Abstract
Osteoprotegerin (OPG) is a kind of tumor necrosis factor, which is related to bone metabolism and vascular calcification. The increase of Osteoprotegerin concentration in serum is related to cardiovascular diseases in humans. The purpose of this study was to figure out the relevance between osteoprotegerin in serum and carotid calcification.
Serum OPG concentrations were compared in 145 patients who underwent carotid sonography (average age: 68 ± 9 years old, male: female = 81:64). A calcified plaque (CP) (37 people [27%]), a noncalcified plaque (NCP) (54 people [37%]), and a nonplaque (NP) (54 people [37%]) were classified for this study.
No significant differences among 3 groups were demonstrated in the distribution of age, diabetes, high blood pressure, and hyperlipidemia. Serum osteoprotegerin concentrations were significantly increased in CP group rather than NCP group or NP group; (median [interquartile range], 4016 [1410] vs 3210 [1802] pg/mL, P < 0.05 and 4016 [1410] vs 3204 [1754] pg/mL, P < 0.05). Serum osteoprotegerin concentrations did not indicate a significant difference between NCP Group or NP Group.
This study had proved that patient group accompanied with carotid calcification in carotid artery disease had an increased serum OPG concentration, so it could consider that OPG plays an important function on calcification related to arteriosclerosis.
INTRODUCTION
Calcification of blood vessels is an important feature of the process of arteriosclerosis, with a direct relationship to the overall degree of arteriosclerotic diseases. Although there might be still controversial,1 several studies reported that circulating osteoprotegerin (OPG) level were found to be significantly higher in the plasma of patients presenting with asymptomatic carotid lesion compared to symptomatic lesions.2
Despite its clinical importance, the mechanism of calcification is not yet known.3 It was believed previously that calcification of blood vessels is caused by the direct movement of calcium from bone to vessel wall; however, many recent proofs point to the presence of complex control mechanisms, which can actively control and promote calcification.4
Jørgensen et al5 demonstrated an inverse correlation between echogenicity of the atheromatous plaque and bone density in humans, so a study was designed to investigate the relation between plaque calcifications and bone density. OPG is a glycoprotein, and is considered as one of the tumor necrosis factor (TNF) receptors. It binds to the receptor activator of nuclear factor kappa-B ligand (RANKL), which is expressed in osteoblasts, competitively blocking the interaction between receptor activator of nuclear factor kappa-B (RANK) and RANKL. This interaction is important for osteoclast differentiation and inhibition of bone resorption.6
In addition, OPG is expressed in various tissues and cells. The cardiovascular system shows a high expression of OPG, for example, in arterial smooth muscles and endothelial cells; hence, it could play an important role in the control of arterial calcification.7
The study showed that OPG concentration increased in proportion to the severity of coronary artery disease.8,9 Moreover, a recent prospective study reported that OPG concentration is an independent risk factor for carotid arteriosclerosis and cardiovascular disease.10
However, the correlation between vessel calcification and OPG concentration, which is important for arteriosclerosis progression, is not yet obvious. Therefore, this study aimed at a deeper understanding of the mechanism of arteriosclerosis through investigating the relation between serum OPG concentration and carotid calcification, opening the door for future research about the clinical efficiency of OPG.
METHODS
Subjects
During the period between September 2003 and July 2006, we retrospectively recruited 1252 consecutive patients who visited St. Mary's Hospital of Catholic Medical College for carotid ultrasonography as a part of routine atherosclerosis screening test.
Based on the results of the carotid ultrasonography, 91 patients were found to have carotid plaque. These patients were classified into a noncalcified plaque (NCP) group (54 patients [37%]), and a calcified plaque (CP) group (37 patients [27%]). A control nonplaque (NP) group (54 participants (37%)) was matched for age, sex, and BMI. Serum OPG concentration was measured in all the 145 patients and participants (average age: 68 ± 9 years, 81 male, 64 female). This study was approved by the Catholic Institutional Review Board and all subjects gave a written informed consent.
