Abstract
Ki-67 is considered as one of prime biomarkers to reflect cell proliferation and immunohistochemical Ki-67 staining has been widely applied in clinical pathology. To solve the widespread controversy whether Ki-67 reactivity significantly predicts clinical prognosis of bladder carcinoma (BC), we performed a comprehensive meta-analysis by combining results from different literature.
A comprehensive search was conducted in the Chinese databases of WanFang, China National Knowledge Infrastructure and Chinese VIP as well as English databases of PubMed, ISI web of science, EMBASE, Science Direct, and Wiley online library. Independent studies linking Ki-67 to cancer-specific survival (CSS), disease-free survival (DFS), overall survival (OS), progression-free survival (PFS), and recurrence-free survival (RFS) were included in our meta-analysis. With the cut-off values literature provided, hazard ratio (HR) values between the survival distributions were extracted and later combined with STATA 12.0.
In total, 76 studies (n = 13,053 patients) were eligible for the meta-analysis. It was indicated in either univariate or multivariate analysis for survival that high Ki-67 reactivity significantly predicted poor prognosis. In the univariate analysis, the combined HR for CSS, DFS, OS, PFS, and RFS were 2.588 (95% confidence interval [CI]: 1.623–4.127, P < 0.001), 2.697 (95%CI: 1.874–3.883, P < 0.001), 2.649 (95%CI: 1.632–4.300, P < 0.001), 3.506 (95%CI: 2.231–5.508, P < 0.001), and 1.792 (95%CI: 1.409–2.279, P < 0.001), respectively. The pooled HR of multivariate analysis for CSS, DFS, OS, PFS, and RFS were 1.868 (95%CI: 1.343–2.597, P < 0.001), 2.626 (95%CI: 2.089–3.301, P < 0.001), 1.104 (95%CI: 1.008–1.209, P = 0.032), 1.518 (95%CI: 1.299–1.773, P < 0.001), and 1.294 (95%CI: 1.203–1.392, P < 0.001), respectively. Subgroup analysis of univariate analysis by origin showed that Ki-67 reactivity significantly correlated with all 5 clinical outcome in Asian and European-American patients (P < 0.05). For multivariate analysis, however, the pooled results were only significant for DFS, OS, and RFS in Asian patients, for CSS, DFS, PFS, and RFS in European-American patients (P < 0.05). In the subgroup with low cut-off value (<20%), our meta-analysis indicated that high Ki-67 reactivity was significantly correlated with worsened CSS, DFS, OS, PFS, and RFS on univariate analysis (P < 0.05). For multivariate analysis, the meta-analysis of literature with low cut-off value (<20%) demonstrated that high Ki-67 reactivity predicted shorter DFS, PFS, and RFS in BC patients (P < 0.05). In the subgroup analysis of high cut-off value (≥20%), our meta-analysis indicated that high Ki-67 reactivity, in either univariate or multivariate analysis, significantly correlated with all five clinical outcomes in BC patients (P < 0.05).
The meta-analysis indicates that high Ki-67 reactivity significantly correlates with deteriorated clinical outcomes in BC patients and that Ki-67 can be considered as an independent indicator for the prognosis by the meta-analyses of multivariate analysis.
INTRODUCTION
Bladder carcinoma (BC) ranks top as the most common malignant tumor in urinary system, with an estimate of 74,000 new cases and 16,000 deaths in America and 429,800 new cases and 165,100 deaths in 2012 worldwide, respectively.1,2 Despite the improved therapeutic strategies nowadays, such as transurethral resection or radical cystectomy, there are still an epic number of BC patients suffering from tumor progression and recurrence. In addition, 5-year relative survival trend and prognosis of BC remain obscure and unfavorable.3,4 According to the National Comprehensive Cancer Network Guidelines of 2013, tumor stages, grades, and metastasis are the major factors to define the individual treatment strategies of BC patients.5 However, conventional established gauges seem inferior to provide accurate prediction for the prognosis of BC patients with diverse and complicated tumor backgrounds. Detecting technique of protein molecules is now an availably novel approach to evaluate the prognosis of tumors, which immensely contributes to more comprehensive and individualized treatments for patients in early stages. Consequently, there is a pressing appeal to identify molecular biomarkers to effectively forecast the clinical outcomes for BC patients.
Ki-67 is a nuclear protein which can be detected during the cell cycles of G1, S, G2, and M stages except G06 and now has been widely applied in immunohistochemistry (IHC) to assess the activities of cell proliferation in various cancers. Compared with other well-identified molecular biomarkers, such as p53,7 survivin,8 and so on, Ki-67 is a preferably convenient biomarker for the proliferation status of tumor cells. Recently, several studies continuously reported that Ki-67 is an independent indicator to predict poor clinical outcomes of both no-muscle invasive and muscle invasive BC patients.9–12 Two studies featuring large sample sizes of tissues, by Margulis et al13 and Wang et al,14 respectively, have concluded that Ki-67 reactivity correlated significantly with poor cancer-specific survival (CSS) and recurrence-free survival (RFS) of BC patients. Nevertheless, there were also studies demonstrating contradictory results.9,15 Considering the controversy of literature, we performed the current comprehensive meta-analysis, which combined results from different literature to confirm whether Ki-67 reactivity is an independent indicator to predicting the prognosis of BC patients.
MATERIALS AND METHODS
Literature Search and Selection
A comprehensive literature search was completed in the Chinese databases of WanFang, China National Knowledge Infrastructure and Chinese VIP as well as English databases of PubMed, ISI web of science, EMBASE, Science Direct, and Wiley online library with closing date marked on September 15th, 2015. Literature were identified by the combination of the following keywords Ki67 OR Ki-67 OR MKi-67 OR MIB1 OR MIB-1 OR proliferation index OR PI OR proliferation activity OR proliferation marker OR mitotic index OR mitotic figure OR mitotic activity OR mitotic count OR labeling index OR LI OR MI; bladder; tumor OR cancer OR carcinoma OR neoplas∗ OR malignan∗; and prognos∗ OR predict OR surviv∗ OR follow-up OR outcome OR mortality.
Two independent investigators (YL and HZ) screened eligible studies by the same multistep procedures. First, investigators reviewed the titles and abstracts of the identified literature prudently. Studies which explored the relationship between Ki-67 reactivity and clinical outcomes of BC patients were deemed to be eligible. Second, full texts of the eligible literature were carefully reviewed and assessed according to the following inclusion criteria: literature must detect the reactivity of Ki-67 by IHC in BC tissues and evaluate the correlation of Ki-67 reactivity with survival outcome of BC patients, and were published in either Chinese or English; hazard ratios (HRs) and its 95% confidence interval (CI) for prognostic marker were provided by authors or could be calculated from available data presented in articles. Accordingly, the following circumstances might result in exclusion for the current meta-analysis: articles investigated the clinical outcomes of BC patients by the combination of Ki-67 with other immunohistochemical marker(s); animals, cell lines, or laboratory studies were not suitable for the meta-analysis; reviews, letters, and case reports were also excluded; and literature failed to estimate HR and its 95%CI by the information offered. Moreover, we tried to contact the authors when survival analysis was conducted in the articles but insufficient data were supplied for HR and 95%CI calculation. To avoid duplications, literature with the most complete data for analysis should be included if the same or overlapping cohorts were used in different studies or the same article published in different journals. Finally, the interesting articles remained were included in our meta-analysis as well. Controversies were solved by discussing with a 3rd investigator (GC).
Data Extraction
The information of the included studies was extracted carefully by 2 independent researchers (YL and CH) with the same criteria and standardized table. The following data from literature were extracted: first author, published year, number of patients, median or mean age of the patients, fold of antibody dilution, whether blind reading or not, cut-off value, tumor stage, treatments for patients, follow-up period, and data for survival analysis. No quality assessment was performed for each study by any scale, since there existed no universally acknowledged quality scoring systems for meta-analyses of follow-up studies.16 Controversial viewpoints were solved by discussion with a 3rd investigator (CG).
Statistical Methods
The HR values were extracted from each study, later synthesized into a meta-analysis, and used to estimate the risk of high Ki-67 reactivity on patients’ survival. For each study, we extracted the survival data with the method by Tierney et al.17 Survival data were either drawn directly or calculated by the calculator of Review Manager 5.3 if required data were available to estimate the HR values and its 95%CI. For the literature only with survival curves, the software, Engauge Digitizer version 4.1 (http://digitizer.sourceforge.net/), was in the charge of calculation. When an HR > 1 and its 95%CI ≤ 1, it indicated that high Ki-67 reactivity was associated with poor survival outcomes in BC patients.
Heterogeneity test for studies included was conducted by χ2 and inconsistency (I2) test, setting a significant level at 0.05% and 50%.18,19 To take all heterogeneity, such as different cut-off value and/or methods of measurement, into consideration, random-effect model was employed to calculate the combined HRs.20–22 Subsequently, subgroup analysis was employed to further analyze the associations of Ki-67 reactivity with clinical outcomes in patients from different origins and under different cut-off values. We have divided the studies into Asian or European-American group by origins and divided the studies into high or low cut-off group with a “cut-off” value of 20%, which were used to classify Ki-67 reactivity in 2 large samples studies published by Margulis et al and Wang et al, respectively.13,14 Sensitivity analysis was conducted to examine the stability of meta-analysis. Moreover, Begg test and funnel plots would be used to examine the publication bias if the numbers of studies were above 10.23 If significant publication bias was detected, trim and fill method would be applied to retrieve “missing” literature.24 The statistical processes were all conducted by STATA version 12.0, and a 2-sided P ≤ 0.05 was considered statistically significant. The current study is a meta-analysis and all the data were extracted from published literature, and therefore ethical approval was waived for this meta-analysis. All the procedures of this meta-analysis were performed according to PRISMA guidelines.25
RESULTS
Summary of Literature’ Characteristics
The search strategy of the current meta-analysis identified a total of 3682 literature, including 2825 studies form English databases and 857 studies from Chinese databases. Overall, 3241 records, identified irrelevant by title and abstract screening, were excluded; and the remained 441 records, which investigated the relationship between Ki-67 reactivity and survival outcomes of BC patients, were evaluated in full text. According to our inclusion criteria, 76 studies (n = 13,053 patients),9,13–15,26–96 published from 1997 to 2015, were eligible and eventually included in our meta-analysis for analysis of the correlation between Ki-67 reactivity and clinical prognosis of BC patients (Figure 1).
FIGURE 1.
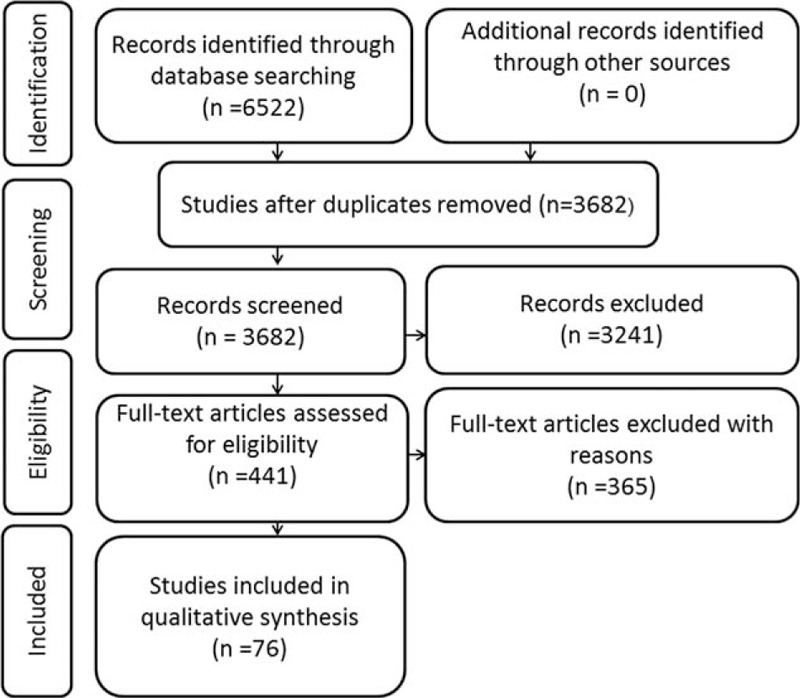
Flow diagram of literature search and selection.
