Abstract
The aim of the study is to determine whether HLA-haploidentical-related donor (HRD) transplant can achieve equivalent outcomes and have stronger GVL compared to HLA-matched sibling donor (MSD) and HLA-matched unrelated donor (MUD) transplants.
A total of 355 consecutive patients with acute leukemia undergoing allogeneic transplant at our single institute between March 2008 and March 2014 were enrolled in this retrospective investigation.
Of the 355 patients, 96 cases received HRD, 153 MSD, and 106 MUD transplants. HRD transplant was associated with higher incidences of grade II to IV aGVHD (40.6%) compared with MSD (23.5%, P = 0.002) and MUD transplants (34.0%, P = 0.049), whereas incidences of grade III to IV aGVHD (11.4%, 7.8%, 10.5%, respectively; P = 0.590) and cGVHD (29.5%, 24.0%, 29.5%, respectively; P = 0.538) did not differ among 3 groups. Five-year relapse rates were 19.2%, 26.8%, and 23.0% in 3 groups, respectively (P = 0.419). However, of 206 high-risk patients, the relapse rate in HRD transplant was lower than in MSD transplant (23.8% vs 41.9%, P = 0.026). Multivariate analysis showed that HRD had beneficial impact on relapse (for MSD: P = 0.006). Five-year transplant-related mortality was lower in MSD transplant compared with those in HRD (17.3% vs 26.4%, P = 0.041) and MUD transplants (17.3% vs 24.1%, P = 0.037). Five-year overall survival were 60.4%, 64.6%, and 61.0%, respectively, in HRD, MSD, and MUD groups (P = 0.371); 5-year disease-free survival were 59.6%, 58.8%, and 54.9%, respectively (P = 0.423).
Our results suggest that HRD transplant results in outcomes equivalent to MSD and MUD transplants. HRD might carry a superior GVL effect compared to MSD for high-risk patients.
Key points.
To determine whether HRD-HSCT can achieve equivalent outcomes and have stronger GVL compared to MSD- and MUD-HSCT.
We find that HRD transplant results in outcomes equivalent to those of MSD and MUD transplants. HRD transplant might carry a superior GVL effect compared to MSD transplant for high-risk patients.
INTRODUCTION
Currently, allogeneic hematopoietic stem cell transplantation (allo-HSCT) remains the only curative therapy for a majority of malignant hematologic diseases, especially acute leukemia. The transplantation from HLA-matched sibling donor (MSD) offers the best results for these diseases, but lack of this donor resource has restricted its wide application. Unrelated donor (MUD) provides another option,1–3 but MUD still cannot satisfy all patients due to unsuccessful donor searches. Almost all patients have an available related donor with whom they share a single HLA haplotype (i.e., haploidentical related donor), and it owns the advantage of immediate availability, especially for those who urgently need transplantation. What is more, the results of transplantation from HLA-haploidentical donor (HRD) have improved significantly over the past few years owing to the development of highly immunosuppressive conditioning, graft manipulation, and prophylaxis of GVHD.4–7 Similar outcomes were observed in patients undergoing HRD transplant compared with those undergoing MUD and MSD transplants in several studies.8–10 However, conflicting data exist regarding this issue.11–12 Particularly, it remains controversial whether HRD transplant might have a stronger graft versus leukemia (GVL) effect than MUD or MSD transplants.8–13 Here we retrospectively compared outcomes of consecutive patients with acute leukemia undergoing HRD transplant by the strategy of using T-cell-replete (TCR) grafts combined with T-cell depletion in vivo performed at our single institute with those undergoing MSD and MUD transplants.
PATIENTS AND METHODS
Patients
All consecutive patients undergoing first allo-HSCT for acute leukemia between March 2008 to March 2014 at our center using HRD (n = 96), MSD (n = 153), or MUD (n = 106) were enrolled in this retrospective study. The endpoint of the last follow-up for all of the surviving patients was March 31, 2015. All living patients had a minimum follow-up of 1 year at the time of analysis. The study was performed in accordance with the modified Helsinki Declaration, and the protocol was approved by our ethical review boards at Nanfang Hospital of Southern Medical University before study initiation. Informed consent was obtained from the donors and recipients.