Methods
All the consecutive patients who underwent carotid ultrasonography for routine health screening test were enrolled in the present study. We excluded the patients with previous history of stroke, overt history of significant coronary artery disease, peripheral arterial diseases, patients who have or had acute myocardial infarction, chronic renal failure under dialysis, thyroid or parathyroid dysfunction, or tumors invading bone tissue.
Diabetes mellitus (DM) was defined as random plasma glucose concentration >200 mg/dL, fasting plasma glucose concentration >126 mg/dL, or a 2-hour post-prandial plasma glucose concentration >200 mg/dL. Hypertension was defined if systolic and diastolic blood pressures were >140 and 90 mmHg, respectively. Hyperlipidemia was defined if fasting serum total cholesterol was >240 mg/dL. Coronary calcium scores were measured by coronary multidetector computed tomography (MDCT). For calcification assessment, unenhanced electrocardiographic (ECG)-gated cardiac CT was performed by using a 64 detector row CT scanner (Lightspeed; General Electric Medical Systems, Milwaukee, WI) with 2.5-mm section thickness (4 × 2.5 mm collimation), pitch of 0.16, 0.35-second rotation time, 140 kV, and 250 mA.
Measurement of Carotid Plaque and its Calcification
A plaque was identified if the intima-media thickness (IMT) in a segment was 50% thicker than that of the adjacent segment, or if it protruded 0.5 mm into the arterial lumen. Calcified plaque was defined by posterior acoustic shadowing at the point of an existing plaque. Plaques and calcification were measured in the segment from both common carotid bulb to the bifurcation.
Measurement of Serum OPG Concentration
Sandwich enzyme linked immune-sorbent assay (ELISA) [DuoSet ELISA, R & D system, Mineapolis, Minnesota] method was used to measure serum OPG in the collected blood samples. All samples were tested in duplicates and then an average values were calculated. The limit of determination was 30 pg/mL and the error ranges within the analysis and among analyses were 3.2% and 5.4%, respectively.
Statistical Analysis
Continuous variables were represented as mean ± standard deviation, or as median. We confirmed the standard normal distribution for the continuous variables statistically. SPSS for Windows version 23.0 (SPSS Inc, Chicago, IL) was used for data analysis. Multiple regression analysis was used for the identification of predictor for carotid arteriosclerosis after univariate analysis was performed. A P value <0.05 was considered statistically significant.
RESULTS
Patients’ Clinical Characteristic
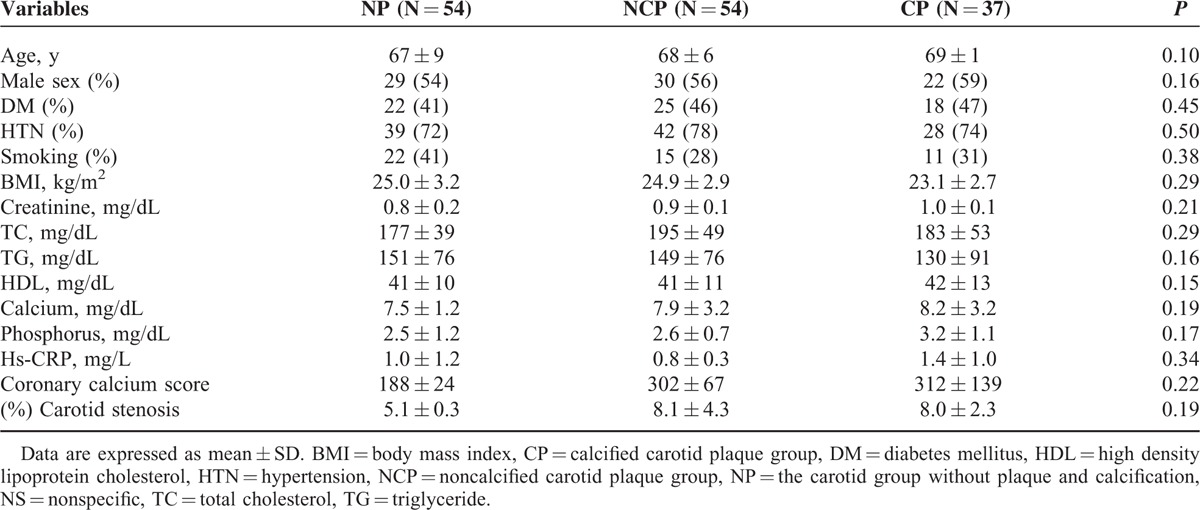
There were no significant differences in age, sex, BMI, presence of DM or hypertension, or in serum levels of total cholesterol, neural fats, or HDL cholesterol between patients at baseline (Table 1).
