion criteria, with a mean MHD duration of 6.36 ± 4.99 years, and were included in the study. Table 1 lists the patient characteristics including age, gender, and BMI, along with the biological, hematological, and biochemical data for the 4 subgroups dividing from age stratification. As increasing with age stratification, this study seemed to comprise patients with a trend of higher BMI, ferritine levels, and hsCRP levels; and higher prevalence of female sex, DM status, and previous CVD status; and lower serum albumin, phosphate, iPTH, nPCR, hemoglobin, and creatinine levels; and lower prevalence of smoking, HDF, malnutrition, HBV infection, arterio-venous (AV) fistula access, and daily residual urine >100 mL (Table 1). The groups did not differ in terms of hypertension, presence of HCV infection, hemodialysis duration, Kt/V (Daugirdas), corrected calcium levels, cholesterol levels, and triglyceride levels.
TABLE 1.
Baseline Characteristics of Hemodialysis Patients in Different Age Stratification
Probability of Having Malnutrition in 1216 MHD Patients by Serum Albumin Level <3.6 and <4 g/dL, Respectively
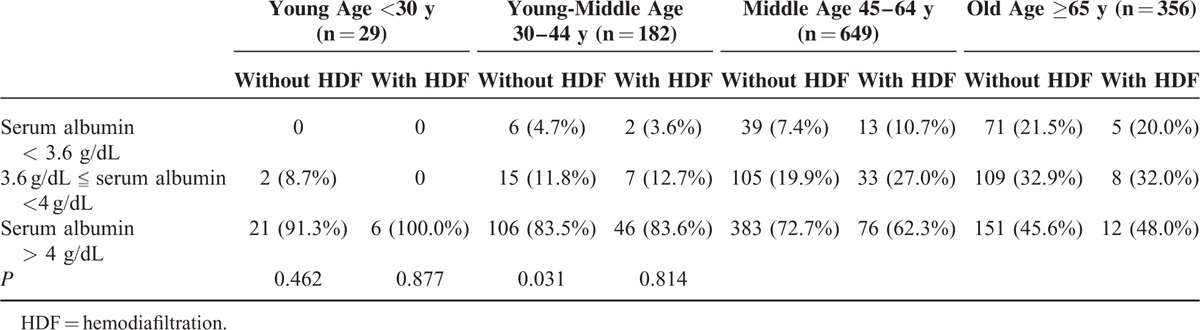
Table 2 shows that among the 4 age stratifications, only in middle-age group, patients with HDF was significantly (P = 0.013) associated with serum albumin <4 g/dL. However, in Table 3, we did not found the association between patients with HDF and serum albumin <3.6 g/dL in the 4 age stratification (P > 0.05). From above finding, in advance, we divided the patients into 3 groups such as serum albumin <3.6 g/dL, 3.6 g/dL
 |
serum albumin <4 g/dL, and serum albumin ≥4 g/dL. Only in middle-age group, number of patients with HDF were significantly higher than without HDF in the range of 3.6 g/dL ≤ serum albumin <4 g/dL (Table 4, P = 0.031).
TABLE 2.
Comparison of the Numbers of Patients With Hypoalbuminemia (<4 g/dL) Between Patients on HDF or Not in Different Age Stratifications
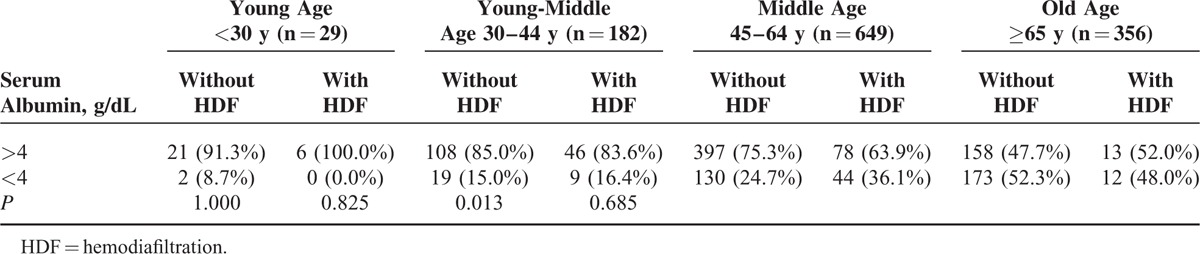
TABLE 3.
