Abstract
Proton pump inhibitors (PPIs) use may be associated with nephritis and acute renal injury. The risk of PPIs and deterioration of renal function, in patients with renal diseases, needs to be investigated.
A case-control study was conducted in a nation-wide data setting from the Taiwan National Health Insurance Research Database (NHIRD). This case-control study used data extracted from NHIRD between the years 2006 and 2011. We used propensity scores to match 3808 patients suffering from renal diseases (ICD-9-CM codes 580–589), with patients (aged ≥20 years) who had had a recent diagnosis of end-stage renal diseases (ESRDs) and had undertaken renal replacement therapy during the period of 2006 to 2011. The 3808 control subjects were selected from people who had a history of renal diseases, but no ESRD. The risk of ESRD in patients with renal diseases and PPIs use was estimated by using odds ratios (ORs) and 95% confidence intervals (CI).
The use of a PPIs was associated with a significantly higher risk of ESRD (adjusted OR = 1.88, 95% CI = 1.71–2.06) in renal disease patients. Of all the types of PPI combined, the adjusted OR was 1.92 (95% CI = 1.74–2.13) for those on <100 cumulative DDD and was 1.74-fold (95% CI = 1.52–2.00) for those on ≥100 cumulative DDD.
PPIs use is associated with the risk of ESRD in patients with renal diseases. It is necessary that appropriate prescription of PPIs coordinated with the close monitoring renal function of patients diagnosed with renal disease.
INTRODUCTION
Gastric acid suppression therapy through the use of proton pump inhibitors (PPIs) is the mainstay for the treatment of acid-related, gastrointestinal disease.1,2 Though PPIs are considered safe, long-term and over-utilization of PPIs has become an important issue and needs to be investigated.3 Gastric mucosa change, enteric infection, outside of gastrointestinal infection, osteoporosis, nutritional deficiency, and hypomagnesemia are all considered to be serious complications resulting from the use of PPIs.4
Regarding concern over renal adverse effects, PPIs therapy has shown to cause an increased risk of acute kidney injury along with acute interstitial nephritis.5 The most common etiology of acute interstitial nephritis is drug-induced diseases, which are believed to underlie 60% to 70% of cases. PPI is also considered one of the drugs producing adverse effects related to nephritis.5–7 PPI-related acute interstitial nephritis is rare, idiosyncratic, and difficult to predict. Till now, most studies have focused on acute interstitial nephritis.5,7–11 There seemed to be lack of evidence for the association of PPIs use and its renal effect among patients with renal diseases, including neprhitis, nephritic syndrome, glomerulonephritis, nephropathy, chronic kidney disease, and renal function impairment. Does PPIs use associated with the risk of deterioration within patients suffering from renal diseases leading to end-stage renal disease (ESRD) need to investigated? And while this condition may be less closely monitored, more attention should be given by the gastroenterologist.12–15
To address this question, we conducted a nationwide case-control study to analyze the risk of developing ESRD among patients with renal diseases and the use of PPIs in Taiwan.
MATERIALS AND METHODS
Data Source
Data analyzed in this case-control study was retrieved from the Taiwan National Health Insurance Research Database (NHIRD). Taiwan launched a compulsory, social insurance program, the NHI program, to provide health care for >99% of the 23.75 million residents in 1995.16 The details of the NHI program have been well documented in previous high-quality studies.17,18 For this study, we used a subset of the NHIRD containing its health care data, including files from the Longitudinal Health Insurance Database 2000 (LHID 2000), the Registry for Catastrophic Illness Patient Database (RCIPD), and the Registry of Beneficiaries. In the NHI program, there are certain subgroups, including cancer, autoimmune diseases, and uremia patients, that possess the catastrophic illness card, which can exempt them from the need to make a co-payment. The application for the catastrophic illness card should be scrutinized by a peer review group according to clinical, laboratory, image, or pathological data. Patients with ESRD who were identified from the RCIPD include those who require long-term renal replacement therapy, such as dialysis or a kidney transplant.
The National Health Research Institute has encrypted all of the patient identification numbers for the protection of their privacy. The criteria of diseases were defined according to the International Classifications of Disease, 9th Revision, Clinical Modification (ICD-9-CM). This study was approved to fulfill the condition for exemption by the Institutional Review Board (IRB) of China Medical University (CMUH-104-REC2–115). The IRB also specifically waived the consent requirement.