Patients were classified as standard- and high-risk based on genetics and clinical response to chemotherapy. High risk was defined by the presence of adverse genetics, and/or failure to achieve complete remission (CR) after 2 cycles of induction chemotherapy, and/or patients in nonremission (NR), and/or patients in CR2 or beyond. Adverse genetic included t (4;11), t (9;22), –5 or del (5), –7 or del (7), del (11), inv3, t (3;3), t (6;9), complex karytype, and Flt3 internal tandem duplication. The high-risk cohorts also included chronic myelogenous leukemia with blast crisis (CML-BP) and acute leukemia secondary to myelodysplastic syndrome (MDS). Others who were ineligible for high risk were all defined as standard risk.
HLA Typing and Donor Selection
High-resolution DNA typing for HLA-A, -B, -C, -DRB1, and -DQB1 were performed in all patients and donors. Donor selection was as follows: if a suitable MSD (i.e., a sibling donor matching >8/10) was available, the donor was chosen. If a suitable MSD was unavailable, a suitably matched MUD was used as the alternative, where a suitable match involved matching >8 of 10 HLA allele loci. If a suitable MSD or MUD was unavailable within the timeframe appropriate for the patient's malignancy and clinical circumstances (i.e., patients in high risk achieved complete remission received 3–4 cycles of consolidation therapy; patients in NR urgently needed allo-HSCT; patients in CR2 or beyond), HRD was administered.
Conditioning and Transplantation
As described previously,14 5 myeloablative conditioning regimens were used, including TBI (total body irradiation)+Cy (cyclophosphamide), Bu (busulfan)+Cy, Bu+Flu (fludarabine), intensified myeloablative conditioning (TBI, Cy and etoposide), and sequential intensified conditioning (Flu, cytarabine, TBI, Cy and etoposide). Generally, the selection of conditioning regimens was based on diagnosis and disease status at transplantation. Acute myeloid leukemia (AML) in CR received BuCy or BuF, and acute lymphoid leukemia (ALL) in CR received TBI+Cy or TBI+Cy+etoposide, and acute biphenotypic leukemia (ABL) or whose diseases were in NR received sequential intensified preparative regimen.
Donor was treated with G-CSF (Gran®, Kirin Kunpeng Bio-pharmaceutical Co. Ltd) given subcutaneously at 5 μg/kg per day for 5 to 6 consecutive days. Bone marrow (BM) were harvested on day 4 of G-CSF, and peripheral blood stem cells (PBSC) collections began on day 5 of G-CSF. CD34+ cell or mononuclear cell counts in the apheresis products were determined and, as needed, consecutive daily collections were performed until CD34 cell yields were >5.0 × 106/kg or mononuclear cell >6.0 × 108/kg. All patients in the HRD group transplanted with the combination of BM and PBSC grafts, whereas most patients in the MSD group and all in the MUD group received PBSC grafts.
GVHD Prophylaxis
Cyclosporin A (CsA) alone or CsA + methotrexate (MTX) (on days +1 and +3) were administered in patients with NR undergoing MSD transplant, and CsA + MTX (on days +1, +3 and +6) were administered in patients with complete remission (CR) undergoing MSD transplant for GVHD prophylaxis. CsA + MTX + antithymocyte globulin (ATG, Thymoglobulin, Genzyme, Cambridge, MA) (total ATG doses of 7.5 mg/kg, on days −3 to −1) used in patients undergoing MUD transplant and CsA + MTX + ATG (total ATG doses of 10 mg/kg, on days −3 to 0) + mycophenolate (MMF) in patients undergoing HRD transplant.15
Infection Prophylaxis
Oral sulfamethoxazole and norfloxacin were used in all cases. Acyclovir and Ganciclovir was given for prophylaxis and treatment of cytomegalovirus (CMV) infection as prescribed in previous literature.15 Anti-CD20 antibody (rituximab, 375 mg/m2) was preemptively administered for Epstein–Barr virus (EBV)-DNA viremia.15 Antifungal agents were used for fungal infection prophylaxis.16
Donor Lymphocyte Infusion (DLI)
For preventing relapse, DLI were administered in patients who were in NR pretransplantation by day 60 to 90 post-transplantation or whose minimal residual disease (MRD) was positive post-transplantation when donor lymphocytes were available if patients did not develop grade II or more than grade II aGVHD. G-CSF mobilized donor lymphocytes were given once to patients in NR pretransplantation regardless of MRD and was then administered based on GVHD and MRD status. DLI was given monthly until GVHD occurred or MRD became negative or for a total of 4 times. Once patients developed GVHD after DLI, DLI would be discontinued.
