Abstract
There has been growing interest in the association between infectious disease and mental disorders, but an association between enterovirus (EV) infection and tic disorders has not been sufficiently explored. Herein, we aim to investigate the association between EV infection and incidence of tic disorders in a nationwide population-based sample using Taiwan's National Health Insurance Research Database.
We identified individuals aged ≤18 years prior to 2005 with an inpatient diagnosis of EV infection and/or history of EV infection. Tic disorder was operationalized using International Classification of Disease, Revision 9, Clinical Modification (ICD-9-CM) codes 307.20–307.23.
A total of 47,998 individuals with history of EV infection were compared to 47,998 sex-, age-, and urbanization-matched controls on incidence of tic disorders. The mean ± standard deviation follow-up period for all subjects was 9.7 ± 3.6 years; the mean latency period between initial EV infection and incident diagnosis of tic disorder diagnosis was 5.4 ± 2.8 years. EV infection was significantly associated with greater incidence of tic disorders (hazard ratio [HR] = 1.24, 95% CI: 1.07–1.45). When subgrouped on the basis of central nervous system (CNS) involvement, EV infection with CNS involvement was not significantly associated with greater incidence of tic disorders when compared to controls (HR = 1.25, 95% CI: 0.64–2.43); EV infection without CNS involvement was significantly associated greater incidence of tic disorders when compared to controls (HR = 1.24, 95% CI: 1.07–1.45). In addition, hospitalization for an EV infection did not increase the hazard for greater incidence of tic disorders (HR = 1.32, 95% CI: 1.04–1.67 with hospitalization and 1.22, 95% CI: 1.04–1.44 without hospitalization).
EV infection is temporally associated with incidence of tic disorders. Our observations add to the growing body of literature implicating immune-inflammatory system in the pathoetiology of brain disorders in a subpopulation of individuals and serve as a clarion call for surveillance of symptoms suggestive of tic disorders in individuals with history of EV infection.
INTRODUCTION
Enteroviruses (EVs) are a group of single-stranded positive-sense RNA viruses, including polioviruses, group A and B coxsackieviruses, echoviruses, and EVs. More than 100 serotypes have been identified.1 EVs are highly infectious, virulent, and associated with approximately 10 to 15 million symptomatic infections each year in the United States and have caused several outbreaks globally.2 For example, the largest EV epidemic in Taiwan in 1998 infected approximately 130,000 individuals during an 8-month period, resulting in 405 cases of severe neurological disease and 78 fatalities attributable to neurogenic pulmonary edema.3 Another large outbreak of EV infection in Western Australia in 1999 resulted in 6000 cases during a 6-month period and 29 cases of severe neurological disease. The rate of neurological disease in this epidemic was estimated to be approximately 1 per 1000 affected cases.4 Among the serotypes of EVs, coxsackieviruses (CV) have a higher fatality rate when compared to other EVs. Infants infected with coxsackieviruses are extremely susceptible to myocarditis, meningitis, and encephalitis with a subsequent mortality rate of 10%.5
EVs can influence brain development by inducing apoptosis. Induction of apoptosis in the cortex, hippocampus, and choroid plexus has been reported in animal studies.6,7 In addition, EV may cause extensive infiltration of leukocytes into the central nervous system (CNS) by activating the inflammatory response and result in neuropathology.5 It is further reported that mental and neurological disorders, such as delayed development, lower intelligence score, schizophrenia, and attention deficit hyperactivity disorder (ADHD) are associated with EV infection.8–12
Tic disorder is one of the most commonly diagnosed movement disorders among in children, with a prevalence rate of 0.2% to 46.3%.13 Tic disorders may persist into late adolescence or early adulthood in a subset of affected. Motor and/or vocal tics associated with tic disorders may cause pain and interfere with daily living.14 In addition to the core symptoms of tic disorders, affected individuals often present with behavioral difficulties, such as comorbid obsessive compulsive disorder or ADHD.15 For example, among young adults with tic disorders, 79% have reported impairments in interpersonal relationships with peers, 39% have reported to have been teased in school, and 28% have reported to have been rejected from their peer groups.16 As with most mental disorders, the etiology of tic disorders is currently not known.
Emerging evidence suggests that neuropsychiatric illnesses (e.g., tic disorders, Gilles de la Tourette syndrome) may, in part, be a result of an infection. For example, it is well established that pediatric autoimmune neuropsychiatric disorders are associated with streptococcus infections.17,18 Patients with tic disorders and Gilles de la Tourette syndrome were reported to have increased serum levels of antibodies against streptococcus19 and streptococcal M protein.20 Having multiple streptococcal infections were associated with increased risk for Gilles de la Tourette syndrome.17 In addition, a recent observational study reported that 3 of 23 boys were diagnosed with tic disorders after an acute EV infection, suggesting an association between EV infection and greater incidence of tic disorders.21 To our knowledge, no previous large-scale study has explored the association between EV infection and tic disorders. The present study aims to investigate the association between EV infection and subsequent diagnoses of tic disorders in a nationwide population-based sample.