Subject Selection
Figure 1 shows the procedure for selecting cases and controls. This case-control study used data extracted from the LHID2000 and RCIPD from the years 2006 to 2011. Subjects with gastroesophageal reflux disease (GERD) (ICD-9-CM codes 530.81, 530.11) or peptic ulcer disease, including gastric ulcers, duodenum ulcers, or other unspecified ulcers (ICD-9-CM codes 531–533), constituted the base population. In Taiwan's NHI system, patients with ESRD undergoing renal replacement therapy are registered in the RCIPD using ICD codes (ICD-9 codes 580–589).
FIGURE 1.
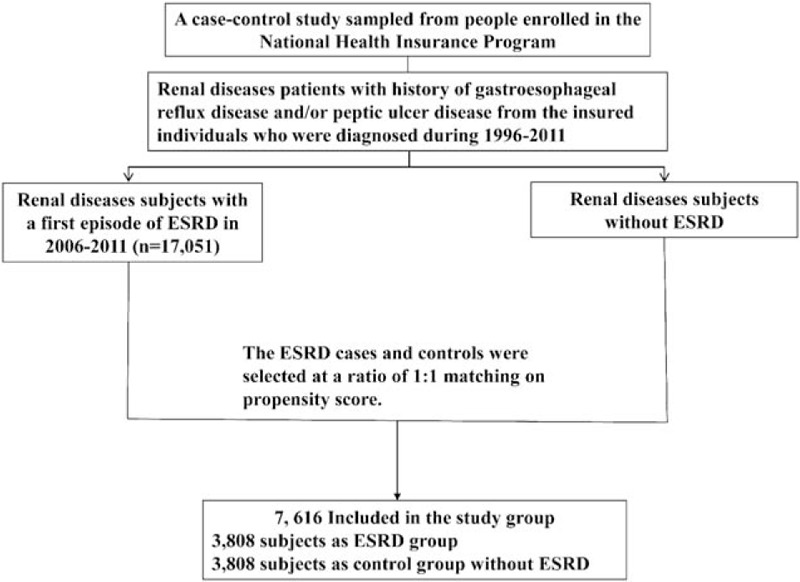
The flow chart for selecting chronic renal disease cases, with end-stage renal disease and without end-stage renal disease.
We identified patients with renal diseases, including neprhitis, nephritic syndrome, glomerulonephritis, nephropathy, chronic kidney disease, and renal function impairment (coded by ICD-9-CM code 580–589), and GERD and/or peptic ulcer diseases. Those renal disease patients (age ≥20 years) who subsequently developed ESRD were identified from RCIPD during the years 2006 to 2011. The ESRD diagnosis date was defined as the index date. Control subjects were selected from people with a history of renal diseases and who did not develop ESRD in the LHID 2000. The ESRD cases and controls were selected using a ratio of 1:1 matching on propensity score. We used a logistic regression model to calculate the propensity score.19 The potential confounders to be considered were gender, age, the years of renal disease diagnosis and ESRD diagnosis, medication with H2 receptor antagonists (H2RA), angiotensin-converting-enzyme inhibitors (ACE-i), angiotensin-receptor blockers (ARB), beta blockers, calcium channel blockers (CCB), aldosterone inhibitors, nonsteroidal anti-inflammatory drugs (NSAID) and comorbidities of diabetes (ICD-9-CM code 250), hypertension (ICD-9-CM codes 401–405), chronic obstructive pulmonary disease (COPD)(ICD-9-CM 491, 492, 496), coronary artery disease (CAD) (ICD-9 code 410–414), congestive heart failure (CHF) (ICD-9 code 428), and cancer (ICD-9 code 140–208).
Measurements of PPIs
Five commercially available PPIs in Taiwan were analyzed, including omeprazole, pantoprazole, lansoprazole, rabeprazole, and esomeprazole. According to the total supply in days and quantity of PPI, we calculated the cumulative defined daily dose (DDD) of each type of PPI for each PPI user, including omeprazole (ATC A02BC01), pantoprazole (ATC A02BC02), lansoprazole (ATC A02BC03), rabeprazole (ATC A02BC04), and esomeprazole (ATC A02BC05). For each type of PPI, the cumulative DDD was partitioned into two levels at the 75th percentile.
