Abstract
Most previous studies have been single case reports, and studies with large samples are presently lacking. In addition, no studies have investigated the associations between the clinical characteristics and prognosis of hepatoid adenocarcinoma of the stomach (HAS).
The aim of this study was to explore the associations of different clinical characteristics with the ages, serum alpha-fetoprotein (AFP) levels, and survival times of HAS patients.
The present study was conducted using the CBM disc, HowNet, Wanfang and VIP data resource systems, and PubMed. According to the PRISMA Flow Diagram, certain case reports from the same center, those that did not provide patient age or sex, and those that did not report serum AFP levels or AFP immunohistochemistry results were excluded. A total of 131 relevant articles, including 124 case reports, 5 reviews, and 2 postgraduate Master's theses, were reported in the above-mentioned five databases. We applied inclusion criteria to case reports on the clinical characteristics and prognosis of HAS, which resulted in the ultimate inclusion of 180 patients from 62 case reports for statistical analyses.
The main finding was that the age of the men was significantly higher than that of the women (P = 0.004). In addition, the serum AFP levels of the participants with antral disease were significantly higher than those with nonantral disease (P = 0.001). The median serum AFP levels and survival times significantly differed among the patients with the three lesion types (P = 0.001 and 0.019, respectively). The serum AFP levels of the participants with ulcerative-upheaval-type tumors and purely ulcerative tumors were significantly higher than those with upheaval-type tumors (P = 0.000 and 0.017, respectively). In addition, the serum AFP levels of the participants with ulcerative-upheaval-type tumors were significantly higher than those with ulcerative-type tumors (P = 0.019), and their survival time was also significantly higher (P = 0.000). The serum AFP levels of the participants without metastasis or liver metastasis were significantly lower than those with metastasis or liver metastasis (P = 0.000 and 0.000, respectively), and their survival time was significantly longer (P = 0.000 and 0.001, respectively). Finally, the survival time of the participants treated with surgery was significantly longer than those treated using nonsurgical methods (P = 0.046). However, survival analysis revealed that the survival time was only significantly associated with the presence of metastasis (P = 0.002) and liver metastasis (P = 0.036).
The main limitations of this study are as follows: it was a retrospective analysis of published case reports, the clinical data were incomplete, and the cases included in subgroup analyses were different.
Our study results have demonstrated that the prognosis of HAS patients is poor. In addition, the survival time is significantly negatively correlated with the presence of metastasis and liver metastasis.
INTRODUCTION
“Hepatoid cancer” refers to an extrahepatic neoplasm with hepatocellular differentiation.1 A wide histological spectrum of extrahepatic carcinomas produce alpha-fetoprotein (AFP) and other peptide hormones. AFP-producing gastric cancer (AFP-GC) is rare and is classified as a special subtype of gastric cancer (GC) called hepatoid adenocarcinoma (HAC). HAC is characterized by aberrant hepatocellular differentiation and it occurs in extrahepatic organs, especially gastrointestinal tract organs, such as the stomach, esophagus, biliary tract, and pancreas. Hepatoid adenocarcinoma of the stomach (HAS) has unique clinicopathological features; it is a rare type of primary GC that exhibits both adenocarcinomatous and hepatocellular differentiation. It is prone to lymph node and liver metastases, and thus, it has an extremely poor prognosis. Because HAS is rare, it is not widely recognized in the clinic. Additionally, it usually produces large amounts of AFP and is sometimes accompanied by hepatic metastasis. A previous study has demonstrated that these tumors are often overlooked or misdiagnosed as hepatic carcinoma.2 Therefore, HAS can be easily missed, leading to delayed diagnosis and treatment, contributing to the poor prognosis. However, most previous studies have been single case reports, and few studies to date have used large samples. In addition, because HAS is a rare type of GC, it is difficult to evaluate in randomized controlled trials (RCTs). Thus far, no studies have investigated the associations between the clinical characteristics and prognosis of HAS. Our study explored the associations of the different clinical characteristics with age, the serum AFP level, and patient survival using data from the four largest medical databases in China (CBM disc, HowNet, Wanfang, and VIP data resource systems) and PubMed; these databases are searchable and contain all published and unpublished medical papers, including doctoral theses, masters theses, and certain conference papers. Moreover, we sought to increase awareness of this disease among physicians to improve early diagnosis and treatment and ultimately to improve patient prognosis.