MATERIALS AND METHODS
Database
The data used in the analysis herein was randomly sampled from the Taiwan National Health Insurance Research Database (NHIRD). The NHIRD provides comprehensive patient data, including demographic data, diagnostic codes according to the International Classification of Disease, Revision 9, Clinical Modification (ICD-9-CM), drug prescriptions, and physician specialties. The NHIRD was launched on March 1, 1995 and is maintained by the Department of Health and the National Health Research Institutes. The NHIRD covers more than 98% of the national population of Taiwan22 and provides valuable information for epidemiological study.23 No statistically significant differences in age, sex, or healthcare utilization were identified between the NHIRD and the randomly sampled population data analyzed herein.24
Study Subjects and Design
The study sample was comprised of insured children under 18 years of age prior to 2005 with EV infection. The study observation was delimited to March 1995 and December 2013. Inclusion criteria included at least 2 ambulatory claims within 1 year or at least 1 inpatient claim with a diagnosis of enteritis due to EV (ICD-9-CM codes 008.67), meningitis due to EV, coxsackievirus, and echovirus (ICD-9-CM codes 047, 047.0, and 047.1), other EV diseases of CNS (ICD-9-CM codes 048), specific diseases related to Coxsackievirus (ICD-9-CM codes 074, 074.1, 074.2, 074.20, 074.21, 074.23, and 074.8), herpangina (ICD-9-CM codes 074.0), hand-foot-and-mouth disease (ICD-9-CM codes 074.3), echovirus and Coxsackievirus infection in conditions classified elsewhere, and of unspecified site (ICD-9-CM codes 079.1 and 079.2). The foregoing definition is consistent with previous research using this database.25
EV encephalitis was operationalized as EV infection with encephalitis (ICD-9-CM codes 323.0, 323.4, and 323.9) within 1 month of infection.26 Subgroup of EV infection with CNS involvement, including meningitis due to EV, meningitis due to Coxsackievirus, meningitis due to echovirus, other EV diseases of CNS, and EV encephalitis were also included in the analysis herein. Children with diagnosis of tic disorders before EV infection were excluded from the analysis. Data from 47,998 individuals with EV infections and 47,998 sex-, age-, and urbanization-matched controls were included in the analysis herein (Figure 1).
FIGURE 1.
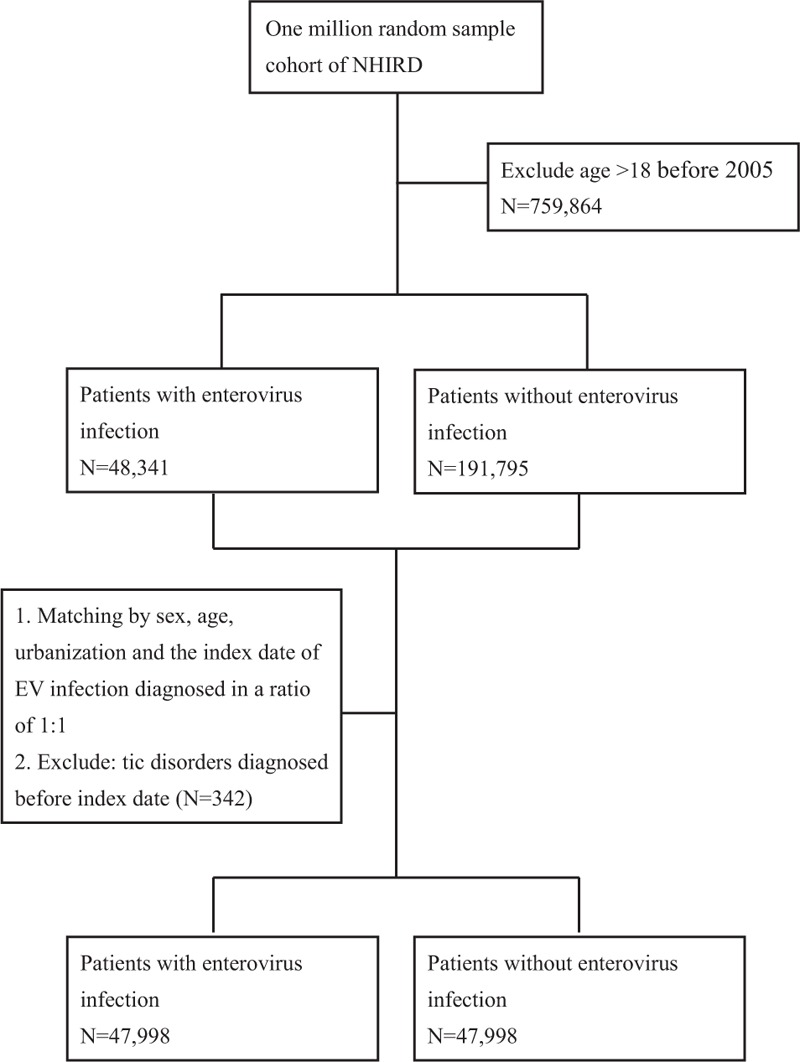
Flow chart of data collection.
Case Identification of Tic Disorders
Cases of tic disorders were identified based on recorded ICD-9-CM codes 307.20–307.23, which includes tic disorder, unspecified, transient tic disorder of childhood, chronic motor tic disorder, and Gilles de la Tourette's disorder. This definition is also consistent with other research.27 At least 1 ambulatory or inpatient claim of tic disorder made under these diagnostic codes were collected for analysis.
Confounding Factors
Although the pathoetiology of tic disorders is not currently known, genetic susceptibility and environmental factors (e.g., prenatal adversities, perinatal complications, and infections) are thought to contribute.28,29 Herein, we adjusted for potential confounds including preterm labor and small for gestational age (ICD-9-CM codes 765–765.19) and perinatal complications (ICD-9-CM codes 760–764, 766–779, and V137). Given the previously reported association between tic disorders and allergic diseases using the NHIRD,30 asthma (ICD-9-CM codes 493, 493.0, 493.1, and 493.9), allergic dermatitis (ICD-9-CM codes 477), atopic dermatitis (ICD-9-CM codes 691, 691.8), and allergic conjunctivitis (ICD-9-CM codes 372.05, 372.14) were also controlled for.