Comparison of the Numbers of Patients With Hypoalbuminemia (<3.6 g/dL) Between Patients on HDF or Not in Different Age Stratifications
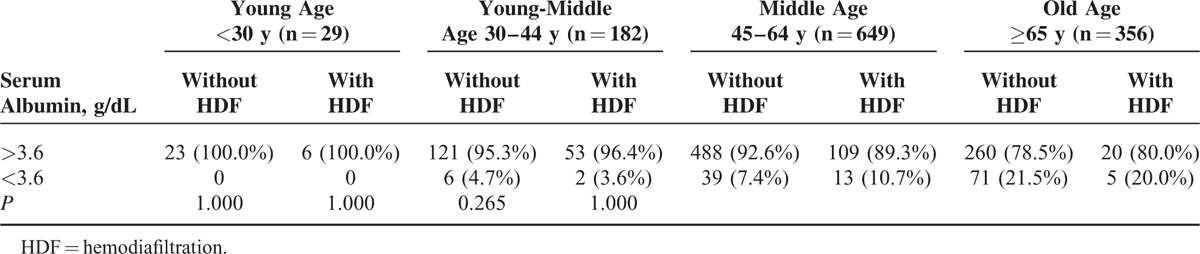
TABLE 4.
Comparison of the Numbers of Patients With Hypoalbuminemia (in Different Range of Serum Albumin Level) Between Patients on HDF or Not in Different Age Stratifications
Comparison of Clinical Variables Between Patients With and Without HDF in Middle-Age Group
In order to clarify the association between patients with HDF and malnutrition, subgroup analysis was used for patients with HDF in middle-age group (Table 5).
TABLE 5.
Baseline Characteristics of Hemodialysis Patients in Middle-Age Patients (n = 649)
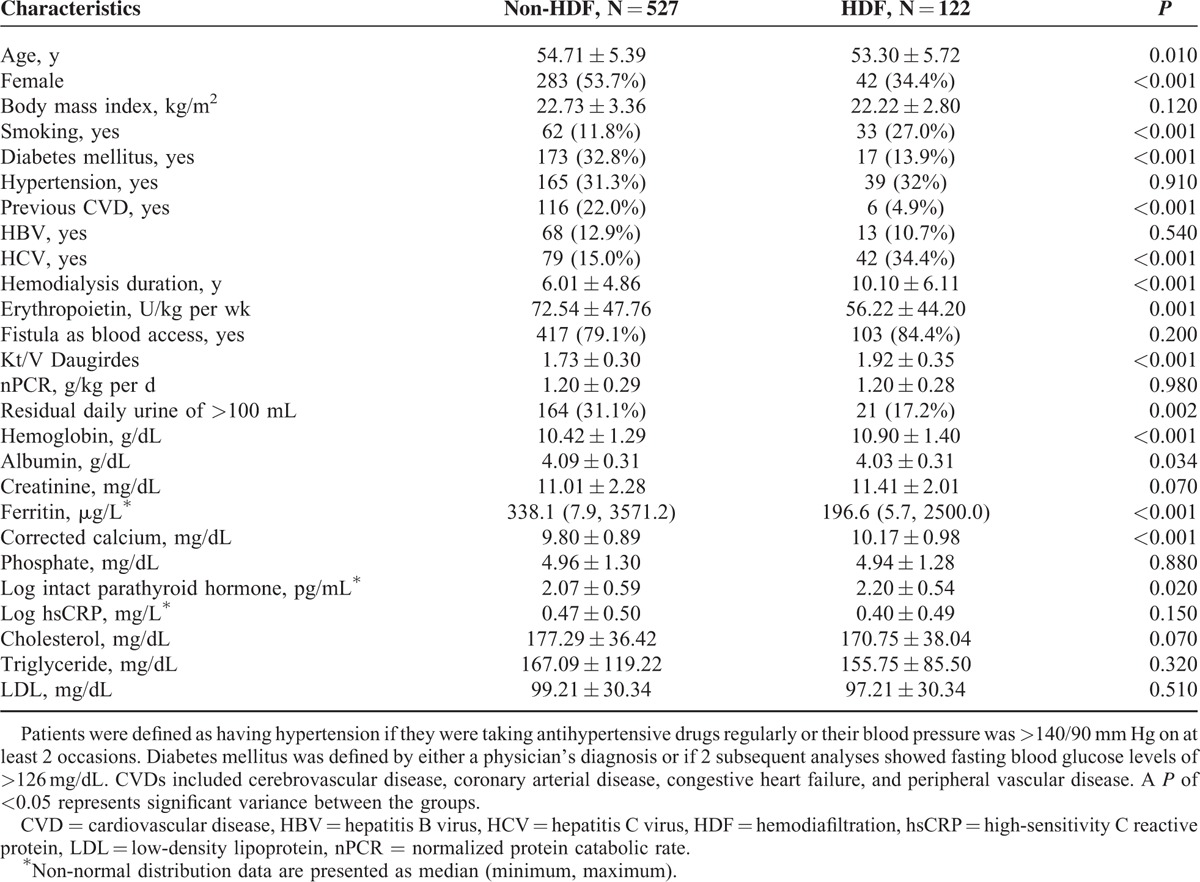
In middle-age group, the patients receiving HDF were younger (53.3 ± 5.72 vs 54.71 ± 5.39 years, P = 0.01), predominantly male (65.6% vs 46.3%, P < 0.001), high incidence of smoking habits (27.0% vs 11.8%, P < 0.001), lower incidence of DM (13.9% vs 32.8%, P < 0.001), lower incidence of CVD (4.9% vs 22.0%, P < 0.001), higher incidence of HCV infection (34.4% vs 15.0%, P < 0.001), longer hemodialysis duration (10.10 ± 6.11 vs 6.01 ± 4.86 years, P < 0.001), low EPO usage (56.22 ± 44.20 vs 72.54 ± 47.76 U/kg weight per wk, P = 0.001), high Kt/V (1.92 ± 0.35 vs 1.73 ± 0.30, P < 0.001), low incidence of daily urine output >100 cc (17.2% vs 31.1%, P = 0.002), high hemoglobin level (10.90 ± 1.40 vs 10.42 ± 1.29 g/dL, P < 0.001), low albumin level (4.03 ± 0.31 vs 4.09 ± 0.31 g/dL, P = 0.034), low ferritine level (196.6 vs 338.1 μg/L, P < 0.001), high corrected calcium level (10.17 ± 0.98 vs 9.80 ± 0.89, P < 0.001), and high log iPTH level (2.20 ± 0.54 vs 2.07 ± 0.59 pg/mL, P = 0.02).
