Abstract
Catheter-related bladder discomfort (CRBD) is a distressing symptom complex after surgery, especially in male patients who have had urinary catheterization under general anesthesia. In this prospective, randomized, controlled trial, we compared dorsal penile nerve block (DPNB) with 0.33% ropivacaine with intravenous tramadol 1.5 mg kg−1 in prevention of CRBD, as well as the incidences of postoperative side effects.
Fifty-eight male patients aged 18 to 50 years, undergoing elective liver surgery and limb surgery with urinary catheterization, were enrolled and divided randomly into 2 groups. In the DPNB group, patients were given dorsal penile nerve block with 15 mL of 0.33% ropivacaine, and in the tramadol intravenous administration (TRAM) group, patients were given 1.5 mg kg−1 tramadol after the completion of surgery before extubation. The primary outcome was the incidence of CRBD, and the secondary outcomes included the severity of CRBD, postoperative side effects, postoperative pain, and the acceptance of urinary catheterization. Patients were evaluated upon arrival to postanesthetic care unit (PACU), at 0.5, 1, 2, 4, and 6 hours after patients’ arrival in the PACU for outcomes.
The incidence of CRBD was significantly lower in the DPNB group than in the TRAM group, either upon arrival to PACU (10.3% vs 37.9%, P = 0.015), or at 0.5 hours (3.4% vs 34.5%, P = 0.003), 1 hours (3.4% vs 37.9%, P = 0.001), 2 hours (6.9% vs 34.5%, P = 0.010), and 4 hours (6.9% vs 27.6%, P = 0.039) after patients’ arrival in PACU. Compared with the TRAM group, the severity of postoperative CRBD upon arrival to PACU (P = 0.011) and at 0.5 hours (P = 0.005), 1 hours (P = 0.002), 2 hours (P = 0.005), 4 hours (P = 0.017), and 6 hours (P = 0.047) after patients’ arrival in PACU were all significantly reduced in the DPNB group. The incidences of postoperative nausea, vomiting, dizziness, and sedation were decreased significantly in the DPNB group compared with the TRAM group (P < 0.05). The acceptance of urinary catheterization was 93.1% (27/29 patients) in the DPNB group and 58.6% (17/29 patients) in the TRAM group, respectively (P < 0.001).
DPNB with ropivacaine has a better effect for CRBD reduction and less side effects than intravenous tramadol administration.
The trial has been registered at www.clinicaltrials.gov (NCT01721031).
INTRODUCTION
Catheter-related bladder discomfort (CRBD) is a distressing symptom complex characterized as stabbing pain or burning sensation with foreign body in urethra, discomfort in the supra-pubic region, or urge to void.1 CRBD is frequently associated with emergence delirium in the postanesthetic care unit (PACU), especially in male patients.2–4 Symptom complex of CRBD may cause patient agitated, with behavioral response such as flailing limb, strong vocal response, or attempt to pull out the catheter,1,2 and lead to exacerbated postoperative pain, as well as prolonged hospital stay.1–4
The pathogenesis of CRBD has been demonstrated to be attributed to muscarinic receptors-mediated activation of the cholinergic nerves, which leads to acetylcholine release and vesicle detrusor muscles involuntary contraction.2,5 Systemic administration of medications with antimuscarinic properties, including tramadol, tolterodine, oxybutynin, pregabalin, and ketamine are the mainstay for CRBD reduction.6–10 However, untoward complications such as postoperative nausea and vomiting (PONV) is not uncommon with tramadol,6,11 sedation may occur with tramadol, ketamine, and prgabalin,6,9–11 and dry mouth, facial flushing, and blurred vision may present with tolterodine and oxybutynin.7,8 It is important to establish an effective method with fewer side effects for CRBD prevention and treatment to meet the clinical requirement.