Statistical Analysis
The distribution of demographic factors and comorbidities between the children with and without EV infection were compared. We used the Kaplan–Meier method to estimate cumulative incidences of tic disorders. The log-rank test was performed to examine differences in the risk for tic disorders in the study population. Finally, Cox proportional hazards models were used to compute the hazard ratio (HR) accompanying 95% confidence intervals (CIs) after adjustment for age, sex, urbanization, preterm labor and small for gestational age, perinatal complications, and atopic diseases. Two-tailed P = 0.05 was considered significant. Individuals who were deceased during the study period and/or were from the beneficiaries register lost to follow-up were omitted from the analysis. All of the foregoing analyses were conducted using SAS statistical software (Version 9.4; SAS Institute, Cary, NC).
Ethics Statement
The study was approved by the Institutional Review Board of Chang Gung Memorial Hospital (Docket Number: 104-7528B). Written informed consent was exempted.
RESULTS
Characteristics of Subjects
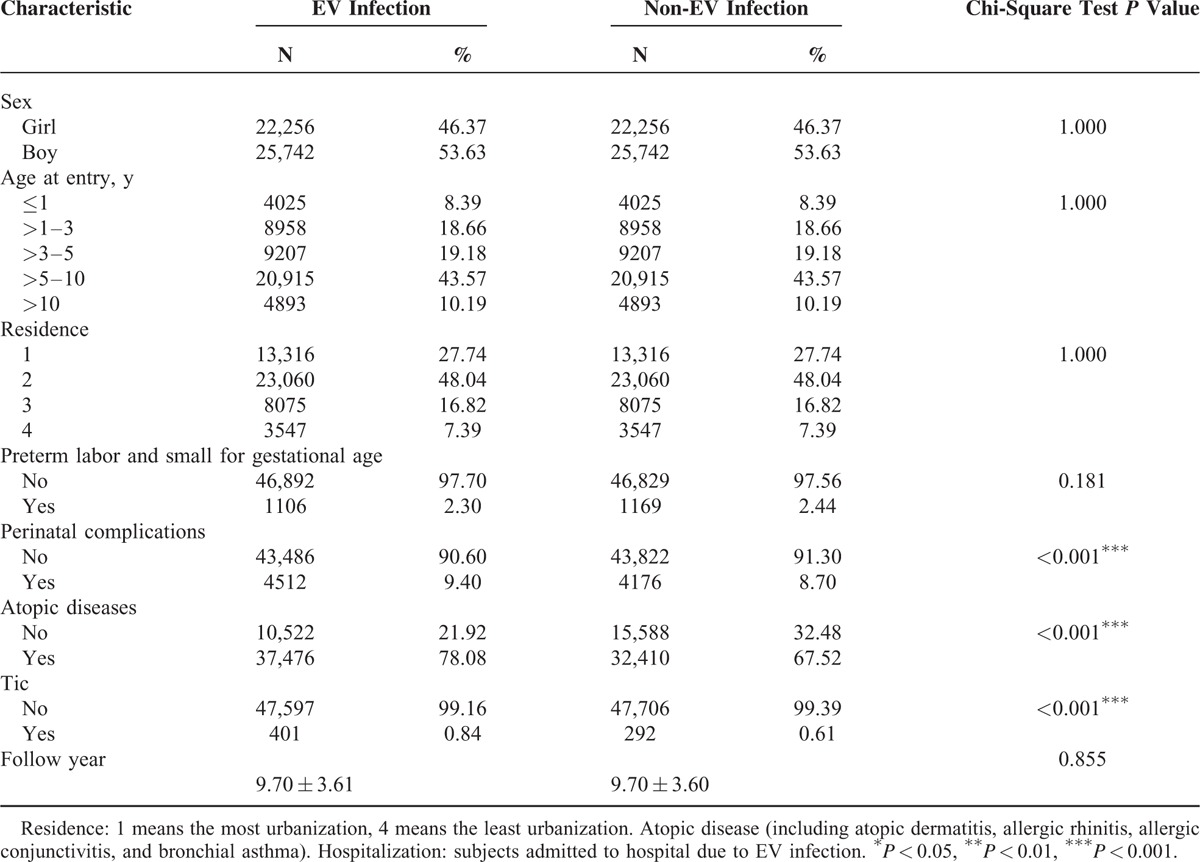
The 2 cohorts were comprised of 47,998 individuals with EV infection and 47,998 age- and sex-matched controls ascertained from the database. Cohort characteristics are described and compared in Table 1. Compared to the control cohort, individuals with prior history of an EV infection had more perinatal complications, atopic diseases, and tic disorders. The mean follow-up interval for all subjects was 9.7 years (standard deviation = 3.6 years). Mean latency period between the initial EV infection and diagnosis of a tic disorder was 5.4 years (standard deviation = 2.8 years).
TABLE 1.
Characteristics of Enterovirus (EV) Infection Cases and Their Matched Controls
Association Between EV Infection and Risk of Tic Disorders
Analyses of associations of interest are summarized in Table 2 and shown in Figure 2. In the fully adjusted Cox regression model for HRs, EV infection was associated with a greater incidence of tic disorders (HR = 1.243, 95% CI: 1.069–1.446) after adjusting for age, sex, urbanization, preterm labor and small for gestational age, perinatal complications, and atopic diseases.
TABLE 2.
