Abstract
Anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA) is a rare congenital coronary abnormality associated with early infant mortality and sudden death in adults. Transthoracic echocardiography (TTE) plays an important role in early detection and diagnosis of ALCAPA as a noninvasive modality. However, its diagnostic value is not well studied. The purpose of this study is to determine the performance of TTE in the diagnostic assessment of ALCAPA as compared with coronary CT and invasive coronary angiography.
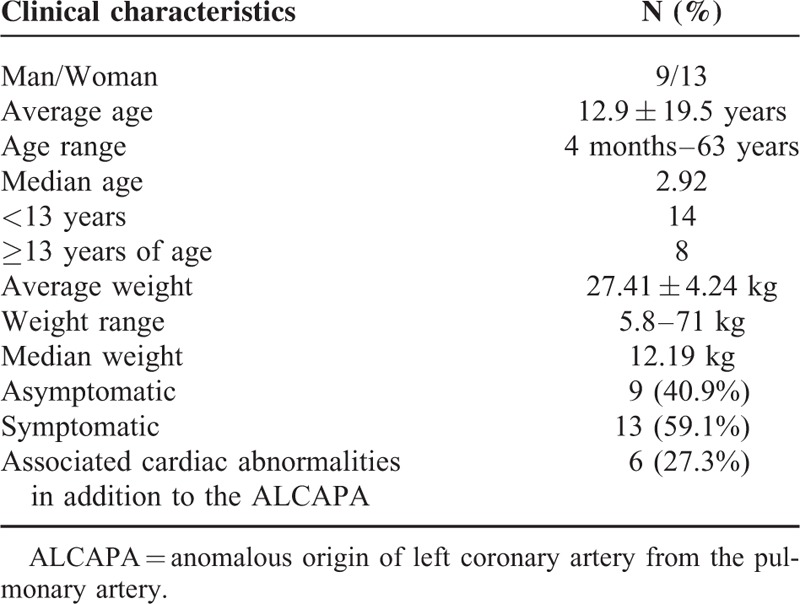
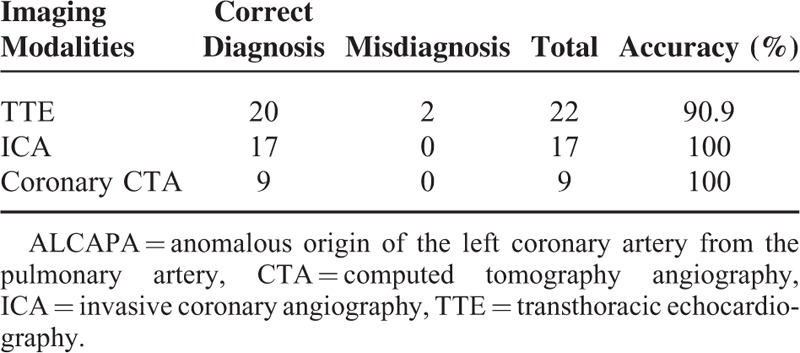
A total of 22 patients (13 women and 9 men, mean age, 12.9 ± 19.5 years) with ALCAPA who underwent echocardiographic examination for clinical diagnosis were retrospectively reviewed and analyzed. Transthoracic echocardiographic features of ALCAPA were analyzed and its diagnostic value was compared with invasive coronary angiography and coronary CT angiography (CTA) with surgical findings serving as the gold standard. Surgery was performed in all of the patients to establish the dual coronary artery system. Five underwent the Takeuchi procedure and 17 had re-implantation of the anomalous left coronary artery. Of 20 patients, echocardiographic diagnoses were in good agreement with findings at surgery, resulting in the diagnostic accuracy of 90.9%. Two cases were misdiagnosed—one as the right coronary artery to pulmonary artery fistula and the other as rheumatic heart disease. The echocardiographic features of these patients with ALCAPA included: abnormal left coronary ostium arising from the pulmonary trunk with retrograde coronary artery flow in 20 patients; enlargement of the right coronary artery in 17 patients; abundant intercoronary septal collaterals in 17 patients; and moderate and significant mitral regurgitation in 14 patients. The diagnostic accuracy of invasive coronary angiography (in 17 patients) and coronary CTA (in 9 patients) was 100%.
This study shows that TTE is an accurate, noninvasive imaging modality for displaying the origin of coronary arteries and demonstrating the coronary courses as well as other associated abnormalities in patients with ALCAPA.
INTRODUCTION
Anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA), a rare congenital coronary abnormality, is associated with early infant mortality and sudden death in adults. The incidence of ALCAPA is estimated at 1/300,000 live births comprising between 0.24% and 0.46% of congenital cardiac disease.1–4 Because of the unfavorable prognosis, it becomes necessary to recognize early and surgically correct the condition before any cardiac dysfunction occurs. ALCAPA was diagnosed exclusively by invasive coronary angiography (ICA).5 However, ICA suffers from disadvantages of radiation exposure (∼6 mSv).6 In recent years coronary computed tomography angiography (CTA) has undergone rapid developments with high diagnostic accuracy in imaging both adult and pediatric patients with coronary artery disease. Coronary CTA provides excellent anatomical information on the whole coronary tree. Therefore, it is a useful modality for diagnosis of coronary artery disease including the ALCAPA.7–10 Despite its superior spatial resolution of visualizing the origin and course of left coronary artery (LCA), the main disadvantages of coronary CTA are relatively high radiation dose, contrast-induced acute kidney injury (CI-AKI) (the third most common cause of acute renal failure among hospitalized patients), high price, and its inability to assess cardiac function such as blood flow.11–15
With the development of ultrasonic imaging, transthoracic echocardiography (TTE) serves as a noninvasive method for diagnosis of ALCAPA by providing important information including the anatomic, functional, and hemodynamic evaluation of the cardiovascular system.16,17 Although significant progress has been made in echocardiographic imaging of cardiovascular disease over the last decade, its diagnostic value in ALCAPA is not well understood. In this article, we present our experience of analyzing echocardiographic findings in a group of patients diagnosed with ALCAPA. We hypothesized that TTE could serve as a reliable imaging modality for the diagnostic evaluation of ALCAPA when compared with coronary CTA and ICA.
