Abstract
Pinato prognostic nutritional index (PNI) adequately predicts long-term outcomes of various malignancies. However, its value in predicting outcomes in laryngeal squamous cell carcinoma (LSCC) is unknown.
All patients newly diagnosed with LSCC presenting to the Department of Head and Neck Oncology at Sun Yat-sen University Cancer Center between January 1, 1990 and July 31, 2010 were eligible. The PNI was calculated as serum albumin (g/L) + 5 × total lymphocyte count/L. The Cutoff Finder software program was used to classify the patients into 3 groups for which the PNI score was at least 70% sensitive, at least 70% specific, or equivocal. Cancer-specific survival was estimated using the Kaplan–Meier method, and predictors were assessed with Cox regression analysis.
Median time between surgery and PNI administration for the 975 eligible patients was 83 months. Index score groups were significantly associated with age, T stage, TNM stage, and type of surgery. Five-year CSS and OS were 57.3% and 56.6% in patients with PNI scores below 48.65 (low-probability of survival), 72.8% and 71.3% with scores between 48.65 and 56.93 (moderate-probability of survival), and 77.6% and 75.3% with scores above 56.93 (high-probability of survival); 10-year CSS and OS were 44.2% and 42.7%, 61.6% and 55.6%, 68.3% and 63.5%, respectively. The PNI score groups significantly predicted CSS and OS (P < 0.001).
The PNI is an inexpensive and readily available score that predicted survival in patients with LSCC after curative laryngectomy.
INTRODUCTION
In 2012, 157,000 new cases of laryngeal cancer were diagnosed in the worldwide, with 138,000 and 19,000 new cases diagnosed in men and women, respectively.1 Despite marked advances in surgery and radiotherapy over the past decades, the 5-year survival rates of patients with laryngeal squamous cell carcinoma (LSCC) have actually decreased in the recent years, from 57.1% to 51.9% in the United States.2 Thus, assessing the prognostic factors in LSCC has become increasingly important.
Outcomes of LSCC patients are currently, if inaccurately, predicted from clinicopathological characteristics, such as primary tumor, regional node, distant metastasis (the tumor–node–metastasis [TNM] components), or the stage, depth of invasion, and differentiation grade of the cancer. Although advances in molecular and cellular biology may lead to the discovery of new biomarkers and new therapeutic targets, the lack of standardization, regional availability, and need for further validation currently limit the routine clinical application of these biomarkers.3 Therefore, clinical characteristics that predict survival are still needed.
Pretreatment nutritional and immunological status has been associated with long-term outcomes in patients with malignant tumors.4,5 Nutritional impairment is correlated with poor performance status, shorter survival, and increased mortality in patients with cancer.6,7 The prognostic nutritional index (PNI) is a score calculated from serum albumin concentration and total lymphocyte count in the peripheral blood. Increasing evidence shows that PNI scores adequately predict long-term outcomes of various malignancies.8–11 However, the prognostic value of the PNI in patients with LSCC is unknown.
As nutritional and immunological status is associated with LSCC prognosis, we hypothesized that the preoperative PNI scores might predict outcomes of patients with this disease. Here, we report the results of a large retrospective study in which preoperative PNI scores predicted long-term outcomes in patients with LSCC who had undergone curative laryngectomy.
METHODS
The institutional review board of the Sun Yat-sen University Cancer Center approved the study. All data were kept anonymous and confidential and were aggregated for analysis.
Patient Selection
We retrospectively analyzed patients who underwent laryngectomy as a 1st curative treatment option for LSCC between January 1, 1990 and July 31, 2010 at the Sun Yat-sen University Cancer Center, Guangzhou, China. All patients had histopathologically proven LSCC without distant metastasis and underwent curative laryngectomy. None had a history of adjuvant or neo-adjuvant therapy or other malignancies. Patients were contacted by telephone every 3 months during the 1st 2 years after surgery and every 6 months thereafter until death.
Study Variables
Patient age, sex, smoking status (never or ever smoker), drinking status (never or ever consumer of alcohol), tumor subsite, tumor stage (T stage), N stage, TNM stage, neck dissection (present or absent), pathological differentiation (poor, moderate, and high), and type of surgery (partial or total laryngectomy) were retrieved from the medical records. The conventional TNM system for laryngeal cancer established by the Union for International Cancer Control and the American Joint Committee on Cancer was used to stage tumors.12 Laboratory data, including the serum albumin concentrations and lymphocyte count used to calculate the PNI, were obtained during preoperative examination. We have no reason to believe that the changes in analytic instruments varied markedly over the period described. Quality assurance protocols were run daily according to the Westgard Rules.
The PNI was calculated from preoperative values as:
PNI = serum albumin (g/L) + 5 × total lymphocyte count (per L).
Cancer-specific survival (CSS) was defined as the time in months from the date of the surgery until death from cancer-related causes. Overall survival (OS) was defined as the time in months from the date of surgery until death from any cause during the follow-up period.
Statistical Methods
The optimal cut-off PNI scores were determined with the Cutoff Finder software program, an R software-engineered, web-based system designed by Budczies et al13 (http://molpath.charite.de/cutoff/). The PNI cut-off value for 2 groups was 44.25, which had a specificity of only 10.4%. We then divided the patients into 3 groups based on their PNI scores: patients in whom the test was 70% sensitive (the high-probability of survival group), patients in whom the test was 70% specific (the low-probability of survival group), and patients in whom the test was equivocal (the mid-probability of survival group).