The DDD is defined by the ATC/DDD system from the WHO's Collaborating Center for Drug Statistics and Methodology. Each product has to be referred to the appropriate ATC code and DDD. The unit of DDD is defined as the assumed average maintenance dose per day for a drug used for its major indication in adults.19 It could provide a fixed unit of measurement independent of price and dosage form, thus the trends in drug consumption could be assessed, the comparisons between population groups could be performed, and standardizing drug dose across multiple types of drugs, thus they could be compared.20 The number of DDDs is calculated as the total amount of drugs divided by amount of drug in a DDD. The cumulative DDD, which expresses dosage and duration of exposure, could be use to estimate the sum of the dispensed DDD of different type of PPIs. Thus, we could correlate PPIs use and the ESRD risk in renal disease patients by cumulative DDD.
Statistical Analysis
The baseline characteristics between ESRD cases and controls were examined using the chi-square test for categorical variables, along with the Student's t test, which was used for continuous variables. The univariable and multivariable unconditional logistic regression analyses were used to calculate odds ratios (ORs) and 95% confidence intervals (95% CIs) for the association between the uses of PPI and ESRD. ORs (95% CIs) associated with the uses of PPI individual were analyzed. The cumulative DDD for all PPIs and each type of PPI were stratified into a third quartile for dose–response relationship analyses. All analyses were performed using SAS statistical software (version 9.4; SAS Institute, Inc., Cary, NC), and the results were considered statistically significant when two-tailed P values were <0.05.