MATERIALS AND METHODS
Clinical Data Collection
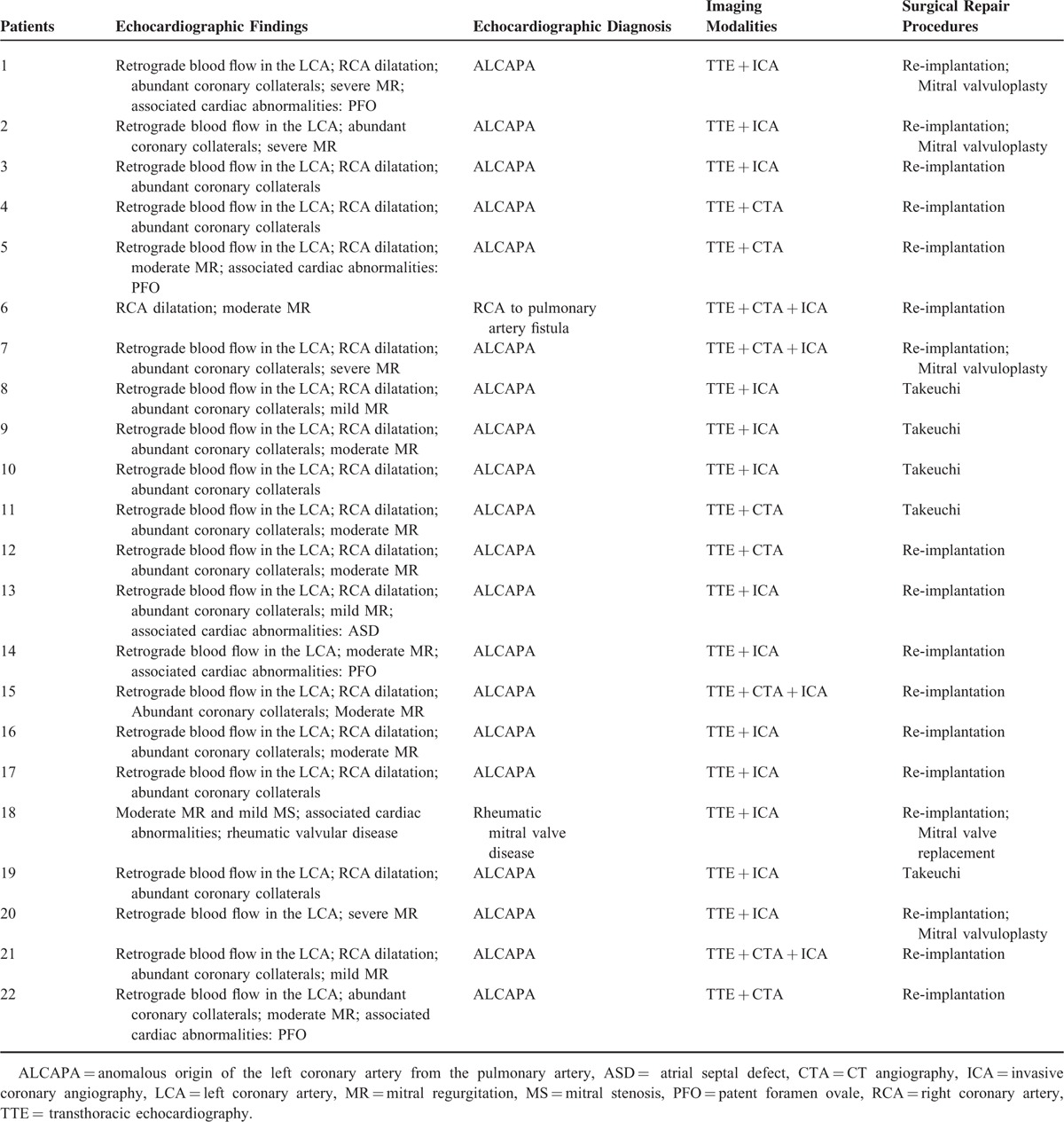
A retrospective review of clinical database from January 2004 to December 2014 was performed and 25 patients were identified with ALCAPA. The study was approved by the local research ethics committee. The inclusion criteria were (1) availability of echocardiographic data in all of the patients; (2) patients with ALCAPA with ICA data or/and CTA data for comparison; (3) surgery done to determine the diagnostic accuracy of these three imaging modalities in all of the patients. Patients with ALCAPA without echocardiographic data or without surgery done to confirm the diagnosis were excluded. Three patients with ALCAPA were excluded because of the following reasons: without echocardiographic data (n = 1) and no surgical treatment data (n = 2, one patient had sudden cardiac death after admission and another one refused further surgical treatment and was discharged from our hospital (Figure 1). After exclusion, 22 cases were included in the analysis. Surgery was performed in all of the patients shortly after the diagnosis. The Takeuchi procedure was performed in 5 patients, and re-implantation of the anomalous coronary into the aorta was done in 17 cases. The clinical characteristics of all of the patients with ALCAPA are shown in Table 1.
