Abstract
Recurrent wheezing (RW) has a significant impact on infants, caregivers, and society, but morbidity and related medical resource utilization (MRU) have not been thoroughly explored. The burden of RW needs to be documented with population-based data. The objective was to assess the characteristics, medical management, and MRU of RW infants identified from national claims data.
Infants aged from 6 to 24 months, receiving ≥2 dispensations of respiratory drugs within 3 months, and presenting a marker of poor control (index date), were selected. During the 6 months after index date, MRU was described in the cohort and among 3 subgroups with more severe RW, defined as ≥4 dispensations of respiratory drugs, ≥3 dispensations of oral corticosteroids (OCS), or ≥1 hospitalization for respiratory symptoms.
A total of 115,489 infants had RW, corresponding to 8.2% of subjects in this age group. During follow-up, 68.7% of infants received inhaled corticosteroids, but only 1.8 U (unit) were dispensed over 6 months, suggesting discontinuous use. Control was mostly inadequate: 61.7% of subjects received OCS, 80.2% antibiotics, and 71.2% short-acting beta-agonists, and medical/paramedical visits were numerous, particularly for physiotherapy. Severe RW concerned 39.0% of the cohort; 32.8% and 11.7% of infants had repeated use of respiratory drugs and OCS, respectively, and 5.5% were hospitalized for respiratory symptoms.
In this real-life nation-wide study, RW was common and infants had poor control and high MRU. Interventions are needed to support adequate use of controller therapy, and to improve medical care.
INTRODUCTION
Diagnosis of asthma is difficult in infants younger than 2 or 3 years,1 although it has been defined as recurrent (≥3) episodes of wheezing dyspnea since birth.2 Wheezing is one of a number of respiratory symptoms that occur in children, and parents often use wheezing as a nonspecific label to describe abnormal respiratory sounds.3 There are several causes of wheeze in childhood and distinct patterns of wheezing can be recognized. In general, these patterns (phenotypes) are assigned retrospectively; they cannot be reliably recognized when a child first presents with wheezing. Furthermore, for a same child, the pattern of symptoms may change with age.2 The common most clinical pattern, especially in pre-school children and in infants, includes episodes of acute wheezing, cough, and difficulty in breathing, associated with upper viral respiratory infections (colds), with no persisting symptoms. A minority of those who wheeze following viral infections in early life will develop wheezing with other triggers, retaining symptoms between acute episodes (interval symptoms), as in older children with classical atopic asthma.4,5,6,7,8 Children with persisting or interval symptoms are most likely to benefit from therapeutic interventions1; cohort studies show a breakpoint at around 2 years, with most children having symptoms before this age becoming asymptomatic by mid-childhood.1
Respiratory symptoms are frequent in childhood; for instance, 7% of children and adolescents self-report asthma and 12% have ever presented wheezing in the European Union.9,10 More specifically, preschool infants with respiratory symptoms, particularly those younger than 24 months, have seldom been studied in the overall population, possibly because of the complex access to subjects when performing field studies, or limited data on medical care. The national French claims database (SNIIR-AM: Système National d’Information Inter-Régimes de l’Assurance Maladie) records all reimbursed medical resource utilization (MRU), including related costs, in primary and secondary care, for the overwhelming majority of the French population.11,12 To describe exhaustive MRU over a 6-month period, this study used the SNIIR-AM database to identify recurrent wheezing (RW) infants for whom there was evidence of poor initial disease control, such as hospital admission for respiratory symptoms or introduction of second-line therapy. Next, groups of infants with more severe RW were identified, and their medical care was detailed.
METHODS
Data Source
The SNIIR-AM,11,12 a nation-wide, population-based database, records anonymized individual data on all reimbursements for healthcare utilization that have been provided by healthcare professionals, including drugs, outpatient medical and nursing care. There is no direct information on the medical indication of reimbursements, but the SNIIR-AM includes information on long-term disease status (LTD status) that allows patients to receive treatment for severe and costly conditions without advancing payment. SNIIR-AM also contains information on free-access-to-care status that enables patients of lower socioeconomic status to receive free medical care. Information from the SNIIR-AM database are cross-referenced with the French hospital discharge database (Programme de Médicalisation des Systèmes d’Information, PMSI)12 that provides medical information about all patients admitted to hospital in France, including discharge diagnoses coded using International Classification of Disease 10th version (ICD-10), medical procedures, and French diagnosis-related groups.
The study was conducted on anonymized data: the French data protection committee (commission nationale de l’informatique et des libertés, CNIL) delivered an authorization to use SNIIR-AM data for this project (approval no. 1589912). The study was performed following approval by the Institute of Health Data (Institut des Données de Santé; approval no. 42, May 2012). Because there was no recognizable individual information in the research data, informed consent was not necessary.