Dorsal penile nerve block (DPNB) is applied in clinical practice for pain relief in neonatal circumcision12,13 and has been shown to provide satisfactory pain reduction.14 Besides, in our experience, the patients receiving DPNB during urethra surgery seldom complained of CRBD and side effects related to systemic drug administration. Therefore, we designed this trial with the hypothesis that DPNB is associated with CRBD reduction effect for male patient with urinary catherization, and compared the effect of DPNB with 0.33% ropivacaine and intravenous tramadol 1.5 mg kg−1 on incidence and severity of CRBD and the postoperative side effects rate.15
METHODS
Patients
The local Institutional Review Board (Biological-Medical Ethical Committee of West China Hospital, Sichuan University, Chengdu, Sichuan, China) approved all study procedures. All patients of this study had provided informed consent with their own signature before enrollment. The trial was registered at www.clinicaltrials.gov with registration number NCT01721031.15
In this prospective, randomized, controlled study, we included adult male patients (18–50 years), ASA physical status I, II, and III, undergoing elective liver surgery or limb surgery under general anesthesia with indwelling urinary catheter left in situ.15 Exclusion criteria were history of over active bladder (frequency > 3 times in the night or > 8 times in 24 hours),5–7,15 neurogenic bladder, bladder outflow obstruction, prostate hyperplasia, renal insufficiency, bleeding disorder, and inability to communicate.15
Patients were divided randomly into 2 groups and received allocated intervention after the completion of surgery before extubation. Patients were given either dorsal penile nerve block with 0.33% ropivacaine 15 mL (Naropin, Ropivacaine Hydrochloride, AstraZeneca, UK) in the DPNB group, or intravenous tramadol 1.5 mg kg−1 (Tramal, Tramadol Hydrochloride Injection, Grunenthal, Germany) in the tramadol intravenous administration (TRAM) group.15 Randomization was performed by application of Statistical Analysis System (SAS 9.1) by a biostatistician, and the result of the group allocation was concealed in a nontransparent envelop and kept by a research nurse. After confirmation of the patient's enrollment, the attending anesthesiologist participated in this trial called the responsible nurse to open the envelop to get the group allocation. The treatment assignment was designed for blinding the staff responsible for follow-up, statisticians, and the patients, but not masking the investigators, physicians, and nurses in charge.15
The primary outcome was the incidence of CRBD. A difference of 30% in incidence of CRBD between groups was considered significant.15 Giving α = 0.05 and 1-β = 0.80, 24 patients in each group were required in this trial.15 We enrolled 30 patients in each group with consideration of possibility of dropout. The severity of CRBD was the most important secondary outcome in this trial, which was assessed according to the following scaling system: no CRBD; mild CRBD (complaint about CRBD when asked); moderate CRBD (complaint about CRBD without enquiring); and severe CRBD (complaint about CRBD without enquiring, with spontaneous behaviors revealing his discomfort).3,15
Other secondary outcomes were evaluated in PACU and ward, as follows:15
Numeric rating scale (NRS) for postoperative pain. Patients were asked for evaluating pain intensity by themselves using this single 11-point numeric scale ranged between 0 and 10, where 0 represented “no pain” and 10 represented “worst imaginable pain.”16
Postoperative sufentanil requirement. If the patient complained about pain with NRS more than 6, intravenous sufentanil 5 μg was given as rescue analgesic treatment in PACU.
Number of patients with postoperative sufentanil treatment.17
Postoperative side effects, as follows:
Postoperative nausea/vomiting (PONV), which was evaluated by a 4-point ordinal scale from 0 to 3, where 0 meant no nausea, 1 meant mild nausea, 2 meant moderate nausea, and 3 meant severe nausea with vomiting. Intravenous ondansetron 4 mg was given to the patients with a PONV scale of 3 as rescue antiemetic treatment.6
Dizziness, which was confirmed by the patient's complaint.
Sedation, which was evaluated by the Ramsay sedation scale (1: anxious, agitated or restless; 2: co-operative, oriented, and tranquil; 3: responds to command; asleep; 4: brisk response to light glabellar tap or loud noise; 5: a sluggish response to light glabellar tap or loud noise; 6: no response). Patients with a sedation scale of >4 were considered sedated.18
Respiratory depression, which was defined as ventilatory frequency was <8 time per minutes and oxygen saturation was <90% without oxygen supplementation.6
Dry mouth, which was confirmed by the patient's complaint.