METHODS
Data Collection
In the present study, searches were performed of related reports published between June 1987 and February 2016 in the four largest medical databases (CBM disc, HowNet, Wanfang and VIP data resource systems) in China and PubMed in Chinese using the keywords “hepatoid adenocarcinomas of the stomach,” “alpha-fetoprotein,” and “China.” We identified 131 related reports, including 124 case reports containing 1 to 28 clinical cases each, 5 reviews containing references to secondary literature and summarizing previous literature, and 2 postgraduate Master's theses. The case reports included descriptions of clinical manifestations as well as diagnostic (results of imaging examinations, laboratory tests, and histologic examinations) and treatment information. We obtained the following information from the 124 case reports. A total of 21407 GC cases were reported across 11 case reports, and 175 HAS patients were identified among these cases. Therefore, the HAS/GC ratio was 0.82% (175/21407, Table 1). Further, a total of 621 HAS patients were included in the 124 case reports; among them, 17 case reports, which included 104 HAS patients, were eliminated because 5 were from the same center and 12 did not provide patient age or sex. A total of 517 HAS patients were included in the remaining 107 case reports (405 men and 112 women; male/female ratio of 3.62:1); among these patients, 337 from 45 case reports were eliminated because their serum AFP levels were not included or were unclear or AFP immunohistochemistry analyses were not performed. Finally, a total of 180 HAS patients from the remaining 62 case reports were included and analyzed in this study (Figure 1). This study was approved by the institutional review board of Taishan Hospital of Shandong Province. However, ethical approval was not required because this study was based on previously published case reports.
TABLE 1.
The References for 175 HAS Cases Identified Among 21,407 GC Cases Reported in 11 Case Reports (n)

FIGURE 1.
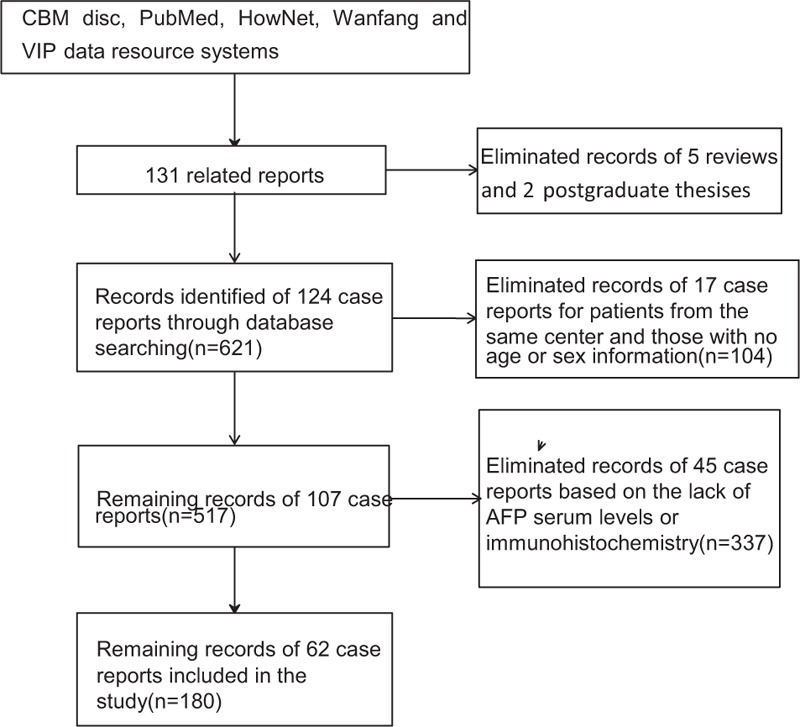
Flow chart of study data collection process.
Statistical Analyses
Statistical analyses were performed using SPSS 19.0 (IBM, Armonk, NY). All of the variables were expressed as the median and range. Kruskal–Wallis ANOVA was used for comparisons among three groups, and the Mann–Whitney U test was used for comparisons between two groups. Survival analyses were performed using the Kaplan–Meier method, and differences between survival curves were examined with the log-rank test. Differences associated with a P < 0.05 were considered significant.