Overall Study Cohort
In the absence of diagnoses (except in case of hospitalization or when subjects benefit from LTD status), studies using French claims data rely on proxies to identify disease and outcomes criteria. For the present study, infants aged 6 to 24 months with wheezing were identified by ≥2 dispensations of respiratory drugs (R03 ATC classification) between March 2010 and December 2011; to include subjects with chronicity of symptoms, the second respiratory drug had to be dispensed between 8 and 91 days after the first one. Among these, infants with RW were identified by a marker of poor control, defined as hospitalization for asthma as primary diagnosis (ICD-10: J45 and J46), new dispensation of oral corticosteroids (OCS, ATC: H02AB), addition of short-acting beta agonists (SABAs—ATC:R03AC) to inhaled corticosteroids (ICS, ATC:R03BA) therapy, switch to higher dose of ICS (ATC:R03BA), or switch from ICS to nebulized corticosteroids (ATC: R03BA), within a timeframe compatible with a worsening of the respiratory symptoms leading to inclusion, that is, within 6 months following dispensation of the first respiratory drug. For all patients, the date of initial marker of poor control was defined as the index date. Only infants with ≥6 months’ follow-up (to allow for assessment of RW management) documented after index date were included. The markers used to identify initial poor control were also used to identify poor control during follow-up.
Identification of 3 Subgroups With Severe RW
MRU in 3 subgroups of subjects with more severe RW was also assessed: repeated use of respiratory therapy (≥4 dispensations of respiratory drugs—ATC: R03—within 6 months), repeated use of OCS (≥3 dispensations—ATC: H02AB—within 6 months), and hospitalization for respiratory symptoms as primary diagnosis (ICD-10 codes: asthma, J45 and J46; acute bronchiolitis, J21; or respiratory failure, J96). Overlap between the 3 subgroups was also assessed.
Data Analyses
Infants were described according to baseline characteristics (age, sex, LTD status, free-access-to-care status and quarter of index date). Markers of poor control and MRU were described during the 6-month follow-up. Markers of poor control included the variables used for inclusion. MRU included number of visits to healthcare professional (general practitioners [GPs], pediatricians, hospital practitioners, nurses, and physiotherapists), procedures (radiology and biology), hospitalizations for respiratory symptoms, and medications. Quantitative variables were described using descriptive statistics: sample size, mean, and standard deviation. Qualitative variables were also described using descriptive statistics: counts and percentage of each modality were computed on the responses expressed, using available data. All analyses were performed using SAS software, version 9.3 (SAS Institute Inc, Cary, NC).
RESULTS
Identification of Infants with RW
Between March 2010 and December 2011, 171,392 infants aged 6 to 24 months met the definition of wheezing (≥2 dispensations of respiratory drugs) in the SNIIR-AM database. Among these, 115,489 infants were considered to suffer from RW, defined as having experienced a marker of poor control (index date), and had ≥6 months of follow-up after the index date (67.4% of wheezing infants and 8.2% of French infants younger than 24 months in 2010).
Description of the Cohort of RW Infants
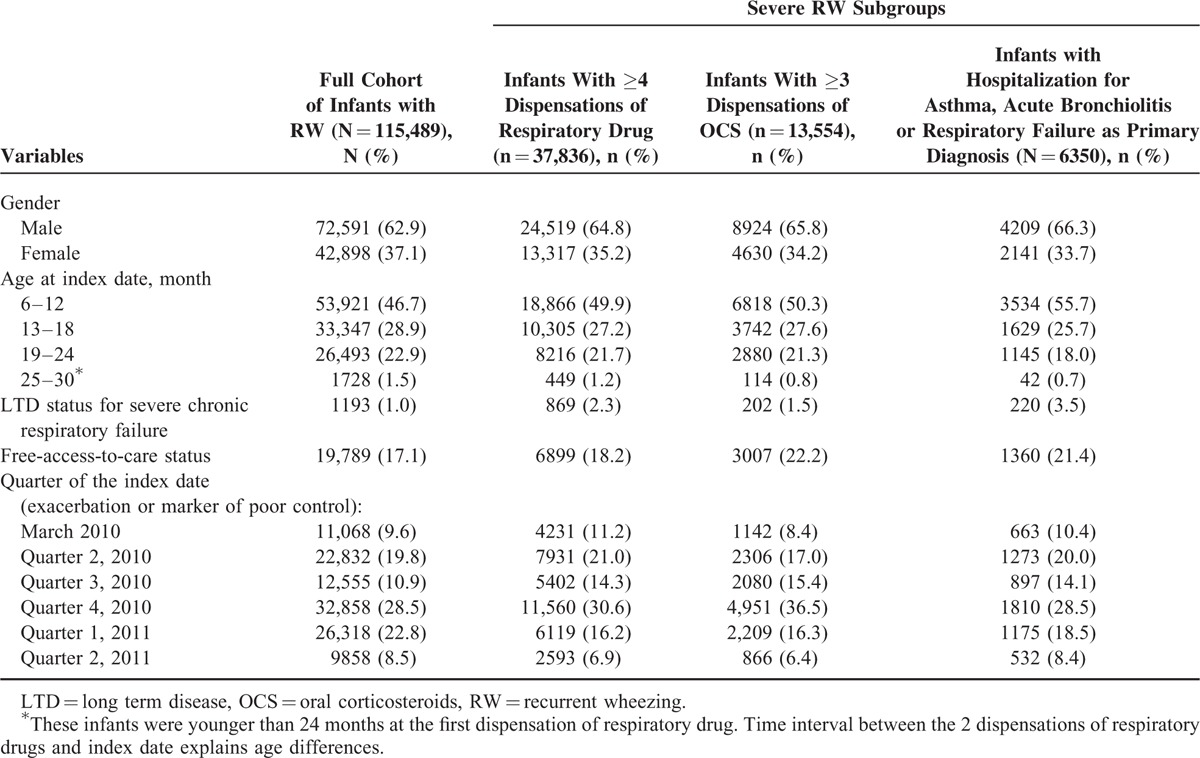
The cohort included a majority of boys (62.9%; Table 1). Mean age was 13.9 months (SD = 5.4). Infants were most frequently aged between 6 and 12 months (46.7%). Few infants (1.0%) benefited from LTD status for severe chronic respiratory failure, whereas a greater proportion had free-access-to-care status (17.1%; Table 1).