In order to clarify the association between variables and adequate nutrition from different reference (serum albumin level <3.6 and <4 g/dL, respectively) in middle-age group, a multivariate-forward logistic regression analysis were used for demonstration. Male sex (2.169 [1.029, 4.574], P = 0.042), inflammation (4.167 [2.043, 8.498], P < 0.001), CVD (2.92 [1.019, 8.402], P = 0.046), serum creatinine level (0.639 [0.538, 0.758], P < 0.001), and cholesterol level (0.984 [0.975, 0.993], P = 0.001) were associated with serum albumin level <3.6 g/dL. HCV infection (1.911 [1.186, 3.077], P = 0.008), HDF (2.143 [1.298, 3.540], P = 0.003), inflammation (2.309 [1.549, 3.440], P < 0.001), use of AV fistula (0.518 [0.327, 0.820], P = 0.005), KT/V (0.395 [0.193, 0.809], P = 0.011), nonanuria (0.542 [0.337, 0.870], P = 0.011), serum creatinine level (0.744 [0.669, 0.828], P < 0.001), and cholesterol level (0.993 [0.987, 0.998], P = 0.013) were associated with serum albumin level <4 g/dL.
In order to compare the difference of patients’ baseline parameters in 4 age groups, 1-way ANOVA showed that there were significant differences in educational levels (P < 0.001) and ultrafiltration amount (P < 0.001) between 4 age groups. Post hoc analysis showed that middle-age group patients had significantly lower educational levels than young middle-age group (average difference: −1.020, standard error: 0.081, P < 0.001) and middle-age group patients had significantly higher ultrafiltration amount than old-age group patients (average difference: 0.4303 kg, standard error: 0.1301, P = 0.001).
