Abstract
The often cited need to achieve ≥95% (nearly perfect) adherence to antiretroviral therapy (ART) for successful virologic outcomes in HIV may present a barrier to initiation of therapy in the early stages of HIV.
This meta-analysis synthesized 43 studies (27,905 participants) performed across >26 countries, to determine the relationship between cut-off point for optimal adherence to ART and virologic outcomes.
Meta-analysis was performed using a random-effect model to calculate pooled odds ratios with corresponding 95% confidence intervals.
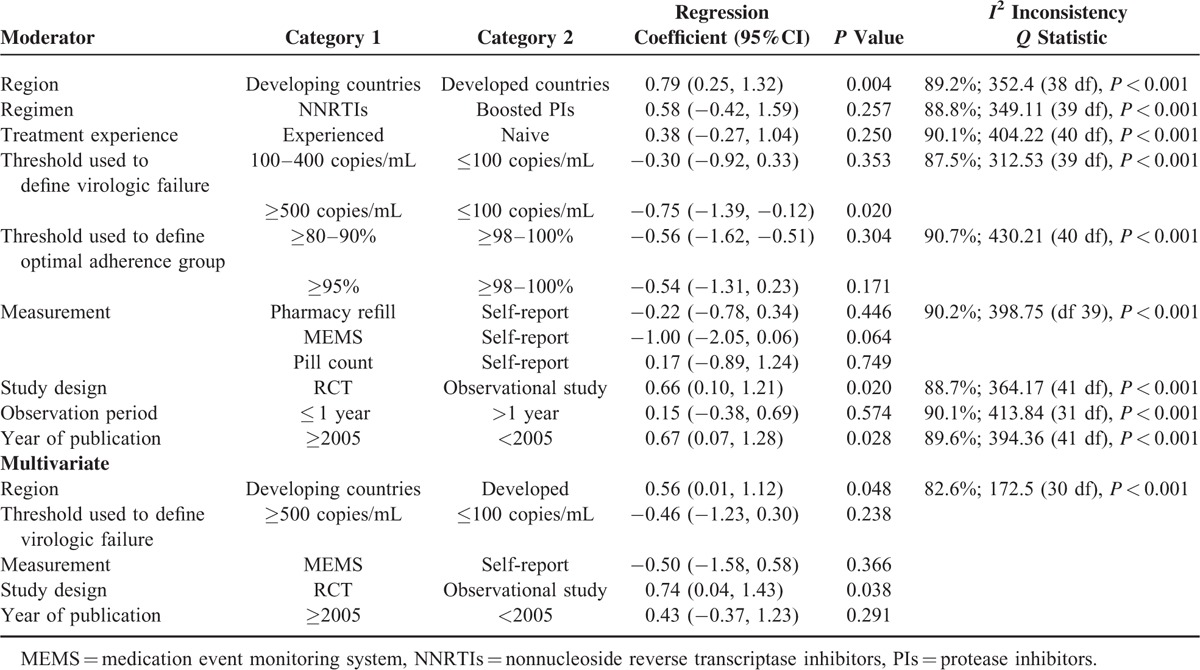
The mean rate of patients reporting optimal adherence was 63.4%. Compared with suboptimal adherence, optimal adherence was associated with a lower risk of virologic failure (0.34; 95% CI: 0.26–0.44). There were no significant differences in the pooled odds ratios among different optimal adherence thresholds (≥98–100%, ≥95%, ≥80–90%). Study design (randomized controlled trial vs observational study) (regression coefficient 0.74, 95% CI: 0.04–1.43, P < 0.05) and study region (developing vs developed countries; regression coefficient 0.56, 95% CI: 0.01–1.12, P < 0.05) remained as independent predictors of between-study heterogeneity, with more patients with optimal adherence from developing countries or randomized controlled trials experiencing virologic failure.
The threshold for optimal adherence to achieve better virologic outcomes appears to be wider than the commonly used cut-off point (≥95% adherence). The cut-off point for optimal adherence could be redefined to a slightly lower level to encourage the prescribing ART at an early stage of HIV infection.
INTRODUCTION
HIV/AIDS has been transformed into a manageable chronic disease with the advent of combination antiretroviral therapy (ART) initiated as the standard of care.1 Three classes of HIV medications have been widely used in combination—nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs), nonnucleoside reverse transcriptase inhibitors (NNRTIs), and protease inhibitors (PIs).1 Despite the availability of effective treatment options, suboptimal adherence to treatment can result in insufficient viral suppression and promote the emergence of drug-resistant viral strains, resulting in regimen failure, progression to AIDS, and death.2–4 Paterson et al suggested that at least 95% adherence to unboosted PIs was required for virologic suppression.5 This 95% adherence cut-off point, based on what is now obsolete therapy, has been widely used as the level of optimal adherence needed to be met by patients taking newer agents and their combinations. The concern that patients may not achieve a near-perfect adherence presents a barrier for initiation of therapy in the early stages of HIV.6
This meta-analysis integrated finding from observational studies on ART adherence with 2 objectives: (a) to critically evaluate the association between optimal adherence to ART and virologic outcomes, and (b) to use meta-regression to determine methodological, regimen, and population factors that could moderate the relationship between adherence and virologic outcomes.
METHODS
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement in conducting this meta-analysis.7 Studies eligible for inclusion were randomized controlled trials, retrospective analyses of data from trials, and cohort studies measuring the relationship between medication adherence to ART and virologic failure.
Search Strategy
WB carried out systematic literature searches of the electronic databases MEDLINE via PubMed, Cochrane Clinical Trials, and EMBASE from their inception date to 17 April 2015. This search used combinations of the following key words: medication adherence, patient compliance, antiretroviral therapy, antiretroviral agent, antiretroviral treatment, protease inhibitors, non-nucleoside reverse transcriptase inhibitors, virologic failure, and viral load. The reference lists of all articles included in this meta-analysis were also searched. Review articles, editorials, commentaries, government reports, and guidelines were excluded from this review. Titles and abstracts of potentially relevant articles were screened independently by WB and YM. Full articles of potentially appropriate citations were screened for inclusion in this review if they fulfilled the following criteria: original research, participants aged 16 years or older, having a clear definition of medication adherence measurement and clear cut-off points for optimal and suboptimal adherence, and virologic failure stratified by optimal and suboptimal medication adherence groups. Ethical approval was not required as this study was based on published data and had no direct access to patient information.