TABLE 1.
Baseline Characteristics of RW Infants and Among the 3 Subgroups of Severe RW
Control of RW Infants at Index Date and During Follow-Up
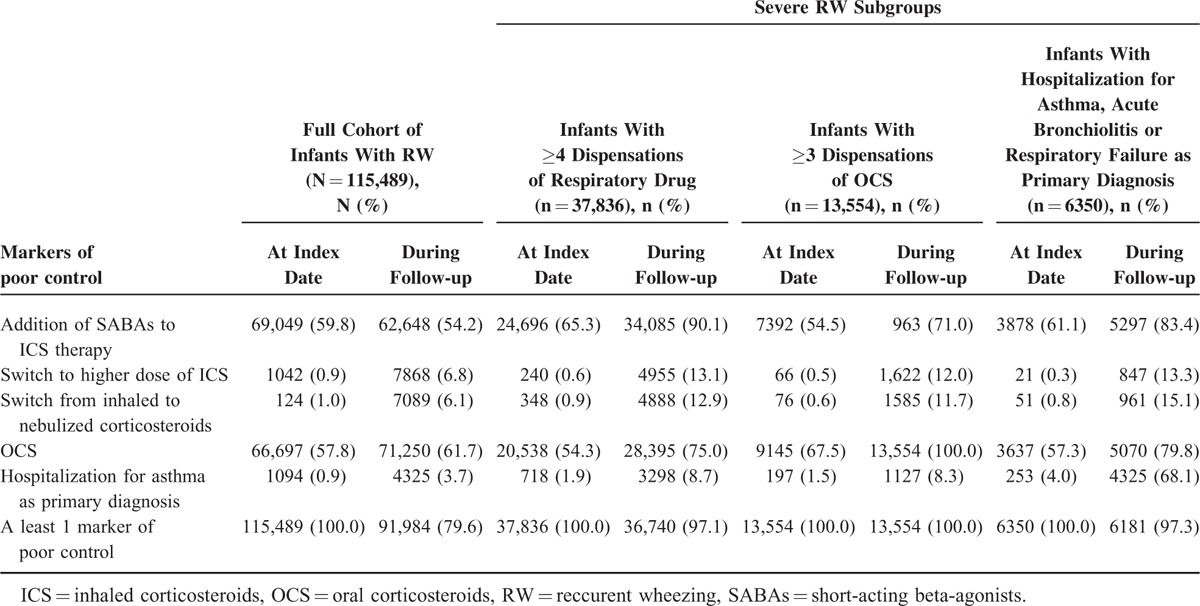
Initial marker of poor control occurred predominantly in the fall and winter (ie, Q1 and Q4; Table 1). OCS (57.8%) and SABAs added to ICS (59.8%) were the most common markers of initial poor control, followed by switch from inhaled to nebulized corticosteroids (1.0%), asthma-related hospitalization (0.9%), and increased dosage of ICS (0.9%) (Table 2). During the 6-month follow-up, 91,984 (79.6%) infants had ≥1 new episode of poor control, and 11.8% had ≥4 episodes; the most common markers of new episodes were OCS dispensation (61.7%) and addition of SABAs (54.2%), followed by asthma-related hospitalization (3.7%). The mean number of new episodes of poor control per infant during follow-up was 1.7 (SD = 1.5). The mean interval between 2 successive episodes was 42.1 days (SD = 34.4) among the 79.6% of infants with >1 episode during follow-up (Table 2).
TABLE 2.