Categorical variables are reported as counts and percentages and were compared using Chi-square or Fisher exact tests. Univariate and multivariate analyses for survival difference were performed using Cox proportional hazards models and were expressed as hazard ratios and 95% confidence intervals. The variables that were shown to be associated with CSS and OS in the univariate analysis were evaluated in the multivariate Cox proportional hazard model. The likelihood ratios forward stepwise method was used for the multivariate Cox proportional analysis. Survival curves were calculated using the Kaplan–Meier method and compared using the log-rank test. All analyses were performed using IBM SPSS statistics software, version 20.0 (SPSS, Inc., Chicago, IL). Two-tailed P values < 0.05 were considered significant.
RESULTS
Patient Characteristics
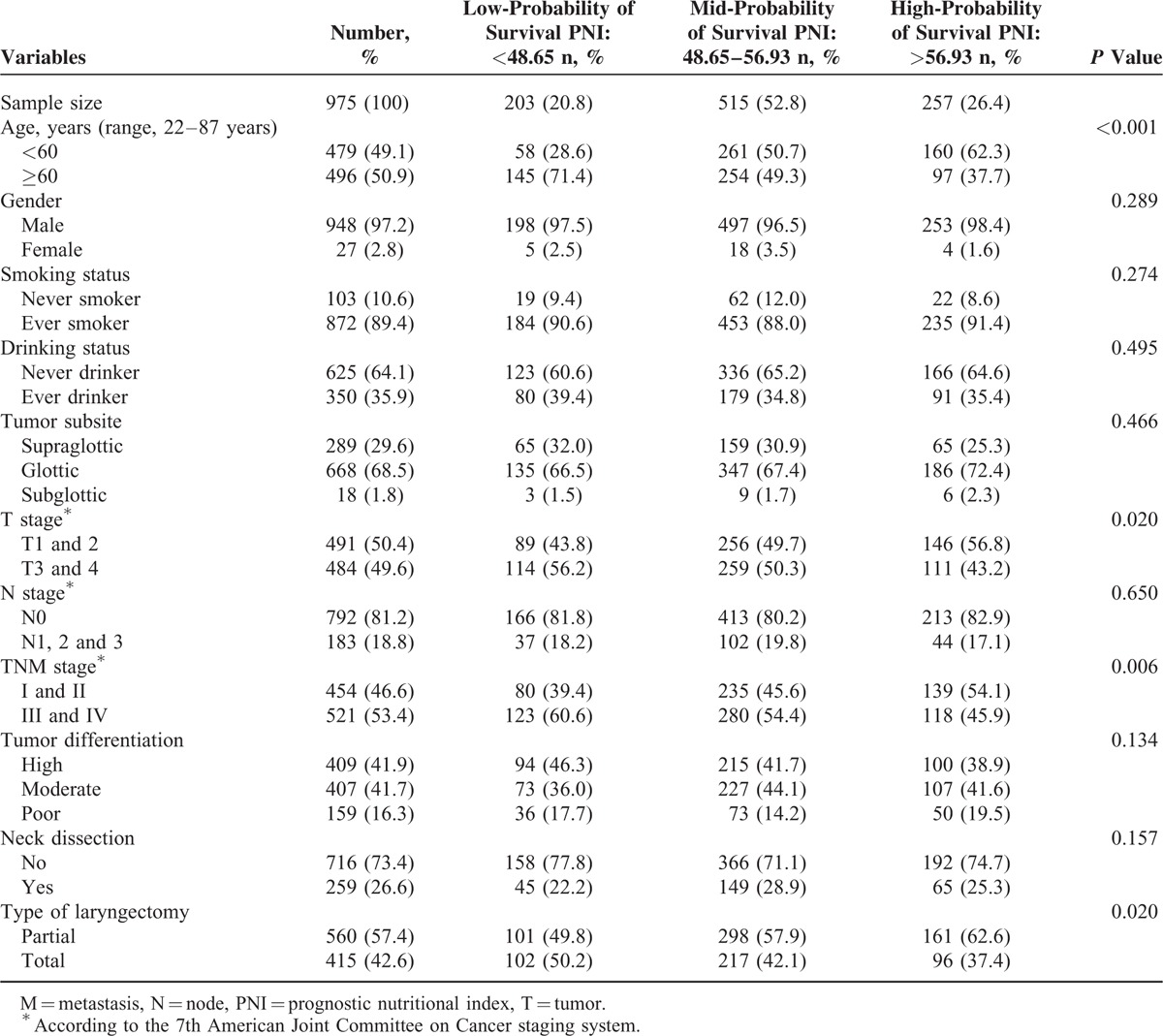
Of 1010 patients identified in the records, 35 with incomplete preoperative laboratory data were excluded, leaving 975 patients (948 men) in the analysis (Table 1). Median follow-up (from the day of surgery to death or final follow-up call) was 83 months (range 0.3–300 months). Median (range) age was 60 (22–87) years. Almost 90% of patients were smokers, and 36% had a history of alcohol intake. Of primary tumors, two-thirds were in the glottis larynx and one-third was in the supraglottic larynx. About half the tumors were T1–2 and half in T3–4. About 20% of patients had lymph node metastasis. For cancer stage, about half the patients were initially diagnosed as early stage and about half as advanced stage.
TABLE 1.