Tables 1 and 2 summarized the main characteristics of the included studies that analyzed the relationships between Ki-67 reactivity and clinical outcome by univariate analysis and multivariate analysis, respectively. For the patient cohorts among 76 studies, 35 were from Asia, 32 from Europe, and 9 from North America. The number of cohorts of each study ranged from 31 to 993, and all the studies detected Ki-67 reactivity by IHC, with an antibody dilution varied from 1:800 to 1:10. Thirty-seven studies assessed the status of immunostaining by pathologist(s) who was/were blind to the clinical outcomes, while another 39 studies did not report whether blind reading was adopted or not. The cut-off value to distinguish high Ki-67 reactivity from low Ki-67 reactivity was set from 5% to 55%. The clinical stage was Ta-T1 in 36 studies and another 40 studies contained tumor stage in T2-T4 stage. As shown in Tables 1 and 2 , the main treatments for BC patients were transurethral resection, radical cystectomy or partial cystectomy, and intravesical chemotherapy. The total follow-up periods range from 12 to 266.8 months. Overall, 45 studies provided data by the calculation of univariate analysis, including 13 for CSS, 5 for disease-free survival (DFS), 12 for overall survival (OS), 21 for progression-free survival (PFS), and 23 for RFS. Fifty-eight studies presented results by the calculation of multivariate analysis, including 18 for CSS, 6 for DFS, 14 for OS, 23 for PFS, and 35 for RFS.
TABLE 1.
Main Characteristics of the Included Studies Estimated Prognosis of High Ki-67 Analyzed by Univariate Analysis
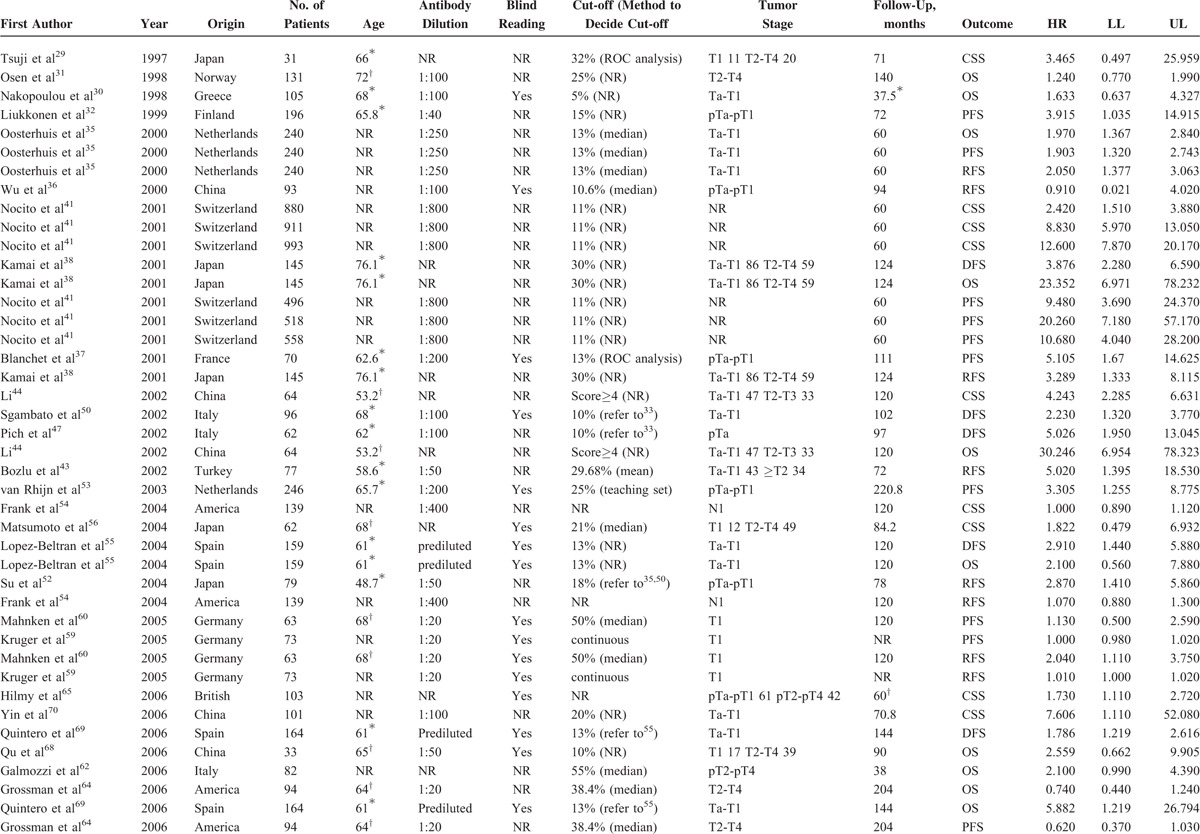
TABLE 1 (Continued).
Main Characteristics of the Included Studies Estimated Prognosis of High Ki-67 Analyzed by Univariate Analysis
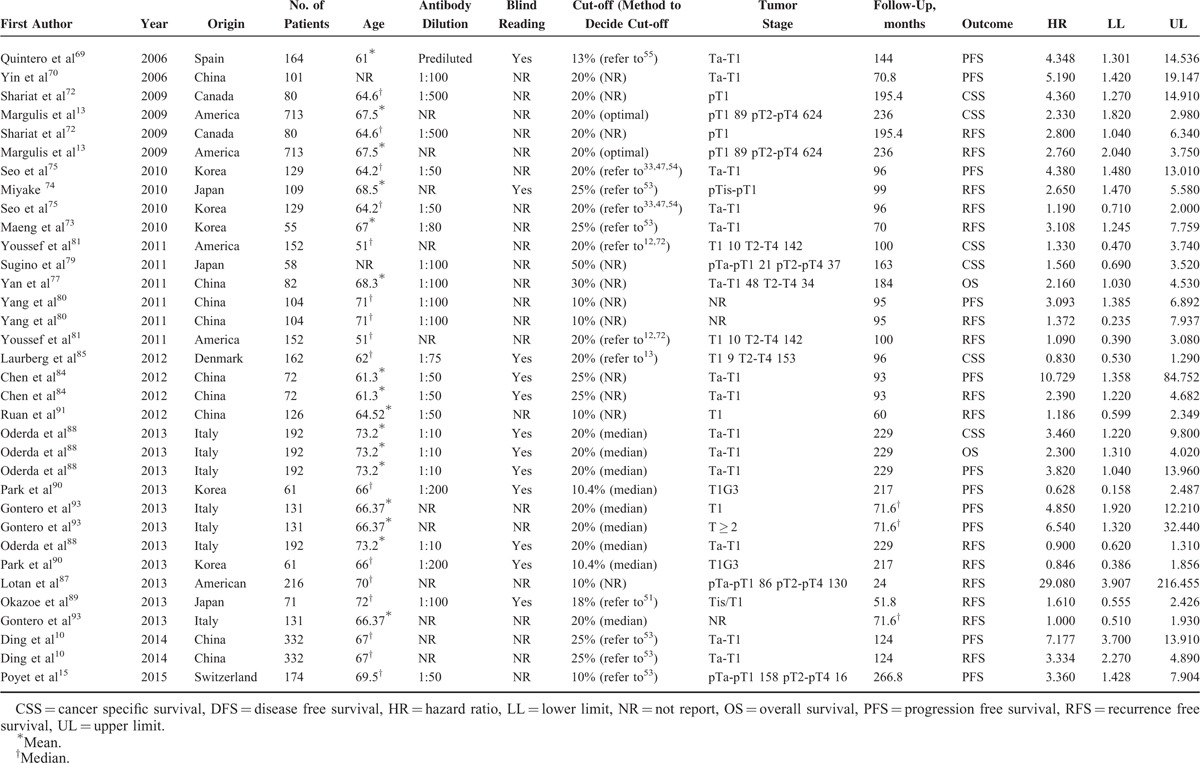
Univariate Analysis of Survival Status
A total of 45 studies (n = 10,083 patients) of univariate analysis for survival status were available for our meta-analysis (Table 3), including 13 studies (n = 4641 patients) for CSS, 5 studies (n = 626 patients) for DFS, 12 studies (n = 1491) for OS, 21 studies (n = 4145) for PFS, and 23 studies (n = 3452) for RFS. Our meta-analysis demonstrated that high Ki-67 reactivity significantly correlated with worsened CSS, DFS, OS, PFS, and RFS in BC patients. The combined HRs were 2.588 (95%CI: 1.623–4.127, P < 0.001) (Figure 2A), 2.697 (95%CI: 1.874–3.883, P < 0.001) (Figure 2B), 2.649 (95%CI: 1.632–4.300, P < 0.001) (Figure 2C), 3.506 (95%CI: 2.231–5.508, P < 0.001) (Figure 2D), and 1.792 (95%CI: 1.409–2.279, P < 0.001) (Figure 2E) for CSS, DFS, OS, PFS, and RFS, respectively (Table 3). Nevertheless, significant heterogeneity existed in all meta-analyses of CSS (P < 0.001, I2 = 94.0%), DFS (P = 0.090, I2 = 50.2%), OS (P < 0.001, I2 = 80.2%), PFS (P < 0.001, I2 = 90.2%), and RFS (P < 0.001, I2 = 85.8%). Thus, it was urgently required to find the main causes of heterogeneity. Consequently, subgroup analysis was performed in accordance with origins of patients and cut-off values to reveal the main factors contributing to significant heterogeneity.
TABLE 2.