Competing Risk Adjusted Cox Regression Analysis
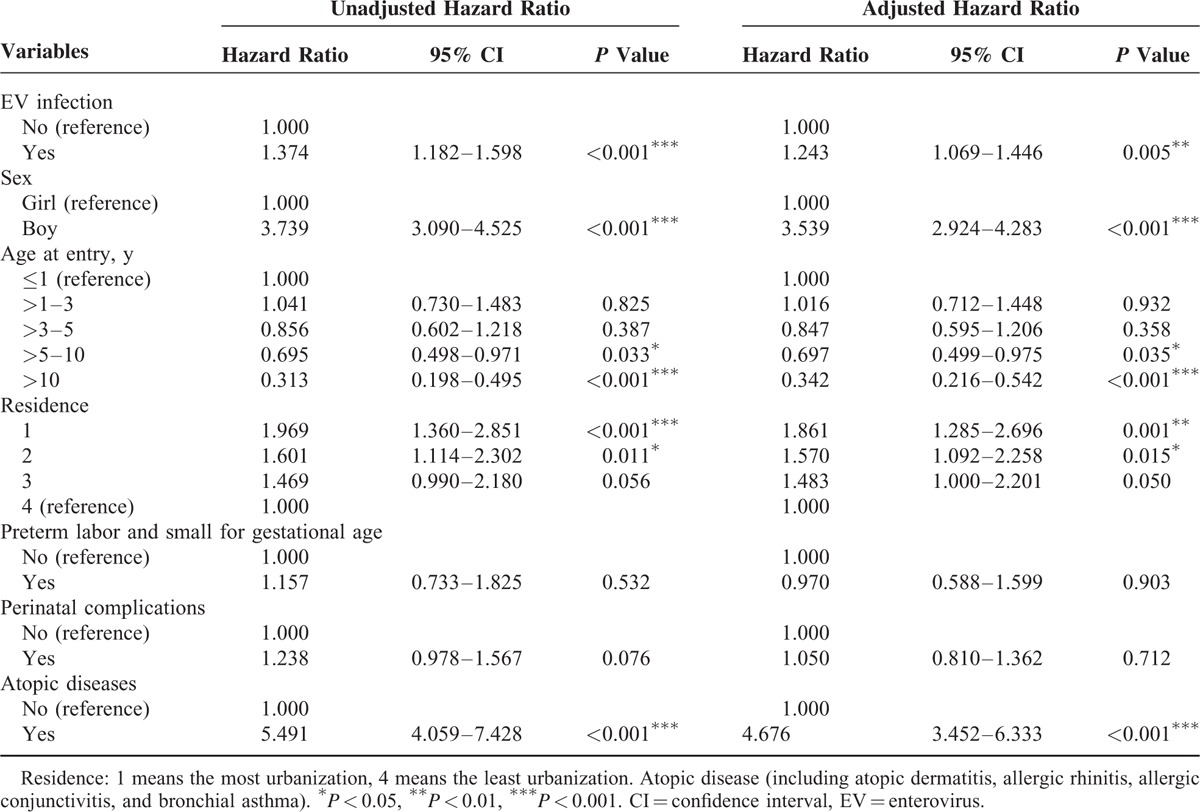
FIGURE 2.
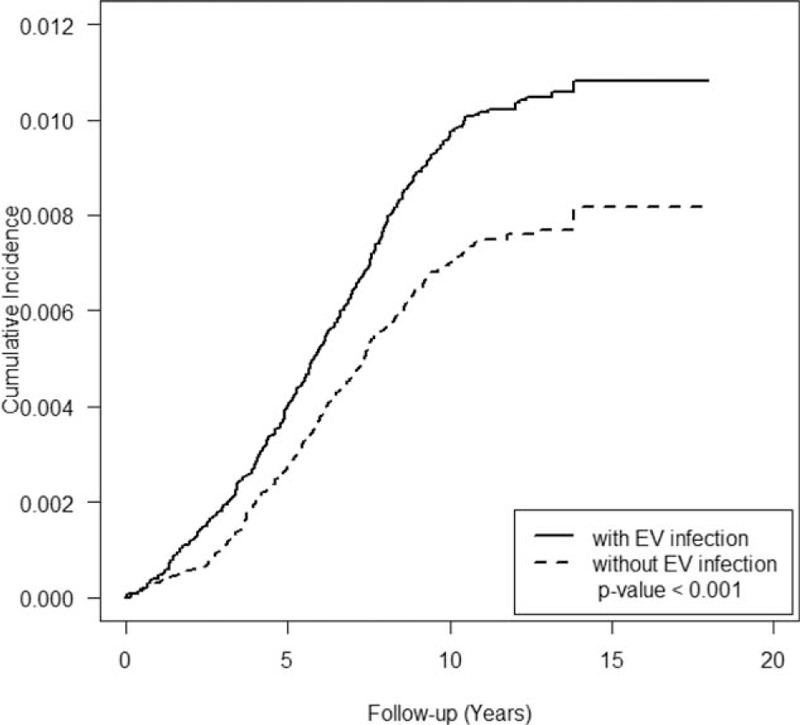
Cumulative incidence of tic disorders in cohorts with/without enterovirus (EV) infection.
Association Between Other Factors and Risk of Tic Disorders
As shown in Table 2, boys were more than 3 times more likely than girls to have tic disorders (HR = 3.539, 95% CI: 2.924–4.283). Compared to children under 1 year of age, children over 5 years of age had lower risk (HR = 0.697, 95% CI: 0.499–0.975 for age 5–10 years and HR = 0.342, 95% CI: 0.216–0.542 for age over 10 years). Within the EV infection cohort, the level of urbanization was associated with a greater incidence of tic disorders (HR = 1.861, 95% CI: 1.285–2.696 for level 1 and HR = 1.570, 95% CI: 1.092–2.258 for level 2). Similar to a previous report,30 atopic diseases were risk factors for tic disorders (HR = 4.676, 95% CI: 3.452–6.333).