Acceptance of indwelling urinary catheter after extraction of the catheter, which was evaluated by the staff responsible for follow-up with an inquiry of a question for the patients that “Will you worry about urinary catheterization if you undergo another operation next time?.” The answer “No” meant acceptance of indwelling urinary catheter, and the answer “Yes” meant unacceptance of indwelling urinary catheter.
At the preoperative interview, patients were educated to differentiate postoperative pain from the discomfort related to urinary catheter. Before induction of anesthesia, no premedication was administered. Patients received a peripheral intravenous line using a 16- or 18-gauge catheter, and routine anesthesia monitoring including electrocardiography, heart rate, noninvasive blood pressure, and pulse oximetry were continuously monitored for each patient. Anesthesia was induced with midazolam 0.05 mg kg−1, sufentanil 0.4 μg kg−1, cis-atracurium 2 mg kg−1, and propofol 1.5 mg kg−1. After induction of anesthesia, capnogram was monitored for the patients. Urinary catheterization was performed with an 18 G Fr Foley catheter, and the catheter balloon was inflated with 10 mL of 0.9% NaCl. Anesthesia was maintained with continuous infusion of remifentanil 3 to 5 μg kg−1 h−1 and inhalation of 2.0% to 2.5% of sevoflurane and was not discontinued until the allocated interventions were completed after the end of surgery.
All patients were transferred to PACU after extubation. In the PACU, patients were enquired to confirm whether it was incision pain or discomfort related to urinary bladder if the patients complained about pain, and sufentanil 5 μg i.v. was given to patients whose NRS for pain was >6 after confirmation of postoperative pain but not CRBD. Patients were evaluated by Dr. Jing-yi Li and 2 responsible nurses for outcomes at 0, 0.5, 1, 2, 4, and 6 hours after patients’ arrival in PACU, and enquired the acceptance of indwelling urinary catheter after extraction of the catheter.
Statistic Package for Social Science (SPSS 18.0, SPSS Inc, Chicago, IL) was used for statistical analysis. The Student t-test was used to analyze demographic data. The incidences of CRBD and side effects between groups were analyzed by the chi-square test, whereas severity of CRBD (mild, moderate, and severe) was analyzed by Fisher's exact test. The NRS scale was analyzed by the Mann–Whitney test. A P value of <0.05 was considered significant.
RESULTS
One-hundred and nine patients were recruited between April 2013 and December 2015, and 49 patients were excluded from the study (Figure 1). After randomization, 1 patient in the DPNB group was excluded because of the surgeon's refusal of participation, and 1 patient in the TRAM group was excluded because of his history of severe vomiting. Therefore, the data of the remaining 58 patients (29 in the DPNB group and 29 in the TRAM group) were analyzed.
FIGURE 1.
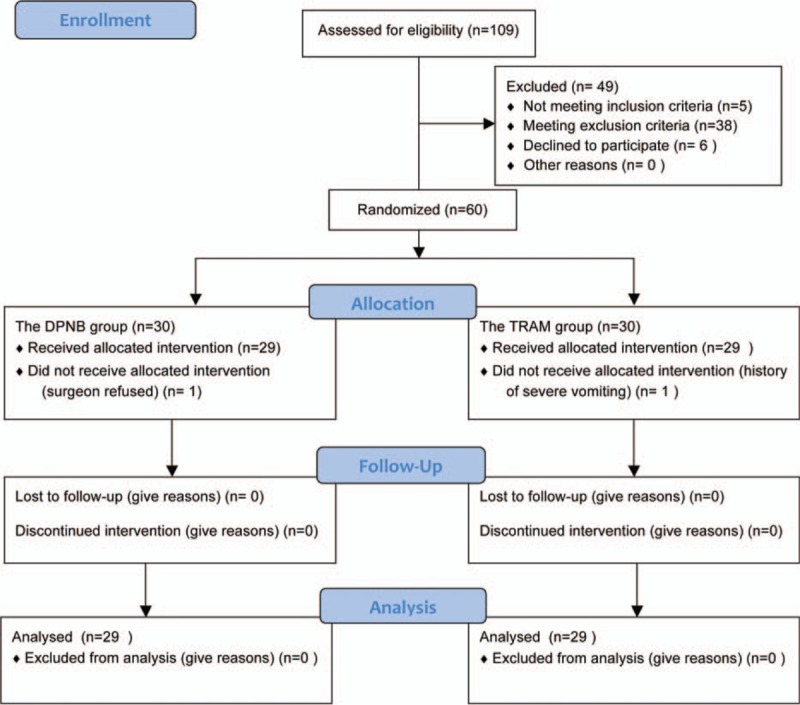
A CONSORT diagram.