RESULTS
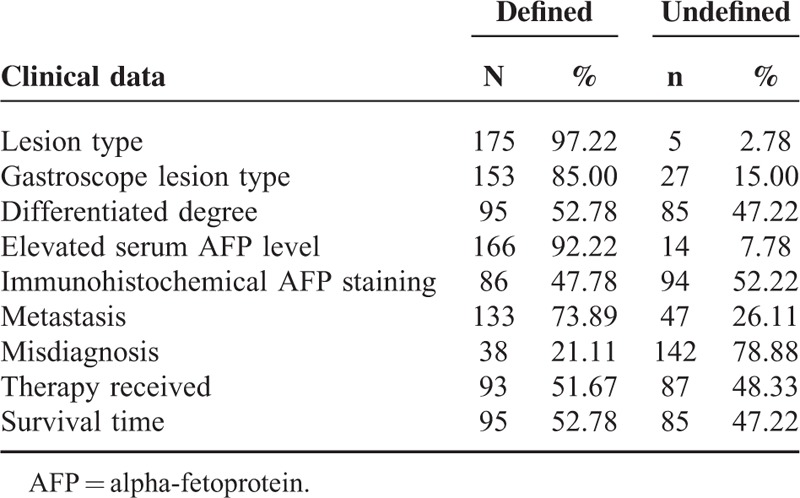
The 180 HAS patients included 139 men and 41 women. The median age at diagnosis was 61.0 years. The chief complaint at the time of the first diagnosis was a stomach ache in 71 patients, melena in 39, epigastric discomfort in 31, abdominal distension in 13, increased AFP in 11, vomiting in 15, hematemesis in 8, nausea in 7, emaciation in 5, anorexia in 4, sour regurgitation in 4, abdominal masses in 3, and anemia, diuresis, polyuria, and polyphagia in 1 patient each. The distribution of the other clinical data at first diagnosis in the 180 HAS patients is presented in Table 2. The disease locations were as follows: 96 (54.86%) patients had tumors in the antrum of the stomach (including 88 patients with tumors in the antrum alone; 4 with tumors in the antrum and corpus of the stomach; 3 with tumors in the antrum and angle of the stomach; and 1 with tumors in both the cardia and antrum of the stomach); and 79 (45.14%) had tumors in nonantrum areas of the stomach (including 41 patients with tumors in the corpus alone; 14 with tumors in the fundus alone; 12 with tumors affecting only the cardia of the stomach; 7 with tumors affecting the angle of the stomach alone; 4 with tumors affecting both the corpus and angle of the stomach; 3 with tumors in the corpus and fundus of the stomach; and 1 with tumors in the fundus and cardia of the stomach). The distribution of the other clinical data is presented in Table 2. The unequivocal lesion types in 153 patients were as follows: 92 (60.13%) patients had tumors of the ulcerative type, 35 (22.88%) had tumors of the upheaval type, and 26 (16.99%) had tumors of a mixed (ulcerative and upheaval) type. The unequivocal degrees of differentiation of the histological-pathological tumors of 95 patients were as follows: 79 were classified as poorly differentiated, 14 were classified as moderately differentiated, and two were classified as highly differentiated. A total of 166 patients had an unambiguous serum AFP level, including 140 positive (AFP > 40 ng/mL) patients and 26 negative (AFP ≤ 40 ng/mL) patients. A total of 86 patients received immunohistochemical staining for AFP, including 75 AFP-positive patients and 11 AFP-negative patients. Among these patients, 94 received serum AFP detection alone (52.22%); 72 underwent both serum and immunohistochemical AFP analyses (40.00%); and 14 underwent AFP immunohistochemical detection alone (7.78%). A total of 86 of the patients who were evaluated via immunohistochemistry were found to be AFP positive; thus, the AFP-positive rate was 87.21% (75/86) and the AFP-negative rate was 12.79% (11/86). A total of 133 patients had unequivocal metastases; 100 had lymph node and distant metastases, including 67 with liver metastases, 7 with widespread celiac metastases, 4 with lung metastases, 2 with pancreatic metastases, 3 with cerebral metastases, 1 with spleen metastasis, 1 with diaphragm muscle metastasis, and 1 with ovarian metastasis. In addition, 14 patients had histopathologically confirmed metastases in only the lymph glands around the stomach. Metastases in distant lymph nodes and remote organs were confirmed using magnetic resonance imaging, computed tomography, or ultrasound. A total of 33 patients were histopathologically confirmed to have no metastasis. In addition, 38 patients were misdiagnosed, including 17 with HAS who were misdiagnosed as having liver carcinoma and 21 who were misdiagnosed as having GC. Of these patients, two had unique manifestations: one HAS patient had neuroendocrine carcinoma14 and another exhibited aldosterone secretion.15 A total of 93 patients received treatment for the disease; 72 patients received surgery, including 59 treated with radical surgery and 13 treated with subtotal gastrectomy, with or without chemotherapy. In addition, 21 patients received nonsurgical therapy, including 16 treated with conservative therapy (15 patients were treated with chemotherapy only and 1 patient was treated with microwave therapy only), and 5 patients withdrew from treatment. The survival times of 95 patients were clearly reported. The 3-year survival rate was 7.36% (7/95), and the median survival time was 10 months.
TABLE 2.