RESULTS
Patient Characteristics
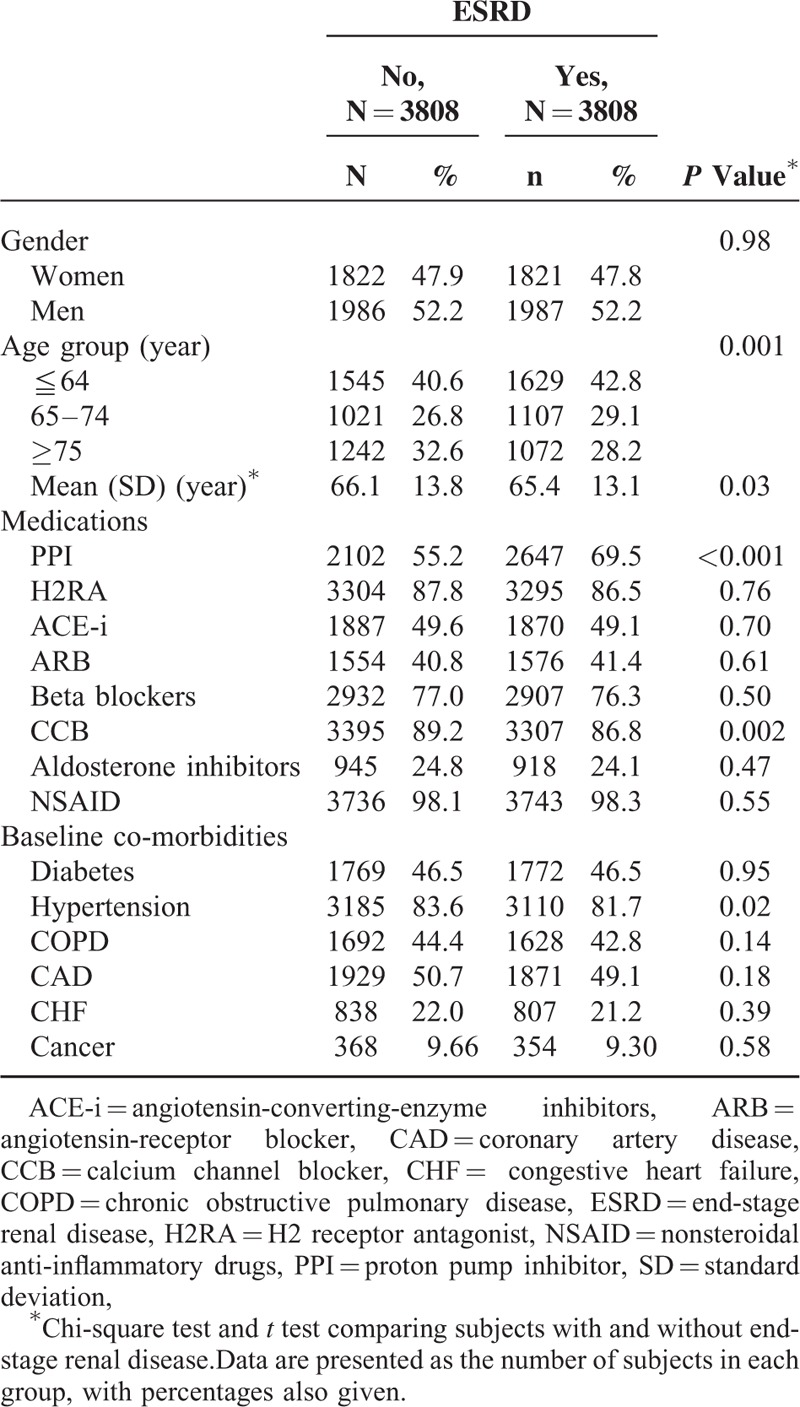
Among patients with renal diseases, we identified 3808 ESRD patients and 3808 controls (without ESRD) between the years 2006 and 2011. The follow-up duration from the diagnosis of renal diseases to the diagnosis of ESRD was 3.99 ± 3.46 years in the ESRD group and 4.02 ± 3.04 years in the control group (P = 0.67). Of the 3808 ESRD patients, 52.2% of them were women and 42.8% were <64 years (Table 1). The mean ages of patients in the ESRD and non-ESRD groups were 65.4 (±13.1) and 66.1 (±13.8) years, respectively. Patients with ESRD tended to have a higher prevalence of PPI use than those in the non-ESRD group (P < 0.001).
TABLE 1.
Baseline Characteristics Between End-Stage Renal Disease Group and Non-End-Stage Renal Disease Group
Risk of ESRD With Individual PPI in Renal Diseases
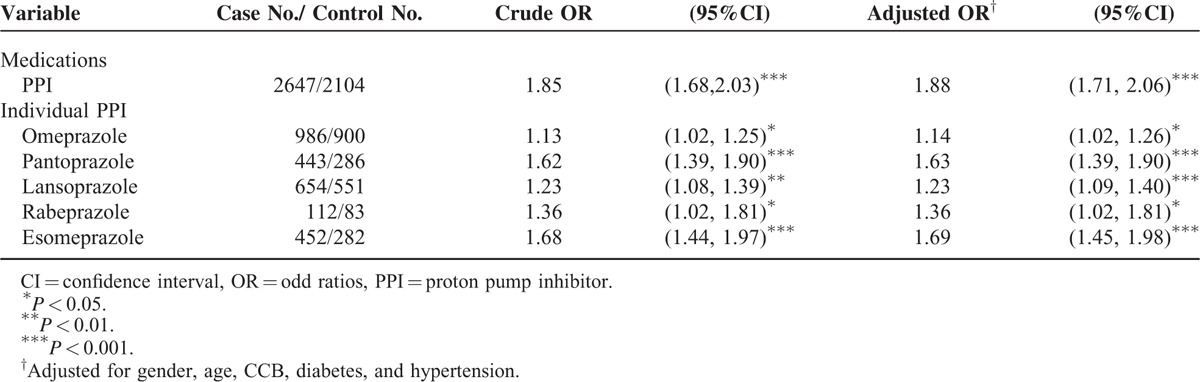
Table 2 shows the ORs of estimated risk of ESRD based on PPI use. Use of a PPI was associated with a significantly higher risk of ESRD (adjusted OR = 1.88, 95% CI = 1.71–2.06). Results that measured for individual PPI were significant for omeprazole (adjusted OR = 1.14, 95% CI = 1.02–1.26), pantoprazole (adjusted OR = 1.63, 95% CI = 1.39–1.90), lansoprazole (adjusted OR = 1.23, 95% CI = 1.02–1.81), rabeprazole (adjusted OR = 1.36, 95% CI = 1.02–1.81), and for esomeprazole (adjusted OR = 1.69, 95% CI = 1.45–1.98).