Main Characteristics of the Included Studies Estimated Prognosis of High Ki-67 Analyzed by Multivariate Analysis
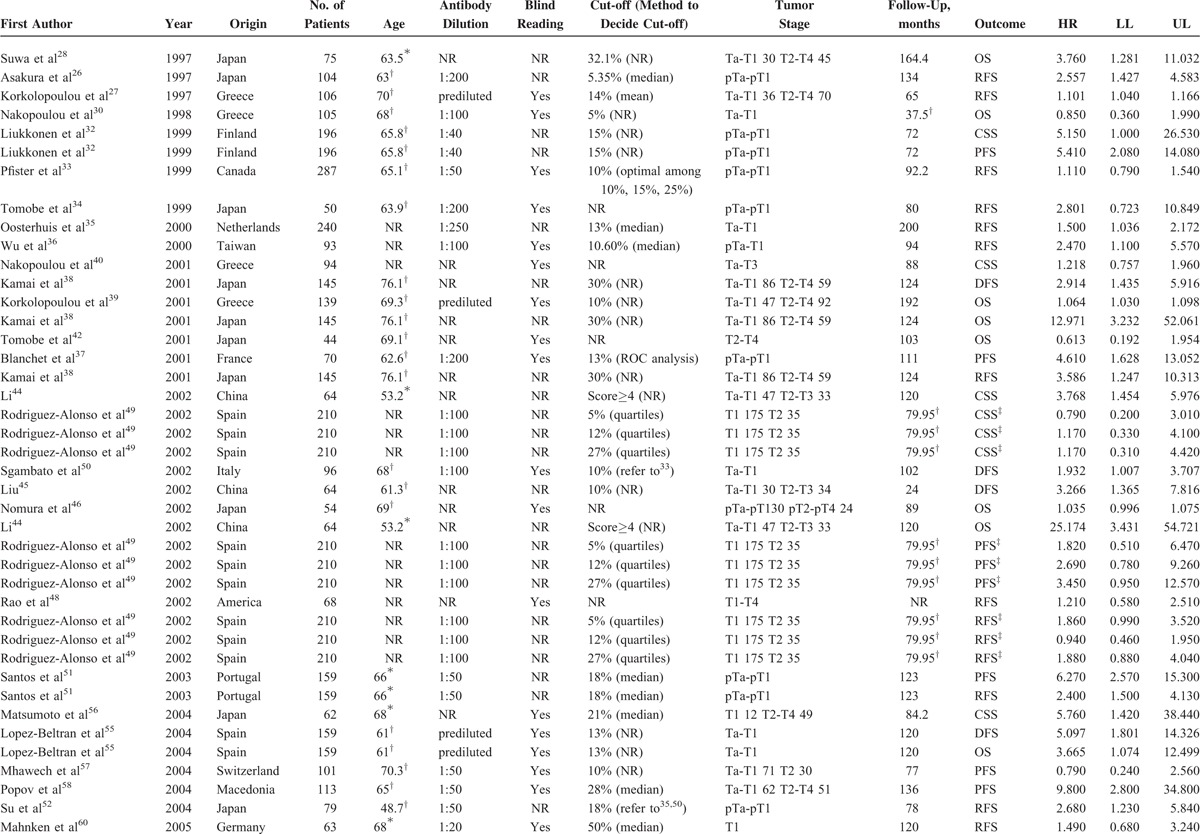
FIGURE 2.

Meta-analyses of studies estimating the correlation of Ki-67 reactivity with survival status by univariate analysis. (A) CSS, (B) DFS, (C) OS, (D) PFS, and (E) RFS. CSS = cancer-specific survival, DFS = disease-free survival, OS = overall survival, PFS = progression-free survival, RFS = recurrence-free survival.
TABLE 3.
Meta-Analysis of Single HR Evaluating the Prognostic Function of Ki-67 Reactivity in BC Patients
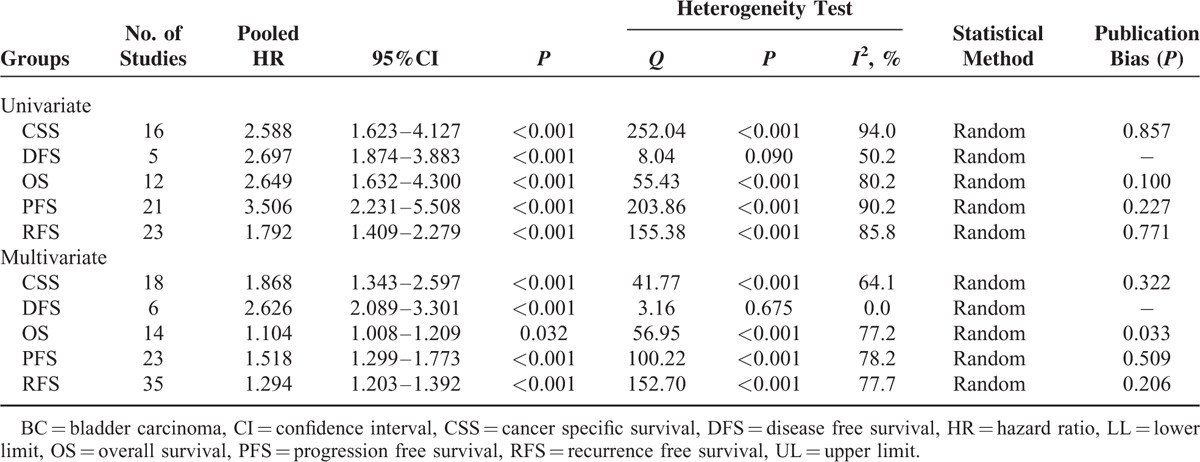
Subgroup analysis by origin indicated that high Ki-67 reactivity was significantly associated with poor CSS (HR = 2.930, 95%CI: 1.687–5.089, P < 0.001), DFS (HR = 3.867, 95%CI: 2.280–6.590), OS (HR = 7.691, 95%CI: 1.832–32.294, P = 0.005), PFS (HR = 3.868, 95%CI: 1.983–7.543, P < 0.001), and RFS (HR = 2.065, 95%CI: 1.526–2.793, P < 0.001) in Asian patients. For European-American patients, the similar results were observed for CSS (HR = 2.930, 95%CI: 1.687–5.089, P < 0.001), DFS (HR = 2.373, 95%CI: 1.649–3.416, P < 0.001), OS (HR = 1.653, 95%CI: 1.161–2.353, P < 0.001), PFS (HR = 3.377, 95%CI: 2.040–5.589, P < 0.001), and RFS (HR = 1.528, 95%CI: 1.134–2.060, P < 0.001). Subgroup analysis by cut-off value demonstrated that high Ki-67 reactivity was significantly associated with deteriorated CSS, DFS, OS, PFS, and RFS regardless of high (<20%)/low cut-off (≥20%) value cut-off values (P < 0.05, Table 4).
TABLE 2 (Continued).
Main Characteristics of the Included Studies Estimated Prognosis of High Ki-67 Analyzed by Multivariate Analysis
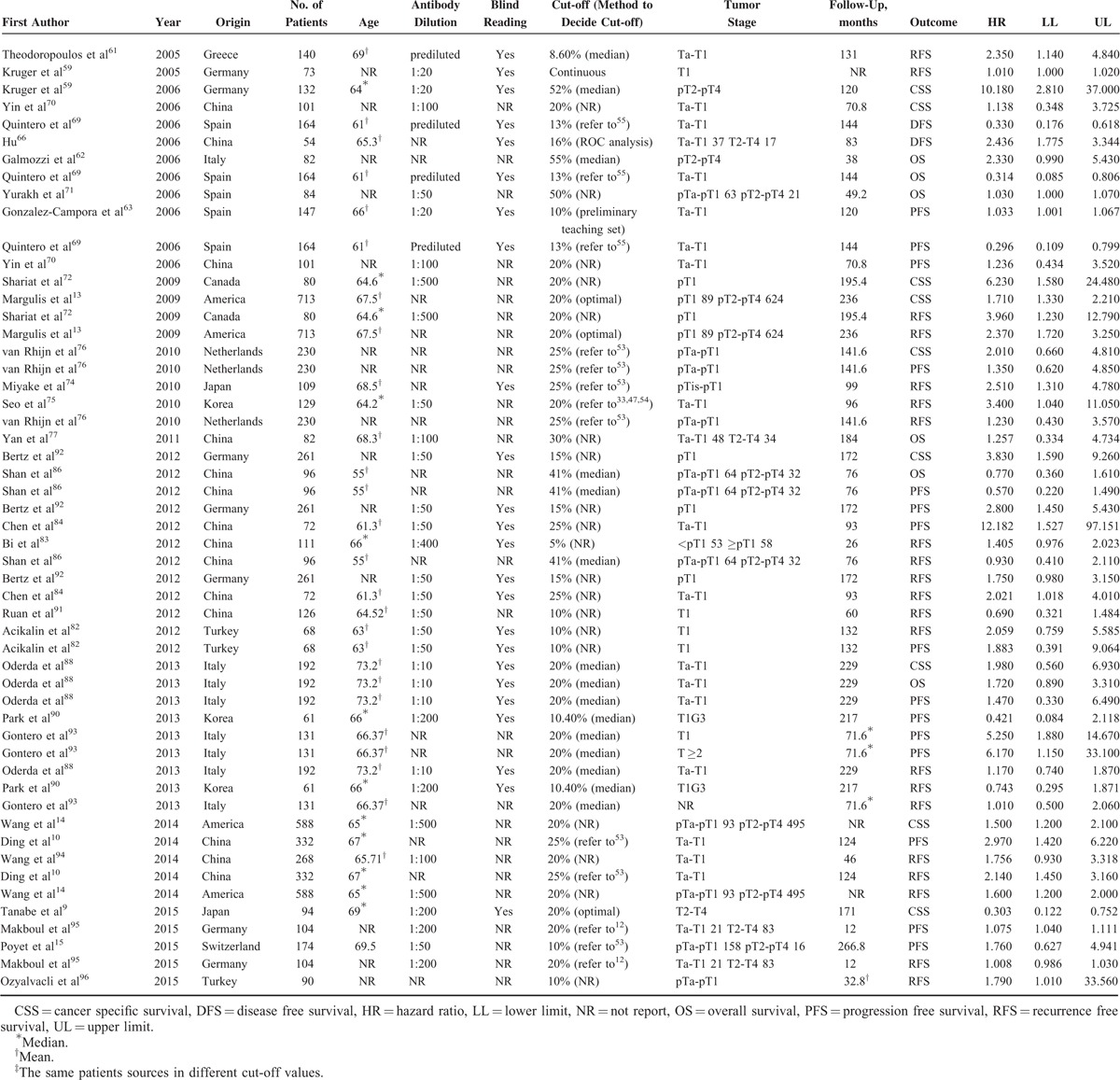
TABLE 4.