The Distribution of Other Clinical Data at Initial Diagnosis for 180 HAS Patients (n, %)
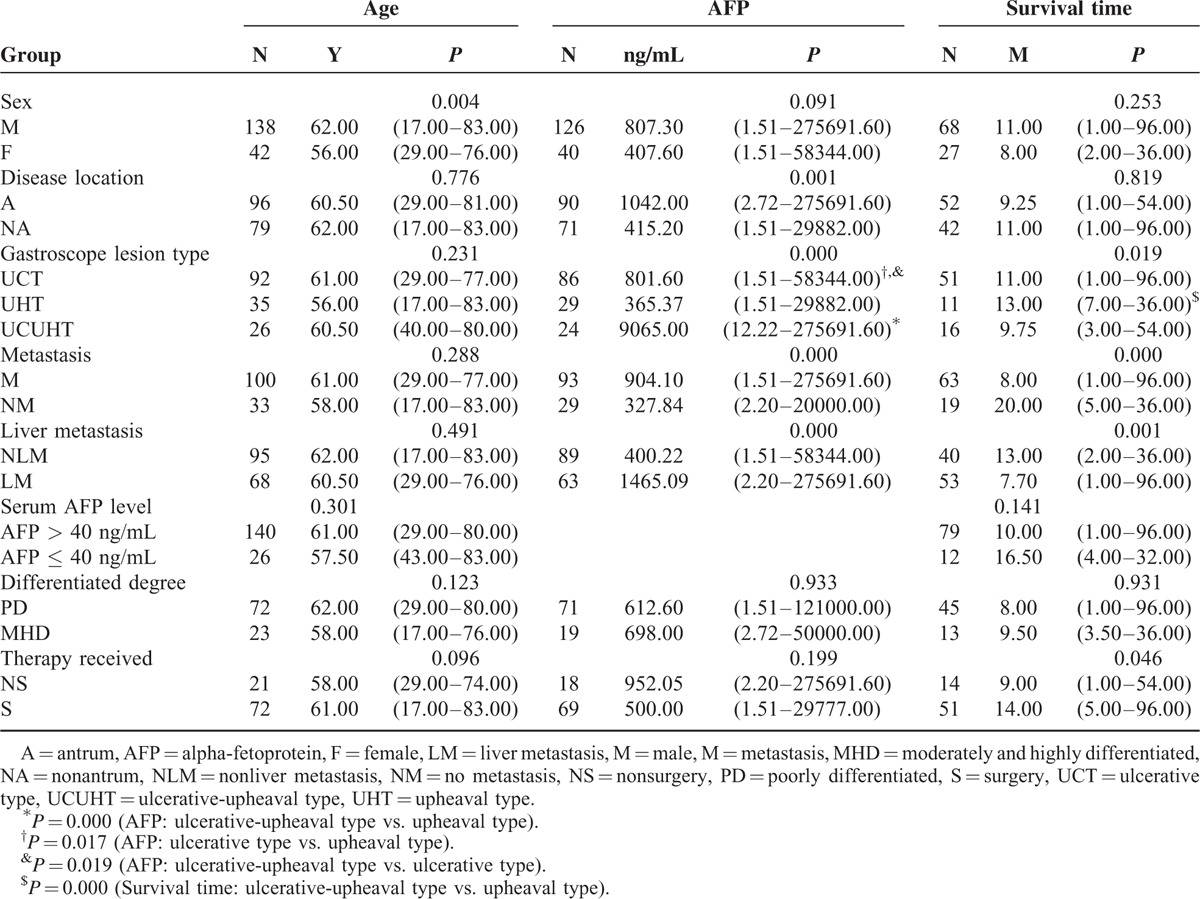
Comparisons of the associations of sex, disease location, the gastroscope lesion type, the presence of metastasis/liver metastasis, the serum AFP level, the differentiated degree, and the therapy received with age, the serum AFP level, and the survival time among the groups are presented in Table 3. The age of the men was significantly higher than that of the women (P = 0.004). No significant differences in the association of sex or the serum AFP level with the survival time were observed among the groups (P = 0.091 and 0.256, respectively). The serum AFP levels of the participants with antral disease were significantly higher than those with nonantral disease (P = 0.001). No differences in age or survival time were found based on tumor location (P = 0.776 and 0.819, respectively). The median serum AFP levels and survival times significantly differed among the three lesion types (P = 0.001 and 0.019, respectively). The serum AFP levels of the participants with ulcerative-upheaval-type tumors and purely ulcerative tumors were significantly higher than those with upheaval-type tumors (P = 0.000 and 0.017, respectively); in addition, the serum AFP levels of the participants with ulcerative-upheaval-type tumors were significantly higher than those with ulcerative-type tumors (P = 0.019). Further, the survival time of the participants with ulcerative-upheaval-type tumors was significantly higher than those with upheaval-type tumors (P = 0.000). However, no significant differences in ages were found among the three groups (P = 0.231). The serum AFP levels of the participants without metastasis or liver metastasis were significantly lower than those with metastasis or liver metastasis (P = 0.000 and 0.000, respectively), and their survival time was significantly longer (P = 0.000 and 0.001, respectively); however, no differences in patient age were found between these two groups (P = 0.288 and 0.491, respectively). Further, no significant differences in age or survival time were found between the patients with AFP levels of ≤40 ng/mL and those with AFP levels of >40 ng/mL (P = 0.301 and 0.141, respectively). In addition, no significant differences were observed in patient age, the serum AFP levels, or survival time among the patients with differing degrees of tumor differentiation (P = 0.123, 0.933, and 0.931, respectively). Finally, the survival time of the participants treated with surgery was significantly longer than those treated using nonsurgical methods (P = 0.046); however, no significant differences in the serum AFP level or age were observed between these two groups of patients (P = 0.096 and 0.199, respectively).