CMV-DNA and EBV-DNA Monitoring
The CMV-DNA and EBV-DNA loads of blood were detected as our previous study 15. Generally, the EBV-DNA of blood was monitored weekly for the first 3 months after transplantation. During the 4th to 9th month post-transplantation, the monitoring frequency was once every 2 weeks; the 10th to 24th month, once a month; the 25th to 36th month, once every 3 months. If positive, virus DNA was monitored twice a week.
Evaluation Points and Definition
This study was mainly focused on engraftment, viral infection, GVHD, transplant-related mortality (TRM), relapse, diseases-free survival (DFS), and overall survival (OS). Hematopoietic engraftment was defined as the first of 3 consecutive days with an absolute neutrophil count exceeding 0.5 × 109/L and the first day of a platelet count >20 × 109/L without platelet transfusion. EBV and CMV-associated diseases were defined as prescribed previously.15 Acute GVHD (aGVHD) and chronic GVHD (cGVHD) were graded as according to literature.17,18 Relapse was defined as hematologic relapse, including re-appearance of blasts in the peripheral blood, any manifestation of leukemia outside the hematopoietic system, or >5% blasts in the BM smear. TRM was defined as death with no relapse. DFS was defined as survival in a state of continuous complete remission.
Statistics
Analysis was performed on March 31, 2015. Variables related to patients, disease, transplant characteristics, as well as time to engraftment among the 3 groups were compared using the Pearson χ2 test or Fisher's exact test for categorical variables and the 1-way ANOVA for continuous variables. Numerical variables were analyzed as categories based on their values being below or above the median of the entire