Markers of Poor Control at Index Date and During Follow-up of RW Infants and Among the 3 Subgroups of Severe RW
Medical Care and MRU of RW Infants
During follow-up, 30.8% of infants were supervised only by GP, whereas 25.4% had a mixed follow-up by both a pediatrician and a GP (with a mean of 7.5 visits); 34.7% had at least 1 outpatient hospital visit. Exclusive follow-up by a community-based pediatrician was not common (8.1%). Biological samplings were prescribed to 20.3% of infants and physiotherapy procedures to 50.4%; infants visiting physiotherapists had an average of 10.4 visits over the 6 months of follow-up. Conversely, nursing procedures were less frequent (6.1% of infants) and radiology procedures were rare (0.1%). The most frequent hospitalization for respiratory symptoms was for asthma (3.7%), followed by bronchiolitis (1.8%), and respiratory failure (0.3%); a total of 6350 infants (5.5%) had ≥1 hospitalization for respiratory symptoms as primary diagnosis (Table 3).
TABLE 3.
MRU of RW Infants and Among the 3 Subgroups of Severe RW During 6 Months’ Follow-up
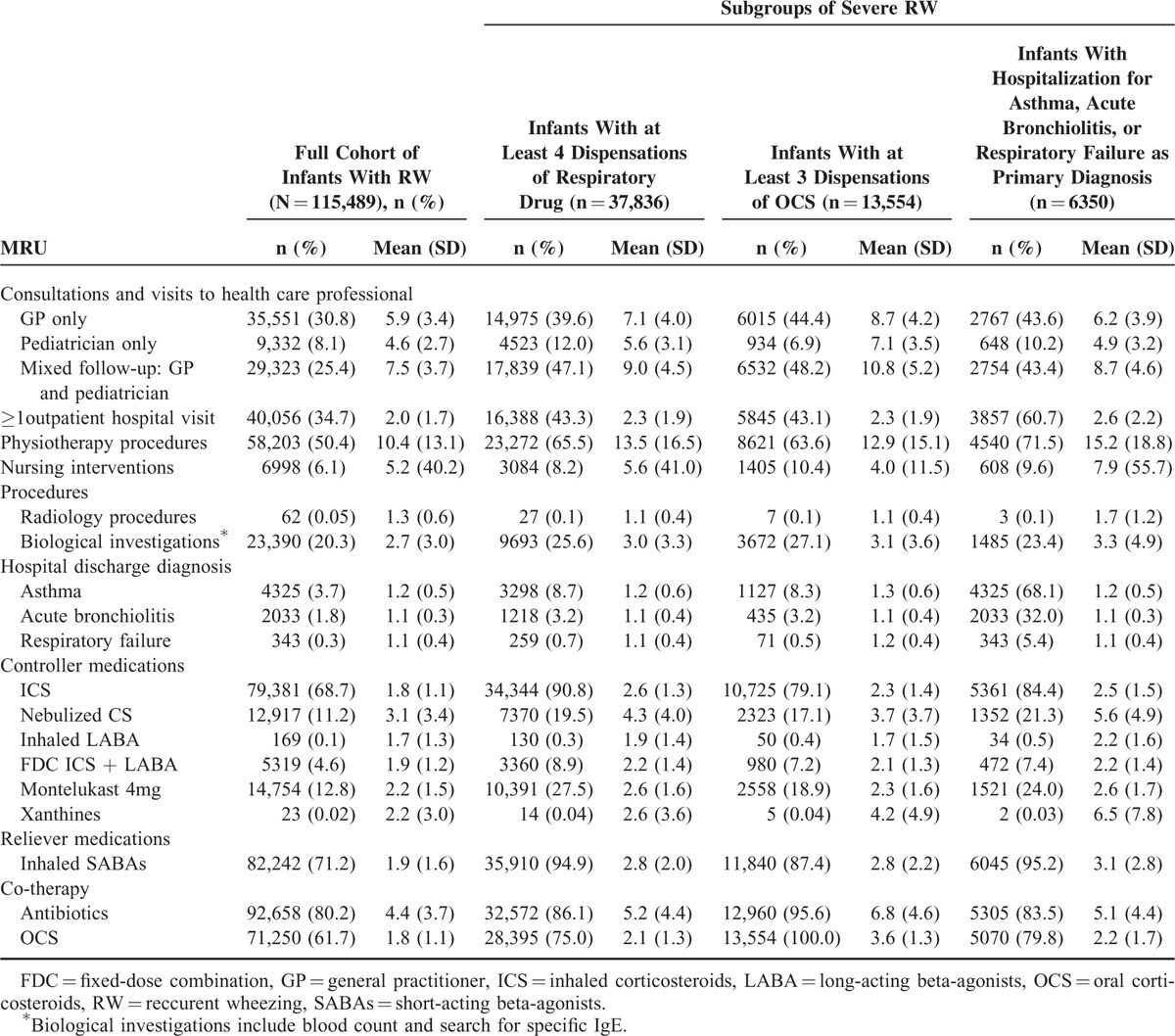
During follow-up, 68.7% of infants received ICS and 11.2% nebulized corticosteroids, whereas 4.6% of infants received fixed-dose combinations (FDCs) of ICS and long-acting β-agonists (LABAs). Montelukast was used by 12.8% of infants, and SABAs were dispensed to 71.2% of infants (Table 3). Following the initial episode, most infants were dispensed a refill of respiratory drugs (90.5%); 70.6% of patients had ≥2 U (unit) during 6-month follow-up (Figure 1). OCSs were prescribed to 61.7% infants, and 11.7% received ≥3 dispensations during 6-month follow-up (Figure 1); antibiotics were used by 80.2% of infants (Table 3), mean 4.4 U per consumer, and 39.9% received ≥4 dispensations during 6-month follow-up (Figure 1).