Association Between EV Infection With CNS Involvement or Hospitalization and Risk for Tic Disorders
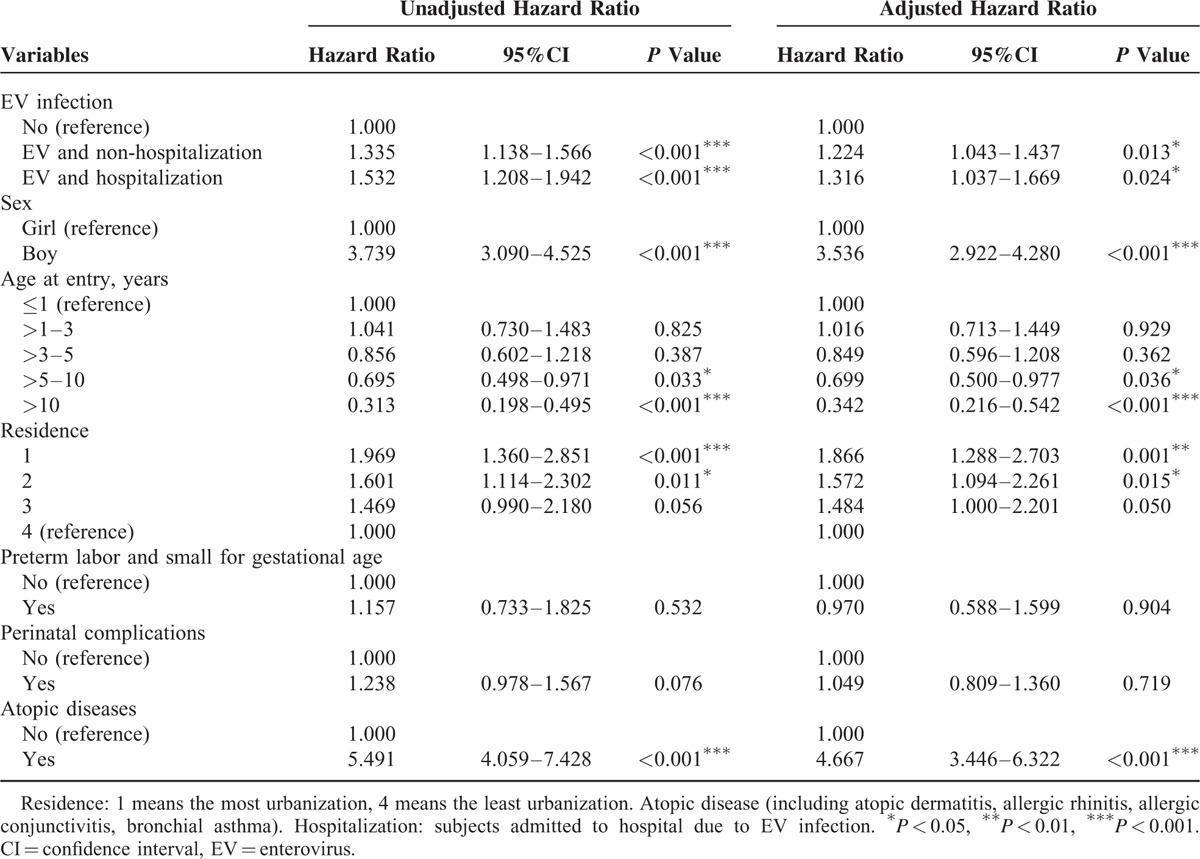
Compared to the control group, the HR for tic disorders was 1.243 (95% CI: 1.068–1.447) among those with an EV infection without CNS involvement and 1.250 (95% CI: 0.642–2.434) among those with an EV infection with CNS involvement. The risk for tic disorders among individuals with EV infection with hospitalization was 1.316 (95% CI: 1.037–1.669) and 1.224 (95% CI: 1.043–1.437) without hospitalization (Tables 3 and 4).
TABLE 3.
Competing Risk Adjusted Cox Regression Analysis
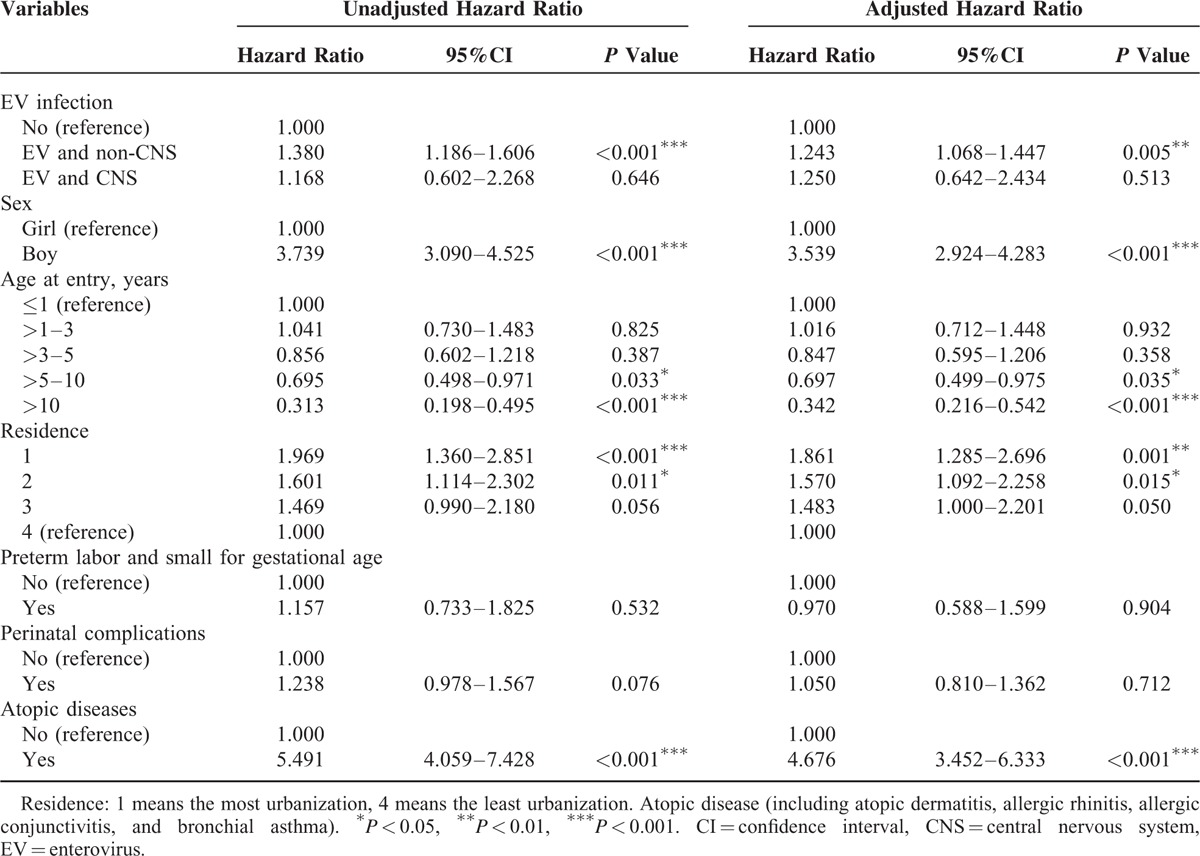
TABLE 4.