TABLE 3.
Comparisons of Sex, Disease Location, Gastroscope Lesion Type, the Presence of Metastasis/Liver Metastasis, the Serum AFP Level, the Differentiated Degree, and the Therapy Received With Age, the Serum AFP Level, and Survival Time Among the Groups (Median and Range)
The results of survival analysis performed using the Kaplan–Meier method revealed that the survival time was not associated with sex (chi-square = 1.203, P = 0.273), the disease location (chi-square = 0.029, P = 0.864), the gastroscope lesion type (chi-square = 1.875, P = 0.392), the serum AFP level (chi-square = 1.106, P = 0.293), the differentiated degree (chi-square = 0.007, P = 0.932) or the therapy received (chi-square = 3.309, P = 0.069). However, the survival time was significantly associated with the presence of metastasis (chi-square = 9.936, P = 0.002) and liver metastasis (chi-square = 4.375, P = 0.036).
DISCUSSION
A previous study reported that 10 (0.22%) out of 4563 patients with primary GC were ultimately diagnosed with HAS.16 The median age at diagnosis of the patients in this previous study was 65.5 years.2 This type of carcinoma often occurs among the elderly, and it most commonly develops in the antrum.17 A large tumor in the upper abdomen associated with GC might be a clinical manifestation of an AFP-producing hepatoid gastric adenocarcinoma.18 Most HAS cases present as Borrmann's type III fungating lesions with purple, berry-like surfaces.16 In the present study, the constituent HAS/GC ratio was 0.82%. In addition, the male/female ratio was 3.62:1, and the median age at diagnosis was 61.0 years. The major manifestations at initial diagnosis were stomach ache, melena, epigastric discomfort, abdominal distension, an elevated AFP level, vomiting, etc. The most common disease location was the antrum of the stomach, and most gastroscopic lesions were of the ulcerative type. Special attention should be paid to patients with specific manifestations of HAS, such as neuroendocrine carcinoma,14 aldosterone secretion,15 and chronic liver disease,19 to prevent misdiagnosis and mistreatment.
A recent study3 has revealed that AFP-GC and HAS have more aggressive behaviors than common GC. In addition, HAS tumors display two major histopathological patterns:17,20,21 hepatoid-like foci of hepatocellular differentiation and adenocarcinomatous differentiation. The clinicopathological features of HAS, a special type of carcinoma that histologically resembles hepatocellular carcinoma (HCC), include the secretion of a large amount of AFP into the serum, which is associated with the clinical prognosis.22 Giant cells and mitotic figures are frequently found in the hepatoid areas.17 Tumor cells in both the hepatoid areas16,20,23 and glandular areas20 might exhibit positive immunoreactivity for AFP. The detection of hepatoid differentiation in tumor cells might also be associated with immunohistochemical positivity for alpha-1 antitrypsin,21 alpha-1 antichymotrypsin,21 SALL4,23 HepPar-1,23 and glypican 3.23 Tumor cell cytoplasm17,24 and hyaline globules24 have been previously shown to be immunohistochemically positive for AFP. Most cases of HAS express large amounts of AFP. Because this disease is rare and is associated with various cytoplasmic types, the true AFP status might not be identified upon first presentation.25 Hence, HAS can be easily misdiagnosed as either HCC or GC based on the findings of general pathological examination, and the serum AFP level should be carefully considered. An elevated serum AFP level might reflect focal hepatoid differentiation only in the metastatic lymph nodes; this condition requires extensive evaluation of metastatic tumors present in regional lymph nodes as in the case of AFP-producing GC.26 Hence, AFP testing is important for the early detection and diagnosis of HAS.27 Although AFP measurement alone should not be used to screen for this type of cancer,28 HAS can be diagnosed using both immunohistochemistry and serum AFP level measurements.29 Notably, a recent study has reported the case of a 69-year-old patient with HCC that was initially mistaken for HAC who had a favorable outcome after neoadjuvant chemotherapy.30 Hence, HCC and HAC can be confused when diagnosing patients. To avoid the misdiagnosis and mistreatment of patients, a strategy for the differentiation between HCC and HAC should be established because early diagnosis and treatment are keys to improving and prolonging the lives of patients with both of these diseases. Recent studies have demonstrated that FNAB and transhepatic mass biopsy can be used for patient diagnosis.25,31 If the results of immunohistochemical staining of transhepatic biopsy tissues and gastric samples are coincident, then the tumor should be diagnosed as HAS, regardless of