Frequency of Clinicopathological Characteristics in 975 Patients With Laryngeal Squamous Cell Carcinoma, by Preoperative Nutritional Index Group
Preoperative Nutritional Index Diagnostic Cut-Off Scores
The optimal cut-off PNI scores for predicting survival were determined to be 48.65 and 56.93 (Figure 1). The level of 48.65 and 56.93 were defined as the cut-off values for CSS and OS in our study. Thus, we classified the patients into 3 groups: those with PNI scores of up to 48.65 (n = 203; 20.8%; the low-probability of survival group), between 48.65 and 56.93 (n = 515; 52.8%; the mid-probability of survival group), and those with scores greater than 56.93 (n = 257; 26.4%; the high-probability of survival group). The 3 groups did not differ significantly except for age (P < 0.001), T stage (P = 0.020), TNM stage (P = 0.006), and type of laryngectomy (P = 0.02; χ2-test).
FIGURE 1.
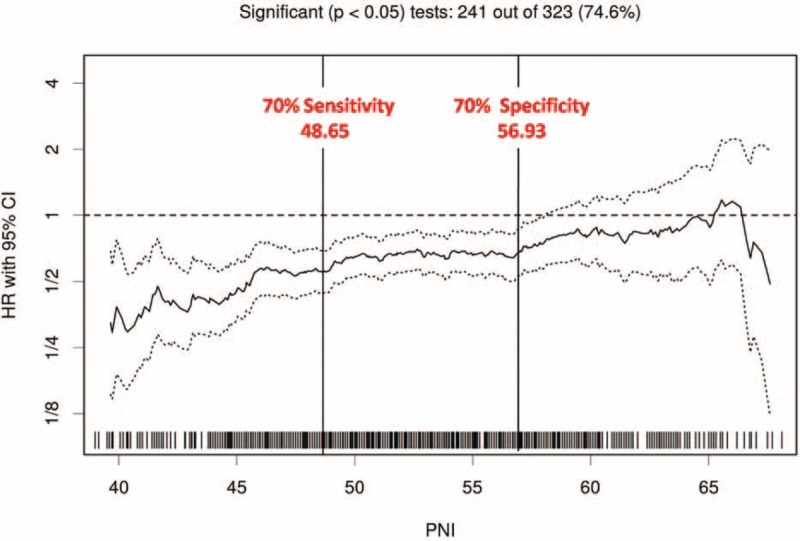
Cut-off points for Preoperative Nutritional Index scores predicting survival for patients with laryngeal squamous cell carcinoma undergoing curative laryngectomy. Scores were set at hazard ratios providing a sensitivity of 70% and a specificity of 70%: scores less than 48.65 (n = 203; 20.8%) indicated a low probability of cancer-specific survival, scores between 48.65 and 56.93 (n = 515; 52.8%), a moderate probability of cancer-specific survival; and scores greater than 56.93 (n = 257; 26.4%), a high probability of cancer-specific survival.