TABLE 2.
Odds Ratio and 95% Confidence Intervals of End-Stage Renal Disease Associated with Individual PPI
Dose–Response Relationship between PPI use and the Risk of ESRD in Renal Diseases
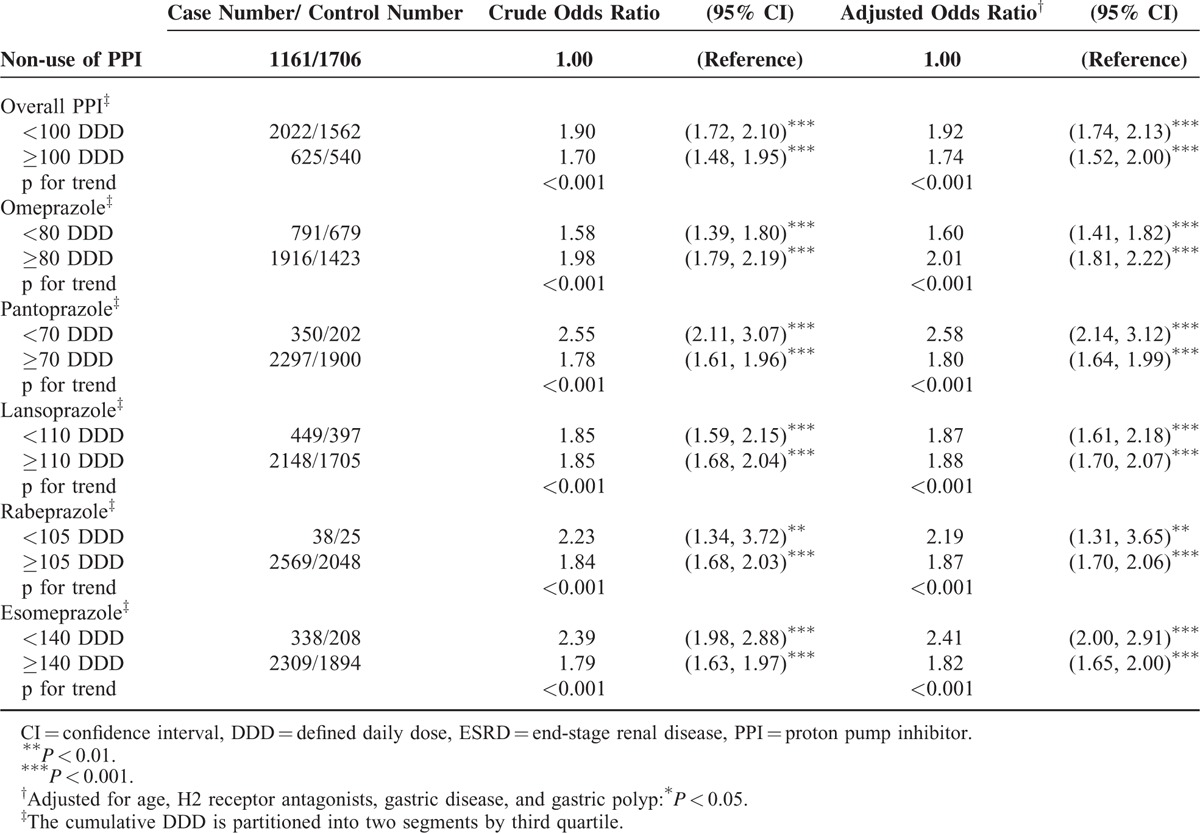
Table 3 shows the dose–response relationship between individual PPI use and the risks of ESRD in patients with renal diseases, compared with controls (non-PPIs users). Of all the types of PPI combined, the OR was 1.92 (95% CI = 1.74–2.13) for those on <100 cumulative DDD and was 1.74-fold (95% CI = 1.52–2.00) for those on ≥100 cumulative DDD. The risk of ESRD of individual PPI in whichever stratification was higher than in the control group.
TABLE 3.