AFP production compared with the histology of GC.31,32 The present study revealed that 140 of the 166 patients (82.96%) who underwent serum AFP detection and were found to have a serum AFP level of >40 ng/mL were AFP positive, with an immunohistochemical AFP-positive rate of 84.34%. The presence of disease in the antrum was more common than in other regions of the stomach. Furthermore, the ulcerative type, with or without upheaval, was more common than the purely upheaval type based on gastroscopy findings among the patients diagnosed with HAS. The detection of serum AFP and immunohistochemical AFP staining are useful for diagnosing HAS. Physicians should be particularly aware of GC patients who have a high serum AFP level. Increasing recognition of the clinical and pathological characteristics of HAS is important, especially among patients without hepatopathy or multiple intrahepatic lesions. Whenever a patient presents with a markedly increased serum AFP level before or after surgery, thorough gastroscopic examination should be performed, and immunohistochemical analysis should be conducted to determine the AFP level. AFP monitoring is crucial for assessing therapeutic efficacy and for predicting tumor relapse or metastasis.
The prognosis of advanced-stage HAS is poor. Previous studies have confirmed that the prognosis of HAS is poorer than that of AFP-GC. In addition, early HAS detection is associated with a high risk of liver metastasis.33 Two previous studies have reported 3-year HAS survival rates of 17.2%4 and 22.6%,22 and the median survival time was 6 months in one study22 and 17 months in another.13 In the present study, the 3-year survival rate was 7.36% (7/95) and the median survival time was 10 months among the 95 patients with an unequivocal survival time. Furthermore, subgroup analysis revealed that survival time was associated with metastasis (especially liver metastasis) and an increased serum AFP level before surgery. In addition, the results of survival analysis conducted using the Kaplan–Meier method showed that the survival time was significantly associated with metastasis and liver metastasis. However, survival was not associated with patient sex, disease location or type, the serum AFP level, the degree of differentiation, or the type of therapy received. The statistical results might have been affected by the small sizes of the subgroups for the degrees of differentiation and types of therapy received; thus, larger sample sizes should be used in sub-sequent studies. Thus far, the main reasons for the poor prognosis of HAS have been reported to likely include the high lymphatic (54.8%) and distant (25.8%) metastasis rates before surgery,22 particularly the early34,35 and frequent2,17 development of liver metastasis and portal vein tumor thrombus (PVTT).3 HAS is more aggressive than other types of adenocarcinoma3,29 because it begins as an aggressive clone featuring extensive loss of heterozygosity (LOH) and high fractional allelic loss.32 HAS tumors that grow in a solid pattern are particularly aggressive.23 Hence, HAS behaves as an aggressive type of adenocarcinoma.21,24,25 In addition, a report of HAC cases has demonstrated that this type of cancer is hypervascular and grows rapidly and that it can result in the spontaneous rupture of metastatic liver lesions.36 All of the above-mentioned factors can result in a poor prognosis.19,22,34,35 Notably, improving disease recognition at initial presentation is crucial for promoting early and appropriate therapy.25 In fact, long-term survival has been achieved among patients with this disease via salvage surgery after chemotherapy.35 Radical surgery and chemotherapy can also positively affect clinical outcome,21 and chemotherapy alone might provide some benefit.16,21 In addition, transarterial embolization might be a feasible option for the treatment of ruptured tumors.36 Therefore, appropriate treatments should be administered as soon as possible. Our study has certain limitations. First, it was a retrospective evaluation of published case reports; the clinical data are incomplete, and the cases included in subgroup analyses are different. The statistical outcomes might have been affected by these differences, as well as the different types of therapy received by the patients. Therefore, larger sample sizes are needed to verify our findings.
CONCLUSIONS
HAS is a rare disease with a poor prognosis that primarily occurs in older individuals. It is more common in men than in women. It primarily presents in the antrum of the stomach and is generally ulcerative. Patient survival is significantly negatively correlated with the likelihood of metastasis and liver metastasis. Hence, early diagnosis is the key to improving and prolonging patients’ lives.