TABLE 1.
Patients’ Baseline Characteristics According to Plaque and Calcification
Relationship Between Carotid Ultrasonography Findings and Serum OPG Concentration
The median serum OPG concentrations were 3204 pg/mL in NP group, 3210 pg/mL in NCP group, and 4106 pg/mL in CP group. Serum OPG concentrations were significantly higher in CP group compared with CP and NCP groups (P < 0.05) and with CP and NP groups (P < .05). However, there was no significant difference in serum OPG concentrations between NP and NCP groups (Figure 1).
FIGURE 1.
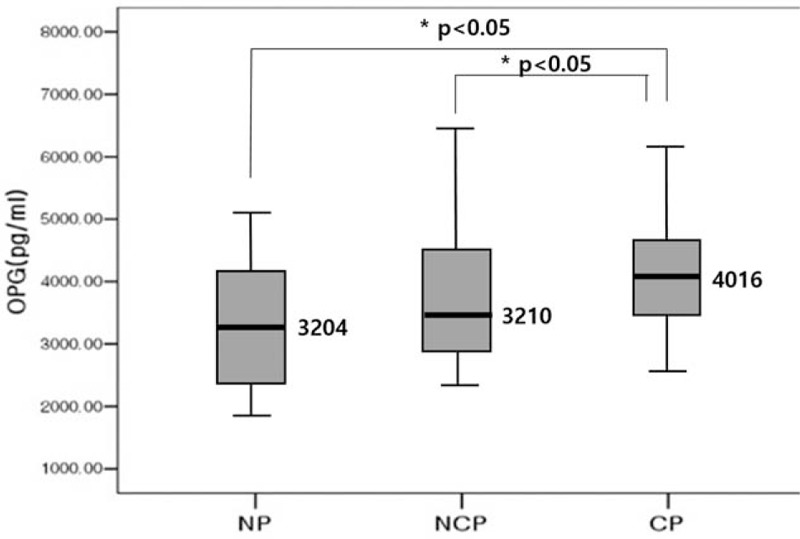
Comparison of serum osteopretegrin level according to the carotid plaque type. Calcified plaque group had higher osteprotegerin level than noncalcified plaque group. The thick solid line represents distribution median, the boxes span from 25th to 75th percentiles, and the error bars extend from 10th to 90th percentiles. NP = the carotid group without plaque and calcification, CP = calcified carotid plaque group, NCP = noncalcified carotid plaque group.
Independent Prediction Factor Related to Calcification in the CP Group
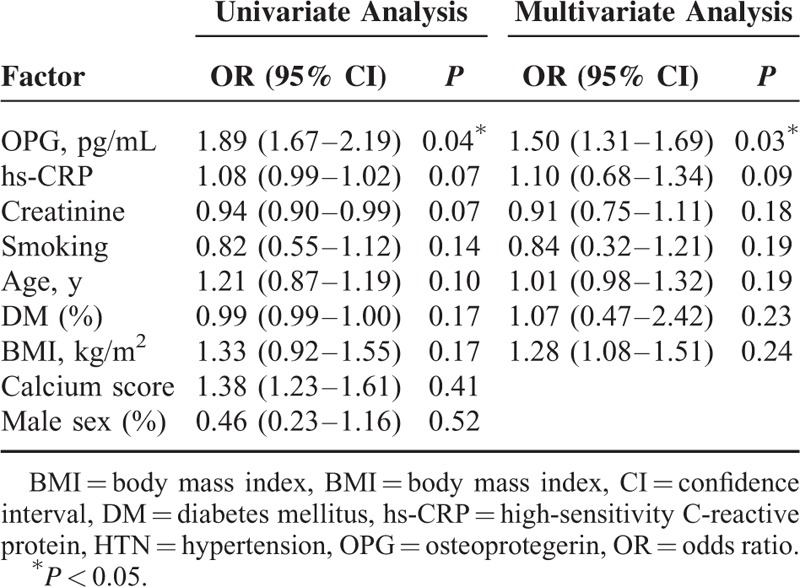
Multiple regression analysis was used to identify a predictor related to carotid calcification. There were no significant relations to age, sex, presence of DM or hypertension, smoking, calcium score or BMI. A statistically significant correlation was found only with serum OPG concentration (P = 0.03; odds ratio = 1.5, 95% CI [1.31–1.69]) (Table 2).