Data Collection and Outcome Measures
WB extracted data using standardized forms, with recording of authors, year of publication, country of study, study type, regimen, method of adherence measurement, cut-off points for good adherence, and virologic failure. The data were verified by a second reviewer (YM). Disagreements between reviewers were resolved through discussion until a consensus was reached. Study authors’ grouping of patients into optimal and suboptimal adherence using the most objective measure was used. When a study reported >1 adherence measurement, the most reliable adherence measurement data was used, with reliability defined in following order: medication event monitoring system (MEMS) > pill count > pharmacy refill > self-reported adherence in the past week > self-reported adherence in the past month. When the number of virologic failures within each adherence group was not reported, we calculated virologic failure from the information provided in the paper or contacted the corresponding author. Studies were excluded when it was not possible to obtain virologic failure data in each adherence group. The United Nations Human Development Index (HDI) ranking was used to categories studies into low and high human development groups.8
Statistical Analyses
The data were analyzed using Review Manager (RevMan) version 5.3 (Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, 2014) and Comprehensive Meta-Analysis (CAM) version 3.3.070 (Biostat, Englewood, NJ). Each class of antiretroviral was considered in a separate analysis of the association between adherence and virologic failure in randomized clinical trials. Results are presented based on 9 categories, including study region, antiretroviral regimen, treatment experience, virologic failure cut-off points, adherence cut-off points, adherence measurement, study design, observation period, and year of publication. Adherence pooled odds ratios and 95% confidence intervals were calculated using a random effect model (DerSimonian and Lard)9 that accommodated the random variation within studies and between-studies.10
Heterogeneity between-studies were examined using the Q and I2 statistics.9,11 The odds ratio was plotted against the inverse of standard error to identify the risk of publication bias by visually assessing the symmetry of funnel plots. Statistical significance was confirmed using Egger's test,12 with a P value <0.05 considered suggestive of publication bias. A meta-regression was performed to examine major moderators of the between-studies heterogeneity. Results with P values <0.1 from univariate analyses were included in the multivariate meta-regression model.
RESULTS
Overall, 1796 studies were identified, of which 1449 were excluded after review of the title and abstract (Figure 1). The full text of the remaining 347 citations was screened, and 43 studies with 27,905 participants met the inclusion criteria. The included studies had wide a variation in sample sizes (range = 34–3607, mean = 649, SD = 805) and a slight majority of participants were men (57%). Twenty-five studies were prospective studies5,13–36 that reported virologic failure according to adherence group. The remaining studies were randomized controlled trials (11)37–47 and retrospective studies (7).48–54 Characteristics of the included studies are shown in Table 1.
FIGURE 1.
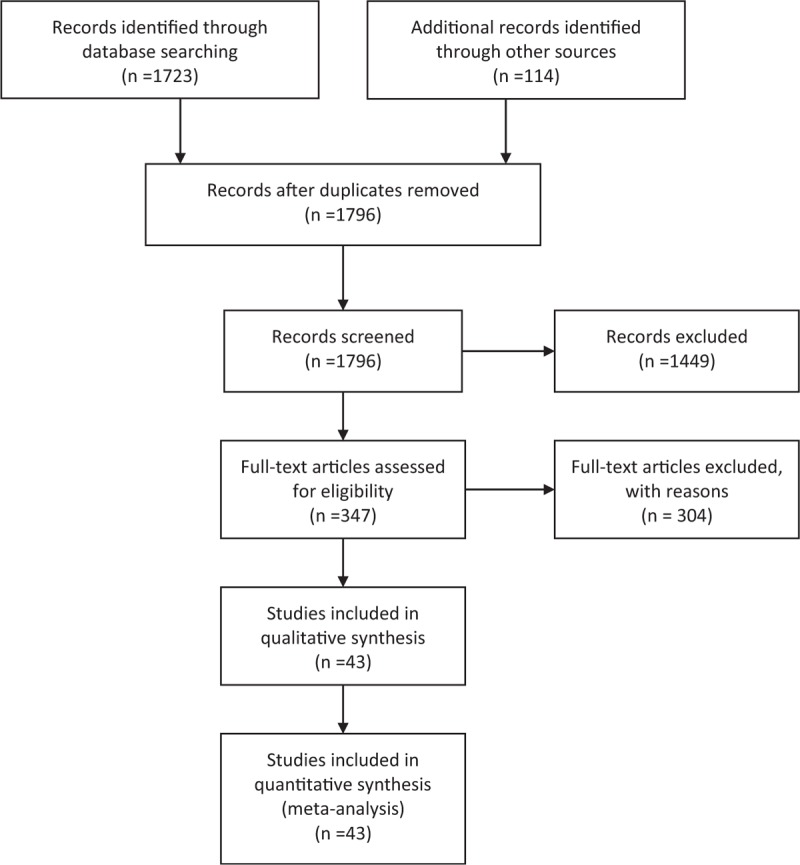
Flow diagram of study selection.
TABLE 1.