Subgroup Analysis Evaluating the Prognostic Function of Ki-67 Reactivity in BC Patients
Multivariate Analyses of Survival Status
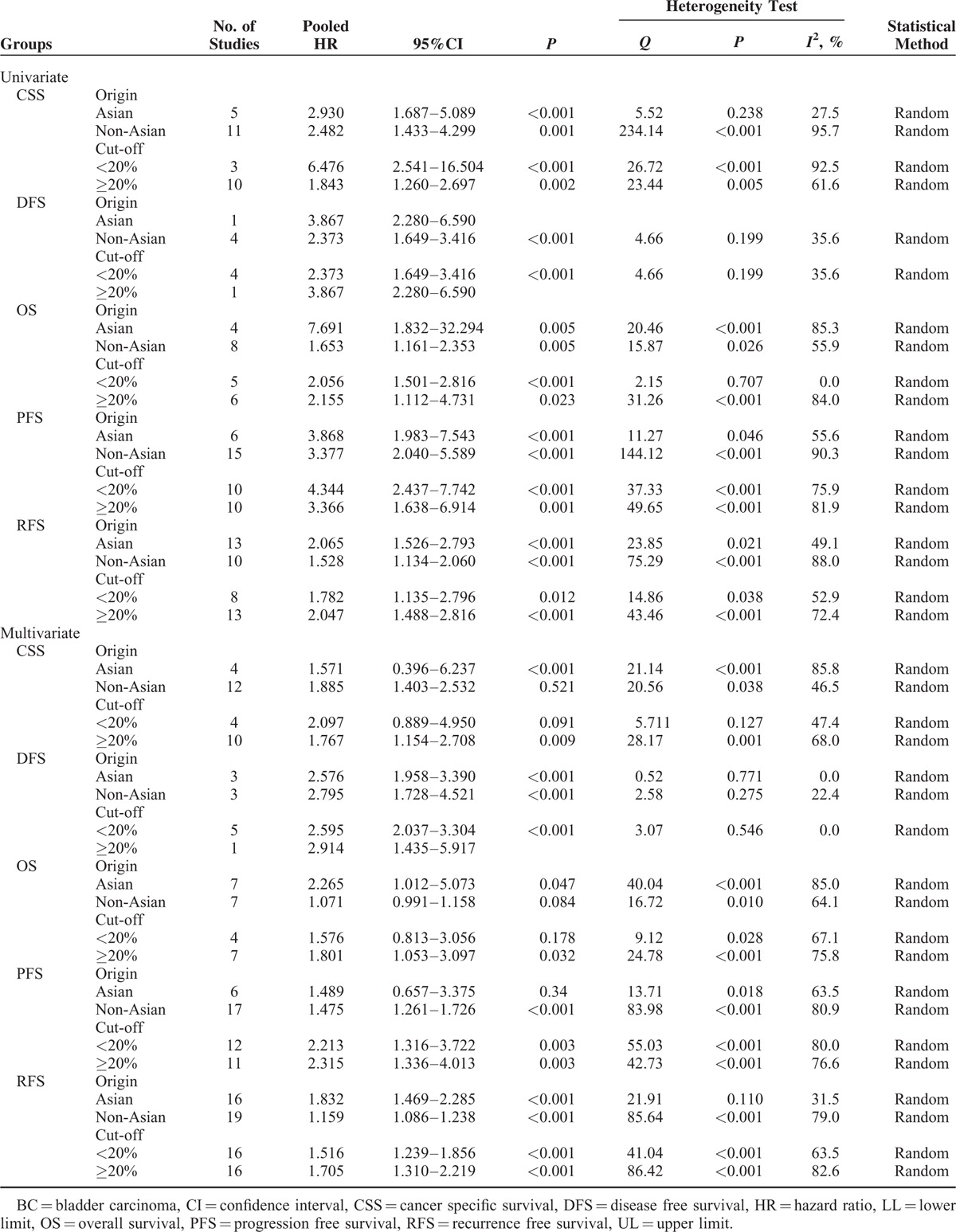
For the meta-analyses of multivariate analysis, 58 studies with 8128 patients enrolled were included. The concomitant variables corrected in the multivariate analysis of each study have list in Table S1 (Additional file 2). Our meta-analysis represented that high Ki-67 reactivity was still significantly associated with shorter CSS 1.868 (95%CI: 1.343–2.597, P < 0.001) (Figure 3A), DFS (HR = 2.626, 95%CI: 2.089–3.301, P < 0.001) (Figure 3B), OS (HR = 1.104, 95%CI: 1.008–1.209, P = 0.032) (Figure 3C), PFS (HR = 1.518, 95%CI: 1.299–1.773, P < 0.001) (Figure 3D), and RFS (HR = 1.294, 95%CI: 1.203–1.392, P < 0.001) (Figure 3E) after adjusting concomitant variables. Significant heterogeneity emerged in CSS (I2 = 64.1%, P < 0.001), OS (I2 = 77.2%, P < 0.001), PFS (I2 = 78.2%, P < 0.001), and RFS (I2 = 77.7%, P < 0.001) but not DFS (I2 = 0.0%, P = 0.675). Identically, we conducted subgroup analyses in accordance with origins of patients and cut-off values.
FIGURE 3.
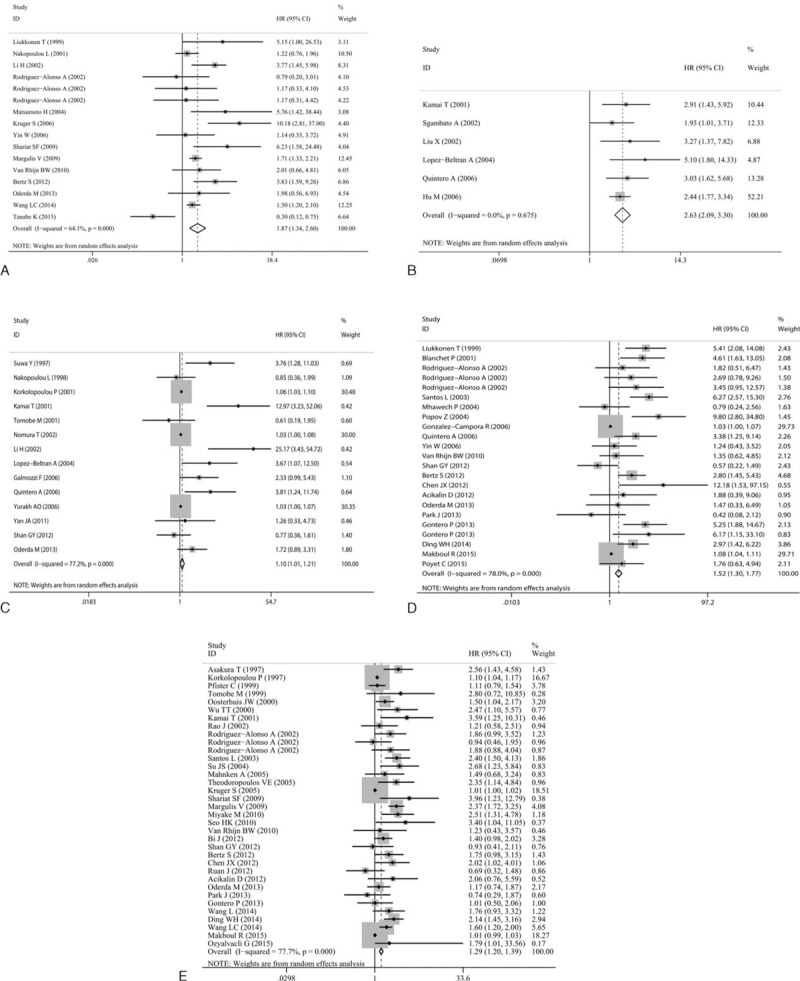
Meta-analyses of studies estimating the correlation of Ki-67 reactivity with survival status by multivariate analysis. (A) CSS, (B) DFS, (C) OS, (D) PFS, and (E) RFS. CSS = cancer-specific survival, DFS = disease-free survival, OS = overall survival, PFS = progression-free survival, RFS = recurrence-free survival.
The results of subgroup analyses demonstrated that, in Asian patients, high Ki-67 reactivity significantly correlated with poorer DFS (HR = 2.576, 95%CI: 1.958–3.390, P < 0.001), OS (HR = 2.265, 95%CI: 1.012–5.073, P = 0.047), and RFS (HR = 1.832, 95%CI: 1.469–2.285, P < 0.001) but not for CSS (HR = 1.571, 95%CI: 0.396–6.237, P = 0.521) and PFS (HR = 1.489, 0.657–3.375, P = 0.34). For European-American patients, significant results were observed in the meta-analyses for CSS (HR = 1.648, 95%CI: 1.279–2.122, P < 0.001), DFS (HR = 2.744, 95%CI: 1.813–4.153, P < 0.001), PFS (HR = 1.475, 95%CI: 1.261–1.726, P < 0.001), and RFS (HR = 1.201, 95%CI: 1.120–1.289, P < 0.001) (Table 4). In terms of both low and high cut-off values to assess high Ki-67 reactivity, subgroup analysis uncovered the significant correlation of high Ki-67 reactivity with worse DFS, PFS, and RFS (P < 0.05, Table 4). For CSS and OS, no significant correlation was observed between Ki-67 reactivity and clinical outcomes when the cut-off value was <20% (HR = 2.097, 95%CI: 0.889–4.950, P = 0.091; HR = 1.576, 95%CI: 0.813–3.056, P = 0.178). However, an increased cut-off value (≥20%) would lead to statistically significant results (HR = 1.767, 95%CI: 1.154–2.708, P = 0.009; HR = 1.801, 95%CI: 1.053–3.097, P = 0.032) (Table 4).
Sensitivity Analysis and Publication Bias
Sensitivity analysis was performed to examine the stability of the current meta-analysis by removing 1 study each time and later pooling the rest. The result of sensitivity analysis indicated that the study published by Frank et al54 in CSS and Kruger et al59 in PFS of univariate analysis were not stable and significantly influenced the pooled HR (Additional file 1: Figures S1–S2). After excluding the study by Frank et al54 and Kruger et al,59 the meta-analyses of the remained studies were stable (Additional file 1: Figures S3–S4). The pooled HRs (random effect model) for CSS of univariate analysis changed from 2.588 (95%CI: 1.623–4.127, P < 0.001) to 2.798 (95%CI: 1.783–4.391, P < 0.001), and the pooled HR (random effect model) for PFS of univariate analysis altered from 3.084 (95%CI: 1.962–4.846, P < 0.001) to 3.331 (95%CI: 2.068–5.365, P < 0.001). For the others meta-analyses, the combined HRs were similar after the exclusion of studies, with the stability of meta-analyses confirmed.
Begg test and funnel plot were conducted to estimate the publication bias of the included studies. For univariate analysis, the results of Begg test and the shape of funnel plot presented us with no significant publication bias for CSS (P = 0.857), OS (P = 0.100), PFS (P = 0.227), and RFS (P = 0.771) (Table 3, Figure 4A–D). As to multivariate analysis, no evidence of publication bias for meta-analyses (P > 0.05) was observed except for OS (P = 0.033) (Table 3, Figure 5A–D). After refilling “missing” studies by trim and fill method, the adjusted pooled HR was not significant for OS (HR = 1.058, 95%CI: 0.940–1.191, P = 0.347) (Additional file 1: Figure S5).
FIGURE 4.
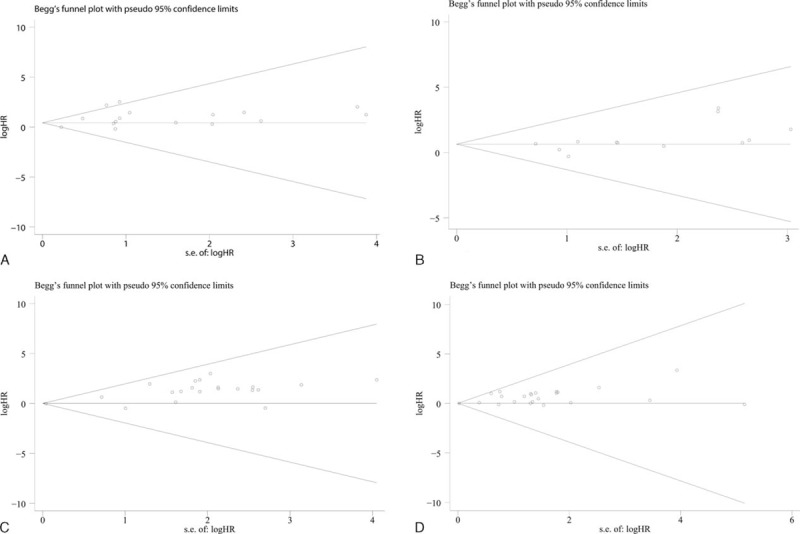
Funnel plots were applied to show potential publication bias in studies estimating the correlation of Ki-67 reactivity with survival status by univariate analysis. (A) CSS, (B) OS, (C) PFS, and (D) RFS. CSS = cancer-specific survival, OS = overall survival, PFS = progression-free survival, RFS = recurrence-free survival.
FIGURE 5.