cohort. The cumulative incidences of CMV viremia, EBV viremia, aGVHD, cGVHD, TRM, relapse, OS and DFS were analyzed with the method of Kaplan–Meyer, comparing the groups using the log-rank test (Mantel–Haenszel). Cox proportional hazards regression model were used for analysis of risk factors for time-to-event variables. The SPSS statistics 17.0 (SPSS, Chicago, IL) was used for all data analysis.
RESULTS
Patient, Donor, and Transplant Characteristics
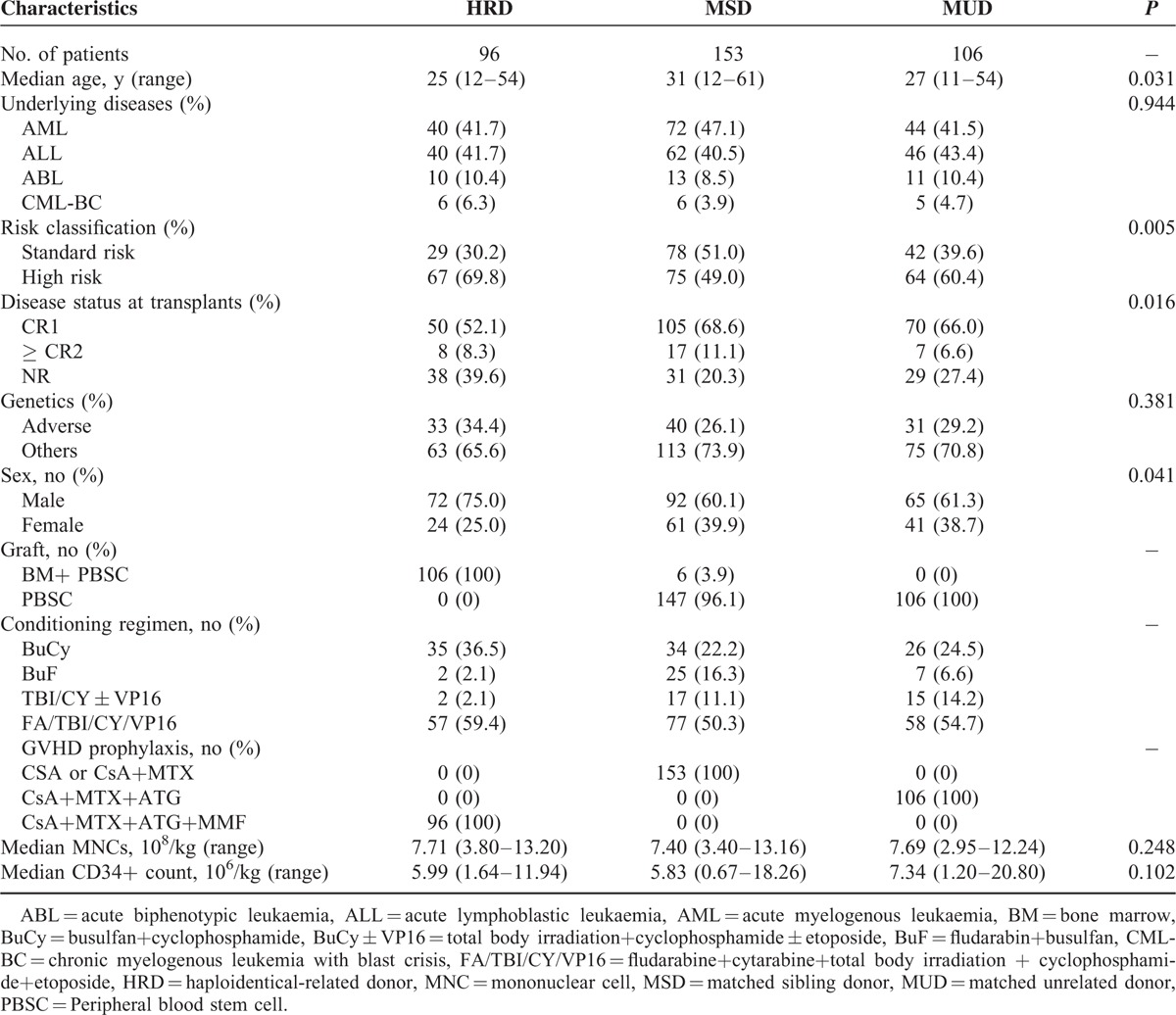
Patient, donor, and transplant characteristics are summarized in Table 1. There were significantly different among the 3 groups in the category of patient age (P = 0.031), patient sex (P = 0.041), disease status at transplantation (P = 0.016), and risk classification (P = 0.005). Compared with MSD and MUD, more patients were high risk in the HRD group.
TABLE 1.
Patients and Transplant Characteristics
Engraftment
Analyses of chimerism showed that all patients achieved full donor chimerism by day +30 post-transplantation except for 2 patients who died of graft rejection and intracranial hemorrhage in HRD and MUD groups, respectively. Of the 353 evaluable patients, median time of neutrophil reconstruction were 13 days (range, 9–47 days), 11 days (range, 8–41 days), and 12 days (range, 9–55 days) in HRD, MSD, and MUD groups (P < 0.001), respectively, and it was faster in the MSD group than in HRD and MUD groups (P < 0.001; P = 0.030, respectively). The median time of platelet engraftment were 15 days (range, 10–90 days), 12 days (range, 9–43 days), and 13 days (range, 9–63 days) in HRD, MSD, and MUD groups (P < 0.001), respectively, and it was significantly faster in MSD and MUD groups than in the HRD group (P = 0.001; P = 0.019, respectively).