Characteristics of Included Studies in Meta-Analysis of Adherence to Antiretroviral Therapy and Virologic Failure
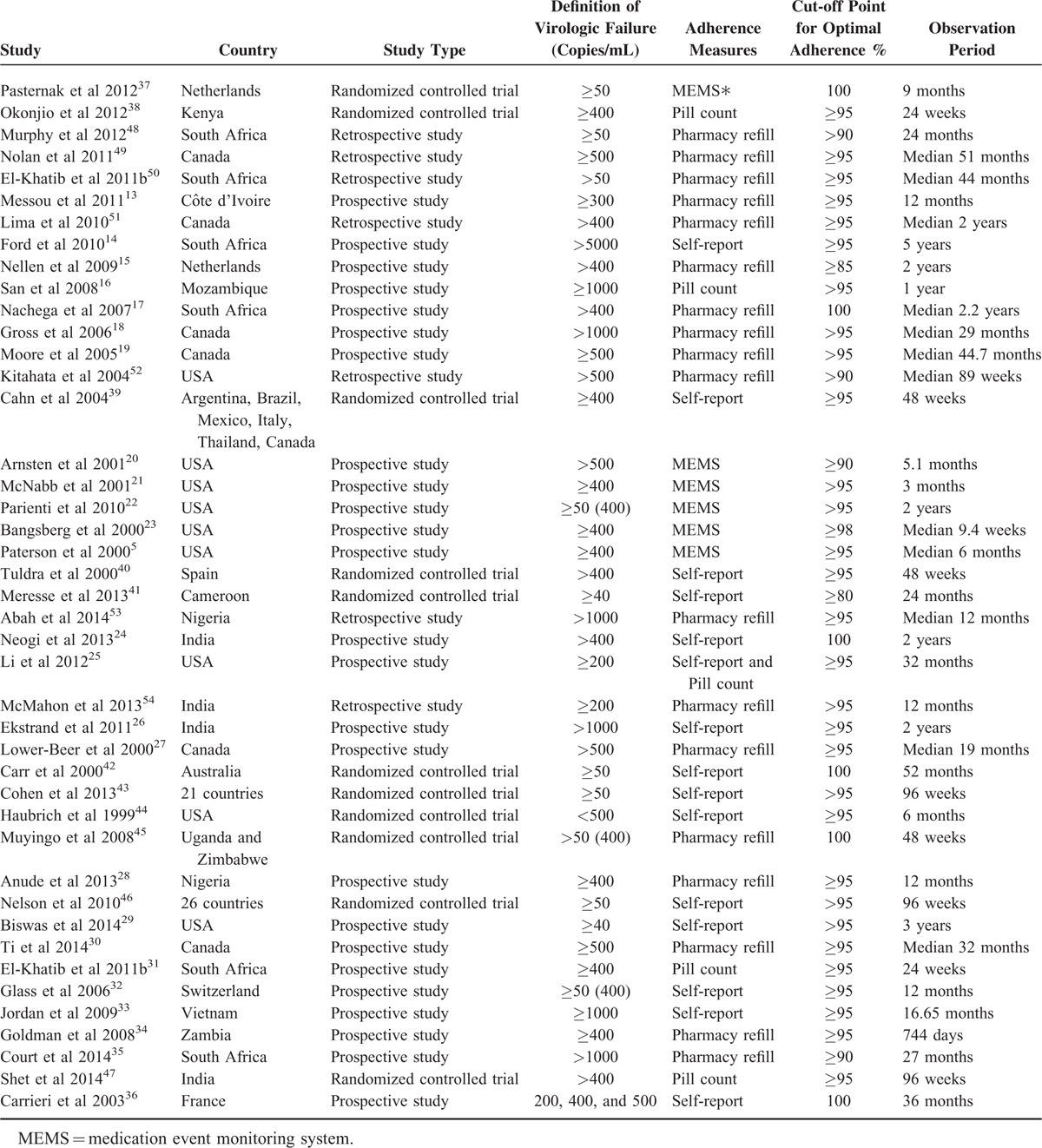
With respect to location, 14 studies were conducted in sub-Saharan Africa; 9 in the US; 6 in Canada; 5 in Europe; 5 in Asia; 1 in Australia; and 3 studies in several countries. Twenty-two (49%) studies included only treatment-naive patients and the remaining 21 studies included both treatment-naive and/or treatment-experienced patients. All studies reported cut-off points for optimal adherence and virologic failure. Thirty studies (70%) defined optimal adherence as ≥95%, with the remainder using 100%, 98%, 90%, 85%, and 80% as the cut-off points. Optimal adherence rates varied greatly across studies, partially due to the use of these different cut-off points and also different methods of measurement to assess adherence. The mean rate of achieving optimal adherence in adults was 63.4% (standard deviation [SD] = 23.7, range 5% to 97%, n = 43).
Meta-analysis and Meta-regression
Of a total 27,905 participants, 22,740 participants had a viral load and adherence measurement; 7056 (31%) had virologic failure. Overall, 3464 of 15,067 participants with optimal adherence to ART (23%), and 3592 of 7673 participants with suboptimal adherence (47%) participants had virologic failure (Figure 2). The pooled odds ratio for virologic failure for optimal adherence compared to suboptimal adherence was 0.34 (95% CI: 0.26–0.44). A high degree of heterogeneity was found: Q statistic P < 0.001 and I2 = 90%. The funnel plot did not show asymmetry (Figure 3), and the result of Egger's test was not statistically significant (P = 0.68). We conducted subgroup analyses to recalculate the pooled odds ratio according to study design, HDI rank, regimen, treatment experience, viral load cut-off points, adherence measurement, and adherence cut-off points (Table 2).
FIGURE 2.
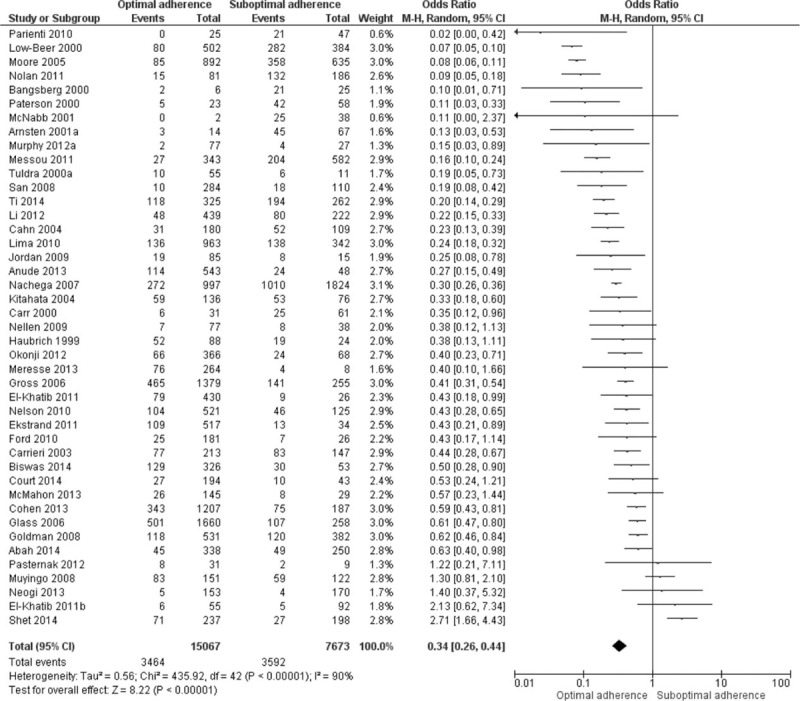
Association between adherence to antiretroviral therapy and virologic failure.