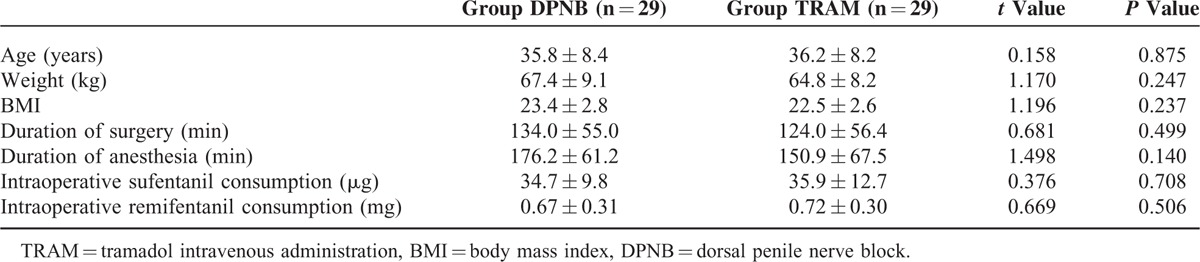
Patient characteristics, duration of surgery and anesthesia, and intraoperative sufentanil and remifentanil consumption were similar between groups (Table 1).
TABLE 1.
Patients’ Characteristics, Duration of Surgery and Anesthesia, and Opioids Requirement
The incidence of CRBD in the DPNB group was significantly lower than in the TRAM group, either upon arrival to PACU (10.3% vs 37.9%, P = 0.015), or at 0.5 hours (3.4% vs 34.5%, P = 0.003), 1 hours (3.4% vs 37.9%, P = 0.001), 2 hours (6.9% vs 34.5%, P = 0.010), and 4 hours (6.9% vs 27.6%, P = 0.039) after patients’ arrival in PACU. Compared with the TRAM group, postoperative CRBD upon arrival to PACU (P = 0.011), and at 0.5 hours (P = 0.005), 1 hours (P = 0.002), 2 hours (P = 0.005), 4 hours (P = 0.017), and 6 hours (P = 0.047) after patients’ arrival in PACU were all less severe in the DPNB group, and no patient felt severe CRBD in the DPNB group at all time points (Table 2). At the time point of 6 hours after patients’ arrival in PACU, when compared with the TRAM group, the incidence of CRBD was decreased without significance (P = 0.072), but the severity was less significantly (P = 0.047) in the DPNB group.
TABLE 2.