DISCUSSION
Previous studies showed that after switching from high-flux HD to HDF, there was a decrease in serum albumin level.8,11,12 But Movilli et al27 recently showed that HDF induces a long-term significant reduction in predialysis β2M and hsCRP concentrations, but no significant serum albumin variation were seen. Vilar et al28 found that there was no difference in serum albumin level between HDF and high-flux hemodialysis 5 years after initiation of hemodialysis. However, these studies did not take age into consideration. In our present study, we demonstrated that HDF was significantly associated with hypoalbuminemia in middle-age MHD patients. In other age stratifications, no significant association was noted. In addition, HDF induced hypoalbuminemia level is mostly in the range of 3.6 g/dL < serum albumin < 4 g/dL. Hence, the effect of age needs to be considered when interpreting the prognostic implications between hypoalbuminemia and HDF.
In our middle-age patients, patients with HDF showed a significant higher Kt/V levels than patients on conventional HD. Multivariate logistic regression in middle-age HD patients also showed significant correlation between Kt/V and serum albumin level <4 g/dL. Bolasco et al29 showed that in longer periods of hemofiltration, a notable correlation between Kt/V and convection volume was noted. Convection volume had been shown to be a negative association with serum albumin.12,30,31 However, there were other studies showed that there was no influence of convection on serum albumin level.32,33 Therefore, the lower serum albumin level in middle-age group might be due to higher convection volume-related higher Kt/V, but the causal effect between serum albumin level and convection volume needs further study.
Our study showed that there was a significant correlation between HCV and serum albumin lower than 4 mg/dL in middle-age HD patients. Tsai et al34 reported that there was an association of HCV infection and malnutrition–inflammation complex syndrome, which included serum albumin, in MHD patients. There was no previous to show that there was association between HCV infection and low serum albumin in middle-age HD patients. Therefore in middle-age HD patients who had HCV infection, we should pay more attention to the serum albumin level.
In our study, we showed that in middle-age patients on HD, 24-h residual urine >100 mL was 1 of the predictors of serum albumin lower than 4 g/dL. It has been demonstrated that residual renal function (RRF) may have a beneficial effect on nutritional parameters, such as serum albumin level, in chronic HD patients.35–37 Schiffl et al38 showed that high efficiency HDF can preserved RRF better than conventional HD. RRF may have a beneficial effect on nutritional parameters, especially serum albumin, and it is important to check RRF over time in chronic HD and HDF patients.
The serum cholesterol level was negatively correlated with serum albumin lower than 4 and 3.6 g/dL in our study. Avram et al39 had demonstrated that levels of serum cholesterol and albumin were higher among long-term survivors of HD patients. Therefore, higher cholesterol level in our study may reflex better nutritional status.
What is the reason that HDF is a predictor of serum albumin <4 g/dL only in the middle-age HDF patients? The patient numbers in the middle-age HDF group was much larger than the other HDF patient group. The small patient numbers in other patient group might be the reason of unsignificance.
We demonstrated that middle-age patients on HDF had significantly lower educational level than young middle-age patients and higher than old-age patients on HDF. However, due to large difference of patient numbers between middle-age and old-age patients on HDF, the significance may not be correct. Therefore, lower educational level may be 1 reason that middle-age patients on HDF were more susceptible to lower albumin level under the influence of HDF. In previous studies, they showed that low educational level and low albumin level were associated with mortality in HD patients.40,41 There might be some correlation between educational level, albumin level, and their correlation with HDF. Darmon and Drewnowski showed that higher-quality diets are, in general, consumed by better-educated and more affluent people. Conversely, lower-quality diets tended to be consumed by groups of lower SES and more limited economic means.42
In our present study, we showed that mean ultrafiltration amount during each HDF session in middle-age HDF patients was significantly higher than old-age HDF. Fournier et al43 showed that albumin loss in postdilution HDF was significantly affected by infusate flow, infusate volume, transmembrane pressure, and ultrafiltration volume.
den Hoedt et al44 reported that compared with women, men had an excess decline of 0.03 g/dL per y in albumin (−0.06 to −0.001; P = 0.05) in HD patients over time. Our middle-age HDF patients have high percentage of males (65.6%) and this may explain the association of HDF and low albumin in this age group.