EBV and CMV Infections
The 1-year cumulative incidence of EBV viremia were 44.8% ± 5.1%, 14.4% ± 2.8%, and 45.3% ± 4.8%, in HRD, MSD, and MUD groups, and it was higher in HRD and MUD groups than in the MSD group (P < 0.001, P < 0.001,respectively). The 3-year cumulative incidence of EBV-associated diseases were 16.7% ± 3.8%, 2.0% ± 1.3%, and 17.9% ± 3.7% in HRD, MSD, and MUD groups, and it was higher in HRD and MUD groups than in the MSD group (P < 0.001, P < 0.001,respectively). But the incidences of EBV viremia and EBV-associated diseases were comparable between HRD and MUD groups (P = 0.670, P = 0.778, respectively). Eleven patients died of EBV-associated diseases (n = 6, 1, and 4 in HRD, MSD, and MUD groups, respectively). The 3-year cumulative mortality of EBV-associated diseases were 7.3% ± 2.9%, 1.8% ± 1.7%, and 5.0% ± 2.5% among 3 groups respectively (P = 0.008 for MSD vs HRD, P = .054 for MSD vs MUD, and P = .468 for MUD vs HRD).
The 1-year cumulative incidence of CMV viremia were 71.9% ± 4.6%, 38.6% ± 3.9%, and 49.1% ± 4.9%, respectively, in HRD, MSD, and MUD groups. Compared with MSD, the incidences of CMV viremia were significantly higher in HRD and MUD groups (P < 0.001 for MSD vs HRD, P = 0.045 for MSD vs MUD and p = .001 for MUD vs HRD). However, 3 year CMV-associated diseases were 6.2% ± 2.5%, 4.6% ± 1.7% and 5.7% ± 2.2%, respectively, in HRD, MSD, and MUD groups (P = 0.829). Eight patients died of CMV-associated diseases (n = 2, 3, and 3 in HRD, MSD, and MUD groups, respectively). The 3-year cumulative mortality of CMV-associated diseases were 2.3% ± 1.3%, 2.2% ± 1.6% and 3.2% ± 1.9% among 3 groups, respectively (P = 0.851).
Prophylactic DLI and GVHD
According to the criteria aforementioned, a total of 157 doses of prophylactic DLI were administered in 99 patients, including 24 (25.0%), 52 (34.0%), and 23 (21.7%), respectively, in HRD, MSD, and MUD groups (P = 0.073), with median doses of 1 (range, 1–4) per patient in HRD group, and also 1 (range, 1–4) in another 2 groups (P = 0.491).
Grade II to IV aGVHD occurred in 135 cases, including 24 cases after prophylactic DLI. After ruling out the effects of DLI, the cumulative incidences of grade II to IV GVHD by day +100 were 40.6% ± 5.0%, 23.5% ± 3.4% and 34.0% ± 4.6%, respectively, in HRD, MSD, and MUD groups. It was higher in these patients undergoing HRD and MUD compared with those undergoing MSD transplant (P = 0.002 for HRD vs MSD; P = 0.049 for MUD vs MSD), and was not different between HRD and MUD transplants (P = 0.301). The incidences of grade III to IV aGVHD were 11.1% ± 3.5%, 7.8% ± 2.3%, and 10.5% ± 3.2%, respectively, in HRD, MSD, and MUD groups (P = 0.590).
One hundred and fifty-one of 329 patients surviving >100 days developed cGVHD, including 89 cases after prophylactic DLI. The overall cumulative incidences of cGVHD at 2 years were 52.3% ± 5.3%, 43.2% ± 4.1%, and 44.2% ± 5.1% (P = 0.409), and extensive cGHVD were 21.7% ± 5.4%, 17.8% ± 3.5%, and 17.8% ± 4.5%, respectively, in HRD, MSD, and MUD groups (P = 0.948). After ruling out the effects of DLI, the 2-year cumulative incidences of cGVHD were 29.5% ± 4.9%, 24.0% ± 3.5%, and 29.5% ± 4.7%, respectively, in HRD, MSD, and MUD groups (P = 0.538).