TABLE 2.
Analysis of the Influence Factors Associated With Calcification in Calcified Plaque Group
DISCUSSION
This study showed that serum OPG concentration was elevated in patients with calcified carotid plaque compared to those with no plaque, or with noncalcified plaque.
Many available reports proposed carotid plaque and its calcification as a predictive factor for cardiovascular and cerebrovascular morbidities.11 The carotid artery is easily approached by ultrasonography, allowing noninvasive evaluation of the type of arterial plaque at an early stage of atherosclerosis. In a carotid ultrasonography, a plaque with low echogenicity has high lipid accumulation, whereas a plaque with high echogenicity has fibrous tissue and calcification.12 Based on the fact that the measurement of plaque categorized in semiquantitative has statistically significant reproducibility, we classified arterial plaque and vessel calcification.13
This study exhibited a relevant increase in the severity of carotid artery arteriosclerosis with higher serum OPG concentration. Park et al14 reported that carotid artery calcification was proportional to serum OPG concentration. They exhibited the same result not only with serum concentration, but also with OPG tissue expression within the vessel wall.15 In addition, another report suggested that serum OPG concentration can act as an independent factor to predict the survival of patients with ischemic heart failure.16 This correlation was represented in a similar way to that in our study; serum OPG concentration was significantly higher in patients with calcified plaque in comparison to patients without calcified carotid plaque. In addition, because of using multiple regression analysis for this, serum OPG concentration was shown to be an independent predictive factor for calcification. On the contrary, age and presence of DM, which were reported to correlate with serum OPG concentration in previous studies, were not found to be independent predictive factors in our study.
Vascular calcification is one of the important clinical characteristics in the process of arteriosclerosis that can be actively and precisely controlled. In many studies, bone morphogenetic protein-2, matrix γ-carboxyglutamic acid protein, osteopontin, OPG, inorganic pyrophosphate, and various other bone matrix regulatory proteins, which control differentiation and maturation of bone cells, have been suggested to control calcification through their expression in the in blood vessels.17
Bucay et al18 reported hypercalcemia, osteoporosis, and calcification of major vessels in OPG knock-out rats. This points to the important role of OPG in preventing blood vessel calcification.
Recent articles19 reported that serum OPG concentration in humans has an inverse correlation with echogenicity of the carotid artery. In other words, patients with more carotid plaques and calcifications had lower serum OPG concentration, which is contradictory to our results. The high level of serum OPG reported by many studies can be considered as the incomplete feedback action according to arteriosclerosis progression.20 In other words, as it is well recognized that arteriosclerosis is a chronic inflammatory process, OPG has been suggested to act as the complementary protective system of blood vessels. OPG is upregulated in any condition that excessively activates arteriosclerosis and inflammatory pathways posing a risk to blood vessels.
The hypothesis of this feedback action is supported by several studies. Symptomatic cardiovascular disease is related to inflammation. This increases OPG synthesis and excretion. An increase in serum OPG concentration in patients with arteriosclerotic disease was related to high serum levels of C-reactive protein (CRP) and other acute inflammatory mediators. In addition, inflammatory cytokines as interleukin-1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α), and growth factors as platelet-derived growth factor (PDGF) induce OPG expression in human smooth muscle cells, which subsequently prevents the inflammatory response. From these results, it can be concluded that the high OPG levels seen in arteriosclerotic diseases is a secondary phenomenon related to the inflammation (Figure 2).
FIGURE 2.