FIGURE 1.
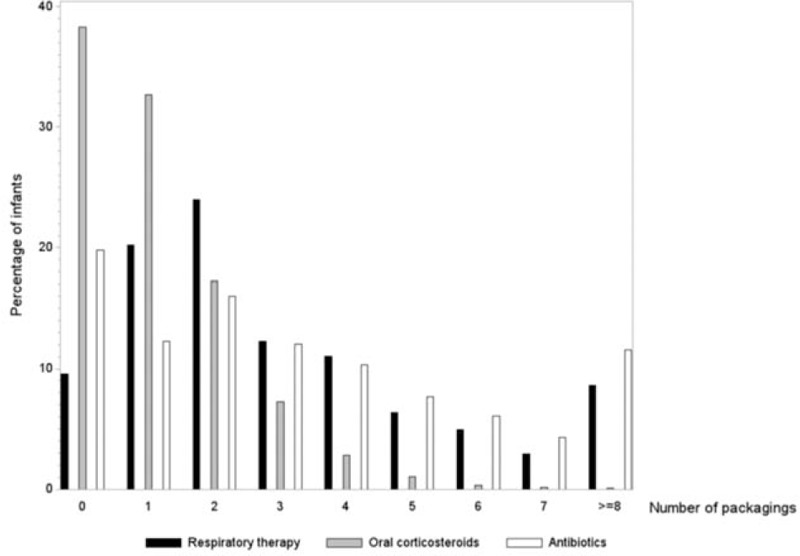
Histogram of frequency of use of respiratory drugs, oral corticosteroids, and antibiotics during 6-month follow-up among recurrent wheezing infants (N = 115,489). The X-axis represents the number of units dispensed and the y-axis the percentage of infants.
Subgroups With Severe RW
Infants with repeated (≥4) dispensations of respiratory drugs during the 6-month follow-up (n = 37,836; 32.8%) were mainly boys (64.8%; Table 1). Addition of SABAs to ICS therapy (65.3%) was the most common marker of initial poor control in this subgroup. During follow-up, 97.1% of infants had ≥1 new episode of poor control; the most common markers of new episodes were addition of SABAs (90.1%), followed by OCS (75.0%). Hospitalization for asthma as primary diagnosis occurred in 8.7% of infants (Table 2). This subgroup of patients was mostly followed by a GP and a pediatrician simultaneously (47.1%) with a mean of 9 visits. Among these infants, 90.8% received ICS, 19.5% received nebulized corticosteroids, 27.5% montelukast, 94.9% SABAs, 75.0% OCS, 86.1% antibiotics, and 8.9% FDC of ICS and LABA (Table 3).
Infants with repeated (≥3) dispensations of OCS during 6-month follow-up (n = 13,554 infants, 11.7% of the cohort) were mainly boys (65.8%), the majority were aged 6 to 12 months (50.3%), and 22.2% had free-access-to-care status (Table 1). In this subgroup, OCSs (67.5%) were the most common marker of poor control at index date (Table 2). During follow-up, new OCS (100%) and addition of SABAs to ICS therapy were the most common markers of poor control (71.0%), and 8.3% of infants were hospitalized for asthma as primary diagnosis. Among these infants, 44.4% were followed up exclusively by a GP with a mean of 8.7 visits; 79.1% received ICS, 17.1% nebulized corticosteroids, 18.9% montelukast, 87.4% SABAs, and 7.2% FDC of ICS and LABA. The majority (95.6%) received antibiotics (Table 3); each consumer received a mean 6.8 U.