The multivariate analysis showed that alternative donors had adverse impact on the risk of grades II to IV aGVHD (for HRD: P = 0.004, RR = 1.978; for MUD: P = 0.039, RR = 1.665), but had no effect on the risk of grades III to IV aGVHD (P = 0.314). aGVHD (P = 0.006, RR = 1.598) and DLI (P < 0.001, RR = 1.934) were the risk factors for cGVHD.
Relapse
Seventy-six patients experienced relapse at a median time of 172 days (range 29–1064) post-transplantation, as a result of HRD in 148 days (range 29–1051), MSD in 187 days (range 46–1064), and MUD in 152 days (range 49–783). The 5-year cumulative incidences of relapse were 19.2% ± 4.9%, 26.8% ± 4.0%, and 23.0% ± 4.7%, respectively, in HRD, MSD, and MUD groups (P = 0.419; Figure 1A).
FIGURE 1.
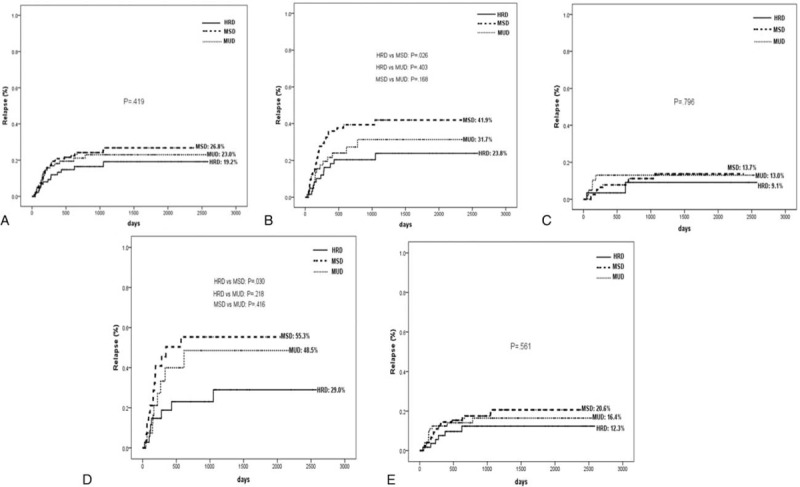
Relapse rates at 5 years after transplantation stratified according to the donor types: (A) all patients, (B) high-risk patients, (C) standard-risk patients, (D) patients in NR, (E) patients in CR. CR = complete remission, NR = nonremission.
The relapse rate, according to risk classification, is shown in Figure 1 (panels B, C, D, and E, respectively). Of 206 high-risk patients, 41.9% experienced relapse in the MSD group, as did 31.7% in the MUD group, but the incidence decreased to 23.8% in the HRD group (MSD vs HRD, P = 0.026; MUD vs HRD, P = 0.403; MSD vs MUD, P = 0.168; Figure 1B). Of 98 patients in NR, the relapse rate in HRD transplant was also significantly lower than in MSD transplant (29.0% vs 55.3%, P = 0.030), both of which were not statistically different compared to relapse rate of 48.5% in MUD transplant (P = 0.218, P = 0.416, respectively; Figure 1D). For standard-risk patients, relapse rates were not significantly different among 3 donors (Figure 1).