FIGURE 1.
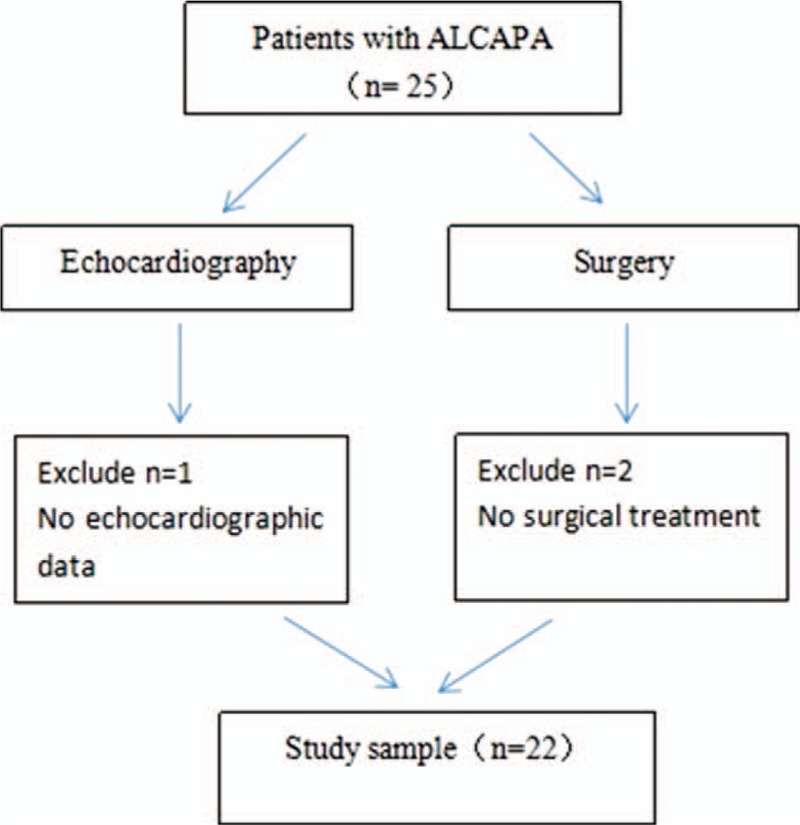
Flow chart showing the strategy of obtaining eligible patients.
TABLE 1.
Clinical Characteristics of 22 Patients with ALCAPA
Imaging Protocols of Transthoracic Echocardiography
TTE was carried out in all of the patients within the preoperative and postoperative week. TTE examinations were performed using a 2.0 to 5.0 MHz transducer with GE Vivid7 and Philips IE33 ultrasound systems. The echocardiographic images included the parasternal left ventricular long axis, large artery short axis, apical four-chamber, suprasternal view, and subcostal views. The anatomical structure of the heart was probed carefully in these views so as to confirm whether there were cardiovascular anomalies. The ostium of the LCA and right coronary artery (RCA) were carefully examined to determine if they originated from the aorta or pulmonary artery. The shapes and inner diameters of the initial segments of main LCA and RCA were carefully visualized and measured according to the criteria established by the Japanese Ministry of Health to classify coronary arteries as abnormal (>3 mm in children <5 years or >4 mm in children ≥5 years).18 The color Doppler imaging (CDI) was utilized to identify blood flow and valvular regurgitation. The left coronary ostium arising from the pulmonary trunk with retrograde coronary artery flow visualized by CDI represented a direct echocardiographic diagnostic parameter of ALCAPA. Abundant coronary collaterals especially intercoronary septal collaterals with retrograde diastolic or continuous flow visualized by CDI represented a main indirect sign of ALCAPA. Continuous Doppler records help to separate septal collateral vessels flow in mid-systole and particularly in diastole from the small muscular ventricular septal defects shunting in the systole. Continuous Doppler was also used to measure the abnormal blood flow speed and shunt differential pressure. Conventional measurements of the left ventricle (LV) in the parasternal left ventricle long-axis view using M-mode image included: left ventricular end-diastolic diameter (LVEDD), end-systolic diameter (LVESD), left ventricular ejection fraction (LVEF), and left ventricular fractional shortening (LVFS). TTE examinations were performed by at least two echocardiography physicians who had >8 and 10 years of experience respectively in echocardiographic imaging. Any discrepancy between the observers was resolved by consensus.
ICA and Coronary CTA
ICA was performed in 17 patients by femoral or radial approach using a 1250-mA DSA system (GE Healthcare). Diagnosis was confirmed in projections clearly showing the origin of coronary artery by a radiologist with >10 years of experience in cardiac imaging. Considering their young age, ICA was not performed in the remaining five cases because of the concern of radiation dose and potential risk associated with ICA procedure.