Infants who were hospitalized for respiratory symptoms (≥1 asthma-related, acute bronchiolitis-related, and/or respiratory failure-related hospitalization; n = 6350 infants, 5.5%) were mainly boys (66.3%), the majority were aged 6 to 12 months (55.7%), 21.4% had free-access-to-care status, and 3.5% of infants had LTD status for severe chronic respiratory failure (Table 1). During follow-up, addition of SABAs to ICS therapy was the most common marker of loss of control (83.4%), followed by OCS (79.8%) (Table 2). In this subgroup, 23.4% had at least 1 biological sampling and 71.5% had at least 1 physiotherapy procedure (mean 15.2 visits among those concerned). Among these infants, 21.3% received nebulized corticosteroids (each consumer received a mean 5.6 U), 7.4% received FDC of ICS and LABA, 83.5% used antibiotics (each consumer received a mean 5.1 packs). ICS were dispensed to 84.4%, SABAs to 95.2%, and OCS to 79.8% of infants (Table 3).
A total 44,984 infants (39.0% of the total cohort) were included in at least 1 of the 3 severe RW subgroups; 25.4% were included in ≥2 of the subgroups (9.9% of the total cohort), and 3% in all 3 subcohorts (1.2% of the total cohort; Figure 2).
FIGURE 2.
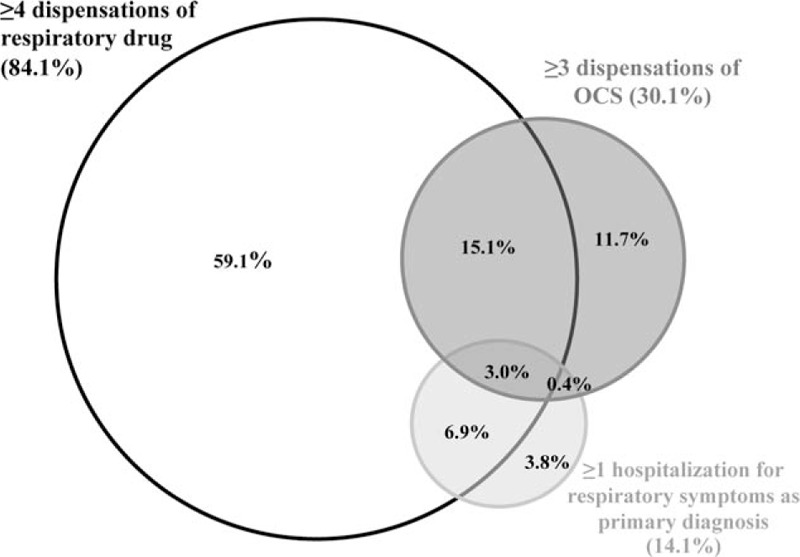
Schematic illustration of the overlap between the 3 sub-groups of severe recurrent wheezing (RW). A total of 44,984 RW infants were included in ≥1 severity sub-groups; the proportion of infants in each subgroup, as well as overlap (% of those with severe RW) is indicated.
DISCUSSION
This nation-wide, real-life study has found RW to be common and the high MRU and morbidity in RW infants indicate frequent poor control.
As diagnoses are not documented in French claims data, the identification of infants with RW relied on a proxy (2 dispensations of respiratory drugs ATC: R03, separated by 8-91 days, and a marker of poor control), which resulted in a population that represented approximately 8.2% of infants in France. Epidemiological data on the prevalence of treated respiratory symptoms in infants are rare, but this is consistent with the available literature (the prevalence of these symptoms in the first year of life is reported to be around 7.5%),13,14 and the observed sex ratio is consistent with published data for young children younger than 5 years, wherein a 2:1 ratio is traditionally observed in favor of boys in asthma.15
Just under 80% of all RW infants experienced poor control over 6 months; SABAs were extensively used in the cohort as a whole and this increased among the “more severe” subgroups. According to the definitions used in the present study, up to 39% of RW infants had more severe disease, and the patterns of reimbursements among these infants indicate that they experienced worse levels of control than other RW infants. During follow-up, 68.7% of RW infants received ICS, but only 1.8 U were dispensed over 6 months, suggesting discontinuous use. Infants with severe RW had slightly higher counts of dispensations (2.3–2.6 U vs 1.8 U), but these figures were surprisingly low. Of particular note is the use of OCS. This class of drug was dispensed to over half of the cohort and just over 10% had at least 3 dispensations during the follow-up period. The frequent use of OCS could be because of the relative inefficiency, irregular, or inadequate use (possibly owing to difficult use of inhalation devices), of ICS in this population.16 The use of OCS could also be due to conditions other than respiratory symptoms, for example, ENT infections. However, the “frequent OCS user” sugroup is of particular interest, as it is characterized by frequent free-access-to-care status, and many infants were followed by GPs exclusively, which might suggest a lower quality of care in this subgroup. Poor control and morbidity were also indicated by hospitalization for respiratory symptoms (asthma, acute bronchiolitis, or respiratory failure) that concerned 5.5% of RW infants, and which is consistent with the literature, as it is reported that 6.3% of wheezing infants required hospital admission for bronchiolitis.17 Overall, our results confirmed the high use of healthcare resources owing to wheezing disorders in infants, already shown in a French study conducted on 560 infants.18 Additionally, it is interesting to note that not only just over half had physiotherapy procedures but also a substantial majority used antibiotics, and this increased further in the severe subgroups. There is however no evidence of clinical benefits from specific chest physiotherapy techniques for infants with RW episodes,19,20 and there is again little evidence to recommend the use of antibiotics to treat or prevent persistent respiratory symptoms,21 especially in view of the role of virus in these infections.22