FIGURE 3.
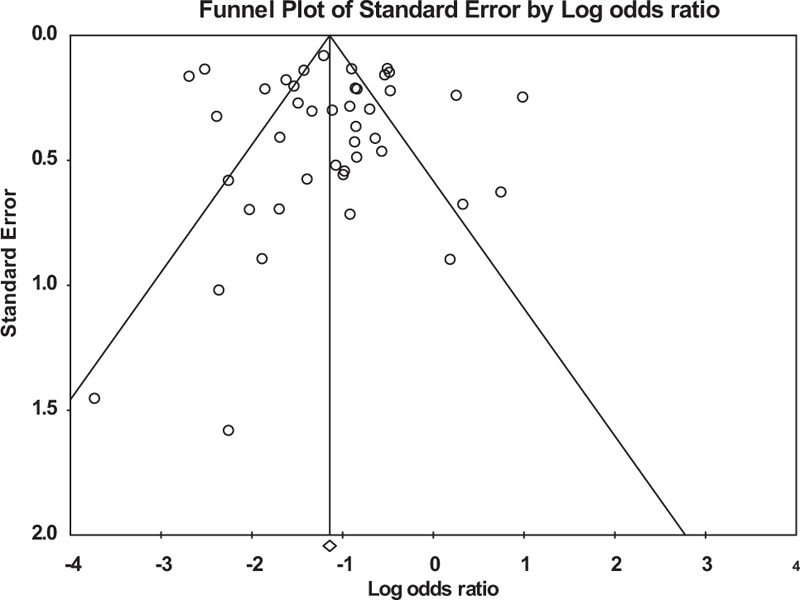
Funnel plot for the association between adherence to antiretroviral therapy and virologic failure (P = 0.68 at Egger's test).
TABLE 2.
Subgroup Analysis Adherence to Antiretroviral Therapy and Virologic Failure
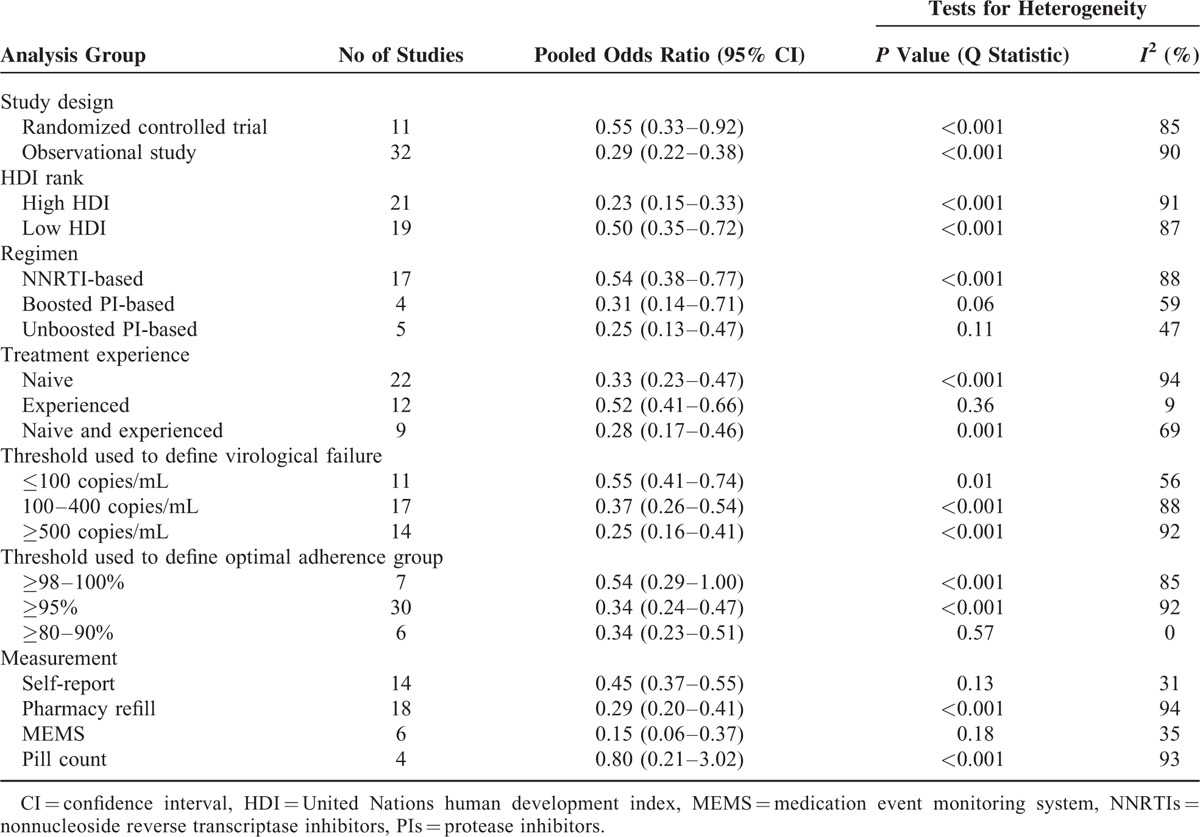
The results of univariate meta-regression analyses for different moderators are shown in Table 3. Based on virologic failure cut-off points, studies were classified into three sets including: ≤100 copies/mL, 11 studies (N = 5646); between 100 copies/mL and 400 copies/mL, 17 studies (N = 9351); and between 500 copies/mL and 1000 copies/mL, 14 studies (N = 7383). The pooled odds ratio for virologic failure for optimal adherence compared to suboptimal adherence for the studies with the lowest virologic failure cut-off was higher (0.55; 95% CI: 0.41–0.74, I2 = 56%) than for the studies with an intermediate virologic failure cut-off (0.37; 95% CI: 0.26–0.54, I2 = 88%). The group using a virologic failure cut-off >500 copies/mL had the lowest pooled odds ratio for virologic failure (0.25; 95% CI: 0.16–0.41, I2 = 92%). Studies with the lowest virologic failure cut-off reported a significantly different pooled odds ratio compared with studies with a virologic failure cut-off > 500 copies/mL (regression coefficient −0.75; 95% CI: −1.39 to −0.12, P = 0.02).
TABLE 3.