Univariate and Multivariate Analysis of Prognostic Factors
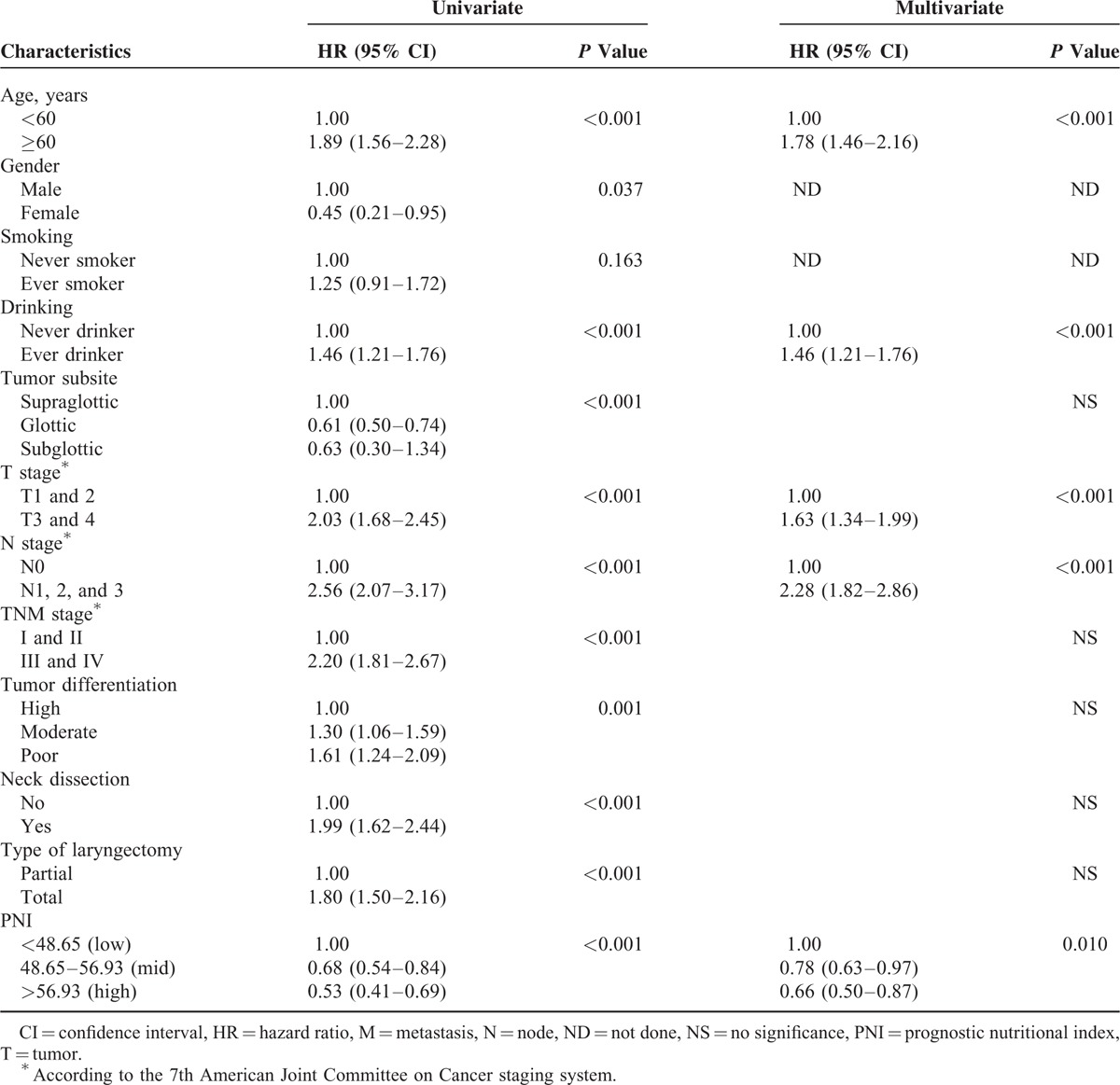
In univariate analyses, age, history of alcohol intake, tumor subsite, T stage, N stage, TNM stage, pathological differentiation, neck dissection, PNI score, and type of surgery were significant predictors of CSS and OS (Tables 2 and 3). In the multivariate Cox proportional hazards model, age, history of alcohol intake, T stage, N stage, and PNI (48.65–56.93 [moderate probability of survival] vs <48.65 [low probability]: hazard ratio [HR], 0.65; 95% CI, 0.51–0.83; P < 0.001; >56.93 [high probability] vs <48.65 [low probability]; HR, 0.54; 95% CI, 0.40–0.73; P < 0.001) remained significant independent predictors of CSS (Table 2). In that model, age, history of alcohol intake, T stage, N stage, and PNI (48.65–56.93 [mid probability] vs <48.65 [low probability]; HR, 0.78; 95% CI, 0.63–0.97; P < 0.001; >56.93 [high probability] vs <48.65 [low probability]; HR, 0.66; 95% CI, 0.50–0.87; P < 0.001) remained significant independent predictors of CSS (Table 3).
TABLE 2.
Results of Cox Regression Analysis for Predictors of Cancer-Specific Survival Among 975 Patients With Laryngeal Squamous Cell Carcinoma
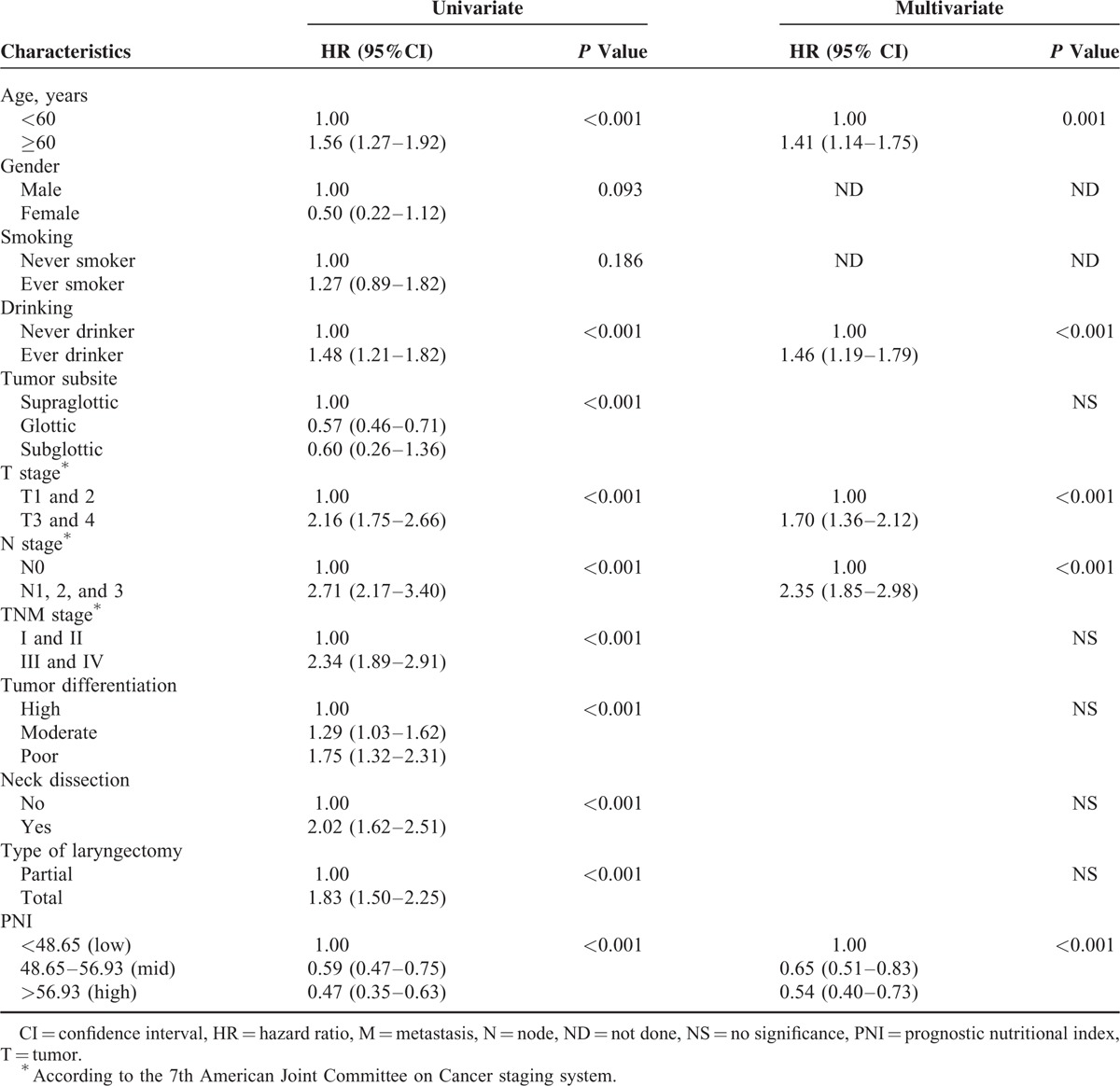
TABLE 3.