Competing Risk Adjusted Cox Regression Analysis
DISCUSSION
To our knowledge, this is the 1st study to investigate the relationship between EV infection and tic disorders using a nationwide longitudinal dataset. The results identified a greater risk for tic disorders with EV infection, after adjusting for confounds including sex, age, urbanization, preterm labor and small for gestational age, perinatal complications, and atopic diseases. In addition, the severity of the EV infection (i.e., as proxied by CNS involvement or hospitalization) did not moderate the risk for tic disorders. In the present study, the mean latency period from initial EV infection to subsequent tic disorders was 5.4 years and to subsequent ADHD was 6.1 years in previous study.26
To our knowledge, there has been only 1 small study of tic disorders and EV infections. The foregoing study reported on the epidemiology of coxsackievirus B4 (CB4) in Taiwan. Twenty-three cases infected with CB4 were randomly selected from clinical specimens isolated in Kaohsiung University Hospital in southern Taiwan between 1993 and 2004; 7 cases were lost to follow-up. Neuropsychological disorders were noted in 5 of 16 subjects, of which 4 had been diagnosed with ADHD and/or tic disorder within 1 to 5 years after CB4 infection.21 Results indicated that EV infection was associated with neuropsychological disorder. However, methodological limitations, such as the lack of a control group, small sample size, and specific select participants, limit the inferences from the study's findings. Results from our study based on longitudinal, representative population-based design provided support that previous EV infection is associated with incident tic disorders.
Perinatal complications, such as delivery complications, low birth weight, Apgar scores at 5 minutes after birth, prolonged labor, umbilical cord round the neck, forceps delivery, neonatal jaundice, and prenatal or neonatal infection have been associated with tic disorders.31–34 Although a recent comprehensive, large-scale, prospective study reported no association between Tourette syndrome/chronic tic disorder and low birth weight, young gestational age as well as complications of delivery,32 the impact of prenatal, or neonatal exposure to infections cannot be excluded. In our study, we did not identify any moderating effects of perinatal complications on tic disorders; however, more research is needed to study the effects of prenatal or neonatal infections on tic disorders.
Several neurostructural alterations have been associated between tic disorders and Gilles de la Tourette syndrome. For example, structural abnormalities in cortical sulci;35 decreased distribution and abnormalities in the distribution of striatal interneurons;36,37 abnormalities cortical thickness in sensorimotor, prefrontal, and temporal cortex;38–40 disturbances in the immune-inflammatory system (e.g., increased serum tumor necrosis factor-α and interleukin-12 at baseline and during symptom exacerbation);41 and decreased numbers of CD4+ and CD8+ T cells have been reported.42 The association between immune response and neurodevelopmental abnormalities subserving tics has been extensively reported on. For example, abnormal brain development as a result of disturbances in the innate immune system is posited to be a proximate cause of tic disorders.
During the past decade, there has been growing evidence documenting an association between virus infection and neuropsychiatric diseases (e.g., cytomegalovirus and Toxoplasma gondii) in the etiopathogenesis of schizophrenia and bipolar disorder.43–45 Viruses may exacerbate neuropsychiatric disease through inflammatory processes (e.g., tumor necrosis factor-α and interleukin-6).46 For example, induction of tumor necrosis factor-α could indirectly increase the production of potentially neurotoxic metabolites (i.e., hydroxykyneurenine) through modulation of neurotransmitter metabolism44 and consequently alter brain development. Elevated levels of interleukin-6 are also associated with cognitive impairment.45 The putative mechanism of virus-induced immune response and its influence on brain development in tic disorders requires further empirical study.
Strength and Limitations
The large nationally representative sample population and longitudinal dataset are key strengths in this study. Limitations in such a database include recall bias and selection bias. Moreover, we could not rule out the possible influence of other confounding variables not assessed and adjusted for, such as maternal smoking status and family history. Second, the period of follow-up is insufficient to confirm all possible cases. Behavioral symptoms of tic disorder may manifest after the end of the study period in some subjects. Third, the diagnosis of EV infection and tic disorders was established by physicians and registered in the database. Differences between healthcare providers in their diagnosis of EV infection cannot be controlled for. Notwithstanding the limitations mentioned above, our results are directionally consistent with the hypothesis that immune inflammatory responses to an infectious agent (e.g., EV) may be relevant to the pathophysiology of tic disorders.
CONCLUSIONS
EV infection is temporally associated with incident tic disorders. Clinicians should advise parents of children with EV infection to attend to symptoms that are associated with the development of tic disorders. Our observations add to growing body of literature implicating the immune-inflammatory process as relevant to the pathoetiology of some mental disorders.
Acknowledgments
This study is based in part on data from the National Health Insurance Research Database provided by the National Health Insurance Administration, Ministry of Health and Welfare and managed by National Health Research Institutes. The interpretation and conclusions contained herein do not represent those of National Health Insurance Administration, Ministry of Health and Welfare or National Health Research Institutes.