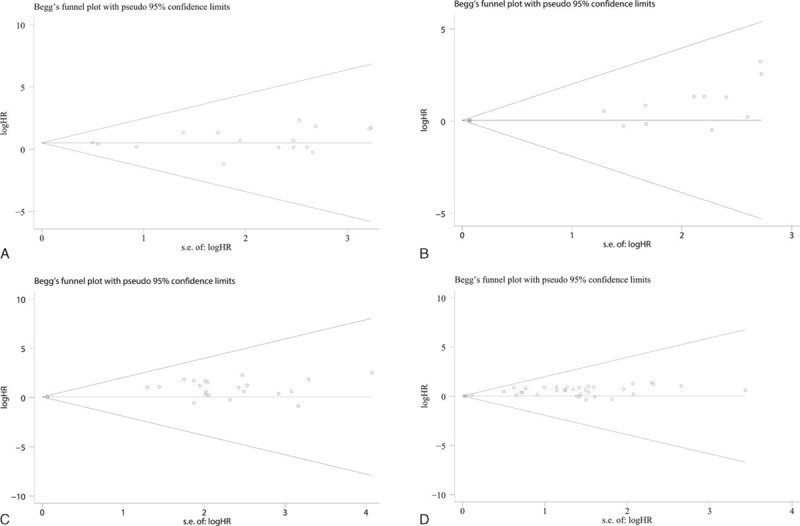
Funnel plots were applied to show potential publication bias in studies estimating the correlation of Ki-67 reactivity with survival status by multivariate analysis. (A) CSS, (B) OS, (C) PFS, and (D) RFS. CSS = cancer-specific survival, OS = overall survival, PFS = progression-free survival, RFS = recurrence-free survival.
Systematic Review
According to the criteria of our meta-analysis, some studies, which investigated the relationship between Ki-67 reactivity and survival status of BC patients, were excluded for failing to estimate HRs and its 95% CIs. The main information of 23 excluded studies97–119 was listed in Table S2 (Additional file 2), including 5 studies for CSS, 5 for DFS, 9 for OS, 9 for PFS, and 10 for RFS. For all 5 survival outcome (CSS, DFS, OS, RFS, and PFS), it can be concluded from 5 studies97,101,109–111 that Ki-67 is an independent indicator to predict prognosis by multivariate analysis, while 7 studies104,105,110,112,113,116,117 reported negative results.
DISCUSSION
Ki-67 is among the well-established proliferation biomarkers, which can be detected by monoclonal antibodies of Ki-67 with IHC.120 Recently, proliferation biomarkers have attracted increasing attention in malignant neoplasms as prognostic indicators. A meta-analysis has demonstrated that Ki-67, MI, proliferating cell nuclear antigen, and thymidine or bromodeoxyuridine labeling index (LI) are significantly associated with worse survival outcomes in early-staged breast cancer.121 Significant correlations of proliferating cell nuclear antigen with poor outcomes were also discovered in BC.27,39,122 It is also reported that minichromosome maintenance 2, a prerequisite protein for initiation and elongation of DNA, is an independent prognostic factor for OS in gastric cancer and nonsmall-cell lung cancer.123,124 In addition, several meta-analyses claimed that Ki-67 exerts its vital influence on the prognosis of hepatocellular carcinoma, breast cancer, lung cancer, upper urinary-tract urothelial carcinomas (UTUC), cervical cancer, gliomas, and lymphoma.125–133 However, conflicting conclusions exist as to the prognostic role of Ki-67 reactivity in BC patients. Considering that a single study might seem unconvincing, we performed the current meta-analysis by combining the results from multiple studies to reach a reasonable conclusion.
The prognostic value of Ki-67 reactivity for tumors in urinary system has been reported in previous meta-analysis published by Lei et al,134 which demonstrated that high Ki-67 reactivity displays significantly higher cancer-specific mortality and shorter 5-year DFS and OS in UTUC. Compared with the previous meta-analysis for UTUC,134 our meta-analysis highlighted a larger cohort of patients and a more comprehensive report for clinical outcomes. Our results have drawn a similar conclusion that high Ki-67 reactivity seems to be an unexceptionable biomarker to predict an unfavorable prognosis for patients with urinary system tumors. The results of univariate analysis strongly supported that Ki-67 reactivity, assessed by IHC, is significantly associated with worse CSS (HR = 2.588, 95%CI: 1.623–4.127), DFS (HR = 2.697, 95%CI: 1.874–3.883), OS (HR = 2.649, 95%CI: 1.632–4.300), PFS (HR = 3.506, 95%CI: 2.231–5.508), and RFS (HR = 1.792, 95%CI: 1.409–2.279). After adjusting the effect of sex, age, tumor stage, and grade as well as other biological variables by multivariate analysis, the pooled HRs were slightly declined but still significant for CSS 1.868 (95%CI: 1.343–2.597), DFS (HR = 2.626, 95%CI: 2.089–3.301), PFS (HR = 1.518, 95%CI: 1.299–1.773), and RFS (HR = 1.294, 95%CI: 1.203–1.392). For multivariate analysis of OS, the pooled HR was of inferior significance with a narrower 95% CI (HR = 1.104, 95%CI: 1.008–1.209), but no significant results were observed after refilling 5 missed studies estimated by trim and fill method (HR = 1.058, 95%CI: 0.940–1.191).
Between-study heterogeneity may be a potential factor which influenced the pooled results, and significant heterogeneity was observed in the major meta-analyses. Although we have established rigorous inclusion criteria, such as that all the included studies should detect Ki-67 reactivity by IHC, there were still a few substantial differences among included studies, which might influence the pooled results. First, sufficient follow-up period is one of the vital factors for a prospective study, but not all the included studies were strictly designed to suit the condition. A case in point would be that 1 study included subjected to a follow-up period of merely 12 months.95 Second, the tumor staging systems adopted for each study were also different, which might swing the clinical outcomes, since patients with more advanced stages are more vulnerable to unfavorable prognosis. Third, the most accurate HR should be calculated by multivariate analysis with original data. In the current meta-analysis, however, some studies failed to provide results of multivariate analysis. Hence, HR values would need to be extracted the from survival curves whenever possible, which is less reliable than data directly provided in the articles. Fourth, the differences in adjusting concomitant variables of each study might also contribute to heterogeneity, though we have pooled the multivariate HRs. Apart from the circumstances mentioned above, there might be other factors, such as scales of the cohort, ages of patients, dilution folds of antibody, and treatment methods, which would contribute to heterogeneity, so a random-effect model was employed to combine the HR values to take all the between-study heterogeneity into consideration.
Subgroup analysis by origin showed that Ki-67 reactivity significantly correlated with unfavorable clinical outcomes in Asian and European-American patients in the univariate analysis. However, after adjusting concomitant variables, such as sex, age, tumor stage, and so on, different impacts of Ki-67 reactivity on clinical outcomes emerged in BC patients of different races. This meta-analysis demonstrated that the impacts of Ki-67 reactivity on survival status vary considerably among Asian patients and European-American patients, which implies that Ki-67 reactivity might exert different effects on tumor variations in patients with diverse ethnic backgrounds. Further perspective researches among different ethnicities would be required to confirm the aforementioned hypothesis.
The cut-off values to define “high” Ki-67 reactivity also differed slightly among studies because of the lack of unified standards, which might potentially contribute to heterogeneity. Apart from this, using optimal cut-points might lead to discrepancy and influence the accurate HRs.135 In the current meta-analysis, we set a threshold at 20% to define low or high cut-off values according to 2 large sample studies published by Margulis et al and Wang et al, respectively.13,14 In the subgroup of low cut-off value (<20%), the meta-analysis identified that high Ki-67 reactivity significantly correlated with worsened CSS, DFS, OS, PFS, and RFS in univariate analysis and was associated with shorter CSS, DFS, PFS, and RFS in multivariate analysis. As to the subgroup analysis of high cut-off value (≥20%), it was well indicated that high Ki-67 reactivity, regardless of univariate or multivariate analysis, was significantly related to all 5 clinical outcomes in BC patients. Therefore, according to the results of our meta-analysis, a higher cut-off value (≥20%) might be more appropriate and a well-established, orthodox threshold would be required to define high Ki-67 reactivity.
Publication bias is also a potential factor which influenced the pooled results. To minimize publication bias, we have conducted a comprehensive search and screening in different databases in English and Chinese. The conflicting conclusions of different literature may impel investigators to publish their research data, whether positive or negative, which will partially limit publication bias. However, publication bias was still detected in the studies of multivariate analysis for DFS and OS. The fact that not all relevant reports were retrieved might attribute to the mentioned bias, since quite a few studies were only included in systematic review due to the lack of proper data to estimate HR. Hence, in order to make results more creditable and reliable, trim and fill method was used for “missing” studies retrieving to minimize publication bias.
The meta-analysis featured several strengths, which are of great significance to clinical practices. First, we have conducted a comprehensive meta-analysis with a relatively large cohort size included (n = 13,053 patients). Second, results of univariate and multivariate analyses were carefully studied and similar results of associations between Ki-67 reactivity and clinical outcomes were observed, which enhanced the reliability of our meta-analysis. Moreover, we have investigated 5 clinical outcomes of tumor prognosis in multiple dimensions, including CSS, DFS, OS, PFS, and RFS, and the results strongly indicated that high Ki-67 reactivity is significantly associated with poor clinical outcomes. Therefore, it is of primal importance to secure patients’ access to personalized treatments in accordance with their individual conditions. A case in point would be for high-risk patients, especially those with high Ki-67 expression, to receive postoperative chemo-/radiotherapy. Meanwhile, the meta-analysis has a few limitations. To begin with, heterogeneity and publication bias still existed in the meta-analysis, which unfavorably influenced the results. Besides, the estimated HRs were extracted from survival curves when the accurate data were not immediately available in articles, which could be easily influenced by investigators despite the same methods applied.
Enlightened by the data extracted and results of the meta-analysis, we would like to kindly provide forthcoming researchers with the following recommendations: provide the full information of patients, such as age, tumor stage, and so on; use blind-reading and a well-received common cut-off value to assess the results of IHC; set aside a more sufficient follow-up period to allow for long-range outcomes; provide the results of univariate and multivariate analysis whether they are positive or not; and design the studies as prospective ones in the setting.
To conclude, it is demonstrated, by the current comprehensive meta-analysis on 82 studies with 13,053 patients involved, that high Ki-67 reactivity is significantly associated with deteriorated clinical outcomes, and that Ki-67 is an independent indicator for the prognosis of BC patients. Prospective studies with a large cohort of patients are demanded to further strengthen our findings on the correlation between Ki-67 and prognosis in BC patients.
Supplementary Material
Acknowledgments
The authors thank the Fund of Guangxi Zhuang Autonomous Region University Student Innovative Plan (No. 201410598003), Guangxi Medical University “Future Academic Star” Research Project (WLXSZX16001), Guangxi Provincial Health Bureau Scientific Research Project (Z2014054), Youth Science Foundation of Guangxi Medical University (GXMUYSF201311), Guangxi University Science and Technology Research Projects (LX2014075), and the Fund of National Natural Science Foundation of China (NSFC81360327) for the support.
Footnotes
Abbreviations: BC = bladder carcinoma, CI = confidence interval, CSS = cancer-specific survival, DFS = disease-free survival, HR = hazard ratio, IHC = immunohistochemistry, MI = mitotic index, OS = overall survival, PFS = progression-free survival, RFS = recurrence-free survival, UTUC = upper urinary-tract urothelial carcinomas.