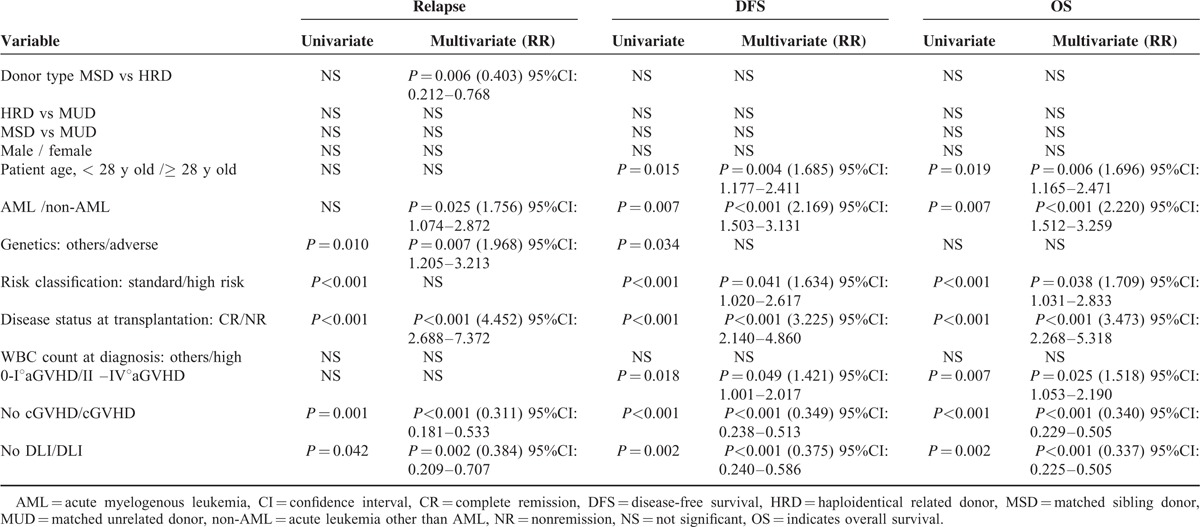
In multivariate analysis for relapse (Table 2), non-AML (P = 0.025, RR = 1.756), NR at transplantation (P < 0.001, RR = 4.452), and adverse genetics (P = 0.007, RR = 1.968) were independently risk factors. HRD had beneficial impact on relapse (for MSD: P = 0.006, RR = 0.403). DLI (P = 0.002, RR = 0.384) and cGVHD (P < 0.001, RR = 0.311) were also protective factors for relapse.
TABLE 2.
Univariate and Multivariate Analysis for Relapse DFS and OS
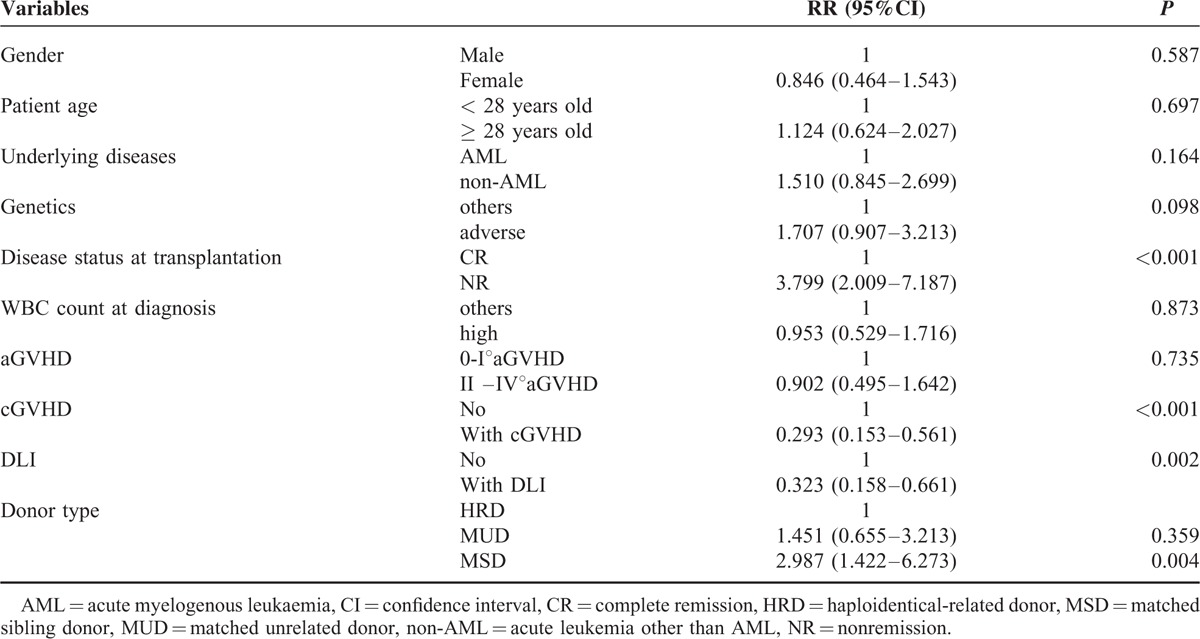
Furthermore, in multivariate analysis for high-risk leukemia (Table 3), HRD (for MSD: P = 0.004, RR = 2.978), cGVHD (P < 0.001, RR = 0.293), and receiving DLI (P = 0.002, RR = 0.323) were protective factors for relapse. NR at transplantation (P < 0.001, RR = 3.799) was the only risk factor. Patient age, underlying diseases, and genetics were not significantly affecting factors for high-risk leukemia relapse.