Footnotes
Abbreviations: ADHD = attention deficit hyperactivity disorder, CI = confidence interval, CNS = central nervous system, EV = enterovirus, HR = hazard ratio, ICD-9-CM = International Classification of Disease, Revision 9, Clinical Modification, NHIRD = National Health Insurance Research Database.
C-ST contributed to the study concept, design, drafted the initial manuscript, and revised the manuscript; Y-HYcarried out the statistical analysis, interpreted the data, and revised the manuscript, and the contribution is equally to C-ST; K-YH and YL interpreted the data and reviewed the manuscript; RSM conceptualized and designed the study, and critically reviewed the manuscript; VC-HC had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; and all authors approved the final manuscript as submitted.
All authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Picornaviridae Study Group. Picornavirus Taxa. http://www.picornastudygroup.com/taxa/taxa.htm [Accessed July 19, 2011]. [Google Scholar]
- 2.Sawyer MH. Enterovirus infections: diagnosis and treatment. Semin Pediatr Infect Dis 2002; 13:40–47. [DOI] [PubMed] [Google Scholar]
- 3.McMinn PC. An overview of the evolution of enterovirus 71 and its clinical and public health significance. FEMS Microbiol Rev 2002; 26:91–107. [DOI] [PubMed] [Google Scholar]
- 4.McMinn P, Stratov I, Nagarajan L, et al. Neurological manifestations of enterovirus 71 infection in children during an outbreak of hand, foot, and mouth disease in Western Australia. Clin Infect Dis 2001; 32:236–242. [DOI] [PubMed] [Google Scholar]
- 5.Rhoades RE, Tabor-Godwin JM, Tsueng G, et al. Enterovirus infections of the central nervous system. Virology 2011; 411:288–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tabor-Godwin JM, Ruller CM, Bagalso N, et al. A novel population of myeloid cells responding to coxsackievirus infection assists in the dissemination of virus within the neonatal CNS. J Neurosci 2010; 30:8676–8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feuer R, Mena I, Pagarigan RR, et al. Coxsackievirus B3 and the neonatal CNS: the roles of stem cells, developing neurons, and apoptosis in infection, viral dissemination, and disease. Am J Pathol 2003; 163:1379–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suvisaari J, Mautemps N, Haukka J, et al. Childhood central nervous system viral infections and adult schizophrenia. Am J Psychiatry 2003; 160:1183–1185. [DOI] [PubMed] [Google Scholar]
- 9.Rantakallio P, Jones P, Moring J, et al. Association between central nervous system infections during childhood and adult onset schizophrenia and other psychoses: a 28-year follow-up. Int J Epidemiol 1997; 26:837–843. [DOI] [PubMed] [Google Scholar]
- 10.Gau SS, Chang LY, Huang LM, et al. Attention-deficit/hyperactivity-related symptoms among children with enterovirus 71 infection of the central nervous system. Pediatrics 2008; 122:e452–458. [DOI] [PubMed] [Google Scholar]
- 11.Chou IC, Lin CC, Kao CH. Enterovirus encephalitis increases the risk of attention deficit hyperactivity disorder: a Taiwanese population-based case-control study. Medicine (Baltimore) 2015; 94:e707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang LY, Huang LM, Gau SS, et al. Neurodevelopment and cognition in children after enterovirus 71 infection. N Engl J Med 2007; 356:1226–1234. [DOI] [PubMed] [Google Scholar]
- 13.Cubo E. Review of prevalence studies of tic disorders: methodological caveats. Tremor Other Hyperkinet Mov (NY) 2012; 2: pii: tre-02-61-349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elstner K, Selai CE, Trimble MR, et al. Quality of Life (QOL) of patients with Gilles de la Tourette's syndrome. Acta Psychiatr Scand 2001; 103:52–59. [DOI] [PubMed] [Google Scholar]
- 15.Robertson MM. Gilles de la Tourette syndrome: the complexities of phenotype and treatment. Br J Hosp Med (Lond) 2011; 72:100–107. [DOI] [PubMed] [Google Scholar]
- 16.Eddy CM, Rizzo R, Gulisano M, et al. Quality of life in young people with Tourette syndrome: a controlled study. J Neurol 2011; 258:291–301. [DOI] [PubMed] [Google Scholar]
- 17.Mell LK, Davis RL, Owens D. Association between streptococcal infection and obsessive-compulsive disorder, Tourette's syndrome, and tic disorder. Pediatrics 2005; 116:56–60. [DOI] [PubMed] [Google Scholar]
- 18.Leslie DL, Kozma L, Martin A, et al. Neuropsychiatric disorders associated with streptococcal infection: a case-control study among privately insured children. J Am Acad Child Adolesc Psychiatry 2008; 47:1166–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cardona F, Orefici G. Group A streptococcal infections and tic disorders in an Italian pediatric population. J Pediatr 2001; 138:71–75. [DOI] [PubMed] [Google Scholar]
- 20.Müller N, Kroll B, Schwarz MJ, et al. Increased titers of antibodies against streptococcal M12 and M19 proteins in patients with Tourette's syndrome. Psychiatry Res 2001; 101:187–193. [DOI] [PubMed] [Google Scholar]
- 21.Chu PY, Tsai YL, Chen HL, et al. Coxsackievirus B4 in southern Taiwan: molecular epidemiology. J Clin Virol 2009; 45:16–22. [DOI] [PubMed] [Google Scholar]