Coronary CTA was performed in nine patients using a 64-MDCT scanner (Aquilion, Toshiba Medical Systems, Otawara, Japan) and Dual-source CT (Siemens Medical Solutions, Forchheim, Germany). In the remaining 13 cases, coronary CTA was not performed because of lack of adequate experience of using coronary CTA for diagnostic assessment of pediatric patients with suspected cardiac disease. The scanning protocols were as follows: detector collimation 64 × 0.5 mm/2 × 32 × 0.6 mm, gantry rotation of 0.4/0.28 s, tube voltage of 100 to 120 kVp (depending the patient's body weight), and automatic tube current modulation for 64-MDCT and dual-source CT. The nonionic contrast medium (Iopamiro or Iopamidol, 370 g/mL, Bracco) was injected using a dual-head power injector. The scan time was determined using bolus tracking technique with a CT attenuation of 200 and 120 HU for 64-MDCT and dual-source CT, respectively, as the threshold in the ascending aorta to trigger the scan. All images were de-identified and transferred to a separate workstation (Vitrea 2, Vital Images) for further image processing and analysis.
Statistical Analysis
Statistical analyses were performed using SPSS (SPSS 19.0, Chicago, IL). Quantitative variables were expressed as mean ± standard deviation and qualitative variables were expressed as a percentage (%). Comparison was performed with the chi-square test or Fisher exact test for categorical data. A P value <0.05 was considered statistically significant.
RESULTS
All of the 22 patients were confirmed to have ALCAPA on surgery, whereas echocardiographic examinations confirmed 20 patients having ALCAPA, leading to the diagnostic accuracy of 90.9%. In the remaining two patients, diagnoses were missed on preoperative TTE. One patient was misdiagnosed as RCA to pulmonary artery fistula by TTE. Subsequently, the ALCAPA was confirmed by ICA and CTA before the operation. Another patient was diagnosed to have mitral regurgitation of rheumatic origin and ALCAPA was missed in the initial diagnosis because the patient's symptoms attracted clinician's attention to focus on mitral valve lesions of rheumatic origin. Before operation ALCAPA was confirmed by ICA. Six (27.3%) patients had other associated cardiac abnormalities in addition to the ALCAPA (Table 1), which included: atrial septal defect (ASD) in one patient, patent foramen ovale (PFO) in four patients, and rheumatic heart disease in another patient. The echocardiographic features included direct signs, indirect signs, abundant coronary collaterals, and mitral regurgitation, which are detailed ahead.
Direct Signs of ALCAPA
The direct signs of ALCAPA were visualized in 20 patients in whom the left coronary ostium arise from the pulmonary trunk with retrograde coronary artery flow. The ostium of the LCA was not visualized to origin from the aorta above the left cusp of the aortic valve by two-dimensional echocardiography. An abnormal vessel inserting into the pulmonary trunk was seen in the diagnoses of ALCAPA and the continuous shunt was shown by CDI to follow the path of this vessel into the pulmonary artery (Figures 2A, 3A, 4A and B Figures 2–4). Pulsed Doppler (PD) showed the retrograde abnormal flow with low-velocity, prominent in the diastolic phase (Figure 2B). In the parasternal aortic root short-axis view, the ostium of the RCA was seen to normally originate above the right cusp of the aortic valve (Figures 2C and 4C Figures 2 and 4). According to these echocardiographic features, the retrograde abnormal flow was considered as the anomalous origin of the LCA from the pulmonary artery.
FIGURE 2.
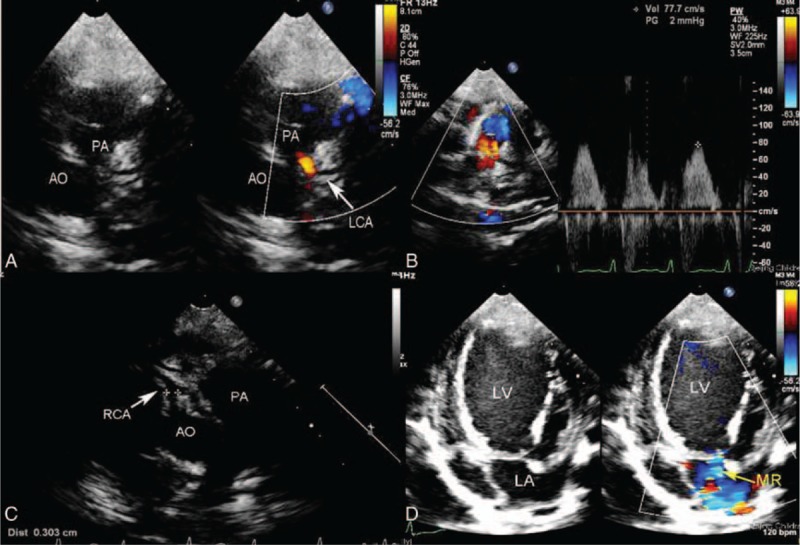
A 3-year-old girl with ALCAPA. (A) Modified parasternal short-axis view identified left main coronary artery arising from the main pulmonary artery and the red shunt from it to the pulmonary artery. (B) Pulse Doppler showed the retrograde red shunt a low-velocity, prominently diastolic flow spectrum. (C) Modified parasternal aortic root short-axis view showed the ostium of the right coronary artery originating above the right cusp of the aortic valve. (D) Apical four-chamber view showed severe mitral regurgitation. ALCAPA = anomalous origin of the left coronary artery from the pulmonary artery.
FIGURE 4.