Meta-Regression Analysis of Moderators for the Association Between Antiretroviral Adherence and Virologic Failure
According to participants’ treatment experience, studies were grouped into 3 sets: treatment-naive patients only, 22 studies (N = 17,010); treatment-experienced patients only, 12 studies (N = 4009), and both treatment-naive and experienced patients, 9 studies (N = 1721). The pooled odds ratio for optimal adherence compared to suboptimal adherence for virologic failure for treatment-experienced patients was the highest (Table 2); however, no statistically significant difference in pooled odds ratio was found between the 3 groups.
The relationship between adherence and virologic outcomes varied with type of adherence measurement. The pooled odds ratio for the self-report adherence measure (0.45; 95% CI: 0.37–0.55, I2 = 31%) was higher than the pooled odds ratio for the pharmacy refill (0.29; 95% CI: 0.20–0.41, I2 = 94%). The group using MEMS adherence measure had the lowest pooled odd ratio (0.15; 95% CI: 0.06–0.37, I2 = 35%) for optimal adherence compared to suboptimal adherence for virologic failure. There was a trend toward significant difference across the odds of virologic failure between self-report and MEMS (regression coefficient −1.00; 95% CI: −2.05, 0.06, P = 0.06), but not between self-report and pharmacy refill (regression coefficient −0.22; 95% CI: −0.78, 0.34, P = 0.45).
The pooled odds ratios were also estimated by grouping studies using cut-off points for optimal adherence studies with a cut-off point between 98% and 100%, 7 studies (N = 3940); studies with a cut-off point of ≥95%, 30 studies (N = 17,779); and studies with a cut-off point of 80% to 90%, 6 studies (N = 1021). The pooled odds ratios for virologic failure for optimal adherence compared to suboptimal adherence for each cut-off point were similar, with no statistically significant differences.
The pooled odds ratio for optimal adherence compared to suboptimal adherence for observational studies was significantly greater than randomized controlled studies (regression coefficient, 0.66; 95% CI: 0.10, 1.21, P = 0.02). Studies were aggregated into three subgroups according to HIV-medication regimens: NNRTI-based, boosted PI-based, and unboosted PI-based. The pooled odds ratio for virologic failure for optimal adherence compared to suboptimal adherence for patients taking NNRTI-containing regimens was the highest, but the differences in pooled odds ratios between the regimens were not statistically significant.
Studies were subgrouped into 2 groups based on the HDI of the country in which the study was performed: very high HDI, 21 studies (N = 10,466); low HDI, 19 studies (N = 9945). The pooled odds ratio for optimal adherence compared to suboptimal adherence for countries with low HDI (0.50; 95% CI: 0.35–0.72) was significantly higher than countries with very high HDI (0.23; 95% CI: 0.15–0.33).
A multivariate meta-regression model was built-in to examine the specific moderators of the between-study heterogeneity, including the following: study region, threshold used to define virologic failure, adherence measurement, study design, and year of publication. Study design (observational study versus randomized controlled trials; regression coefficient 0.74, 95% CI: 0.04–1.43, P < 0.05) and study region (developed versus developing countries; regression coefficient 0.56, 95% CI: 0.01–1.12, P < 0.05) remained as independent predictors of between-study heterogeneity.
DISCUSSION
This meta-analysis of 43 studies, involving 27,905 participants, addresses a gap in the current HIV treatment adherence literature with a quantitative evaluation of the association between level of adherence and virologic outcomes among adults taking ART. This study revealed that adherence levels as low as 80% to 90% may be adequate for viral suppression in patients taking newer antiretroviral drugs. Our data also showed that pooled odds ratios for virologic failure for optimal adherence compared to suboptimal adherence were similar between NNRTI-based and boosted PI-based regimens. The effectiveness of newer antiretroviral agents at the lower level of adherence may encourage the prescribing of ART at an early stage of HIV infection.
The findings indicated that the mean proportion of patients who were reported to demonstrate optimal adherence worldwide was 63.4%, which is similar to a meta-analysis of 84 studies that reported 62% of patients take ≥90% of their prescribed ART.55 The results of this study demonstrate that adherence is robustly associated with virologic outcomes across the various types of adherence measure, ART regimen, study population, and reporting. The odds of virologic failure were almost 3 times higher for participants with suboptimal adherence compared with those with optimal adherence. This confirms that achieving long-term optimal adherence is indeed Achilles’ heel of successful virologic outcomes.56 The need for clinicians to exert concerted efforts to maintain continuing optimal adherence to antiretroviral therapy is indisputable.
Classifying patients according to various optimal adherence thresholds (≥98–100%, ≥95%, and 80–90%) did not result in statistically significant differences in the odds of virologic failure. This finding is consistent with a meta-analysis of 37 studies in children that reported no significant group differences in virologic outcomes between different thresholds of good adherence.57 This suggests that patients who achieved “perfect” (100%) or “near perfect” (≥95%) adherence did not necessarily have better virologic outcomes than patients who had achieved “good enough” (≥80–90%) adherence. This finding has clinical importance and is in line with previous studies58,59 that indicated that although the need to maintain high levels of adherence to achieve long-term virologic suppression is clear, the level of adherence behavior capable of sustaining viral suppression is broader than previously thought.
Considerable variation in the relationship between adherence and virologic outcomes was found based on the type of adherence measurement used in the studies we reviewed. For studies using self-reported adherence, the odds of virologic failure in participants with optimal adherence was about half that of participants with suboptimal adherence. The odds of virologic failure for optimal adherence were about one-third and one-seventh that of the participants with suboptimal adherence using pharmacy refill and MEMS, respectively. Our meta-analysis undermines the validity of using self-reported adherence to distinguish virologic outcomes. A high proportion of patients with optimal self-reported adherence experienced virologic failure. Self-reported adherence is potentially confounded by social desirability and recall bias, which leads patients to overestimate their actual adherence;60 this method is inferior to MEMS in its ability to explain virologic outcomes.
Despite the findings17,61 of previous studies suggesting the need for different levels of optimal adherence between antiretroviral regimens for achieving similar virologic outcomes, classifying patients based on regimen did not result in statistically significant differences in the odds of virologic outcomes in this meta-analysis.