Incidence and Severity of CRBD
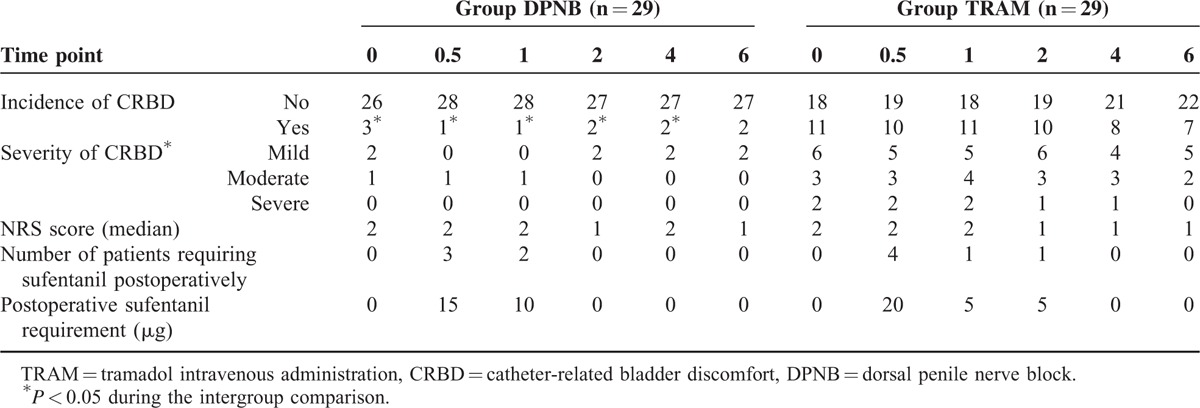
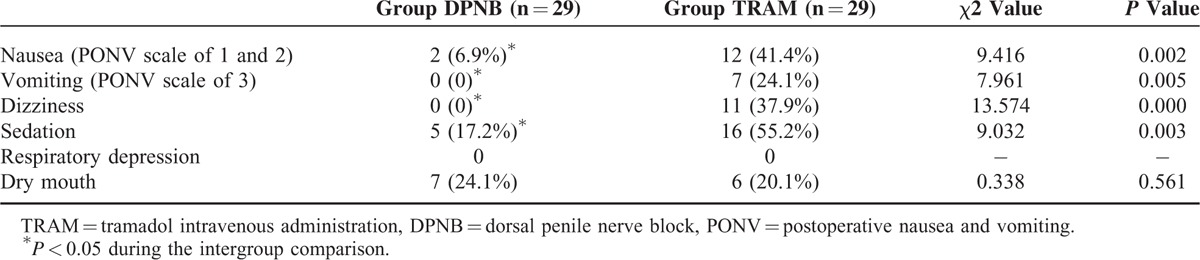
There were no differences in the postoperative NRS score in PACU, postoperative sufentanil requirement, and number of patients who required postoperative sufentanil between groups (Table 2). The incidences of postoperative nausea, vomiting, dizziness, and sedation were decreased significantly in the DPNB group compared with the TRAM group (P < 0.05), and the incidences of dry mouth was comparable between groups (P > 0.05) (Table 3). No respiratory depression was found in the both groups (Table 3). After the urinary catheter was extracted, the acceptance of urinary catheterization was 93.1% (27/29 patients) in the DPNB group and 58.6% (17/29 patients) in the TRAM group (P < 0.001). In the DPNB group, none of the patients complained of pain, and no patient was observed bleeding at the injection site.
TABLE 3.
Incidences of Side Effects
DISCUSSION
The present study demonstrated that DPNB with ropivacaine reduced the incidence and severity of CRBD, as well as the side effects associated with tramadol, including PONV, sedation, and dizziness.
Tramadol is a commonly used clinical medication for postoperative pain relief with anticholinergic effect to decrease the incidence rate of postoperative CRBD,6,11 so we chose it to be the control group to test our hypothesis that dorsal penile nerve block is associated with effective CRBD reduction as well as decreased systemic side effects.15 As a local anesthetic technique, DPNB has been applied for years in patients underwent penile surgery such as circumcision, penile hypospadias repair, and prosthesis placement.12–14,19–23 Its safety and effectiveness have been proved in a system review with meta-analysis.14 As well as the analgesia effect, patients received DPNB rarely complain about CRBD even with urinary catheter left in-situ in our clinical practice. In the present study, consistent with the previous study concerning about tramadol for CRBD reduction,6 we found about only 40% (11/29) of patients experienced CRBD with tramadol administration after the completion of surgery. As the incidence of CRBD ranges from 47% to 90% in the postoperative period,3 these findings suggested the incidence of CRBD was reduced by tramadol. Moreover, we found that CRBD happened in only about 10% (3/29) of patients who received DPNB at all time points, the incidence of CRBD in patients with DPNB was reduced significantly when compared with the patients received tramadol, and no patient felt severe CRBD in patients with DPNB at all time points. It is suggested that DPNB is associated with a better effect of CRBD prevention than intravenous tramadol according to our findings.