The study was partly supported by the Fund of Guangxi Zhuang Autonomous Region University Student Innovative Plan (No. 201410598003), Guangxi Medical University “Future Academic Star” Research Project (WLXSZX16001), Guangxi Provincial Health Bureau Scientific Research Project (Z2014054), Youth Science Foundation of Guangxi Medical University (GXMUYSF201311), Guangxi University Science and Technology Research Projects (LX2014075), and the Fund of National Natural Science Foundation of China (NSFC81360327).
The funders had no role in study design, data collection and analysis, preparation of the manuscript, or decision to publish.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Siegel RL, Fedewa SA, Miller KD, et al. Cancer statistics for Hispanics/Latinos, 2015. CA Cancer J Clin 2015; 65:457–480. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65:87–108. [DOI] [PubMed] [Google Scholar]
- 3.Cho KS, Seo HK, Joung JY, et al. Lymphovascular invasion in transurethral resection specimens as predictor of progression and metastasis in patients with newly diagnosed T1 bladder urothelial cancer. J Urol 2009; 182:2625–2630. [DOI] [PubMed] [Google Scholar]
- 4.Schmid SC, Zahel T, Haller B, et al. Prognostic value of computed tomography before radical cystectomy in patients with invasive bladder cancer: imaging predicts survival. World J Urol 2016; 34:569–576. [DOI] [PubMed] [Google Scholar]
- 5.Clark PE, Agarwal N, Biagioli MC, et al. Bladder cancer. J Natl Compr Canc Netw 2013; 11:446–475. [DOI] [PubMed] [Google Scholar]
- 6.Gerdes J, Lemke H, Baisch H, et al. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol 1984; 133:1710–1715. [PubMed] [Google Scholar]
- 7.Zhou X, Zhang G, Tian Y. p53 Status correlates with the risk of recurrence in non-muscle invasive bladder cancers treated with Bacillus Calmette-Guerin: a meta-analysis. PLoS One 2015; 10:e0119476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeon C, Kim M, Kwak C, et al. Prognostic role of survivin in bladder cancer: a systematic review and meta-analysis. PLoS One 2013; 8:e76719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanabe K, Yoshida S, Koga F, et al. High Ki-67 expression predicts favorable survival in muscle-invasive bladder cancer patients treated with chemoradiation-based bladder-sparing protocol. Clin Genitourin Cancer 2015; 13:e243–e251. [DOI] [PubMed] [Google Scholar]
- 10.Ding W, Gou Y, Sun C, et al. Ki-67 is an independent indicator in non-muscle invasive bladder cancer (NMIBC); combination of EORTC risk scores and Ki-67 expression could improve the risk stratification of NMIBC. Urol Oncol 2014; 32:13–19. [DOI] [PubMed] [Google Scholar]
- 11.Seo HK, Cho KS, Chung J, et al. Prognostic value of p53 and Ki-67 expression in intermediate-risk patients with nonmuscle-invasive bladder cancer receiving adjuvant intravesical mitomycin C therapy. Urology 2010; 76:1–7. [DOI] [PubMed] [Google Scholar]
- 12.Margulis V, Shariat SF, Ashfaq R, et al. Ki-67 is an independent predictor of bladder cancer outcome in patients treated with radical cystectomy for organ-confined disease. Clin Cancer Res 2006; 12:7369–7373. [DOI] [PubMed] [Google Scholar]
- 13.Margulis V, Lotan Y, Karakiewicz PI, et al. Multi-institutional validation of the predictive value of Ki-67 labeling index in patients with urinary bladder cancer. J Natl Cancer Inst 2009; 101:114–119. [DOI] [PubMed] [Google Scholar]
- 14.Wang LC, Xylinas E, Kent MT, et al. Combining smoking information and molecular markers improves prognostication in patients with urothelial carcinoma of the bladder. Urol Oncol 2014; 32:433–440. [DOI] [PubMed] [Google Scholar]
- 15.Poyet C, Buser L, Roudnicky F, et al. Connexin 43 expression predicts poor progression-free survival in patients with non-muscle invasive urothelial bladder cancer. J Clin Pathol 2015; 68:819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altman DG. Systematic reviews of evaluations of prognostic variables. BMJ 2001; 323:224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007; 8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 19.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med 1997; 127:820–826. [DOI] [PubMed] [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials 2015; 45 (Pt A):139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959; 22:719–748. [PubMed] [Google Scholar]
- 22.Riley RD, Elia EG, Malin G, et al. Multivariate meta-analysis of prognostic factor studies with multiple cut-points and/or methods of measurement. Stat Med 2015; 34:2481–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50:1088–1101. [PubMed] [Google Scholar]
- 24.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000; 56:455–463. [DOI] [PubMed] [Google Scholar]
- 25.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asakura T, Takano Y, Iki M, et al. Prognostic value of Ki-67 for recurrence and progression of superficial bladder cancer. J Urol 1997; 158:385–388. [PubMed] [Google Scholar]
- 27.Korkolopoulou P, Christodoulou P, Kapralos P, et al. The role of p53, MDM2 and c-erb B-2 oncoproteins, epidermal growth factor receptor and proliferation markers in the prognosis of urinary bladder cancer. Pathol Res Pract 1997; 193:767–775. [DOI] [PubMed] [Google Scholar]
- 28.Suwa Y, Takano Y, Iki M, et al. Prognostic significance of Ki-67 expression in transitional cell bladder carcinoma after radical cystectomy. Pathol Res Pract 1997; 193:551–556. [DOI] [PubMed] [Google Scholar]
- 29.Tsuji M, Kojima K, Murakami Y, et al. Prognostic value of Ki-67 antigen and p53 protein in urinary bladder cancer: immunohistochemical analysis of radical cystectomy specimens. Br J Urol 1997; 79:367–372. [DOI] [PubMed] [Google Scholar]
- 30.Nakopoulou L, Vourlakou C, Zervas A, et al. The prevalence of bcl-2, p53, and Ki-67 immunoreactivity in transitional cell bladder carcinomas and their clinicopathologic correlates. Hum Pathol 1998; 29:146–154. [DOI] [PubMed] [Google Scholar]
- 31.Osen I, Fossa SD, Majak B, et al. Prognostic factors in muscle-invasive bladder cancer treated with radiotherapy: an immunohistochemical study. Br J Urol 1998; 81:862–869. [DOI] [PubMed] [Google Scholar]
- 32.Liukkonen T, Rajala P, Raitanen M, et al. Prognostic value of MIB-1 score, p53, EGFr, mitotic index and papillary status in primary superficial (Stage pTa/T1) bladder cancer: a prospective comparative study. The Finnbladder Group. Eur Urol 1999; 36:393–400. [DOI] [PubMed] [Google Scholar]
- 33.Pfister C, Lacombe L, Vezina MC, et al. Prognostic value of the proliferative index determined by Ki-67 immunostaining in superficial bladder tumors. Hum Pathol 1999; 30:1350–1355. [DOI] [PubMed] [Google Scholar]
- 34.Tomobe M, Shimazui T, Uchida K, et al. Argyrophilic nucleolar organizer region in proliferating cell has a predictive value for local recurrence in superficial bladder tumor. J Urol 1999; 162:63–68. [DOI] [PubMed] [Google Scholar]
- 35.Oosterhuis JW, Schapers RF, Janssen-Heijnen ML, et al. MIB-1 as a proliferative marker in transitional cell carcinoma of the bladder: clinical significance and comparison with other prognostic factors. Cancer 2000; 88:2598–2605. [DOI] [PubMed] [Google Scholar]
- 36.Wu TT, Chen JH, Lee YH, et al. The role of bcl-2, p53, and ki-67 index in predicting tumor recurrence for low grade superficial transitional cell bladder carcinoma. J Urol 2000; 163:758–760. [DOI] [PubMed] [Google Scholar]
- 37.Blanchet P, Droupy S, Eschwege P, et al. Prospective evaluation of Ki-67 labeling in predicting the recurrence and progression of superficial bladder transitional cell carcinoma. Eur Urol 2001; 40:169–175. [DOI] [PubMed] [Google Scholar]
- 38.Kamai T, Takagi K, Asami H, et al. Decreasing of p27(Kip1)and cyclin E protein levels is associated with progression from superficial into invasive bladder cancer. Br J Cancer 2001; 84:1242–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Korkolopoulou P, Christodoulou P, Lazaris A, et al. Prognostic implications of aberrations in p16/pRb pathway in urothelial bladder carcinomas: a multivariate analysis including p53 expression and proliferation markers. Eur Urol 2001; 39:167–177. [DOI] [PubMed] [Google Scholar]
- 40.Nakopoulou L, Zervas A, Lazaris AC, et al. Predictive value of topoisomerase II alpha immunostaining in urothelial bladder carcinoma. J Clin Pathol 2001; 54:309–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nocito A, Bubendorf L, Tinner EM, et al. Microarrays of bladder cancer tissue are highly representative of proliferation index and histological grade. J Pathol 2001; 194:349–357. [DOI] [PubMed] [Google Scholar]
- 42.Tomobe M, Shimazui T, Uchida K, et al. AgNOR count in resting cells (resting NOR) is a new prognostic marker in invasive bladder tumor. Anal Cell Pathol 2001; 22:193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bozlu M, Orhan D, Baltaci S, et al. The prognostic value of proliferating cell nuclear antigen, Ki-67 and nucleolar organizer region in transitional cell carcinoma of the bladder. Int Urol Nephrol 2002; 33:59–66. [DOI] [PubMed] [Google Scholar]
- 44.Li H. The relationshiu among P27KIP1, P21WAF1, Cyclin E, Ki-67 in transitional cell bladder cancer [master's thesis]. Fuzhou, China: Fujian Medical University; 2002. [Google Scholar]
- 45.Liu X. Expression and interaction of p53, p21WAF1/CIP1, cyclin E genes in Human bladder carcinoma and predictive value in tumor recurrence [master's thesis]. Shenyang, China: China Medical University; 2002. [Google Scholar]
- 46.Nomura T, Nakagawa M, Fujita Y, et al. Clinical significance of thymidylate synthase expression in bladder cancer. Int J Urol 2002; 9:368–376. [DOI] [PubMed] [Google Scholar]
- 47.Pich A, Chiusa L, Formiconi A, et al. Proliferative activity is the most significant predictor of recurrence in noninvasive papillary urothelial neoplasms of low malignant potential and grade 1 papillary carcinomas of the bladder. Cancer 2002; 95:784–790. [DOI] [PubMed] [Google Scholar]
- 48.Rao J, Seligson D, Visapaa H, et al. Tissue microarray analysis of cytoskeletal actin-associated biomarkers gelsolin and E-cadherin in urothelial carcinoma. Cancer 2002; 95:1247–1257. [DOI] [PubMed] [Google Scholar]
- 49.Rodriguez-Alonso A, Pita-Fernandez S, Gonzalez-Carrero J, et al. Multivariate analysis of survival, recurrence, progression and development of mestastasis in T1 and T2a transitional cell bladder carcinoma. Cancer 2002; 94:1677–1684. [DOI] [PubMed] [Google Scholar]