LIMITATIONS
This is a cross-sectional study and further randomized controlled studies should be performed to confirm the relation between causal and effect of age and hypoalbuminemia in HDF patients. The patient numbers were different in different age groups and varied a lot with only 29 patients in the young-age group and 649 patients in the middle-age group. Small patient number will increase the possibility of type II errors. We should enroll more patients of young and young-middle-age groups by cooperation with other HD centers to correct this bias. We did not measure the albumin level in the dialysate to access the level of albumin loss in the HDF process.
CONCLUSION
HDF is a predictor of serum albumin level <4 g/dL in middle-age HD patients. The effect of age needs to be considered when interpreting the prognostic implications between hypoalbuminemia and HDF.
Acknowledgments
We thank the members of the Statistic Center in Chang Gung Memorial Hospital and Hemodialysis Center in Chang Gung Memorial Hospital for their invaluable and dedicated assistance.
Footnotes
Abbreviations: ALP = alkaline phosphatase, AV fistula = arterio-venous fistula, CI = confidence interval, CVDs = cardiovascular diseases, DM = diabetes mellitus, EPO = erythropoietin, HBV = hepatitis B virus, HCV = hepatitis C virus, HD = hemodialysis, HDF = hemodiafiltration, hsCRP = high-sensitivity C-reactive protein, iPTH = intact parathyroid hormone, MHD = maintenance hemodialysis, nPCR = normalized protein catabolism rate, OR = odds ratio, PTH = parathyroid hormone, RRF = residual renal function.
W-HH and T-HY were funded by research grants from the Chang Gung Memorial Hospital, Linkou (CMRPG3D0322, G3D0012) and C-HW was funded by research grants from the Chang Gung Memorial Hospital, Linkou (CMRPG5D0081).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Filiopoulos V, Hadjiyannakos D, Metaxaki P, et al. Inflammation and oxidative stress in patients on hemodiafiltration. Am J Nephrol 2008; 28:949–957. [DOI] [PubMed] [Google Scholar]
- 2.Maduell F, Moreso F, Pons M, et al. High-efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J Am Soc Nephrol 2013; 24:487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carracedo J, Merino A, Nogueras S, et al. On-line hemodiafiltration reduces the proinflammatory CD14+CD16+ monocyte-derived dendritic cells: a prospective, crossover study. J Am Soc Nephrol 2006; 17:2315–2321. [DOI] [PubMed] [Google Scholar]
- 4.Ward RA, Schmidt B, Hullin J, et al. A comparison of on-line hemodiafiltration and high-flux hemodialysis: a prospective clinical study. J Am Soc Nephrol 2000; 11:2344–2350. [DOI] [PubMed] [Google Scholar]
- 5.Ahrenholz PG, Winkler RE, Michelsen A, et al. Dialysis membrane-dependent removal of middle molecules during hemodiafiltration: the beta2-microglobulin/albumin relationship. Clin Nephrol 2004; 62:21–28. [DOI] [PubMed] [Google Scholar]
- 6.Combarnous F, Tetta C, Cellier CC, et al. Albumin loss in on-line hemodiafiltration. Int J Artif Organs 2002; 25:203–209. [DOI] [PubMed] [Google Scholar]
- 7.Vega A, Quiroga B, Abad S, et al. Albumin leakage in online hemodiafiltration, more convective transport, more losses? Ther Apher Dial 2015; 19:267–271. [DOI] [PubMed] [Google Scholar]
- 8.Munoz R, Gallardo I, Valladares E, et al. Online hemodiafiltration: 4 years of clinical experience. Hemodial Int 2006; 10 Suppl 1:S28–S32. [DOI] [PubMed] [Google Scholar]
- 9.Imamovic G, Hrvacevic R, Kapun S, et al. Survival of incident patients on high-volume online hemodiafiltration compared to low-volume online hemodiafiltration and high-flux hemodialysis. Int Urol Nephrol 2014; 46:1191–1200. [DOI] [PubMed] [Google Scholar]