Univariate and Multivariate Analyses for Predictors of Overall Survival Among 975 Laryngeal Squamous Cell Carcinoma
Analysis of Cancer-Specific Survival and Prognostic Factors
Overall, 5- and 10-year CSS rates were 70.8% and 59.6%, respectively. The 5-year CSS rate was 57.3% in the low-probability group, 72.8% in the mid-probability group, and 77.6% in the high-probability group. The 10-year CSS rates were 44.2%, 61.6%, and 68.3%, respectively (P < 0.001; Figure 2). During follow-up, 112 patients (55.2%) in the low-probability group, 186 (36.1%) in the mid-probability group, and 78 (30.4%) in the high-probability group died of tumor-related causes (P < 0.001; Figure 3).
FIGURE 2.
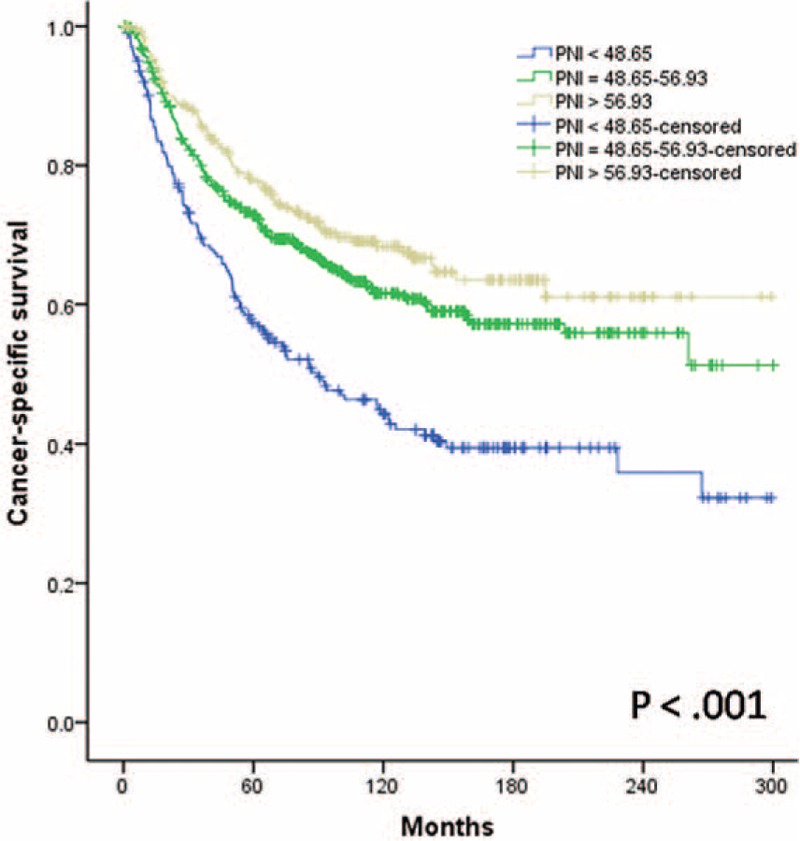
Relationship between the Preoperative Nutritional Index scores and cancer-specific survival in patients with laryngeal squamous cell carcinoma after curative laryngectomy, P < 0.001.
FIGURE 3.
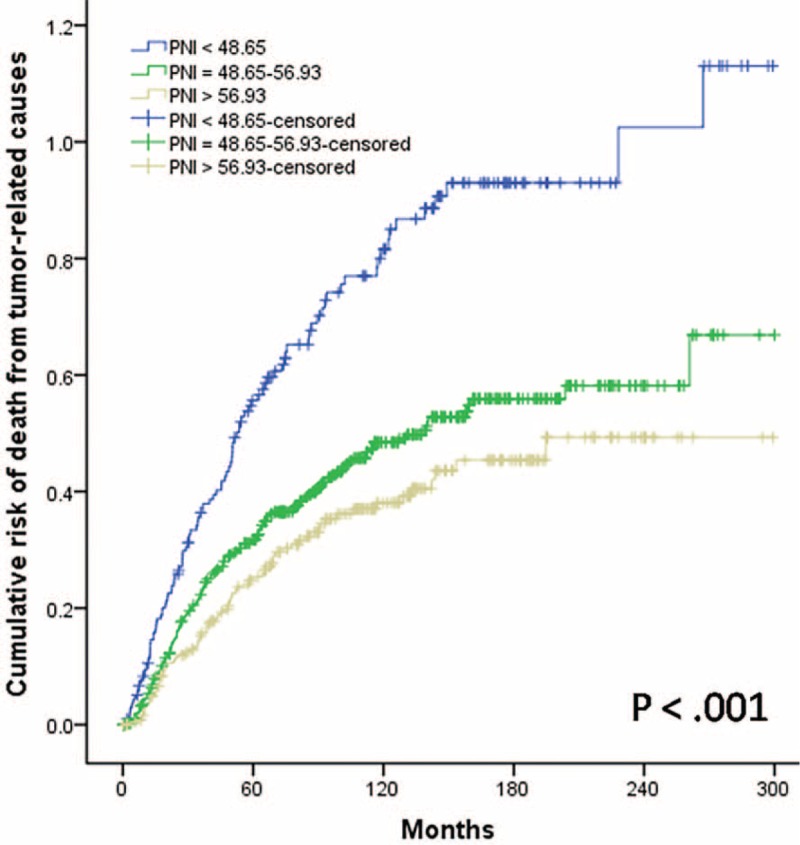
Cumulative risk of death from tumor-related causes in patients with laryngeal squamous cell carcinoma after curative laryngectomy, by Preoperative Nutritional Index score, P < 0.001.