The pooled odds ratio for optimal adherence compared to suboptimal adherence for virologic failure for studies with virologic failure cut-offs < 100 copies/mL were significantly higher than studies with virologic failure cut-offs between 500 copies/mL and 1000 copies/mL. The rate of virologic failure detected in patients with good adherence increased when studies defined a virologic failure at a low level of HIV-1 ribonucleic acid (RNA). The relationship between adherence and viral load improved when the level of detection of HIV-1 RNA increased. The tighter the definition of virologic failure the more likely it is to unmask suboptimal adherence.
The odds of virologic failure for optimal adherence were about half and one-third that of the patients with suboptimal adherence in countries with a low HDI and high HDI, respectively. More patients with optimal adherence experienced virologic failure in countries with low HDI than in countries with high HDI. This review indicates that patients with equal or better levels of optimal adherence in developing countries compared to developed countries62 does not necessarily translate into better virologic outcomes. This might be associated with the increase in pretreatment antiretroviral drug resistance63 and unavailability of baseline HIV drug resistance testing before initiation of ART64 in resource-limited settings that have a potential to contribute to the increasing rates of virologic failure in optimally adherent patients. We support moves toward the use viral load monitoring at the point of care in resource-limited settings65 to improve treatment outcomes.
Study design (observational study vs randomized controlled trials) was an independent predictor of between-study heterogeneity. More patients with optimal adherence experienced virologic failure in randomized controlled trials than in observational studies. Differences in estimated magnitude of treatment effect are very common between randomized controlled trials and observational studies.66 This difference in virologic outcomes between study designs might be related with selection bias in observational studies67 and higher quality and rigor of randomized controlled trials.
This meta-analysis shares the limitations intrinsic to meta-analysis in general and with studies of adherence in particular. We only included studies published in English, so we may have missed studies that were relevant to our research question during the literature search. When the included studies were stratified and analyzed based on regimen, virologic failure cut-off, adherence cut-off and type of adherence measurement, heterogeneity between-studies remained high for most of the subgroups. Because of this high degree of heterogeneity, which was not entirely described either by subgroup analysis or by meta-regression, our pooled results need to be viewed with caution.
CONCLUSION
Irrespective of the cut-off point for optimal adherence, our findings support the tenet that optimal adherence to ART is associated with positive clinical outcomes. The threshold for optimal adherence to achieve better virologic outcomes appears to be wider than the commonly used cut-off point (≥95% adherence). Though patients taking ART should be instructed to attain ≥95% adherence, apprehensions of slightly lower adherence should not deter prescribing ART regimens at an early stage of HIV infection.
Acknowledgments
The authors would like to acknowledge Yihienew Mequanint for his assistance in screening articles and verifying the extracted data, and all study authors who responded to inquiries about relevant papers.
This meta-analysis was conducted in partial fulfillment of a PhD in Pharmacy at School of Medicine, University of Tasmania. The authors received no specific funding for this research.
Footnotes
Abbreviations: AIDS = acquired immunodeficiency syndrome, ART = antiretroviral therapy, CAM = comprehensive meta-Analysis, HDI = United Nations human development index, HIV = human immunodeficiency virus, MEMS = medication event monitoring system, NNRTIs = nonnucleoside reverse transcriptase inhibitors, NRTIs = nucleoside/nucleotide reverse transcriptase inhibitors, PIs = protease inhibitors, PRISMA = preferred reporting items for systematic reviews and meta-analyses, RevMan = review manager, RNA = ribonucleic acid, SD = standard deviation.
Author contributions: WB, LC, LB, and GP conceived of and designed the meta-analysis. WB and YM reviewed abstracts and full articles. WB and YB extracted the data. WB performed the meta-analysis and wrote the first draft. All authors have read and approved the final draft.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998; 338:853–860. [DOI] [PubMed] [Google Scholar]
- 2.Delaugerre C, Rohban R, Simon A, et al. Resistance profile and cross-resistance of HIV-1 among patients failing a non-nucleoside reverse transcriptase inhibitor-containing regimen. J Med Virol 2001; 65:445–448. [PubMed] [Google Scholar]
- 3.Parienti JJ, Massari V, Descamps D, et al. Predictors of virologic failure and resistance in HIV-infected patients treated with nevirapine- or efavirenz-based antiretroviral therapy. Clin Infect Dis 2004; 38:1311–1316. [DOI] [PubMed] [Google Scholar]
- 4.Bangsberg DR, Perry S, Charlebois ED, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS 2001; 15:1181–1183. [DOI] [PubMed] [Google Scholar]
- 5.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med 2000; 133:21–30. [DOI] [PubMed] [Google Scholar]
- 6.Kurth AE, Mayer K, Beauchamp G, et al. Clinician practices and attitudes regarding early antiretroviral therapy in the United States. J Acquir Immune Defic Syndr 2012; 61:e65–e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.United Nations Development Programme. Human development report. New York, USA 2014. [Google Scholar]
- 9.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188. [DOI] [PubMed] [Google Scholar]
- 10.Hedges LV, Vevea JL. Fixed-and random-effects models in meta-analysis. Psychol Methods 1998; 3:486–504. [Google Scholar]
- 11.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Messou E, Chaix ML, Gabillard D, et al. Association between medication possession ratio, virologic failure and drug resistance in HIV-1-infected adults on antiretroviral therapy in Cote d’Ivoire. J Acquir Immune Defic Syndr 2011; 56:356–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ford N, Darder M, Spelman T, et al. Early adherence to antiretroviral medication as a predictor of long-term HIV virological suppression: five-year follow up of an observational cohort. PLoS One 2010; 5:e10460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nellen JF, Nieuwkerk PT, Burger DM, et al. Which method of adherence measurement is most suitable for daily use to predict virological failure among immigrant and non-immigrant HIV-1 infected patients? AIDS Care 2009; 21:842–850. [DOI] [PubMed] [Google Scholar]