- 10.den Hoedt CH, Bots ML, Grooteman MP, et al. Online hemodiafiltration reduces systemic inflammation compared to low-flux hemodialysis. Kidney Int 2014; 86:423–432. [DOI] [PubMed] [Google Scholar]
- 11.Orasan RA, Patiu IM, Anghel D, et al. Variation of clinical and laboratory features in chronic dialysis patients treated with high-flux hemodialysis after switching to online hemodiafiltration. Int Urol Nephrol 2013; 45:1415–1422. [DOI] [PubMed] [Google Scholar]
- 12.Jean G, Hurot JM, Deleaval P, et al. Online-haemodiafiltration vs. conventional haemodialysis: a cross-over study. BMC Nephrol 2015; 16:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pifer TB, McCullough KP, Port FK, et al. Mortality risk in hemodialysis patients and changes in nutritional indicators: DOPPS. Kidney Int 2002; 62:2238–2245. [DOI] [PubMed] [Google Scholar]
- 14.Kanda E, Bieber BA, Pisoni RL, et al. Importance of simultaneous evaluation of multiple risk factors for hemodialysis patients’ mortality and development of a novel index: Dialysis Outcomes and Practice Patterns Study. PLoS ONE 2015; 10:e0128652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kucirka LM, Grams ME, Lessler J, et al. Association of race and age with survival among patients undergoing dialysis. JAMA 2011; 306:620–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lertdumrongluk P, Lau WL, Park J, et al. Impact of age on survival predictability of bone turnover markers in hemodialysis patients. Nephrol Dial Transplant 2013; 28:2535–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Husten CG. How should we define light or intermittent smoking? Does it matter? Nicotine Tob Res 2009; 11:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taiwan NHRIo. Nutrition and Health Survey in Taiwan; 2010. http://nahsit.nhri.org.tw/node/7 Accessed June 20, 2015. [Google Scholar]
- 19.Yen TH, Lin JL, Lin-Tan DT, et al. Blood cadmium level's association with 18-month mortality in diabetic patients with maintenance haemodialysis. Nephrol Dial Transplant 2011; 26:998–1005. [DOI] [PubMed] [Google Scholar]
- 20.Daugirdas JT. The post: pre-dialysis plasma urea nitrogen ratio to estimate Kt/V and NPCR: mathematical modeling. Int J Artif Organs 1989; 12:411–419. [PubMed] [Google Scholar]
- 21.Sargent JA. Control of dialysis by a single-pool urea model: the National Cooperative Dialysis Study. Kidney Int Suppl 1983; 23:S19–S25. [PubMed] [Google Scholar]
- 22.Kalantar-Zadeh K. Recent advances in understanding the malnutrition-inflammation-cachexia syndrome in chronic kidney disease patients: what is next? Semin Dial 2005; 18:365–369. [DOI] [PubMed] [Google Scholar]
- 23.Lin JL, Lin-Tan DT, Yen TH, et al. Blood lead levels, malnutrition, inflammation, and mortality in patients with diabetes treated by long-term hemodialysis. Am J Kidney Dis 2008; 51:107–115. [DOI] [PubMed] [Google Scholar]
- 24.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39 Suppl 1:S1–S266. [PubMed] [Google Scholar]
- 25.Pupim LB, Caglar K, Hakim RM, et al. Uremic malnutrition is a predictor of death independent of inflammatory status. Kidney Int 2004; 66:2054–2060. [DOI] [PubMed] [Google Scholar]
- 26.Wanner C, Metzger T. C-reactive protein a marker for all-cause and cardiovascular mortality in haemodialysis patients. Nephrol Dial Transplant 2002; 17 Suppl 8:29–32.discussion 39–40. [DOI] [PubMed] [Google Scholar]
- 27.Movilli E, Camerini C, Gaggia P, et al. Total convection affects serum beta2 microglobulin and C-reactive protein but not erythropoietin requirement following post-dilutional hemodiafiltration. Am J Nephrol 2015; 41:494–501. [DOI] [PubMed] [Google Scholar]