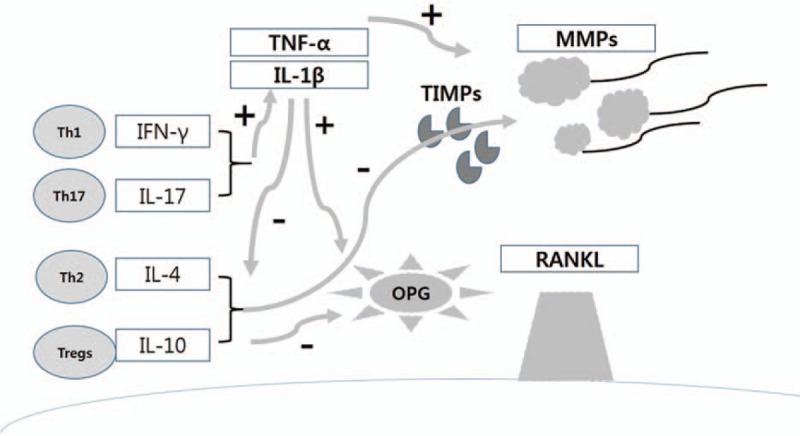
Initial production of proinflammatory cytokines, such as TNF-α and IL-1β, which stimulate expression and activation of MMPs that degrade extracellular connective tissue matrix. Cytokines such as TNF-α can stimulate osteoclastogenesis independently while other cytokines stimulate RANKL expression that leads to formation of osteoclasts and osteoclast activity. Conversely, cytokines produced by Th2 cells and Tregs, such as IL-4 and IL-10, have the opposite effect, in part, through stimulating production of TIMPs and OPG as well as restrain inflammatory cytokine production. IL = interleukin, MMPs = matrix metalloproteinases, OPG = osteoprotegerin, RANKL = receptor activator of nuclear factor kappa-B ligand, TIMPs = tissue inhibitors of matrix metalloproteinases, TNF = tumor necrosis factor.
In our study, the degree of calcification was not measured directly in a quantitative method, but previous studies showed that a semiquantitative test represented the reproducibility for measuring plaques.
This study has several limitations. First, it is a retrospective study, so we did not expect subjects to recall all the risk factors for carotid artery disease. Second, there were not enough data on the degrees of osteoporosis, or on hormonal effect on bone metabolism in female patients. Third, we could not compare several arteriosclerotic factors (eg, RANKL, etc.) which are related to OPG.
The results of this study showed a higher serum OPG concentration in patients with carotid calcification in coronary artery disease when compared with the control group. This could indicate that OPG plays an important function in calcification related to arteriosclerosis.
More future studies are needed for better understanding of blood vessel calcification and feedback action of OPG. These studies are expected to have clinical applications such as in the diagnosis and treatment of cardiovascular diseases such as arteriosclerosis.
Footnotes
Abbreviations: CP = calcified plaque, DM = Diabetes mellitus, ELISA = enzyme linked immune-sorbent assay, IMT = intima-media thickness, MDCT = multi-detector computed tomography, NCP = non calcified plaque, NFκB = nuclear factor kappa-B ligand, OPG = Osteoprotegerin, PDGF = platelet derived growth factor, TNF = the tumor necrosis factor.
The authors report no conflicts of interest.