Odds Ratio and 95% Confidence Intervals of ESRD Associated with Cumulative DDD of Individual Proton Pump Inhibitors
DISCUSSION
In the population-based nationwide study, we demonstrated a significant difference of PPIs use in patients with renal diseases who developed ESRD, which was not associated with H2RA. PPIs use was associated with the risk of ESRD in renal disease patients who possessed an adjusted OR = 1.88 (95% CI = 1.71–2.06) upon measuring all PPIs combined. ORs measured by the cumulative DDD of individual PPIs also showed an increased risk of developing ESRD for most subgroups within the individual PPIs. There is also increased ESRD risk for those who have taken omeprazole, lansoprazole, rabeprazole, or esomeprazole. Our results demonstrated that PPIs use is associated with an increased risk of developing ESRD in patients afflicted with renal diseases. The estimated risk for ESRD in PPI users was 1.88, but when risk was analyzed for individual PPI it was <1.88. It could be explained that some patients may took more than one kind of PPIs.
GERD is more common in patients with renal diseases than those in the general population. However, there is limited data for PPIs in patients with renal diseases.14 The study demonstrated the association between PPI and ESRD in patients with renal diseases, including neprhitis, nephritic syndrome, glomerulonephritis, nephropathy, chronic kidney disease, and renal function impairment. The NHRID houses a large computerized database, making the studied population sufficient in size and highly representative. The details, including stage or glomerular filtration rates of chronic renal diseases and creatinine levels, could not be obtained from the NHRID. We could only follow extracted patients with renal diseases coded by ICD-9 580-589. With the same follow-up duration from the diagnosis of renal diseases to whether the patient was diagnosed with ESRD or not, the variations of chronic renal function stage may be minimized. There is an association between PPIs use and the risk of deterioration of renal diseases becoming ESRD. The data on PPIs use was collected from a computerized database containing all available prescription information, rendering the potential for bias with regard to medication as being minimized. The PPIs prescriptions were required to be in accordance with the National Health Insurance regulations, assuring that there was a definite diagnosis of peptic ulcer disease, including gastric ulcer or duodenal ulcer, reflux esophagitis, or gastro-esophagus reflux disease diagnosed by an upper gastrointestinal endoscopy.
We were able to demonstrate a dose-dependent risk for ESRD by OR of cumulative DDDs in omeprazole and lansoprazole. However, the results demonstrated a variation of OR in pantorazole, rabeprazole, and esomeprazole. This may be explained by a reduction in the prescription dosage for patients with renal diseases. Though dose reductions of PPI in patients with renal diseases is recommended, it is not very restricted owing to the rare complications stemming from PPIs.21 The variable OR of cumulative DDD for pantoprazole could be explained by both a wide therapeutic range along with the prescription writing behavior of the clinical physicians.
The knowledge of PPIs causing acute interstitial nephritis and acute renal injury was first reported in 1992,21 and a case series was subsequently reported in 2006.8,9 Acute interstitial nephritis is considered as an immunologically mediated renal injury that is estimated to account for up to one quarter of the cases of acute kidney injury and is considered to be an idiosyncratic result.6 In our data setting, the risk of patients with renal disease, subsequently developing ESRD, is associated with PPIs.