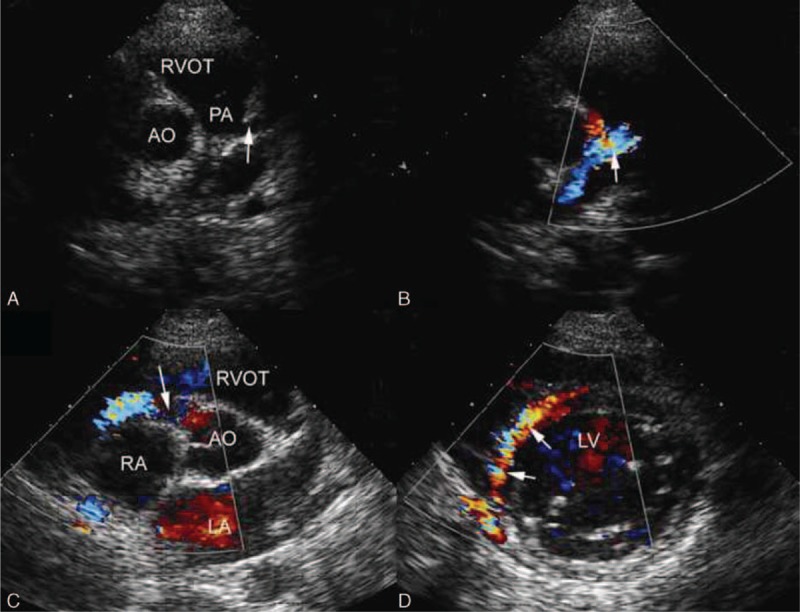
A 20-year-old boy with ALCAPA. (A) Modified parasternal short-axis view identified LCA originating from the main pulmonary artery. (B) CDI showed the blood flow of LCA in the opposite direction of normal LCA and the blue shunt from it to the pulmonary artery. (C) CDI showed the dilated blue flow of the right coronary artery arising from the aorta. (D) Parasternal short-axis view showed abundant reversed septal collateral signals within the ventricular septum from right to left coronary artery. ALCAPA = anomalous origin of the left coronary artery from the pulmonary artery, CDI = color Doppler imaging, LCA = left coronary artery.
FIGURE 3.
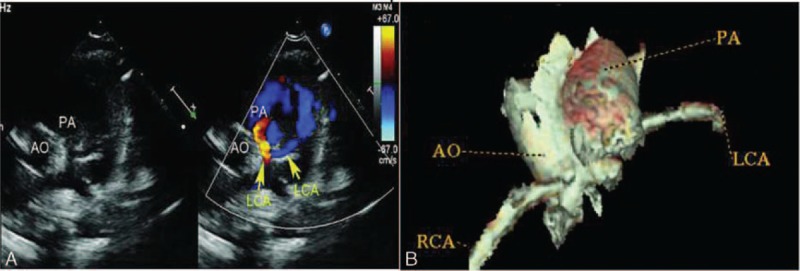
A 1-year-old boy with ALCAPA. (A) Modified parasternal short-axis view identified left main coronary artery arising from the main pulmonary artery and the retrograde shunt from it to the pulmonary artery. (B) Coronary CT angiography showed left coronary artery originated from the posterior aspect of the main pulmonary artery. ALCAPA = anomalous origin of the left coronary artery from the pulmonary artery, CT = computed tomography.
Indirect Signs of ALCAPA
Enlargement of the RCA
The proximal RCA is usually dilated. The diameter of the RCA was noted to be enlarged in 17 (77.3%) of 22 patients. The diameter of the LCA measured by TTE ranged from 1.3 to 9.0 mm with a mean value of 3.52 ± 2.94 mm. The diameter of RCA ranged from 2.3 to 11.0 mm with a mean value of 4.45 ± 3.16 mm, which was significantly higher than those of LCA (P <0.01).
Abundant Coronary Collaterals
Of 22 patients, 17 (77.3%) showed multivessel myocardial collateral blood flow signals at the left ventricular wall and ventricular septum by CDI (Figure 4D). The dilated septal branch had reversed blood flow with high velocity, indicating that a large blood volume flew into the LAD through the septal branch from the RCA in these patients.
Mitral Regurgitation (MR)
CDI showed different degrees of mitral regurgitation in this group of patients. Of 22 patients, 14 (63.6%) had moderate to severe mitral regurgitation (Figure 2D). Three (13.6%) had mild mitral regurgitation. The remaining five patients (22.7%) did not have mitral regurgitation.
Comparison of Diagnostic Value of TTE with ICA and Coronary CTA
The diagnostic accuracy of ICA and coronary CTA was 100% showing excellent correlation of these image findings with surgery (Figure 2B). Table 2 shows details of the diagnostic value of these three imaging modalities for detection of ALCAPA.
TABLE 2.
Comparison of Diagnostic Value of TTE in ALCAPA when Compared With Invasive Coronary Angiography and Coronary
Surgical Findings
Of 22 patients who were treated by surgical repairs, Takeuchi procedure was performed on 5 patients and re-implantation of the anomalous coronary into the aorta was done on 17 cases. Mitral valvuloplasty for severe mitral regurgitation was performed on four patients with ALCAPA re-implantation. One patient with ALCAPA re-implantation underwent mitral valve replacement for rheumatic mitral valve disease. In this group of patients, the left ventricular systolic and diastolic diameters were significantly improved in the first postoperative week compared with those in pre-operation diameters (P <0.01). However, the left ventricular ejection fraction and shortening fraction did not change significantly following operation (P >0.05) (Table 3). Two patients died after operation, although the cause of death was unknown. Table 4 shows details of cardiac image findings in each patient with ALCAPA.