This study was conducted using the SNIIR-AM database linked to the PMSI database12 that has the advantage to provide an extensive access to reimbursed MRU including drugs, medical procedures, medical and paramedical visits, as well as individual medical resource utilization, in primary and secondary care. However, there were certain limitations as few variables in the SNIIR-AM are available to describe patients: age, sex, free-access-to-care status, and LTD status in case of more severe disease. SNIIR-AM does not include clinical data (ie, results from blood tests), nor information on family history. Prescriptions without dispensation are not recorded, and dispensation does not ensure drug utilization by the patient. Doses associated with prescriptions, or their durations, are also not recorded.12 Finally, RW infants were identified using at least 2 distinct respiratory drug dispensations followed by a marker of poor control, as SNIIR-AM has no diagnostic data. Nonetheless, considering the complexity of reaching valid diagnoses in these very young subjects, the criteria used to identify RW may be considered to be relevant.1
The rate of outcomes during 6-month follow-up may have been overestimated; as the proxy for the recurrence (of wheezing) was initial poor control, these criteria may limit the generalizability of the findings. However, RW infants represented two-thirds of wheezing infants (as defined by 2 distinct respiratory drug dispensations alone), and the importance of this aspect is limited. An alternative could be to perform a field study, but besides the practical limitations, for instance, complex organization, such a design would be exposed to selection (eg, compliant caregivers) and classification biases (eg, identification of outcomes), which are avoided by using claims data.
In conclusion, RW in infants was common and frequently poorly controlled; further research is needed to better understand the high MRU and to test methods for optimizing medical care in this young population, for instance, regarding a more efficient use of ICS, and a more parsimonious use of OCS, SABAs, and antibiotics.
Acknowledgments
We thank the French National Health Service (Caisse Nationale de l’Assurance Maladie des Travailleurs Salariés) and the Institute of Health Data (Institut des Données de Santé) for data extraction. We warmly thank Philip Robinson for help in manuscript preparation.
Footnotes
Abbreviations: ATC = Anatomical Therapeutic Chemical, CNIL = Commission Nationale Informatique et Libertés, ENT = Ears Nose Throat, FDC = Fixed-dose combination, GP = General Practitioner, ICD-10 = International Classification of Disease 10th version, ICS = inhaled corticosteroids, IDS = Institut des Données de Santé, LABAs = Long Acting Beta-Agonists, LTD = Long Term Disease, MRU = Medical Resource Utilization, OCS = oral corticosteroids, PMSI = Programme de Médicalisation des Systèmes d’Information, RW = Recurrent Wheezing, SABAs = Short Acting Beta-Agonists, SNIIR-AM = Système National d’Information Inter-Régimes de l’Assurance Maladie, U = unit.
Funding Source: MSD France.
Financial relationship: MB is a PhD student and part-time employee of MSD France; VL, LL, and CC-V are full-time employees of MSD France. Others authors have no financial relationships relevant to this article to disclose.
Authors’ contributions: MB designed and oversaw the study, performed the statistical analysis, and wrote the manuscript; JdB, member of the study Scientific Committee, made suggestions to improve the study, wrote the manuscript; LL designed and oversaw the study; VL made suggestions to improve the study, and revised the manuscript.
CC-V made suggestions to improve the study, and revised the manuscript; LL made suggestions to improve the study, and revised the manuscript.
JB, Member of the study Scientific Committee, made suggestions to improve the study, and revised the manuscript; BF, Member of the study Scientific Committee, made suggestions to improve the study, and revised the manuscript; GdP, Member of the study Scientific Committee, Read and approved the final manuscript; MG performed the statistical analysis; EVG designed and oversaw the study, and wrote the manuscript. All the authors have read and approved the final manuscript.
GdP, JB, JdB, and BF were members of the study Scientific Committee. Others authors have no conflicts of interest relevant to this article to disclose.