One additional concern is the possible mechanism of PPIs on renal functions. The kidney is not the major site where PPIs are metabolized, as PPIs are metabolized via the cytochrome P450 system, thus there are important differences in individual PPIs.15 Along with acute nephritis, PPIs directly cause renal function impairment, which is not mentioned when discussing most safety issues.4,22 Clinically, renal function deterioration in patients is less frequently observed with normal renal function after the use of PPIs. Drug interaction could be considered where both drugs or PPIs combination may increase the risk of renal function impairment in patients diagnosed with renal diseases.15,23 Drug deterioration renal function may be considered as a possibility because of medications becoming accumulated in the kidney, a reduction in renal function, or nephrotoxicity.24 Hypomeganesium is one of the adverse side effects of PPIs use,22 where the major clinical consequences include electrolyte imbalance, neuromuscular, neurological, and cardiovascular.22,24,25 Thus, PPI-related hypomeganesium may be not the important factor in the development of ESRD in renal disease cases. However, it should be noted that the ability to excrete magnesium is impaired in patients who have been diagnosed with renal diseases, particularly those with PPIs use.26
There are several limitations in this study. First, the chronic renal disease stage and creatinine level are not available through the NHIRD. Therefore, we selected patients diagnosed with renal diseases, who were experiencing ERSD, as opposed to those diagnosed with renal disease who had no developing ESRD during the same follow-up period. Secondly, there were variable ranges within the PPIs dosage, where some patients received reduced dose PPIs, while some received a full or even double dose. Thirdly, as most, patients would be expected to ingest their medication as written on their prescription. Owing to the rules of the NHRI, most patients taking PPI would receive an endoscopy examination. Over-the-counter (OTC) PPIs might to some extent impact these results, so the information on OTC drugs remains unavailable within the NHIRD. We assumed that OTC PPIs are only a small portion of all PPIs, and felt this issue should be mentioned.27 Fourth, there are several important factors related to renal function, including blood pressure, proteinuria, hemoglobin A1c, creatinine, sodium, postassium, uric acid, magnesium, calcium, phosphate, intact parathyroid hormone, lipids, iron parameters, and blood counts, but all these data are not available through the NHIRD. Fifth, the average cutoff period form renal disease defined to ESRD is also an important for caring renal disease patients with PPIs use. However, to be retrospective case-control study, it is difficult to define a cutoff duration for PPIs use to ESRD.
In the present study, we have demonstrated that PPIs use is associated with the risk of developing ESRD in patients with renal disease, but the definite mechanism could not be illuminated through data analysis. Moreover, our attention should move towards the appropriate prescription of PPIs, coordinated with the close monitoring renal function of patients diagnosed with renal disease. For patients with renal diseases who require long-term PPIs therapy on demand or intermittent therapy should be considered.
Footnotes
Abbreviations: CI = confidence interval, HR = hazard ratios, IRR = incidence rate ratio, NHIRD = National Health Insurance Research Database, PPIs = proton pump inhibitors.
Author contributions: conception and design: Y-CP, C-HK; administrative support: C-HK; collection and assembly of data: all authors; data analysis and interpretation: all authors; manuscript writing: all authors; final approval of manuscript: all authors.
Funding: This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW105-TDU-B-212–133019), China Medical University Hospital, Academia Sinica Taiwan Biobank Stroke Biosignature Project (BM10501010037), NRPB Stroke Clinical Trial Consortium (MOST 104–2325-B-039 -005), Tseng-Lien Lin Foundation, Taichung, Taiwan, Taiwan Brain Disease Foundation, Taipei, Taiwan, and Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Vela MF. Medical treatments of GERD: the old and new. Gastroenterol Clin North Am 2014; 43:121–133. [DOI] [PubMed] [Google Scholar]
- 2.Fortinsky KJ, Bardou M, Barkun AN. Role of medical therapy for nonvariceal upper gastrointestinal bleeding. Gastrointest Endosc Clin N Am 2015; 25:463–478. [DOI] [PubMed] [Google Scholar]