TABLE 3.
Comparison of the Echocardiographic Parameters Before and After Operation in 22 Patients with ALCAPA
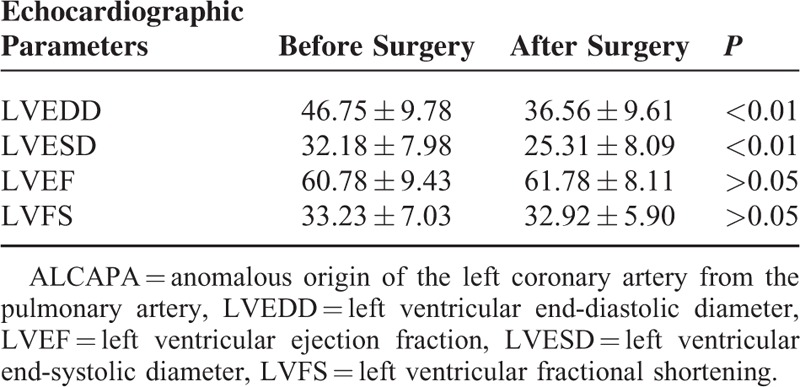
TABLE 4.
Transthoracic Echocardiography Findings, Diagnostic Imaging Modalities, and Surgical Repair Procedures
DISCUSSION
ALCAPA, first reported by Brooks in 1886,19 is a rare congenital heart disease. In this study, we reported echocardiographic features of ALCAPA consisting of direct and indirect signs. Findings of this study confirmed TTE as a reliable imaging modality for the diagnostic evaluation of ALCAPA as compared with ICA and coronary CTA. To the best of our knowledge, only a few studies about the diagnostic usefulness of echocardiography in patients with ALCAPA have been published, with most of them reporting single cases.20–23 This study represents the largest number of surgically treated patients with ALCAPA from a single medical center with the aim of investigating diagnostic the value of TTE.
The typical and direct echocardiographic feature of ALCAPA is the ostium of the LCA originating from the pulmonary artery in parasternal aortic root short-axis view. It is difficult to distinguish LCA originating from the pulmonary artery or the aorta based on anatomical image analysis, because the LCA is close to the aorta, and sometimes it even crosses through the aortic wall in patients with ALCAPA. This limitation can be overcome by CDI as it provides hemodynamic flow information which cannot be obtained by other imaging modalities such as ICA or coronary CTA. The blood flow in the abnormal vessel from the pulmonary trunk is in the opposite direction of normal LCA and this is prominent in the diastolic phase. In some cases, the two branches of LCA and retrograde flow can be visualized. However, when visualizing a dilated RCA and diastolic or continuous retrograde flow from the abnormal vessel into the main pulmonary artery, it was easily misdiagnosed as RCA to pulmonary artery fistula. One patient in our series was misdiagnosed as the RCA to pulmonary artery fistula because of the absence of a careful visualization of the origin of LCA. Therefore, when coronary anomalies are suspected, the origin, size, and course of the coronary arteries as well as the direction of flow should be evaluated carefully. The retrograde flow from the LCA into the pulmonary artery trunk is an important feature in the diagnosis of ALCAPA.
In this study, TTE showed enlargement of the proximal RCA in 77.3% patients, which showed the multivessel myocardial collateral blood flow signals in the left ventricular wall and ventricular septum by CDI. Retrograde flow of LCA and intercoronary septal collaterals suggest left-to-right shunting from RCA via LCA to main pulmonary artery through highly developed collateral vessels. These signs are because of the low pulmonary vascular resistance, leading to a coronary steal phenomenon.24 It is worth noting that intercoronary septal collaterals should not be confused with multiple muscular ventricular septal defects.25 Mitral regurgitation is frequently reported in ALCAPA cases. This is confirmed in this study as 63.6% of patients with ALCAPA had moderate to severe mitral regurgitation. Mitral regurgitation is likely caused by ischemia of the myocardium and the papillary muscle in the preoperative period. Consequently, the combination of left ventricular dysfunction and significant mitral valve insufficiency leads to irreversible congestive heart failure. Of note, one of the patients in this group was diagnosed as mitral regurgitation of rheumatic origin and ALCAPA was missed in the diagnosis. The diagnosis of ALCAPA was eventually established by coronary CTA before surgery.
ICA is recognized as the “gold standard” for diagnosis of coronary artery disease because of its excellent spatial and temporal resolution.5 However, the procedure is invasive with ionizing radiation exposure and the use of expensive potentially nephrotoxic contrast media which are inappropriate for patients with allergic history to iodine in the contrast media. CTA has been increasingly used to assess the coronary anatomy in recent years with very good diagnostic accuracy and is considered as a reliable alternative to ICA for diagnostic evaluation of the coronary anatomy.26 Nine patients in whom coronary CTA was performed in this study were accurately diagnosed as ALCAPA. The main disadvantages are relatively high radiation dose, contrast-induced acute kidney injury, inability to assess flow, and expensiveness. Furthermore, the post-processing of coronary CTA images is quite complex and rather time consuming.11–15 In contrast, TTE is noninvasive, inexpensive, and safe and may be repeated as frequently as necessary. TTE findings of ALCAPA include visualization of the origin of the LCA from the pulmonary artery, proximal dilated and tortuous RCA, and visualization of abundant coronary collaterals especially, intercoronary septal collaterals. In addition, TTE can reveal any abnormal ventricular wall motion, decreased cardiac function because of inadequate myocardial perfusion, hemodynamic status, and other cardiac abnormalities with ALCAPA. TTE is also very useful in the postoperative evaluation of patients with ALCAPA. The echocardiographic diagnosis of 20 patients was in good agreement with findings at surgery. Therefore, it can be used as a primary method for diagnosis of ALCAPA, although this needs to be confirmed in a larger group of patients.