Besides, in this study, we found the incidences of postoperative nausea, vomiting, dizziness, and sedation decreased significantly in patients received DPNB when compared with intravenous tramadol, and the rate of acceptance of urinary catheterization was higher in patients given DPNB than tramadol. These findings suggested that in addition to CRBD reduction, DPNB provided less side effects related to systemic administration.
However, in another study concerning about the effect of DPNB with bupivacaine on urethral catheter-related pain after robotic-assisted radical prostatectomy (RARP), there was no difference in reported catheter-related discomfort or bladder spasm-associated discomfort when comparing to DPNB with placebo.24 The difference between this study and our study may be contributed to the routine usage of oxybutynin, ketorolac, and opiates in postoperative period in the study, resulted in relative lower level of discomfort in both groups, and covered the effectiveness of DPNB with bupivacaine. But indeed, the afferent nerves of the urethra and bladder triangle are derived from sacral somatic (S2–4)5 and theoretically, prevention of CRBD should be achieved by blocking sacral plexus or pudendal nerve other than DPNB. Based on these findings, we speculated that CRBD could be caused by stimulation of urethra in some degree other than the mechanism of overactive bladder, and some patients’ complain of CRBD could be only urethra discomfort.
Several limitations of this study should be considered. First, because of different route of administration (nerve block vs intravenously), double-blind could not be achieved. Second, the patients we enrolled in this trial were all men, and DPNB cannot be applied for female patients who suffered from CRBD. The third, although none of the patients who received DPNB complained of pain or observed bleeding at the injection site, DPNB does have complications related to nerve block such as vascular puncture, hematoma, local anesthetic toxicity, and so on. In addition, the fact that ∼10% (3/29) of patients who received DPNB complained of CRBD suggested that the full prevention of CRBD could not achieved by DPNB, and further study could be focused on the efficacy of pudendal nerve block or caudal block, a fully block for the afferent nerves of the urethra and bladder triangle, for CRBD prevention.
In summary, DPNB with ropivacaine has a better effect for CRBD reduction and less side effects than intravenous tramadol administration.
Acknowledgments
The authors thank Mr. Liang Zhao, a statistician and research assistant in Department of Anesthesiology, West China Hospital of Sichuan University, for his support for this study, and special thanks is due to all staff and participants in this study.
Footnotes
Abbreviations: CRBD = catheter-related bladder discomfort, DPNB = dorsal penile nerve block, NRS = numeric rating scale, PACU = postanesthetic care unit, PONV = postoperative nausea and vomiting, RARP = robotic-assisted radical prostatectomy, SAS = Statistical Analysis System, SPSS = Statistic Package for Social Science, TRAM = tramadol intravenous administration.
J-yL and M-lY contributed equally to this study and should be considered co-first authors.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Wilson M. Causes and management of indwelling urinary catheter-related pain. Br J Nurs 2008; 17:232–239. [DOI] [PubMed] [Google Scholar]
- 2.Binhas M, Motamed C, Hawajri N, et al. Predictors of catheter-related bladder discomfort in the post-anaesthesia care unit. Ann Fr Anesth Reanim 2011; 30:122–125. [DOI] [PubMed] [Google Scholar]
- 3.Bai Y, Wang X, Li X, et al. Management of catheter-related bladder discomfort in patients who underwent elective surgery. J Endourol 2015; 29:640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lepouse C, Lautner CA, Liu L, et al. Emergence delirium in adults in the post-anaesthesia care unit. Br J Anaesth 2006; 96:747–753. [DOI] [PubMed] [Google Scholar]