- 3.Heidelbaugh JJ, Goldberg KL, Inadomi JM. Overutilization of proton pump inhibitors: a review of cost-effectiveness and risk [corrected]. Am J Gastroenterol 2009; 104 Suppl 2:S27–32. [DOI] [PubMed] [Google Scholar]
- 4.Lodato F, Azzaroli F, Turco L, et al. Adverse effects of proton pump inhibitors. Best Pract Res Clin Gastroenterol 2010; 24:193–201. [DOI] [PubMed] [Google Scholar]
- 5.Antoniou T, Macdonald EM, Hollands S, et al. Proton pump inhibitors and the risk of acute kidney injury in older patients: a population-based cohort study. CMAJ Open 2015; 3:E166–E171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perazella MA, Markowitz GS. Drug-induced acute interstitial nephritis. Nat Rev Nephrol 2010; 6:461–470. [DOI] [PubMed] [Google Scholar]
- 7.Sierra F, Suarez M, Rey M, et al. Systematic review: proton pump inhibitor-associated acute interstitial nephritis. Aliment Pharmacol Ther 2007; 26:545–553. [DOI] [PubMed] [Google Scholar]
- 8.Geevasinga N, Coleman PL, Webster AC, et al. Proton pump inhibitors and acute interstitial nephritis. Clin Gastroenterol Hepatol 2006; 4:597–604. [DOI] [PubMed] [Google Scholar]
- 9.Simpson IJ, Marshall MR, Pilmore H, et al. Proton pump inhibitors and acute interstitial nephritis: report and analysis of 15 cases. Nephrology (Carlton) 2006; 11:381–385. [DOI] [PubMed] [Google Scholar]
- 10.Ray S, Delaney M, Muller AF. Proton pump inhibitors and acute interstitial nephritis. BMJ (Clinical research ed) 2010; 341: [DOI] [PubMed] [Google Scholar]
- 11.Klepser DG, Collier DS, Cochran GL. Proton pump inhibitors and acute kidney injury: a nested case-control study. BMC Nephrol 2013; 14:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cekin AH, Boyacioglu S, Gursoy M, et al. Gastroesophageal reflux disease in chronic renal failure patients with upper GI symptoms: multivariate analysis of pathogenetic factors. Am J Gastroenterol 2002; 97:1352–1356. [DOI] [PubMed] [Google Scholar]
- 13.Fallone CA, Mayrand S. Gastroesophageal reflux and hyperacidity in chronic renal failure. Perit Dial Int 2001; 21 Suppl 3:S295–S299. [PubMed] [Google Scholar]
- 14.Kawaguchi Y, Mine T, Kawana I, et al. Gastroesophageal reflux disease in chronic renal failure patients: evaluation by endoscopic examination. Tokai J Exp Clin Med 2009; 34:80–83. [PubMed] [Google Scholar]
- 15.Brewster UC, Perazella MA. Proton pump inhibitors and the kidney: critical review. Clin Nephrol 2007; 68:65–72. [DOI] [PubMed] [Google Scholar]
- 16.Database NHIR. Taiwan, http://nhird.nhri.org.tw/en/Background.html 2015. [Google Scholar]
- 17.Peng YC, Lin CL, Hsu WY, et al. Statins are associated with a reduced risk of cholangiocarcinoma: a population-based case-control study. Br J Clin Pharmacol 2015; 80:755–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung WS, Peng CL, Lin CL, et al. Rheumatoid arthritis increases the risk of deep vein thrombosis and pulmonary thromboembolism: a nationwide cohort study. Ann Rheum Dis 2014; 73:1774–1780. [DOI] [PubMed] [Google Scholar]
- 19.WHO International Working Group for Drug Statistics Methodology, W.C.C.f.D.S.M., WHO Collaborating Centre for Drug Utilization Research and Clinical Pharmacological Services, Introduction to Drug Utilization Research. 2003, Geneva, Switzerland: World Health Organization. [Google Scholar]
- 20.WHO Collaborating Center for Drugs Statistics Methodology:ATC/DDD Index, http://www.whocc.no/atc_ddd_index/?code=A10BH 2015. [Google Scholar]
- 21.Ruffenach SJ, Siskind MS, Lien YH. Acute interstitial nephritis due to omeprazole. Am J Med 1992; 93:472–473. [DOI] [PubMed] [Google Scholar]
- 22.Yang YX, Metz DC. Safety of proton pump inhibitor exposure. Gastroenterology 2010; 139:1115–1127. [DOI] [PubMed] [Google Scholar]
- 23.Bell J, Blacker N, LeBlanc V, et al. Prescribing for older people with chronic renal impairment. Aust Fam Physician 2013; 42:24–28. [PubMed] [Google Scholar]
- 24.Choudhury D, Ahmed Z. Drug-associated renal dysfunction and injury. Nat Clin Pract Neph 2006; 2:80–91. [DOI] [PubMed] [Google Scholar]
- 25.Martin KJ, Gonzalez EA, Slatopolsky E. Clinical consequences and management of hypomagnesemia. J Am Soc Nephrol 2009; 20:2291–2295. [DOI] [PubMed] [Google Scholar]
- 26.Cunningham J, Rodríguez M, Messa P. Magnesium in chronic kidney disease stages 3 and 4 and in dialysis patients. Clin Kidney J 2012; 5 Suppl 1:i39–i51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsing AW, Ioannidis JP. Nationwide population science: lessons from the Taiwan National Health Insurance Research Database. JAMA Intern Med 2015; 175:1527–1529. [DOI] [PubMed] [Google Scholar]