REFERENCES
- 1.Nandalur KR, Baskurt E, Hagspiel KD, et al. Carotid artery calcification on CT may independently predict stroke risk. Am J Roentgenol 2006; 186:547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davaine JM, Quillard T, Brion R, et al. Osteoprotegerin, pericytes and bone-like vascular calcification are associated with carotid plaque stability. PLoS One 2014; 9:e107642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joshi FR, Rajani NK, Abt M, et al. Does Vascular Calcification Accelerate Inflammation?: A Substudy of the dal-PLAQUE Trial. J Am Coll Cardiol 2016; 67:69–78. [DOI] [PubMed] [Google Scholar]
- 4.Dhore CR, Cleutjens JP, Lutgens E, et al. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol 2001; 21:1998–2003. [DOI] [PubMed] [Google Scholar]
- 5.Jørgensen L, Joakimsen O, Rosvold Berntsen GK, et al. Low bone mineral density is related to echogenic artery plaques: a population based study. Am J Epidemiol 2004; 160:549–556. [DOI] [PubMed] [Google Scholar]
- 6.Rattazzi M, Faggin E, Buso R, et al. Atorvastatin reduces circulating osteoprogenitor cells and T-cell RANKL expression in osteoporotic women: implications for the bone-vascular axis. Cardiovasc Ther 2016; 34:13–20. [DOI] [PubMed] [Google Scholar]
- 7.Davaine JM, Quillard T, Chatelais M, et al. Bone like arterial calcification in femoral atherosclerotic lesions: prevalence and role of osteoprotegerin and pericytes. Eur J Vasc Endovasc Surg 2016; 51:259–267. [DOI] [PubMed] [Google Scholar]
- 8.Jono S, Ikari Y, Shioi A, et al. Serum osteoprotegerin levels are associated with the presence and severity of coronary artery disease. Circulation 2002; 106:1192–1194. [DOI] [PubMed] [Google Scholar]
- 9.Fojt R, Pirk J, Kamenický P, et al. Values of osteoprotegerin in aortic valve tissue in patients with significant aortic stenosis depend on the existence of concomitant coronary artery disease. Cardiovasc Pathol 2015; 25:181–184. [DOI] [PubMed] [Google Scholar]
- 10.Pérez de Ciriza C, Moreno M, Restituto P, et al. Circulating osteoprotegerin is increased in the metabolic syndrome and associates with subclinical atherosclerosis and coronary arterial calcification. Clin Biochem 2014; 47:272–278. [DOI] [PubMed] [Google Scholar]
- 11.Kalogeropoulos A, Terzis G, Chrysanthopoulou A, et al. Risk for transient ischemic attacks is mainly determined by intima-media thickness and carotid plaque echogenicity. Atherosclerosis 2007; 192:190–196. [DOI] [PubMed] [Google Scholar]
- 12.Erlöv T, Cinthio M, Edsfeldt A, et al. Determining carotid plaque vulnerability using ultrasound center frequency shifts. Atherosclerosis 2016; 246:293–300. [DOI] [PubMed] [Google Scholar]
- 13.Xenikou MF, Golemati S, Gastounioti A, et al. Using ultrasound image analysis to evaluate the role of elastography imaging in the diagnosis of carotid atherosclerosis. Conf Proc IEEE Eng Med Biol Soc 2015; 2015:6313–6316. [DOI] [PubMed] [Google Scholar]
- 14.Park CS, Chung WS, Youn HJ, et al. Association between the serum osteoprotegerin level and target lesion calcium in coronary artery disease. Korean Circ J 2006; 36:337–342. [Google Scholar]
- 15.Golledge J, McCann M, Mangan S, et al. Osteoprotegerin and osteopontin are expressed at high concentrations within symptomatic carotid atherosclerosis. Stroke 2004; 35:1636–1641. [DOI] [PubMed] [Google Scholar]
- 16.Sung KC, Kwon CH, Rhee EJ, et al. The association of osteoprotegerin with cardiovascular disease and the New York Heart Association Classification. Korean Circ J 2007; 37:353–358. [Google Scholar]
- 17.Feng ZY, He ZN, Zhang B, et al. Osteoprotegerin promotes the proliferation of chondrocytes and affects the expression of ADAMTS-5 and TIMP-4 through MEK/ERK signaling. Mol Med Rep 2013; 8:1669–1679. [DOI] [PubMed] [Google Scholar]
- 18.Bucay N, Sarosi I, Dunstan CR, et al. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev 1998; 12:1260–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kadoglou NP, Gerasimidis T, Kapelouzou A, et al. Beneficial changes of serum calcification markers and contralateral carotid plaques echogenicity after combined carotid artery stenting plus intensive lipid-lowering therapy in patients with bilateral carotid stenosis. Eur J Vasc Endovasc Surg 2010; 39:258–265. [DOI] [PubMed] [Google Scholar]
- 20.Park CS, Youn HJ, Choi YS, et al. Plasma osteoprotegerin level, a new biochemical marker of atherosclerosis, is associated with diabetes mellitus and age in coronary artery disease. Korean Circ J 2006; 36:543–548. [Google Scholar]