- 5.Andersson KE, Wein AJ. Pharmacology of the lower urinary tract: basis for current and future treatments of urinary incontinence. Pharmacol Rev 2004; 56:581–631. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal A, Yadav, Gupta D, et al. Evaluation of intra-operative tramadol for prevention of catheter-related bladder discomfort: a prospective, randomized, double-blind study. Br J Anaesth 2008; 101:506–510. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal A, Raza M, Singhal V, et al. Evaluation of efficacy of tolterodine for prevention of catheter related bladder discomfort: a prospective, randomized, placebo-controlled double blind study. Anesth Analg 2005; 101:1065–1067. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal A, Dhiraaj S, Singhal V, et al. Comparison of efficacy of oxybutynin and tolterodine for prevention of catheter related bladder discomfort: a prospective, randomized, placebo-controlled, double-blind study. Br J Anaesth 2006; 96:377–380. [DOI] [PubMed] [Google Scholar]
- 9.Srivastava VK, Agrawal S, Kadiyala VN, et al. The efficacy of pregabalin for prevention of catheter-related bladder discomfort: a prospective, randomized, placebo-controlled double-blind study. J Anesth 2015; 29:212–216. [DOI] [PubMed] [Google Scholar]
- 10.Shariat Moharari R, Lajevardi M, Khajavi M, et al. Effects of intra-operative ketamine administration on postoperative catheter-related bladder discomfort: a double-blind clinical trial. Pain Pract 2014; 14:146–150. [DOI] [PubMed] [Google Scholar]
- 11.Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet 2004; 43:879–923. [DOI] [PubMed] [Google Scholar]
- 12.Butler-O’Hara M, LeMoine C, Guillet R. Analgesia for neonatal circumcision: a randomized controlled trial of EMLA cream versus dorsal penile nerve block. Pediatrics 1998; 101:E5. [DOI] [PubMed] [Google Scholar]
- 13.Howard CR, Howard FM, Fortune K, et al. A randomized, controlled trial of a eutectic mixture of local anesthetic cream (lidocaine and prilocaine) versus penile nerve block for pain relief during circumcision. Am J Obstet Gynecol 1999; 181:1506–1511. [DOI] [PubMed] [Google Scholar]
- 14.Brady-Fryer B, Wiebe N, Lander JA. Pain relief for neonatal circumcision. Cochrane Database Syst Rev 2004; CD004217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li JY, Liao R. Dorsal penile nerve block with ropivacaine versus intravenous tramadol for the prevention of catheter-related bladder discomfort: study protocol for a randomized controlled trial. Trials 2015; 16:596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Downie WW, Leatham PA, Rhind VM, et al. Studies with pain rating scales. Ann Rheum Dis 1978; 37:378–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agarwal A, Gautam S, Gupta D, et al. Evaluation of a single preoperative dose of pregabalin for attenuation of postoperative pain after laparoscopic cholecystectomy. Br J Anaesth 2008; 101:700–704. [DOI] [PubMed] [Google Scholar]
- 18.Ramsay MA, Savege TM, Simpson BR, et al. Controlled sedation with alphaxalone-alphadolone. Br Med J 1974; 2:656–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGowan PR, May II, Molnar A, et al. A comparison of three methods of analgesia in children having day case circumcision. Paediatr Anaesth 1998; 8:403–407. [DOI] [PubMed] [Google Scholar]
- 20.Salgado Filho MF, Gonçalves HB, Pimentel Filho LH, et al. Assessment of pain and hemodynamic response in older children undergoing circumcision: comparison of eutectic lidocaine/prilocaine cream and dorsal penile nerve block. J Pediatr Urol 2013; 9:638–642. [DOI] [PubMed] [Google Scholar]
- 21.Chhibber AK, Perkins FM, Rabinowitz R, et al. Penile block timing for postoperative analgesia of hypospadias repair in children. J Urol 1997; 158 (3 Pt 2):1156–1159. [DOI] [PubMed] [Google Scholar]
- 22.Flores S, Herring AA. Ultrasound-guided dorsal penile nerve block for ED paraphimosis reduction. Am J Emerg Med 2015; 33:863. e3-5. [DOI] [PubMed] [Google Scholar]
- 23.Raynor MC, Smith A, Vyas SN, et al. Dorsal penile nerve block prior to inflatable penile prosthesis placement: a randomized, placebo-controlled trial. J Sex Med 2012; 9:2975–2979. [DOI] [PubMed] [Google Scholar]
- 24.Weinberg AC, Woldu SL, Bergman A, et al. Dorsal penile nerve block for robot-assisted radical prostatectomy catheter related pain: a randomized, double-blind, placebo-controlled trial. Springerplus 2014; 3:181. [DOI] [PMC free article] [PubMed] [Google Scholar]