- 16.San Lio MM, Carbini R, Germano P, et al. Evaluating adherence to highly active antiretroviral therapy with use of pill counts and viral load measurement in the drug resources enhancement against AIDS and malnutrition program in Mozambique. Clin Infect Dis 2008; 46:1609–1616. [DOI] [PubMed] [Google Scholar]
- 17.Nachega JB, Hislop M, Dowdy DW, et al. Adherence to nonnucleoside reverse transcriptase inhibitor-based HIV therapy and virologic outcomes. Ann Intern Med 2007; 146:564–573. [DOI] [PubMed] [Google Scholar]
- 18.Gross R, Yip B, Lo Re V, 3rd, et al. A simple, dynamic measure of antiretroviral therapy adherence predicts failure to maintain HIV-1 suppression. J Infect Dis 2006; 194:1108–1114. [DOI] [PubMed] [Google Scholar]
- 19.Moore DM, Hogg RS, Yip B, et al. Discordant immunologic and virologic responses to highly active antiretroviral therapy are associated with increased mortality and poor adherence to therapy. J Acquir Immune Defic Syndr 2005; 40:288–293. [DOI] [PubMed] [Google Scholar]
- 20.Arnsten JH, Demas PA, Farzadegan H, et al. Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: comparison of self-report and electronic monitoring. Clin Infect Dis 2001; 33:1417–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNabb J, Ross JW, Abriola K, et al. Adherence to highly active antiretroviral therapy predicts virologic outcome at an inner-city human immunodeficiency virus clinic. Clin Infect Dis 2001; 33:700–705. [DOI] [PubMed] [Google Scholar]
- 22.Parienti JJ, Ragland K, Lucht F, et al. Average adherence to boosted protease inhibitor therapy, rather than the pattern of missed doses, as a predictor of HIV RNA replication. Clin Infect Dis 2010; 50:1192–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bangsberg DR, Hecht FM, Charlebois ED, et al. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS 2000; 14:357–366. [DOI] [PubMed] [Google Scholar]
- 24.Neogi U, Heylen E, Shet A, et al. Long-term efficacy of first line antiretroviral therapy in Indian HIV-1 infected patients: a longitudinal cohort study. PLoS One 2013; 8:e55421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li JZ, Paredes R, Ribaudo HJ, et al. Relationship between minority nonnucleoside reverse transcriptase inhibitor resistance mutations, adherence, and the risk of virologic failure. AIDS 2012; 26:185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ekstrand ML, Shet A, Chandy S, et al. Suboptimal adherence associated with virological failure and resistance mutations to first-line highly active antiretroviral therapy (HAART) in Bangalore, India. Int Health 2011; 3:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Low-Beer S, Yip B, O'Shaughnessy MV, et al. Adherence to triple therapy and viral load response. J Acquir Immune Defic Syndr 2000; 23:360–361. [DOI] [PubMed] [Google Scholar]
- 28.Anude CJ, Eze E, Onyegbutulem HC, et al. Immuno-virologic outcomes and immuno-virologic discordance among adults alive and on anti-retroviral therapy at 12 months in Nigeria. BMC Infect Dis 2013; 13:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biswas B, Spitznagel E, Collier AC, et al. Characterizing HIV medication adherence for virologic success among individuals living with HIV/AIDS: experience with the CNS HIV Antiretroviral Therapy Effects Research (CHARTER) cohort. J HIV AIDS Soc Serv 2014; 13:8–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ti L, Milloy MJ, Shannon K, et al. Suboptimal plasma HIV-1 RNA suppression and adherence among sex workers who use illicit drugs in a Canadian setting: an observational cohort study. Sex Transm Infect 2014; 90:418–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El-Khatib Z, Ekstrom AM, Coovadia A, et al. Adherence and virologic suppression during the first 24 weeks on antiretroviral therapy among women in Johannesburg, South Africa—a prospective cohort study. BMC Public Health 2011; 11:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glass TR, De Geest S, Weber R, et al. Correlates of self-reported nonadherence to antiretroviral therapy in HIV-infected patients: the Swiss HIV Cohort Study. J Acquir Immune Defic Syndr 2006; 41:385–392. [DOI] [PubMed] [Google Scholar]
- 33.Jordan MR, La H, Nguyen HD, et al. Correlates of HIV-1 viral suppression in a cohort of HIV-positive drug users receiving antiretroviral therapy in Hanoi, Vietnam. Int J STD AIDS 2009; 20:418–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldman JD, Cantrell RA, Mulenga LB, et al. Simple adherence assessments to predict virologic failure among HIV-infected adults with discordant immunologic and clinical responses to antiretroviral therapy. AIDS Res Hum Retroviruses 2008; 24:1031–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Court R, Leisegang R, Stewart A, et al. Short term adherence tool predicts failure on second line protease inhibitor-based antiretroviral therapy: an observational cohort study. BMC Infect Dis 2014; 14:664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carrieri MP, Raffi F, Lewden C, et al. Impact of early versus late adherence to highly active antiretroviral therapy on immuno-virological response: a 3-year follow-up study. Antivir Ther 2003; 8:585–594. [DOI] [PubMed] [Google Scholar]
- 37.Pasternak AO, de Bruin M, Jurriaans S, et al. Modest nonadherence to antiretroviral therapy promotes residual HIV-1 replication in the absence of virological rebound in plasma. J Infect Dis 2012; 206:1443–1452. [DOI] [PubMed] [Google Scholar]
- 38.Okonji JA, Zeh C, Weidle PJ, et al. CD4, viral load response, and adherence among antiretroviral-naive breast-feeding women receiving triple antiretroviral prophylaxis for prevention of mother-to-child transmission of HIV in Kisumu, Kenya. J Acquir Immune Defic Syndr 2012; 61:249–257. [DOI] [PubMed] [Google Scholar]
- 39.Cahn P, Vibhagool A, Schechter M, et al. Predictors of adherence and virologic outcome in HIV-infected patients treated with abacavir- or indinavir-based triple combination HAART also containing lamivudine/zidovudine. Curr Med Res Opin 2004; 20:1115–1123. [DOI] [PubMed] [Google Scholar]
- 40.Tuldra A, Fumaz CR, Ferrer MJ, et al. Prospective randomized two-arm controlled study to determine the efficacy of a specific intervention to improve long-term adherence to highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2000; 25:221–228. [DOI] [PubMed] [Google Scholar]