Overall, the 5- and the 10-year OS rates were 69.3% and 55.0%, respectively. The 5-year OS rate was 56.6% in the low-probability group, 71.3% in the mid-probability group, and 75.3% in the high-probability group. The 10-year OS rates were 42.7%, 55.6%, and 63.5%, respectively (P < 0.001; Figure 4).
FIGURE 4.
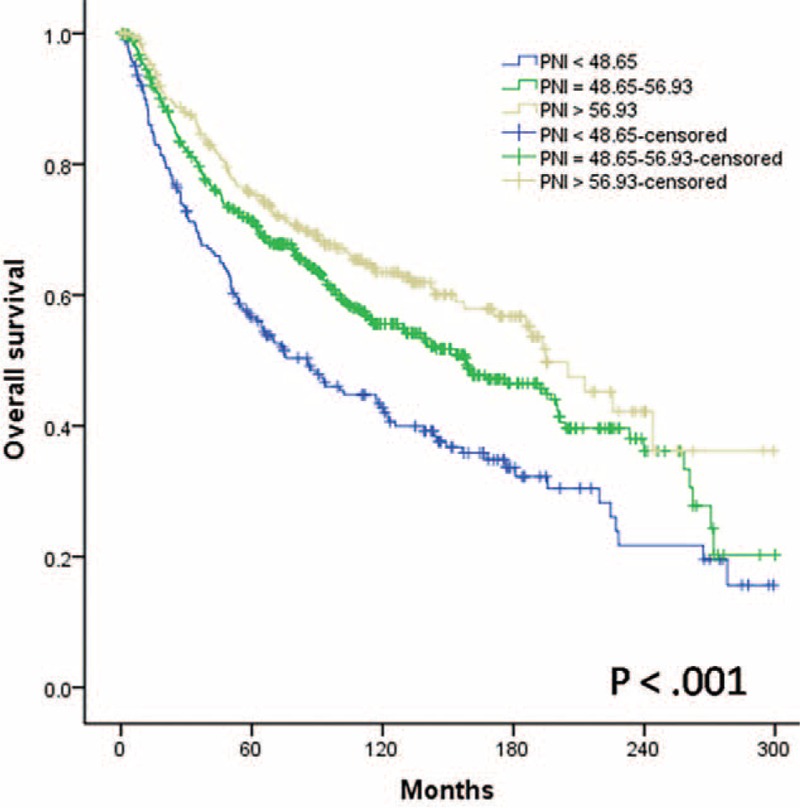
Relationship between the Preoperative Nutritional Index scores and overall survival in patients with laryngeal squamous cell carcinoma after curative laryngectomy, P < 0.001.
DISCUSSION
We used the Cutoff Finder software program to determine the optimal PNI scores for predicting cancer survival. The preoperative PNI groups predicted long-term outcome of LSCC, independent of age, history of alcohol intake, T stage, and N stage. The low-probability group had a significantly lower CSS and OS rate and more often died of tumor-related causes than the other 2 groups.
Two important factors characterize head and neck cancer patients: malnutrition and immunosuppression.14 Clinically, most laboratory assessments could reflect both malnutrition and immunosuppression, but they are not ideal because they are inaccurate, insensitive, or inconvenient to perform. However, albumin is a widely used indicator of nutrition and the host's inflammatory reaction, whereas lymphocytes are key elements of the immune system.4,11 The PNI, a prognostic score based on lymphocyte count and albumin levels, was first used to assess the immunological and nutritional aspects of patients undergoing surgery of the gastrointestinal tract, predominantly as an indicator of the nutritional status of any given patient.15 Nutritional status has recently been linked to the prognosis of various cancer types.8,16–18 A low PNI indicates lower in albumin concentrations, lymphocyte counts, or both. The presence of cancer cachexia, partly reflected by a lower albumin concentration, is driven by a sustained inflammatory response, either from the tumor itself or as a host reaction.19
Albumin is an objective measure often used in clinical studies to reflect malnutrition in the patient. On one hand, in some studies, malnutrition prolonged hospital stay,20,21 increased the number of medical complications,22 and sometimes increased the death rate.22,23 Furthermore, ecological and observational studies suggest that low serum albumin concentration is associated with higher mortality from cancer.24–26 Albumin is important in assessing malnutrition, indicating that these nutritional assessments identify different at-risk groups. On the other hand, malnutrition impairs host immunity.27 Patients with tumors have decreased host immunity that nearly always worsens coexisting malnutrition, and the outcomes of decreased host immunity maybe more dangerous in a malnourished host than in a well-nourished one.