REFERENCES
- 1.GINA 2015. GINA Report, Global Strategy for Asthma Management and Prevention Documents /Resources GINA. Available at: http://www.ginasthma.org/documents/4 Accessed on April 14, 2015. [Google Scholar]
- 2.Brand PLP, Baraldi E, Bisgaard H, et al. Definition, assessment and treatment of wheezing disorders in preschool children: an evidence-based approach. Eur Respir J 2008; 32:1096–1110.doi:10.1183/09031936.00002108. [DOI] [PubMed] [Google Scholar]
- 3.Asthma diagnosis and control. Lancet 2015; 385:482.doi: 10.1016/S0140-6736(15)60184-2. [DOI] [PubMed] [Google Scholar]
- 4.Dodge R, Martinez FD, Cline MG, et al. Early childhood respiratory symptoms and the subsequent diagnosis of asthma. J Allergy Clin Immunol 1996; 98:48–54. [DOI] [PubMed] [Google Scholar]
- 5.Martinez FD, Wright AL, Taussig LM, et al. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med 1995; 332:133–138.doi:10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 6.Park ES, Golding J, Carswell F, et al. Preschool wheezing and prognosis at 10. Arch Dis Child 1986; 61:642–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sporik R, Holgate ST, Cogswell JJ. Natural history of asthma in childhood--a birth cohort study. Arch Dis Child 1991; 66:1050–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strachan DP, Butland BK, Anderson HR. Incidence and prognosis of asthma and wheezing illness from early childhood to age 33 in a national British cohort. BMJ 1996; 312:1195–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van den Akker-van Marle ME, Bruil J, Detmar SB. Evaluation of cost of disease: assessing the burden to society of asthma in children in the European Union. Allergy 2005; 60:140–149.doi:10.1111/j.1398-9995.2005.00692.x. [DOI] [PubMed] [Google Scholar]
- 10.Pearce N, Aït-Khaled N, Beasley R, et al. Worldwide trends in the prevalence of asthma symptoms: phase III of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax 2007; 62:758–766.doi:10.1136/thx.2006.070169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuppin P, de Roquefeuil L, Weill A, et al. French national health insurance information system and the permanent beneficiaries sample. Rev Dépidémiologie Santé Publique 2010; 58:286–290.doi:10.1016/j.respe.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Martin-Latry K, Bégaud B. Pharmacoepidemiological research using French reimbursement databases: yes we can!. Pharmacoepidemiol Drug Saf 2010; 19:256–265.doi:10.1002/pds.1912. [DOI] [PubMed] [Google Scholar]
- 13.=Société Pédiatrique de Pneumologie et Allergologie. FRA, =Haute Autorit de Sant. (H.A.S.). Saint-Denis. FRA. Asthme de L’enfant de Moins de 36 Mois: Diagnostic, Prise En Charge et Traitement En Dehors Des pisodes Aigus. Saint Denis La Plaine: HAS; 2009:110p. [Google Scholar]
- 14.Dela Bianca ACC, Wandalsen GF, Mallol J, et al. Prevalence and severity of wheezing in the first year of life. J Bras Pneumol 2010; 36:402–409. [DOI] [PubMed] [Google Scholar]
- 15.AFRITE A, ALLONIER C, COM-RUELLE L, LE GUEN N, =Institut de Recherche et de Documentation en Economie de la Sant. (I.R.D.E.S.). Paris. FRA. L’asthme En France En 2006: Pr valence, Contrle et D terminants. Paris: IRDES; 2011:122p. [Google Scholar]
- 16.Clavenna A, Sequi M, Cartabia M, et al. Effectiveness of nebulized beclomethasone in preventing viral wheezing: an RCT. Pediatrics 2014; 133:e505–e512.doi:10.1542/peds.2013-2404. [DOI] [PubMed] [Google Scholar]
- 17.Pérez-Yarza EG, Moreno-Galdó A, Ramilo O, et al. Risk factors for bronchiolitis, recurrent wheezing, and related hospitalization in preterm infants during the first year of life. Pediatr Allergy Immunol 2015; 26:797–804.doi:10.1111/pai.12414. [DOI] [PubMed] [Google Scholar]
- 18.Herr M, Nikasinovic L, Foucault C, et al. Prise en charge des sifflements chez le nourrisson dans la cohorte de nouveau-nés PARIS. Rev Mal Respir 2012; 29:52–59.doi:10.1016/j.rmr.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 19.Castro-Rodriguez JA, Rodrigo GJ, Rodriguez-Martinez CE. Principal findings of systematic reviews for chronic treatment in childhood asthma. J Asthma 2015; 52:407–416.doi:10.3109/02770903.2014.971968. [DOI] [PubMed] [Google Scholar]
- 20.Gajdos V, Katsahian S, Beydon N, et al. Effectiveness of chest physiotherapy in infants hospitalized with acute bronchiolitis: a multicenter, randomized, controlled trial. PLoS Med 2010; 7:e1000345.doi:10.1371/journal.pmed.1000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCallum BJ, Amrol D, Horvath J, et al. A case of allergic bronchopulmonary aspergillosis leading to pneumonia with unusual organisms. South Med J 2005; 98:1135–1138.doi:10.1097/01.smj.0000184788.50353.83. [DOI] [PubMed] [Google Scholar]
- 22.Dawson-Caswell M, Muncie HL. Respiratory syncytial virus infection in children. Am Fam Physician 2011; 83:141–146. [PubMed] [Google Scholar]