- 41.Meresse M, Carrieri MP, Laurent C, et al. Time patterns of adherence and long-term virological response to non-nucleoside reverse transcriptase inhibitor regimens in the Stratall ANRS 12110/ESTHER trial in Cameroon. Antivir Ther 2013; 18:29–37. [DOI] [PubMed] [Google Scholar]
- 42.Carr A, Chuah J, Hudson J, et al. A randomised, open-label comparison of three highly active antiretroviral therapy regimens including two nucleoside analogues and indinavir for previously untreated HIV-1 infection: the OzCombo1 study. AIDS 2000; 14:1171–1180. [DOI] [PubMed] [Google Scholar]
- 43.Cohen CJ, Molina JM, Cassetti I, et al. Week 96 efficacy and safety of rilpivirine in treatment-naive, HIV-1 patients in two Phase III randomized trials. AIDS 2013; 27:939–950. [DOI] [PubMed] [Google Scholar]
- 44.Haubrich RH, Little SJ, Currier JS, et al. The value of patient-reported adherence to antiretroviral therapy in predicting virologic and immunologic response. California Collaborative Treatment Group. AIDS 1999; 13:1099–1107. [DOI] [PubMed] [Google Scholar]
- 45.Muyingo SK, Walker AS, Reid A, et al. Patterns of individual and population-level adherence to antiretroviral therapy and risk factors for poor adherence in the first year of the DART trial in Uganda and Zimbabwe. J Acquir Immune Defic Syndr 2008; 48:468–475. [DOI] [PubMed] [Google Scholar]
- 46.Nelson M, Girard PM, Demasi R, et al. Suboptimal adherence to darunavir/ritonavir has minimal effect on efficacy compared with lopinavir/ritonavir in treatment-naive, HIV-infected patients: 96 week ARTEMIS data. J Antimicrob Chemother 2010; 65:1505–1509. [DOI] [PubMed] [Google Scholar]
- 47.Shet A, De Costa A, Kumarasamy N, et al. Effect of mobile telephone reminders on treatment outcome in HIV: evidence from a randomised controlled trial in India. BMJ 2014; 349:g5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murphy RA, Sunpath H, Castilla C, et al. Second-line antiretroviral therapy: long-term outcomes in South Africa. J Acquir Immune Defic Syndr 2012; 61:158–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nolan S, Milloy MJ, Zhang R, et al. Adherence and plasma HIV RNA response to antiretroviral therapy among HIV-seropositive injection drug users in a Canadian setting. AIDS Care 2011; 23:980–987. [DOI] [PubMed] [Google Scholar]
- 50.El-Khatib Z, Katzenstein D, Marrone G, et al. Adherence to drug-refill is a useful early warning indicator of virologic and immunologic failure among HIV patients on first-line ART in South Africa. PLoS One 2011; 6:e17518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lima VD, Bangsberg DR, Harrigan PR, et al. Risk of viral failure declines with duration of suppression on highly active antiretroviral therapy irrespective of adherence level. J Acquir Immune Defic Syndr 2010; 55:460–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kitahata MM, Reed SD, Dillingham PW, et al. Pharmacy-based assessment of adherence to HAART predicts virologic and immunologic treatment response and clinical progression to AIDS and death. Int J STD AIDS 2004; 15:803–810. [DOI] [PubMed] [Google Scholar]
- 53.Abah IO, Ojeh VB, Musa J, et al. Clinical utility of pharmacy-based adherence measurement in predicting virologic outcomes in an adult HIV-infected cohort in jos, North Central Nigeria. J Int Assoc Provid AIDS Care 2014; 15:77–83. [DOI] [PubMed] [Google Scholar]
- 54.McMahon JH, Manoharan A, Wanke CA, et al. Pharmacy and self-report adherence measures to predict virological outcomes for patients on free antiretroviral therapy in Tamil Nadu, India. AIDS Behav 2013; 17:2253–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ortego C, Huedo-Medina TB, Llorca J, et al. Adherence to highly active antiretroviral therapy (HAART): a meta-analysis. AIDS Behav 2011; 15:1381–1396. [DOI] [PubMed] [Google Scholar]
- 56.Simoni JM, Frick PA, Pantalone DW, et al. Antiretroviral adherence interventions: a review of current literature and ongoing studies. Top HIV Med 2003; 11:185–198. [PubMed] [Google Scholar]
- 57.Kahana SY, Rohan J, Allison S, et al. A meta-analysis of adherence to antiretroviral therapy and virologic responses in HIV-infected children, adolescents, and young adults. AIDS Behav 2013; 17:41–60. [DOI] [PubMed] [Google Scholar]
- 58.Rosenblum M, Deeks SG, van der Laan M, et al. The risk of virologic failure decreases with duration of HIV suppression, at greater than 50% adherence to antiretroviral therapy. PLoS One 2009; 4:e7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Viswanathan S, Justice AC, Alexander GC, et al. Adherence and HIV RNA suppression in the current era of highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2015; 69:493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wagner G, Miller LG. Is the influence of social desirability on patients’ self-reported adherence overrated? J Acquir Immune Defic Syndr 2004; 35:203–204. [DOI] [PubMed] [Google Scholar]
- 61.Bangsberg DR. Less than 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clin Infect Dis 2006; 43:939–941. [DOI] [PubMed] [Google Scholar]
- 62.Mills EJ, Nachega JB, Buchan I, et al. Adherence to antiretroviral therapy in sub-Saharan Africa and North America: a meta-analysis. JAMA 2006; 296:679–690. [DOI] [PubMed] [Google Scholar]
- 63.Gupta RK, Jordan MR, Sultan BJ, et al. Global trends in antiretroviral resistance in treatment-naive individuals with HIV after rollout of antiretroviral treatment in resource-limited settings: a global collaborative study and meta-regression analysis. Lancet 2012; 380:1250–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gunthard HF, Aberg JA, Eron JJ, et al. Antiretroviral treatment of adult HIV infection: 2014 recommendations of the International Antiviral Society—USA Panel. JAMA 2014; 312:410–425. [DOI] [PubMed] [Google Scholar]
- 65.Haas AD, Keiser O, Balestre E, et al. Monitoring and switching of first-line antiretroviral therapy in sub-Saharan Africa: collaborative analysis of adult treatment cohorts. Lancet HIV 2015; 2:e271–e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ioannidis JP, Haidich A-B, Pappa M, et al. Comparison of evidence of treatment effects in randomized and nonrandomized studies. JAMA 2001; 286:821–830. [DOI] [PubMed] [Google Scholar]
- 67.Glesby MJ, Hoover DR. Survivor treatment selection bias in observational studies: examples from the AIDS literature. Ann Intern Med 1996; 124:999–1005. [DOI] [PubMed] [Google Scholar]