On the other hand, lymphocytes are involved in cytotoxic cell death and cytokine production, which inhibit the proliferation and metastatic capacity of tumor cells by starting an immune response against the tumor.28 Cytotoxic T Lymphocytes (CTL) induce apoptosis of cancer cells and inhibit tumor growth, whereas CD8+ T lymphocyte infiltration is associated with better overall patient outcomes. Furthermore, immunologic mediators (such as IL-10 and transforming growth factor-b) are released, which can have a marked immunosuppressive effect with consequent impaired lymphocyte function and reduced lymphocyte counts.29 Thus, a low lymphocyte count is associated with an immunosuppressed condition, suggesting that the host has an insufficient antitumor immunological reaction.30 Thus, albumin concentrations and lymphocyte counts, taken together, may indicate chronic inflammation, immunity, and nutritional status, all of which are of prognostic value.
Malnutrition and immunosuppression are often problems in elderly surgical patients. Among our patients, 71.4% (145/203) of those with a low-probability of survival (PNI scores <48.65) were 60 years old or older, whereas only 37.7% (97/257) of those with a high-probability of survival (PNI scores >56.93) were 60 years old or older. Thus, the overall prevalence of malnutrition and immunosuppression was indeed higher among our elderly patients.
We found that PNI score was also related to T stage and TNM stage. Further, advanced T stage (T3–4) and advanced TNM stage (TNM III–IV) patients were preferentially treated with total laryngectomy.2 The TNM stage comprises the effects of T and N stages, which generally reflects the prominent impact of local invasion of LSCC and cervical lymph node metastasis on prognosis. More than 60% of our patients with TNM III–IV stage disease were in the low-probability-of-survival group. These patients were also more likely to experience malnutrition and immunosuppression.
Various PNI cut-off scores selected with different methods have been used in different cancers.5,11,31 The cut-off score is usually set at 45, which is defined as moderate-to-severe malnutrition.31 Yao et al32 showed that a cut-off score of 44.6 predicted reduced survival. Hong et al11 used 52.48 and found the same result.
However, the optimal PNI cut-off score for predicting the long-term outcomes of LSCC remains unclear. In the present study, the optimal PNI cut-off scores were determined to be 48.65 and 56.93 by the Cutoff Finder software program. Based on these cut-off values, we classified the patients into 3 groups according to their PNI scores: below 48.65, between 48.65 and 56.93, and above 56.93. CSS was lower in patients with PNI scores less than 48.65 than it was in those with PNI scores between 48.65 and 56.93 and in those with PNI greater than 56.93 (5-year CSS, 57.3% vs 72.8% and 77.6%, respectively; 10-year CSS, 44.2% vs 61.6% and 68.3%, respectively; log-rank test, P < 0.001). OS was lower in patients with PNI scores less than 48.65 than it was in those with PNI scores between 48.65 and 56.93 and in those with PNI greater than 56.93 (5-year OS, 56.6% vs 71.3% and 75.3%, respectively; 10-year OS, 42.7% vs 55.6% and 63.5%, respectively; log-rank test, P < 0.001).
Limitations of the Study
It is a retrospective, single-institution observational study based on only 975 patients. A prospective study would provide a better evaluation of prognostic factors and would allow serial PNI scores to be obtained so that they could be correlated with declines in other clinical variables. Hence, these analyses need to be validated in a larger cohort of patients.
CONCLUSIONS
Although our results warrant further validation in independent prospective studies, we found that the PNI is a simple, reproducible, inexpensive, and reliable measure of systemic inflammation response. In our study, the PNI score significantly predicted CSS and OS. Therefore, we suggest that the PNI should be included in the routine assessment of LSCC patients.
Acknowledgments
The authors thank Tom Lang of Tom Lang Communications and Training International for substantial editing of earlier versions of the manuscript. The authors also thank the anonymous reviewers for their insightful comments and great efforts to help improve our manuscript.