- 50.Sgambato A, Migaldi M, Faraglia B, et al. Cyclin D1 expression in papillary superficial bladder cancer: its association with other cell cycle-associated proteins, cell proliferation and clinical outcome. Int J Cancer 2002; 97:671–678. [DOI] [PubMed] [Google Scholar]
- 51.Santos L, Amaro T, Costa C, et al. Ki-67 index enhances the prognostic accuracy of the urothelial superficial bladder carcinoma risk group classification. Int J Cancer 2003; 105:267–272. [DOI] [PubMed] [Google Scholar]
- 52.Su JS, Arima K, Hasegawa M, et al. Proliferative status is a risk index for recurrence in primary superficial (pTa/T1) low-grade urothelial bladder carcinoma. Hinyokika Kiyo 2003; 49:649–658. [PubMed] [Google Scholar]
- 53.van Rhijn BW, Vis AN, van der Kwast TH, et al. Molecular grading of urothelial cell carcinoma with fibroblast growth factor receptor 3 and MIB-1 is superior to pathologic grade for the prediction of clinical outcome. J Clin Oncol 2003; 21:1912–1921. [DOI] [PubMed] [Google Scholar]
- 54.Frank I, Cheville JC, Blute ML, et al. Prognostic value of p53 and MIB-1 in transitional cell carcinoma of the urinary bladder with regional lymph node involvement. Cancer 2004; 101:1803–1808. [DOI] [PubMed] [Google Scholar]
- 55.Lopez-Beltran A, Luque RJ, Alvarez-Kindelan J, et al. Prognostic factors in survival of patients with stage Ta and T1 bladder urothelial tumors – the role of G(1)-S modulators (p53, p21Waf1, p27Kip1, cyclin D1, and cyclin D3), proliferation index, and clinicopathologic parameters. Am J Clin Pathol 2004; 122:444–452. [DOI] [PubMed] [Google Scholar]
- 56.Matsumoto H, Wada T, Fukunaga K, et al. Bax to Bcl-2 ratio and Ki-67 index are useful predictors of neoadjuvant chemoradiation therapy in bladder cancer. Jpn J Clin Oncol 2004; 34:124–130. [DOI] [PubMed] [Google Scholar]
- 57.Mhawech P, Greloz V, Oppikofer C, et al. Expression of cell cycle proteins in T1a and T1b urothelial bladder carcinoma and their value in predicting tumor progression. Cancer 2004; 100:2367–2375. [DOI] [PubMed] [Google Scholar]
- 58.Popov Z, Gil-Diez-De-Medina S, Ravery V, et al. Prognostic value of EGF receptor and tumor cell proliferation in bladder cancer: therapeutic implications. Urol Oncol 2004; 22:93–101. [DOI] [PubMed] [Google Scholar]
- 59.Kruger S, Mahnken A, Kausch I, et al. P16 immunoreactivity is an independent predictor of tumor progression in minimally invasive urothelial bladder carcinoma. Eur Urol 2005; 47:463–467. [DOI] [PubMed] [Google Scholar]
- 60.Mahnken A, Kausch I, Feller AC, et al. E-cadherin immunoreactivity correlates with recurrence and progression of minimally invasive transitional cell carcinomas of the urinary bladder. Oncol Rep 2005; 14:1065–1070. [PubMed] [Google Scholar]
- 61.Theodoropoulos VE, Lazaris AC, Kastriotis I, et al. Evaluation of hypoxia-inducible factor 1alpha overexpression as a predictor of tumour recurrence and progression in superficial urothelial bladder carcinoma. BJU Int 2005; 95:425–431. [DOI] [PubMed] [Google Scholar]
- 62.Galmozzi F, Rubagotti A, Romagnoli A, et al. Prognostic value of cell cycle regulatory proteins in muscle-infiltrating bladder cancer. J Cancer Res Clin Oncol 2006; 132:757–764. [DOI] [PubMed] [Google Scholar]
- 63.Gonzalez-Campora R, Davalos-Casanova G, Beato-Moreno A, et al. Apoptotic and proliferation indexes in primary superficial bladder tumors. Cancer Lett 2006; 242:266–272. [DOI] [PubMed] [Google Scholar]
- 64.Grossman HB, Tangen CM, Cordon-Cardo C, et al. Evaluation of Ki67, p53 and angiogenesis in patients enrolled in a randomized study of neoadjuvant chemotherapy with or without cystectomy: a Southwest Oncology Group Study. Oncol Rep 2006; 16:807–810. [PubMed] [Google Scholar]
- 65.Hilmy M, Campbell R, Bartlett JM, et al. The relationship between the systemic inflammatory response, tumour proliferative activity, T-lymphocytic infiltration and COX-2 expression and survival in patients with transitional cell carcinoma of the urinary bladder. Br J Cancer 2006; 95:1234–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hu M. Expression of Ki-67 protein in bladder transitional cell cancer and its relation to angiogenesis and expression of Bcl-2 [master's thesis]. Tianjin, China: Nankai University; 2006. [Google Scholar]
- 67.Kruger S, Mahnken A, Kausch I, et al. Value of clusterin immunoreactivity as a predictive factor in muscle-invasive urothelial bladder carcinoma. Urology 2006; 67:105–109. [DOI] [PubMed] [Google Scholar]
- 68.Qu W, Duan J, Qin D, et al. Research of c- FLIP, BCL-2 and Ki-67 expression in bladder urothelial carcinomas and their relation with clinical pathology and prognosis. China J Mod Med 2006; 16:1353–1357. [Google Scholar]
- 69.Quintero A, Alvarez-Kindelan J, Luque RJ, et al. Ki-67 MIB1 labelling index and the prognosis of primary TaT1 urothelial cell carcinoma of the bladder. J Clin Pathol 2006; 59:83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yin W, Chen N, Zhang Y, et al. Survivin nuclear labeling index: a superior biomarker in superficial urothelial carcinoma of human urinary bladder. Mod Pathol 2006; 19:1487–1497. [DOI] [PubMed] [Google Scholar]
- 71.Yurakh AO, Ramos D, Calabuig-Farinas S, et al. Molecular and immunohistochemical analysis of the prognostic value of cell-cycle regulators in urothelial neoplasms of the bladder. Eur Urol 2006; 50:506–515.discussion 515. [DOI] [PubMed] [Google Scholar]
- 72.Shariat SF, Bolenz C, Godoy G, et al. Predictive value of combined immunohistochemical markers in patients with pT1 urothelial carcinoma at radical cystectomy. J Urol 2009; 182:78–84.discussion 84. [DOI] [PubMed] [Google Scholar]
- 73.Maeng YH, Eun SY, Huh JS. Expression of fibroblast growth factor receptor 3 in the recurrence of non-muscle-invasive urothelial carcinoma of the bladder. Korean J Urol 2010; 51:94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miyake M, Fujimoto K, Anai S, et al. Clinical significance of heme oxygenase-1 expression in non-muscle-invasive bladder cancer. Urol Int 2010; 85:355–363. [DOI] [PubMed] [Google Scholar]
- 75.Seo HK, Cho KS, Chung J, et al. Prognostic value of p53 and Ki-67 expression in intermediate-risk patients with nonmuscle-invasive bladder cancer receiving adjuvant intravesical mitomycin C therapy. Urology 2010; 76:512.e511–e517. [DOI] [PubMed] [Google Scholar]
- 76.van Rhijn BW, Zuiverloon TC, Vis AN, et al. Molecular grade (FGFR3/MIB-1) and EORTC risk scores are predictive in primary non-muscle-invasive bladder cancer. Eur Urol 2010; 58:433–441. [DOI] [PubMed] [Google Scholar]
- 77.Yan JA, Xiao H, Ji HX, et al. Cathepsin L is associated with proliferation and clinical outcome of urothelial carcinoma of the bladder. J Int Med Res 2010; 38:1913–1922. [DOI] [PubMed] [Google Scholar]
- 78.Behnsawy HM, Miyake H, Abdalla MA, et al. Expression of cell cycle-associated proteins in non-muscle-invasive bladder cancer: correlation with intravesical recurrence following transurethral resection. Urol Oncol 2011; 29:495–501. [DOI] [PubMed] [Google Scholar]
- 79.Sugino T, Baba K, Hoshi N, et al. Overexpression of fatty acid synthase in human urinary bladder cancer and combined expression of the synthase and Ki-67 as a predictor of prognosis of cancer patients. Med Mol Morphol 2011; 44:146–150. [DOI] [PubMed] [Google Scholar]
- 80.Yang G, Zhang L, Bo X, et al. Expression of Ki67 protein in non-muscle-invasive bladder cancer and its significance. J Chin Oncol 2011; 17:212–215. [Google Scholar]
- 81.Youssef RF, Shariat SF, Kapur P, et al. Expression of cell cycle-related molecular markers in patients treated with radical cystectomy for squamous cell carcinoma of the bladder. Hum Pathol 2011; 42:347–355. [DOI] [PubMed] [Google Scholar]
- 82.Acikalin D, Oner U, Can C, et al. Predictive value of maspin and Ki-67 expression in transurethral resection specimens in patients with T1 bladder cancer. Tumori 2012; 98:344–350. [DOI] [PubMed] [Google Scholar]
- 83.Bi J, Chen X, Zhang Y, et al. Fascin is a predictor for invasiveness and recurrence of urothelial carcinoma of bladder. Urol Oncol 2012; 30:688–694. [DOI] [PubMed] [Google Scholar]
- 84.Chen JX, Deng N, Chen X, et al. A novel molecular grading model: combination of Ki67 and VEGF in predicting tumor recurrence and progression in non-invasive urothelial bladder cancer. Asian Pac J Cancer Prev 2012; 13:2229–2234. [DOI] [PubMed] [Google Scholar]
- 85.Laurberg JR, Brems-Eskildsen AS, Nordentoft I, et al. Expression of TIP60 (tat-interactive protein) and MRE11 (meiotic recombination 11 homolog) predict treatment-specific outcome of localised invasive bladder cancer. BJU Int 2012; 110 (11 Pt C):E1228–E1236. [DOI] [PubMed] [Google Scholar]
- 86.Shan GY, Zhang Z, Chen QG, et al. Overexpression of RIN1 associates with tumor grade and progression in patients of bladder urothelial carcinoma. Tumour Biol 2012; 33:847–855. [DOI] [PubMed] [Google Scholar]
- 87.Lotan Y, Bagrodia A, Passoni N, et al. Prospective evaluation of a molecular marker panel for prediction of recurrence and cancer-specific survival after radical cystectomy. Eur Urol 2013; 64:465–471. [DOI] [PubMed] [Google Scholar]
- 88.Oderda M, Ricceri F, Pisano F, et al. Prognostic factors including Ki-67 and p53 in Bacillus Calmette-Guerin-treated non-muscle-invasive bladder cancer: a prospective study. Urol Int 2013; 90:184–190. [DOI] [PubMed] [Google Scholar]
- 89.Okazoe H, Zhang X, Liu D, et al. Expression and role of GPR87 in urothelial carcinoma of the bladder. Int J Mol Sci 2013; 14:12367–12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Park J, Song C, Shin E, et al. Do molecular biomarkers have prognostic value in primary T1G3 bladder cancer treated with bacillus Calmette-Guerin intravesical therapy? Urol Oncol 2013; 31:849–856. [DOI] [PubMed] [Google Scholar]
- 91.Ruan J, Wei B, Xu Z, et al. Predictive value of Sox2 expression in transurethral resection specimens in patients with T1 bladder cancer. Med Oncol 2013; 30:445. [DOI] [PubMed] [Google Scholar]
- 92.Bertz S, Otto W, Denzinger S, et al. Combination of CK20 and Ki-67 immunostaining analysis predicts recurrence, progression, and cancer-specific survival in pT1 urothelial bladder cancer. Eur Urol 2014; 65:218–226. [DOI] [PubMed] [Google Scholar]