Surgical correction is highly recommended for all of the patients with ALCAPA regardless of the presence or absence of stress myocardial ischemia.27 The treatment of choice is to establish a dual coronary artery system, which can be achieved by the use of extracardiac arterial blood supply, re-implantation of the anomalous coronary ostia, or by creation of an aorticopulmonary window with an intrapulmonary baffle (Takeuchi procedure).28 Presently, re-implantation has become the procedure of choice whenever possible with an excellent medium-term survival rate and a low morbidity rate.29,30 Our study findings are in accordance with the literature as 77% of our patients (17/22) underwent this procedure. If the artery cannot be mobilized sufficiently, another option for the repair of ALCAPA is the intrapulmonary tunnel (Takeuchi) procedure, which was performed in five patients in our study. In this study, the left ventricular diameter showed improvement after the first week of operation. Two recent reviews reported only 4% perioperative death in ALCAPA and 8% in the origin of the RCA from the pulmonary artery (ARCAPA).5,31 Two patients in our series (9%) died after operation, which was comparable to those reported in the literature.
This study has some limitations which need to be acknowledged. First, the sample size is small, and it is based on a single center experience. This is because of the rare nature of ALCAPA. Second, although ICA and coronary CTA showed 100% accuracy, these two imaging modalities were not performed in all of the patients because of either the invasive nature of the ICA or the radiation risk associated with coronary CTA. Thus, results have to be interpreted with caution. Finally, although echocardiographic parameters were analyzed in this study, no detailed quantitative assessment of myocardial function was performed. Further studies based on a large cohort are required to enable a more comprehensive analysis of patients with ALCAPA.
In conclusion, TTE, as a noninvasive modality plays an important role in the accurate diagnosis of LCA originating from the pulmonary artery. TTE can clearly display the origin of coronary arteries, their courses, hemodynamic changes, and other associated malformations. This study suggests that TTE could be used as the first-line method for the diagnosis of patients with ALCAPA.
Footnotes
Abbreviations: ALCAPA = anomalous origin of the left coronary artery from the pulmonary artery, ASD = atrial septal defect, CDI = color Doppler imaging, CTA = computed tomography angiography, ICA = invasive coronary angiography, LCA = left coronary artery, LV = left ventricle, LVEDD = left ventricular end-diastolic diameter, LVEF = left ventricular ejection fraction, LVESD = left ventricular end-systolic diameter, LVFS = left ventricular fractional shortening, MR = mitral reguritation, PD = pulsed Doppler, PFO = patent foramen ovale, RCA = right coronary artery, TTE = transthoracic echocardiography.
Funding: this work was supported by grants from the Natural Science Foundation (No. 81201110) and the Basic-Clinical Scientific Research Foundation Program of the Capital Medical University in China (No.12JL55).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Keith JD. The anomalous origin of the left coronary artery from the pulmonary artery. Br Heart J 1959; 21:149–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamanaka O, Hobbs RE. Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Cathet Cardiovasc Diagn 1990; 21:28–40. [DOI] [PubMed] [Google Scholar]
- 3.Hauser M. Congenital anomalies of the coronary arteries. Heart 2005; 91:1240–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frescura C, Basso C, Thiene G, et al. Anomalous origin of coronary arteries and risk of sudden death: a study based on an autopsy population of congenital heart disease. Hum Pathol 1998; 29:689–695. [DOI] [PubMed] [Google Scholar]
- 5.Frommelt MA, Miller E, Williamson J, et al. Detection of septal coronary collaterals by color flow Doppler mapping is a marker for anomalous origin of a coronary artery from the pulmonary artery. J Am Soc Echocardiogr 2002; 15:259–263. [DOI] [PubMed] [Google Scholar]
- 6.Coles DR, Smail MA, Negus IS, et al. Comparion of radiation dose form multislise computed tomography coronary angiography and conventional diagnostic angiography. J Am Coll Cardiol 2006; 47:1840–1845. [DOI] [PubMed] [Google Scholar]
- 7.Sun Z, Choo GH, Ng KH. Coronary CT angiography: current status and continuing challenges. Br J Radiol 2012; 85:495–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun Z, Wang YL, Hsieh IC, et al. Coronary CT angiography in the diagnosis of coronary artery disease. Curr Med Imaging Rev 2013; 9:184–193. [Google Scholar]
- 9.Meinel FG, Bayer IIRR, Zwerner PL, et al. Coronary computed tomographic angiography in clinical practice: state of the art. Radio Clin N Am 2015; 53:287–296. [DOI] [PubMed] [Google Scholar]