Footnotes
Abbreviations: CSS = cancer-specific survival, LSCC = laryngeal squamous cell carcinoma, OS = overall survival, PNI = prognostic nutritional index, T stage = tumor stage, TNM = tumor–node–metastasis
This study was supported by grants from the Guangdong Provincial Department of Science and Technology (program series 2010B031600061).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136:E359–E386. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman HT, Porter K, Karnell LH, et al. Laryngeal cancer in the United States: changes in demographics, patterns of care, and survival. Laryngoscope 2006; 116:1–13. [DOI] [PubMed] [Google Scholar]
- 3.Guan GF, Zheng Y, Wen LJ, et al. Gene expression profiling via bioinformatics analysis reveals biomarkers in laryngeal squamous cell carcinoma. Mol Med Rep 2015; 12:2457–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shibutani M, Maeda K, Nagahara H, et al. The prognostic significance of the postoperative prognostic nutritional index in patients with colorectal cancer. BMC Cancer 2015; 15:521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinato DJ, North BV, Sharma R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI). Br J Cancer 2012; 106:1439–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dewys WD, Begg C, Lavin PT, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med 1980; 69:491–497. [DOI] [PubMed] [Google Scholar]
- 7.Rey-Ferro M, Castano R, Orozco O, et al. Nutritional and immunologic evaluation of patients with gastric cancer before and after surgery. Nutrition 1997; 13:878–881. [DOI] [PubMed] [Google Scholar]
- 8.Chen KL, Liu YH, Li WY, et al. The prognostic nutritional index predicts survival for patients with extranodal natural killer/T cell lymphoma, nasal type. Ann Hematol 2015; 94:1389–1400. [DOI] [PubMed] [Google Scholar]
- 9.Chan AW, Chan SL, Wong GL, et al. Prognostic Nutritional Index (PNI) predicts tumor recurrence of very early/early stage hepatocellular carcinoma after surgical resection. Ann Surg Oncol 2015; 22:4138–4148. [DOI] [PubMed] [Google Scholar]
- 10.Jeon HG, Choi DK, Sung HH, et al. Preoperative Prognostic Nutritional Index is a significant predictor of survival in renal cell carcinoma patients undergoing nephrectomy. Ann Surg Oncol 2016; 23:321–327. [DOI] [PubMed] [Google Scholar]
- 11.Hong S, Zhou T, Fang W, et al. The prognostic nutritional index (PNI) predicts overall survival of small-cell lung cancer patients. Tumour Biol 2015; 36:3389–3397. [DOI] [PubMed] [Google Scholar]
- 12.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010; 17:1471–1474. [DOI] [PubMed] [Google Scholar]
- 13.Budczies J, Klauschen F, Sinn BV, et al. Cutoff Finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS One 2012; 7:e51862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerrebijn JD, Simons PJ, Tas M, et al. The effects of thymostimulin on immunological function in patients with head and neck cancer. Clin Otolaryngol Allied Sci 1996; 21:455–462. [DOI] [PubMed] [Google Scholar]
- 15.Buzby GP, Mullen JL, Matthews DC, et al. Prognostic nutritional index in gastrointestinal surgery. Am J Surg 1980; 139:160–167. [DOI] [PubMed] [Google Scholar]
- 16.Geng Y, Qi Q, Sun M, et al. Prognostic nutritional index predicts survival and correlates with systemic inflammatory response in advanced pancreatic cancer. Eur J Surg Oncol 2015; 41:1508–1514. [DOI] [PubMed] [Google Scholar]
- 17.Migita K, Takayama T, Saeki K, et al. The prognostic nutritional index predicts long-term outcomes of gastric cancer patients independent of tumor stage. Ann Surg Oncol 2013; 20:2647–2654. [DOI] [PubMed] [Google Scholar]
- 18.Mohri Y, Inoue Y, Tanaka K, et al. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg 2013; 37:2688–2692. [DOI] [PubMed] [Google Scholar]
- 19.Esper DH, Harb WA. The cancer cachexia syndrome: a review of metabolic and clinical manifestations. Nutr Clin Pract 2005; 20:369–376. [DOI] [PubMed] [Google Scholar]
- 20.Virtaniemi JA, Kumpulainen EJ, Hirvikoski PP, et al. The incidence and etiology of postlaryngectomy pharyngocutaneous fistulae. Head Neck 2001; 23:29–33. [PubMed] [Google Scholar]
- 21.Smale BF, Mullen JL, Buzby GP, et al. The efficacy of nutritional assessment and support in cancer surgery. Cancer 1981; 47:2375–2381. [DOI] [PubMed] [Google Scholar]
- 22.Weingrad DN, Spiro RH. Complications after laryngectomy. Am J Surg 1983; 146:517–520. [DOI] [PubMed] [Google Scholar]
- 23.Redaelli DZL, Ferrari L, Tomenzoli D, et al. Postlaryngectomy pharyngocutaneous fistula: incidence, predisposing factors, and therapy. Head Neck 1999; 21:131–138. [DOI] [PubMed] [Google Scholar]
- 24.Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J 2010; 9:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li G, Gao J, Liu ZG, et al. Influence of pretreatment ideal body weight percentile and albumin on prognosis of nasopharyngeal carcinoma: long-term outcomes of 512 patients from a single institution. Head Neck 2014; 36:660–666. [DOI] [PubMed] [Google Scholar]
- 26.Boonpipattanapong T, Chewatanakornkul S. Preoperative carcinoembryonic antigen and albumin in predicting survival in patients with colon and rectal carcinomas. J Clin Gastroenterol 2006; 40:592–595. [DOI] [PubMed] [Google Scholar]
- 27.Mainous MR, Deitch EA. Nutrition and infection. Surg Clin North Am 1994; 74:659–676. [PubMed] [Google Scholar]
- 28.Ownby HE, Roi LD, Isenberg RR, et al. Peripheral lymphocyte and eosinophil counts as indicators of prognosis in primary breast cancer. Cancer 1983; 52:126–130. [DOI] [PubMed] [Google Scholar]
- 29.Salazar-Onfray F, Lopez MN, Mendoza-Naranjo A. Paradoxical effects of cytokines in tumor immune surveillance and tumor immune escape. Cytokine Growth Factor Rev 2007; 18:171–182. [DOI] [PubMed] [Google Scholar]
- 30.Ray-Coquard I, Cropet C, Van Glabbeke M, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res 2009; 69:5383–5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng JF, Chen QX. Significance of the prognostic nutritional index in patients with esophageal squamous cell carcinoma. Ther Clin Risk Manag 2014; 10:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yao ZH, Tian GY, Wan YY, et al. Prognostic nutritional index predicts outcomes of malignant pleural mesothelioma. J Cancer Res Clin Oncol 2013; 139:2117–2123. [DOI] [PubMed] [Google Scholar]