- 28.Vilar E, Fry AC, Wellsted D, et al. Long-term outcomes in online hemodiafiltration and high-flux hemodialysis: a comparative analysis. Clin J Am Soc Nephrol 2009; 4:1944–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bolasco P, Altieri P, Sorba G, et al. Adequacy in pre-dilution haemofiltration: Kt/V or infusion volume? The Sardinian Collaborative Study Group on Haemofiltration On-line. Nephrol Dial Transplant 2000; 15 Suppl 2:60–64. [DOI] [PubMed] [Google Scholar]
- 30.Krieter DH, Hackl A, Rodriguez A, et al. Protein-bound uraemic toxin removal in haemodialysis and post-dilution haemodiafiltration. Nephrol Dial Transplant 2010; 25:212–218. [DOI] [PubMed] [Google Scholar]
- 31.Pedrini LA, Cozzi G, Faranna P, et al. Transmembrane pressure modulation in high-volume mixed hemodiafiltration to optimize efficiency and minimize protein loss. Kidney Int 2006; 69:573–579. [DOI] [PubMed] [Google Scholar]
- 32.Canaud B, Barbieri C, Marcelli D, et al. Optimal convection volume for improving patient outcomes in an international incident dialysis cohort treated with online hemodiafiltration. Kidney Int 2015; 88:1108–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chapdelaine I, Mostovaya IM, Blankestijn PJ, et al. Treatment policy rather than patient characteristics determines convection volume in online post-dilution hemodiafiltration. Blood Purif 2014; 37:229–237. [DOI] [PubMed] [Google Scholar]
- 34.Tsai HB, Chen PC, Liu CH, et al. Association of hepatitis C virus infection and malnutrition-inflammation complex syndrome in maintenance hemodialysis patients. Nephrol Dial Transplant 2012; 27:1176–1183. [DOI] [PubMed] [Google Scholar]
- 35.Suda T, Hiroshige K, Ohta T, et al. The contribution of residual renal function to overall nutritional status in chronic haemodialysis patients. Nephrol Dial Transplant 2000; 15:396–401. [DOI] [PubMed] [Google Scholar]
- 36.Vilar E, Wellsted D, Chandna SM, et al. Residual renal function improves outcome in incremental haemodialysis despite reduced dialysis dose. Nephrol Dial Transplant 2009; 24:2502–2510. [DOI] [PubMed] [Google Scholar]
- 37.Yang PY, Lin JL, Lin-Tan DT, et al. Residual daily urine volume association with inflammation and nutrition status in maintenance hemodialysis patients. Ren Fail 2009; 31:423–430. [DOI] [PubMed] [Google Scholar]
- 38.Schiffl H, Lang SM, Fischer R. Effects of high efficiency post-dilution on-line hemodiafiltration or conventional hemodialysis on residual renal function and left ventricular hypertrophy. Int Urol Nephrol 2013; 45:1389–1396. [DOI] [PubMed] [Google Scholar]
- 39.Avram MM, Bonomini LV, Sreedhara R, et al. Predictive value of nutritional markers (albumin, creatinine, cholesterol, and hematocrit) for patients on dialysis for up to 30 years. Am J Kidney Dis 1996; 28:910–917. [DOI] [PubMed] [Google Scholar]
- 40.Huang WH, Lin JL, Lin-Tan DT, et al. Education level is associated with mortality in male patients undergoing maintenance hemodialysis. Blood Purif 2013; 35:316–326. [DOI] [PubMed] [Google Scholar]
- 41.Xu R, Han QF, Zhu TY, et al. Impact of individual and environmental socioeconomic status on peritoneal dialysis outcomes: a retrospective multicenter cohort study. PLoS ONE 2012; 7:e50766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Darmon N, Drewnowski A. Does social class predict diet quality? Am J Clin Nutr 2008; 87:1107–1117. [DOI] [PubMed] [Google Scholar]
- 43.Fournier A, Birmele B, Francois M, et al. Factors associated with albumin loss in post-dilution hemodiafiltration and nutritional consequences. Int J Artif Organs 2015; 38:76–82. [DOI] [PubMed] [Google Scholar]
- 44.den Hoedt CH, Bots ML, Grooteman MP, et al. Clinical predictors of decline in nutritional parameters over time in ESRD. Clin J Am Soc Nephrol 2014; 9:318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]