- 93.Gontero P, Gillo A, Fiorito C, et al. Prognostic factors of ‘high-grade’ Ta bladder cancers according to the WHO 2004 classification: are these equivalent to ‘high-risk’ non-muscle-invasive bladder cancer? Urol Int 2014; 92:136–142. [DOI] [PubMed] [Google Scholar]
- 94.Wang L, Feng C, Ding G, et al. Ki67 and TP53 expressions predict recurrence of non-muscle-invasive bladder cancer. Tumour Biol 2014; 35:2989–2995. [DOI] [PubMed] [Google Scholar]
- 95.Makboul R, Refaiy AE, Badary FA, et al. Expression of survivin in squamous cell carcinoma and transitional cell carcinoma of the urinary bladder: a comparative immunohistochemical study. Korean J Urol 2015; 56:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ozyalvacli G, Ozyalvacli ME, Astarci HM, et al. Evaluation of different p16 immunostaining methods and the prognostic role of p16/Ki-67 combined expression in non-muscle invasive bladder cancers. Pol J Pathol 2015; 66:57–66. [DOI] [PubMed] [Google Scholar]
- 97.Saiki S, Kinouchi T, Meguro N, et al. A multivariate analysis of prognostic factors related to recurrence and progression of superficial bladder cancer. Urol Oncol 1996; 2:152–157. [DOI] [PubMed] [Google Scholar]
- 98.Inagaki T, Ebisuno S, Uekado Y, et al. PCNA and p53 in urinary bladder cancer: correlation with histological findings and prognosis. Int J Urol 1997; 4:172–177. [DOI] [PubMed] [Google Scholar]
- 99.Siu LL, Banerjee D, Khurana RJ, et al. The prognostic role of p53, metallothionein, P-glycoprotein, and MIB-1 in muscle-invasive urothelial transitional cell carcinoma. Clin Cancer Res 1998; 4:559–565. [PubMed] [Google Scholar]
- 100.Zlotta AR, Noel JC, Fayt I, et al. Correlation and prognostic significance of p53, p21WAF1/CIP1 and Ki-67 expression in patients with superficial bladder tumors treated with bacillus Calmette-Guerin intravesical therapy. J Urol 1999; 161:792–798. [PubMed] [Google Scholar]
- 101.Liukkonen T, Lipponen P, Raitanen M, et al. Evaluation of p21(WAF1/CIP1) and cyclin D-1 expression in the progression of superficial bladder cancer. Urol Res 2000; 28:285–292. [DOI] [PubMed] [Google Scholar]
- 102.Haitel A, Posch B, El-Baz M, et al. Bilharzial related, organ confined, muscle invasive bladder cancer: prognostic value of apoptosis markers, proliferation markers, p53, E-cadherin, epidermal growth factor receptor and c-erbB-2. J Urol 2001; 165:1481–1487. [PubMed] [Google Scholar]
- 103.Moonen L, Ong F, Gallee M, et al. Apoptosis, proliferation and p53, cyclin D1, and retinoblastoma gene expression in relation to radiation response in transitional cell carcinoma of the bladder. Int J Radiat Oncol Biol Phys 2001; 49:1305–1310. [DOI] [PubMed] [Google Scholar]
- 104.Ong F, Moonen LM, Gallee MP, et al. Prognostic factors in transitional cell cancer of the bladder: an emerging role for Bcl-2 and p53. Radiother Oncol 2001; 61:169–175. [DOI] [PubMed] [Google Scholar]
- 105.Sagol O, Yorukoglu K, Tuna B, et al. Expression of pS2 protein and its relation with the Ki-67 proliferative indices and tumor recurrence in superficial bladder carcinomas. Eur Urol 2001; 40:163–168. [DOI] [PubMed] [Google Scholar]
- 106.Wolf HK, Stober C, Hohenfellner R, et al. Prognostic value of p53, p21/WAF1, Bcl-2, Bax, Bak and Ki-67 immunoreactivity in pT1 G3 urothelial bladder carcinomas. Tumour Biol 2001; 22:328–336. [DOI] [PubMed] [Google Scholar]
- 107.Bol MG, Baak JP, Rep S, et al. Prognostic value of proliferative activity and nuclear morphometry for progression in TaT1 urothelial cell carcinomas of the urinary bladder. Urology 2002; 60:1124–1130. [DOI] [PubMed] [Google Scholar]
- 108.Korkolopoulou P, Lazaris A, Konstantinidou AE, et al. Differential expression of bcl-2 family proteins in bladder carcinomas. Relationship with apoptotic rate and survival. Eur Urol 2002; 41:274–283. [DOI] [PubMed] [Google Scholar]
- 109.Liu X, Yang C, Sun Z, et al. Relationship among the expression and interaction of p53, p21WAF1/CIP1 and cyclinE genes and human and bladder carcinoma recurrence. Chin J Exp Surg 2002; 16–17. [Google Scholar]
- 110.Stavropoulos NE, Filiadis I, Ioachim E, et al. Prognostic significance of p53, bcl-2 and Ki-67 in high risk superficial bladder cancer. Anticancer Res 2002; 22 (6b):3759–3764. [PubMed] [Google Scholar]
- 111.Bol MGW, Baak JPA, van Diermen B, et al. Proliferation markers and DNA content analysis in urinary bladder TaT1 urothelial cell carcinomas: identification of subgroups with low and high stage progression risks. J Clin Pathol 2003; 56:447–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gakiopoulou-Givalou H, Nakopoulou L, Panayotopoulou EG, et al. Non-endothelial KDR/flk-1 expression is associated with increased survival of patients with urothelial bladder carcinomas. Histopathology 2003; 43:272–279. [DOI] [PubMed] [Google Scholar]
- 113.Hoskin PJ, Sibtain A, Daley FM, et al. GLUTI and CAIX as intrinsic markers of hypoxia in bladder cancer: relationship with vascularity and proliferation as predictors of outcome of ARCON. Br J Cancer 2003; 89:1290–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fromont G, Roupret M, Amira N, et al. Tissue microarray analysis of the prognostic value of E-cadherin, Ki67, p53, p27, survivin and MSH2 expression in upper urinary tract transitional cell carcinoma. Eur Urol 2005; 48:764–770. [DOI] [PubMed] [Google Scholar]
- 115.Gonul II, Akyurek N, Dursun A, et al. Relationship of Ki67, TP53, MDM-2 and BCL-2 expressions with WHO 1973 and WHO/ISUP grades, tumor category and overall patient survival in urothelial tumors of the bladder. Pathol Res Pract 2008; 204:707–717. [DOI] [PubMed] [Google Scholar]
- 116.Eltze E, Wild PJ, Wulfing C, et al. Expression of the endothelin axis in noninvasive and superficially invasive bladder cancer: relation to clinicopathologic and molecular prognostic parameters. Eur Urol 2009; 56:837–845. [DOI] [PubMed] [Google Scholar]
- 117.Ben Abdelkrim S, Rammeh S, Ziadi S, et al. Expression of topoisomerase II alpha, ki67, and p53 in primary non-muscle-invasive urothelial bladder carcinoma. J Immunoassay Immunochem 2014; 35:358–367. [DOI] [PubMed] [Google Scholar]
- 118.Mangrud OM, Gudlaugsson E, Skaland I, et al. Prognostic comparison of proliferation markers and World Health Organization 1973/2004 grades in urothelial carcinomas of the urinary bladder. Hum Pathol 2014; 45:1496–1503. [DOI] [PubMed] [Google Scholar]
- 119.Go H, Kim PJ, Jeon YK, et al. Sphingosine-1-phosphate receptor 1 (S1PR1) expression in non-muscle invasive urothelial carcinoma: association with poor clinical outcome and potential therapeutic target. Eur J Cancer 2015; 51:1937–1945. [DOI] [PubMed] [Google Scholar]
- 120.Gerdes J, Li L, Schlueter C, et al. Immunobiochemical and molecular biologic characterization of the cell proliferation-associated nuclear antigen that is defined by monoclonal antibody Ki-67. Am J Pathol 1991; 138:867–873. [PMC free article] [PubMed] [Google Scholar]
- 121.Stuart-Harris R, Caldas C, Pinder SE, et al. Proliferation markers and survival in early breast cancer: a systematic review and meta-analysis of 85 studies in 32,825 patients. Breast 2008; 17:323–334. [DOI] [PubMed] [Google Scholar]
- 122.El-kott AF, El-baz MA, Mokhtar AA. Proliferating cell nuclear antigen (PCNA) overexpression and microvessel density predict survival in the urinary bladder carcinoma. Int Urol Nephrol 2006; 38:237–242. [DOI] [PubMed] [Google Scholar]
- 123.Yang C, Wen Y, Li H, et al. Overexpression of minichromosome maintenance 2 predicts poor prognosis in patients with gastric cancer. Oncol Rep 2012; 27:135–142. [DOI] [PubMed] [Google Scholar]
- 124.Ramnath N, Hernandez FJ, Tan DF, et al. MCM2 is an independent predictor of survival in patients with non-small-cell lung cancer. J Clin Oncol 2001; 19:4259–4266. [DOI] [PubMed] [Google Scholar]
- 125.Luo Y, Ren F, Liu Y, et al. Clinicopathological and prognostic significance of high Ki-67 labeling index in hepatocellular carcinoma patients: a meta-analysis. Int J Clin Exp Med 2015; 8:10235–10247. [PMC free article] [PubMed] [Google Scholar]
- 126.Petrelli F, Viale G, Cabiddu M, et al. Prognostic value of different cut-off levels of Ki-67 in breast cancer: a systematic review and meta-analysis of 64,196 patients. Breast Cancer Res Treat 2015; 153:477–491. [DOI] [PubMed] [Google Scholar]
- 127.Wen S, Zhou W, Li CM, et al. Ki-67 as a prognostic marker in early-stage non-small cell lung cancer in Asian patients: a meta-analysis of published studies involving 32 studies. BMC Cancer 2015; 15:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pan D, Wei K, Ling Y, et al. The prognostic role of Ki-67/MIB-1 in cervical cancer: a systematic review with meta-analysis. Med Sci Monit 2015; 21:882–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen WJ, He DS, Tang RX, et al. Ki-67 is a valuable prognostic factor in gliomas: evidence from a systematic review and meta-analysis. Asian Pac J Cancer Prev 2015; 16:411–420. [DOI] [PubMed] [Google Scholar]
- 130.He X, Chen Z, Fu T, et al. Ki-67 is a valuable prognostic predictor of lymphoma but its utility varies in lymphoma subtypes: evidence from a systematic meta-analysis. BMC Cancer 2014; 14:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu Y, Yin W, Yan T, et al. The clinical significance of Ki-67 as a marker of prognostic value and chemosensitivity prediction in hormone-receptor-positive breast cancer: a meta-analysis of the published literature. Curr Med Res Opin 2013; 29:1453–1461. [DOI] [PubMed] [Google Scholar]
- 132.de Azambuja E, Cardoso F, de Castro G, Jr, et al. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer 2007; 96:1504–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Martin B, Paesmans M, Mascaux C, et al. Ki-67 expression and patients survival in lung cancer: systematic review of the literature with meta-analysis. Br J Cancer 2004; 91:2018–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lei Y, Li Z, Qi L, et al. The prognostic role of Ki-67/MIB-1 in upper urinary-tract urothelial carcinomas: a systematic review and meta-analysis. J Endourol 2015; 29:1302–1308. [DOI] [PubMed] [Google Scholar]
- 135.Altman DG, Lausen B, Sauerbrei W, et al. Dangers of using “optimal” cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst 1994; 86:829–835. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