Footnotes
Abbreviations: AFP = alpha-fetoprotein, AFP-GC = alpha-fetoprotein-producing gastric cancer, GC = gastric cancer, HAC = hepatoid adenocarcinoma, HAS = hepatoid adenocarcinoma of the stomach, HCC = hepatocellular carcinoma, LOH = loss of heterozygosity, PVTT = portal vein tumor thrombus.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Iida M, Imura J, Furuichi T, et al. Alteration of the AT motif binding factor-1 expression in alpha-fetoprotein producing gastric cancer: is it an event for differentiation and proliferation of the tumors? Oncol Rep 2004; 11:3–7. [PubMed] [Google Scholar]
- 2.Xie Y, Zhao Z, Li P, et al. Hepatoid adenocarcinoma of the stomach is a special and easily misdiagnosed or missed diagnosed subtype of gastric cancer with poor prognosis but curative for patients of pN0/1: the experience of a single center. Int J Clin Exp Med 2015; 8:6762–6772. [PMC free article] [PubMed] [Google Scholar]
- 3.Liu X, Sheng W, Wang Y. An analysis of clinicopathological features and prognosis by comparing hepatoidadenocarcinoma of the stomach with AFP-producing gastric cancer. J Surg Oncol 2012; 106:299–303. [DOI] [PubMed] [Google Scholar]
- 4.Zhang JF, Shi SS, Shao YF, et al. Clinicopathological and prognostic features of hepatoid adenocarcinoma of the stomach. Chin Med J (Engl) 2011; 124:1470–1476. [PubMed] [Google Scholar]
- 5.Chen NH, Shen DP, Liu GH, et al. Clinical pathologic features and prognosis of hepatoid adenocarcinoma of the stomach. Guangdong Med J (in Chinese) 2014; 35:2248–2251. [Google Scholar]
- 6.Li XY, Zhong DR, Liu HF, et al. Hepatoid adenocarcinoma of stomach: a report of 11 cases. Oncol Prog 2012; 10:185–186.198. [Google Scholar]
- 7.Zhang X, Li X, Wang C, et al. Study of p21 ras gene expression in hepatoid adenocarcinoma of stomach. J Clin Exp Pathol 1998; 14:264–265. [Google Scholar]
- 8.Nie XQ. Gastric hepatoid adenocarcinoma: 9 cases report and review of the literature. Chin J Clin Gastroenterol (in Chinese) 2012; 24:146–148.155. [Google Scholar]
- 9.Zhang BL. Gastric hepatoid adenocarcinoma. Chin J Clin Gastroenterol (in Chinese) 1992; 19:236–237. [Google Scholar]
- 10.Li N, Feng ZZ, Gu CZ, et al. Clinicopathologic features of hepatoid adenocarcinoma of the stomach. Chin J Histochem Cytochem (in Chinese) 2013; 22:75–78. [Google Scholar]
- 11.Song HF, Li SQ, Yang Y, et al. Pathologic study of hepatoid adenocarcinoma of the stomach. Chin J Oncol (in Chinese) 1995; 17:56–58. [PubMed] [Google Scholar]
- 12.Yang CK, Zhao WJ, Dai QB, et al. Clinical characteristics, diagnosis and therapy of hepatoid adenocarcinoma of the stomach. Chin J Gastrointest Surg (in Chinese) 2007; 10:245–248. [PubMed] [Google Scholar]
- 13.Gao YB, Zhang DF, Jin XL, et al. Preliminary study on the clinical and pathological relevance of gastric hepatoid adenocarcinoma. J Dig Dis 2007; 8:23–28. [DOI] [PubMed] [Google Scholar]
- 14.Yin QQ, Jiang B, Wang ML, et al. A case report of hepatoid adenocarcinomas of the stomach with neuroendocrine carcinoma. Prac J Med Pharm 2014; 31:432. [Google Scholar]
- 15.Yang Z, Yang SG. A case report of hepatoid adenocarcinomas of the stomach with aldosterone secretion functions. Chin J Oncol (in Chinese) 1996; 18:394. [Google Scholar]
- 16.Lin CY, Yeh HC, Hsu CM, et al. Clinicopathologial features of gastric hepatoid adenocarcinoma. Author information. Biomed J 2015; 38:65–69.doi: 10.4103/23 19-4170.126860. [DOI] [PubMed] [Google Scholar]
- 17.Wang XL. Hepatoid adenocarcinoma of the stomach. Zhonghua Zhong Liu Za Zhi (in Chinese) 1988; 10:455–457. [PubMed] [Google Scholar]
- 18.Iso Y, Sawada T, Shimoda M, et al. Solitary AFP- and PIVKA-II-producing hepatoid gastric cancer with giant lymph node metastasis. Hepatogastroenterology 2005; 52:1930–1932. [PubMed] [Google Scholar]