- 22.Chen CY, Liu CY, Su WC, et al. Factors associated with the diagnosis of neurodevelopmental disorders: a population-based longitudinal study. Pediatrics 2007; 119:e435–e443. [DOI] [PubMed] [Google Scholar]
- 23.Tsai CJ, Loh el W, Lin CH, et al. Correlation of antidepressive agents and the mortality of end-stage renal disease. Nephrology (Carlton) 2012; 17:390–397. [DOI] [PubMed] [Google Scholar]
- 24.National Health Research Institutes (NHRI). Introduction to the National Health Insurnce Research Database (NHIRD). 2013; http://nhird.nhri.org.tw/date_01.htm [Accessed July 31, 2015]. [Google Scholar]
- 25.Lin HC, Wang CH, Tsai FJ, et al. Enterovirus infection is associated with an increased risk of childhood type 1 diabetes in Taiwan: a nationwide population-based cohort study. Diabetologia 2015; 58:79–86. [DOI] [PubMed] [Google Scholar]
- 26.Chou IC, Lin CC, Kao CH. Enterovirus encephalitis increases the risk of attention deficit hyperactivity disorder: a Taiwanese population-based case-control study. Medicine (Baltimore) 2015; 94:e707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen CY, Liang HY, Chang CM, et al. Factors associated with newly diagnosed tic disorders among children in Taiwan: a 10-year nationwide longitudinal study. J Psychiatr Res 2013; 47:1013–1018. [DOI] [PubMed] [Google Scholar]
- 28.Chao TK, Hu J, Pringsheim T. Prenatal risk factors for Tourette Syndrome: a systematic review. BMC Pregnancy Childbirth 2014; 14:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoekstra PJ, Dietrich A, Edwards MJ, et al. Environmental factors in Tourette syndrome. Neurosci Biobehav Rev 2013; 37:1040–1049. [DOI] [PubMed] [Google Scholar]
- 30.Chen MH, Su TP, Chen YS, et al. Attention deficit hyperactivity disorder, tic disorder, and allergy: is there a link? A nationwide population-based study. J Child Psychol Psychiatry 2013; 54:545–551. [DOI] [PubMed] [Google Scholar]
- 31.Saccomani L, Fabiana V, Manuela B, et al. Tourette syndrome and chronic tics in a sample of children and adolescents. Brain Dev 2005; 27:349–352. [DOI] [PubMed] [Google Scholar]
- 32.Mathews CA, Scharf JM, Miller LL, et al. Association between pre- and perinatal exposures and Tourette syndrome or chronic tic disorder in the ALSPAC cohort. Br J Psychiatry 2014; 204:40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martino D, Zis P, Buttiglione M. The role of immune mechanisms in Tourette syndrome. Brain Res 2015; 1617:126–143. [DOI] [PubMed] [Google Scholar]
- 34.Bos-Veneman NG, Kuin A, Minderaa RB, et al. Role of perinatal adversities on tic severity and symptoms of attention deficit/hyperactivity disorder in children and adolescents with a tic disorder. J Dev Behav Pediatr 2010; 31:100–106. [DOI] [PubMed] [Google Scholar]
- 35.Muellner J, Delmaire C, Valabregue R, et al. Altered structure of cortical sulci in gilles de la Tourette syndrome: further support for abnormal brain development. Mov Disord 2015; 30:655–661. [DOI] [PubMed] [Google Scholar]
- 36.Kalanithi PS, Zheng W, Kataoka Y, et al. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proc Natl Acad Sci U S A 2005; 102:13307–13312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haber SN, Kowall NW, Vonsattel JP, et al. Gilles de la Tourette's syndrome. A postmortem neuropathological and immunohistochemical study. J Neurol Sci 1986; 75:225–241. [DOI] [PubMed] [Google Scholar]
- 38.Worbe Y, Gerardin E, Hartmann A, et al. Distinct structural changes underpin clinical phenotypes in patients with Gilles de la Tourette syndrome. Brain 2010; 133:3649–3660. [DOI] [PubMed] [Google Scholar]
- 39.Sowell ER, Kan E, Yoshii J, et al. Thinning of sensorimotor cortices in children with Tourette syndrome. Nat Neurosci 2008; 11:637–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fahim C, Yoon U, Das S, et al. Somatosensory-motor bodily representation cortical thinning in Tourette: effects of tic severity, age and gender. Cortex 2010; 46:750–760. [DOI] [PubMed] [Google Scholar]
- 41.Leckman JF, Katsovich L, Kawikova I, et al. Increased serum levels of interleukin-12 and tumor necrosis factor-alpha in Tourette's syndrome. Biol Psychiatry 2005; 57:667–673. [DOI] [PubMed] [Google Scholar]
- 42.Kawikova I, Leckman JF, Kronig H, et al. Decreased numbers of regulatory T cells suggest impaired immune tolerance in children with tourette syndrome: a preliminary study. Biol Psychiatry 2007; 61:273–278. [DOI] [PubMed] [Google Scholar]
- 43.Leweke FM, Gerth CW, Koethe D, et al. Antibodies to infectious agents in individuals with recent onset schizophrenia. Eur Arch Psychiatry Clin Neurosci 2004; 254:4–8. [DOI] [PubMed] [Google Scholar]
- 44.Prossin AR, Yolken RH, Kamali M, et al. Cytomegalovirus antibody elevation in bipolar disorder: relation to elevated mood states. Neural Plast 2015; 2015:939780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamdani N, Daban-Huard C, Lajnef M, et al. Cognitive deterioration among bipolar disorder patients infected by Toxoplasma gondii is correlated to interleukin 6 levels. J Affect Disord 2015; 179:161–166. [DOI] [PubMed] [Google Scholar]
- 46.Jelinek J, Adkins I, Mikulkova Z, et al. In vitro activation of CMV-specific human CD8(+) T cells by adenylate cyclase toxoids delivering pp65 epitopes. Bone Marrow Transplant 2012; 47:243–250. [DOI] [PubMed] [Google Scholar]