- 10.Miglioretti DL, Johnson E, Williams A, et al. The use of computed tomography in paediatrics and associated radiation exposure and estimated cancer risk. JAMA Paediatr 2013; 167:700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shapiro MD, Dodd JD, Kalva S, et al. A comprehensive electrocardiogram-gated 64-slice multidetector computed tomography imaging protocol to visualize the coronary arteries, thoracic aorta, and pulmonary vasculature in a single breath hold. J Comput Assist Tomogr 2009; 33:225–232. [DOI] [PubMed] [Google Scholar]
- 12.Achenbach S, Goroll T, Seltmann M, et al. Detection of coronary artery stenoses by low-dose, prospectively ECG-triggered, high-pitch spiral coronary CT angiography. JACC Cardiovasc Imaging 2011; 4:328–337. [DOI] [PubMed] [Google Scholar]
- 13.Shen Y, Sun Z, Xu L, et al. High pitch dual-source whole aorta CT angiography in the detection of coronary arteries: a feasibility study of using iodixanol 270 and 100 kVp with Iterative. J Med Imaging Health Inf 2015; 5:117–125. [Google Scholar]
- 14.Yau JM, Singh R, Halpern EJ, et al. Anomalous origin of the left coro-nary artery from the pulmonary artery in adults: a comprehensive review of 151 adult cases and a new diagnosis in a 53-year-old woman. Clin Cardiol 2011; 34:204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mizouni H, Ben Moussa N, Ouarda F, et al. Infant-type anoma-lous origin of the left coronary artery from the main pulmonary arterydiagnosed with sixty-four multislice computed tomography. Pediatr Cardio 2011; 32:1202–1203. [DOI] [PubMed] [Google Scholar]
- 16.Chen S, Chen Z, Zheng B. Diagnostic usefulness of quantitative tissue velocity imaging and anatomic M-mode echocardiography for coronary artery disease: a pilot study. J Clin Ultrasound 2014; 43:346–352. [DOI] [PubMed] [Google Scholar]
- 17.Shah BN. Echocardiography in the era of multimodality cardiovascular imaging. Biomed Res Int 2013; 2013:310483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manlhiot C, Millar K, Golding F, et al. Improved classification of coronary artery abnormalities based only on coronary artery z-scores after Kawasaki disease. Pediatr Cardiol 2010; 31:242–249. [DOI] [PubMed] [Google Scholar]
- 19.Brooks HS. Two cases of an abnormal coronary artery of the heart arising from the pulmonary artery: with some remarks upon the effect of this anomaly in producing cirsoid dilatation of the vessels. J Anat Physiol 1885; 20:26–29. [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen MS, Herlong RJ, Silverman NH. Echocardiographic imaging of anomalous origin of the coronary arteries. Cardiol Young 2010; 20:26–34. [DOI] [PubMed] [Google Scholar]
- 21.Drighil A, Chraibi S, Bennis A. Adult type anomalous origin of the left coronary artery from the pulmonary artery: when should we be aware? Int J Cardiol 2006; 113:E119–E121. [DOI] [PubMed] [Google Scholar]
- 22.Yang YL, Nanda NC, Wang XF, et al. Echocardiographic diagnosis of anomalous origin of the left coronary artery from the pulmonary artery. Echocardiography 2007; 24:405–411. [DOI] [PubMed] [Google Scholar]
- 23.Alva C, Gomez FD, Jimenez-Arteaga S, et al. Anomalous origin of the left coronary artery from the pulmonary artery. Echocardiographic diagnosis. Arch Cardiol Mex 2009; 79:274–278. [PubMed] [Google Scholar]
- 24.Edwards JE. The direction of blood flow in coronary arteries arising from the pulmonary trunk. Circulation 1964; 29:163–166. [DOI] [PubMed] [Google Scholar]
- 25.Courand PY, Bozio A, Ninet J, et al. Focus on echocardiographic and Doppler analysis of coronary artery abnormal origin from the pulmonary trunk with mild myocardial dysfunction. Echocardiography 2013; 30:829–836. [DOI] [PubMed] [Google Scholar]
- 26.Neves PO, Andrade J, Monção H. Coronary anomalies: what the radiologist should know. Radiol Bras 2015; 48:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedman AH, Fogel MA, Stephens P, Jr, et al. Identification, imaging, functional assessment and management of congenital coronary arterial abnormalities in children. Cardiol Young 2007; 17 Suppl 2:56–67. [DOI] [PubMed] [Google Scholar]
- 28.Bunton R, Jonas R, Lang P, et al. Anomalous origin of left coronary artery from pulmonary artery: ligation versus establishment of a two coronary artery system. J Thorac Cardiovasc Surg 1987; 93:103–108. [PubMed] [Google Scholar]
- 29.Ramirez S, Curi-Curi PJ, Calderon-Colmenero J, et al. Outcomes of coronary reimplantation for correction of anomalous origin of left coronary artery from pulmonary artery. Rev Esp Cardiol 2011; 64:681–687. [DOI] [PubMed] [Google Scholar]
- 30.Kottayil BP, Jayakumar K, Dharan BS, et al. Anomalous origin of left coronary artery from pulmonary artery in older children and adults: direct aortic implantation. Ann Thorac Surg 2011; 91:549–553. [DOI] [PubMed] [Google Scholar]
- 31.Williams IA, Gersony WM, Hellenbrand WE. Anomalous right coronary artery arising from the pulmonary artery: a report of 7 cases and a review of the literature. Am Heart J 2006; 152:1004.e9–1004.e17. [DOI] [PubMed] [Google Scholar]