- 19.Cholinesterase PJ, Voutsadakis IA, Barbanis S, et al. AFP-producing hepatoid adenocarcinoma of the stomach: a case report. Cases J 2009; 2:9296.doi: 10.1186/1757-1626-2-9296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoshida Y, Nagakawa T, Mano S, et al. Hepatoid adenocarcinoma of the endometrium associated with alpha-fetoprotein production. Int J Gynecol Pathol 1996; 15:266–269. [DOI] [PubMed] [Google Scholar]
- 21.Ye MF, Tao F, Liu F, et al. Hepatoid adenocarcinoma of the stomach: a report of three cases. World J Gastroenterol 2013; 19:4437–4442.doi: 0.3748/wjg.v19.i27.4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang J, Wang R, Zhang W, et al. Clinicopatholo gical and prognostic characteristics of hepatoid adenocarcinoma of the stomach. Gastroenterol Res Pract 2014; 2014:140587.doi: 10.1155/2014/140587. Epub 2014 Feb 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osada M, Aishima S, Hirahashi M, et al. Combination of hepato-cellular markers is useful for prognostication in gastric hepatoid adenocarcinoma. Hum Pathol 2014; 45:1243–1250. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi Y, Inoue T. Hepatoid carcinoma of the uterus that collided with carcinosarcoma. Pathol Int 2003; 53:323–326. [DOI] [PubMed] [Google Scholar]
- 25.Wee A, Thamboo TP, Thomas A. Alpha-fetoprotein-producing liver carcinomas of primary extrahepatic origin. Acta Cytol 2003; 47:799–808. [DOI] [PubMed] [Google Scholar]
- 26.Kang GH, Kim YI. Alpha-fetoprotein-producing gastric carcinoma presenting focal hepatoid differentiation in metastatic lymph nodes. Virchows Arch 1998; 432:85–87. [DOI] [PubMed] [Google Scholar]
- 27.Yao L, Xu H. Clinical characteristics and prognosis of hepatoid adenocarcinoma of the stomach. Zhonghua Wei Chang Wai Ke Za Zhi (in Chinese) 2014; 17: doi:10.3760/cma.j.issn. 1671-0274.2014.02.023. [PubMed] [Google Scholar]
- 28.Van Roon AH, ter Borg PC, Zondervan PE, et al. A patient with an alpha-foetoprotein producing tumour. Ned Tijdschr Geneeskd 2009; 153:A364. [PubMed] [Google Scholar]
- 29.Bakir T, Aliyazicioglu Y, Bektas A, et al. Hepatoid adenocarcinoma of the stomach: report of five cases and review of the literature. Acta Gastroenterol Belg 2006; 69:330–337. [PubMed] [Google Scholar]
- 30.Becq A, Mateescu C, Khayat D, et al. Atypical Ppesentation of hepatocellular carcinoma mimicking a gastric hepatoid adenocarcinoma: a case report. Medicine (Baltimore) 2015; 94:e1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moon JY, Kim GH, Cheong JH, et al. A case of hepatic metastasis of gastric hepatoid adenocarcinoma mistaken for primary hepatocellular carcinoma. Korean J Gastroenterol 2012; 60:262–266. [DOI] [PubMed] [Google Scholar]
- 32.Supriatna Y, Kishimoto T, Uno T, et al. Evidence for hepatocellular differentiation in alpha-fetoprotein-negative gastric adenocarcinoma with hepatoid morphology: a study with in situ hybridisation for albumin mRNA. Pathology 2005; 37:211–215. [DOI] [PubMed] [Google Scholar]
- 33.Aoyagi K, Koufuji K, Yano S, et al. Alpha-fetoprotein-producing early gastric cancer: report of two cases. Kurume Med J 2003; 50:63–66. [DOI] [PubMed] [Google Scholar]
- 34.Jeong EH, Kim DH, Ma SH, et al. A case of liver metastasis of gastric hepatoid adenocarcinoma. Korean J Hepatol 2009; 15:201–208. [DOI] [PubMed] [Google Scholar]
- 35.Nakao S, Nakata B, Tendo M, et al. Salvage surgery after chemotherapy with S-1 plus cisplatin for α-fetoprotein-producing gastric cancer with a portal vein tumor thrombus: a case report. BMC Surg 2015; 15:5.doi: 10.1186/1471-24 82-15-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takeda H, Kita R, Kanesaka T, et al. A case of α-fetoprotein-producing hepatoid adenocarcinoma of the stomach with spontaneous rupture of multiple liver metastases. Nihon Shokakibyo Gakkai Zasshi 2013; 110:1625–1632. [PubMed] [Google Scholar]