TABLE 3.
Multivariate Analysis for High-Risk Leukemia Relapse
Survival
One hundred and twenty-three patients were dead at a median follow-up of 22.1 months (range, 0.1–83.1 months) post-transplantation. Causes of death included relapse (n = 56), infectious diseases (n = 35, including 6 EBV-associated PTLD), aGVHD (n = 18, including 2 after prophylactic DLI), cGVHD (n = 6, including 2 after DLI), multiple organ failure (n = 2), hepatic veno-occlusive disease (n = 1), thrombotic microangiopathy (n = 1), hemorrhagic cystitis (n = 1), intracranial hemorrhage (n = 2), and graft rejection (n = 1).
A total of 67 patients died of TRM. Infections were main causes of TRM, as a result of 15 infection-related deaths in HRD, 13 in MUD, and 7 in MSD (P = 0.004 for HRD vs MSD; P = 0.046 for MUD vs MSD). The 5-year cumulative incidences of TRM were significantly lower in MSD transplant compared with those in HRD (17.3% vs 26.4%, P = 0.041), and MUD transplants (17.3% vs 24.1%, P = 0.037; Figure 2), whereas the patients between HRD and MUD groups had comparable TRM rates (P = 0.978).
FIGURE 2.
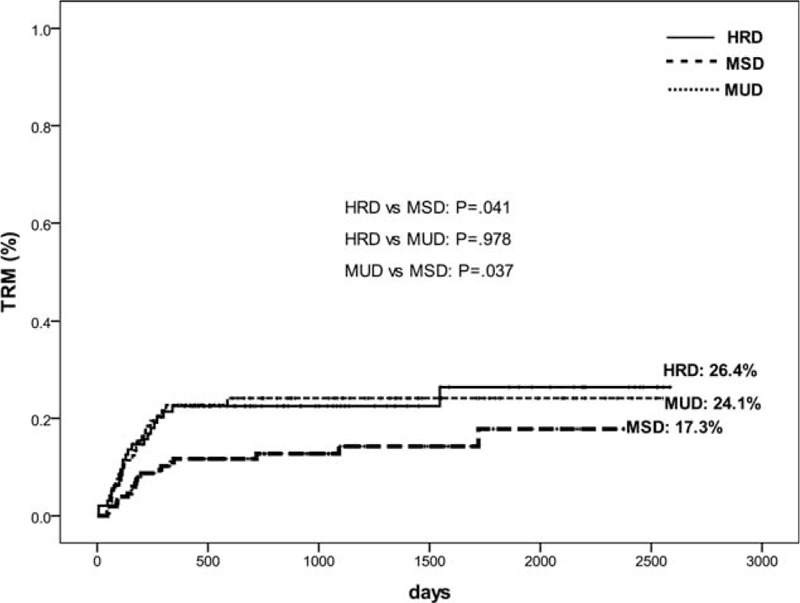
Cumulative incidence of TRM stratified according to the donor types for all patients.
The 5-year cumulative incidences of OS post-transplantation were 60.4% ± 5.7%, 64.6% ± 4.7%, and 61.0% ± 4.9% (P = 0.371; Figure 3A), and the 5-year cumulative incidences of DFS were 59.6% ± 5.9%, 58.8% ± 4.8%, and 54.9% ± 5.0% (P = 0.423; Figure 4A) in HRD, MSD, and MUD groups, respectively. OS and DFS, according to risk classification, are shown in Figure 3 and Figure 4, respectively.
FIGURE 3.
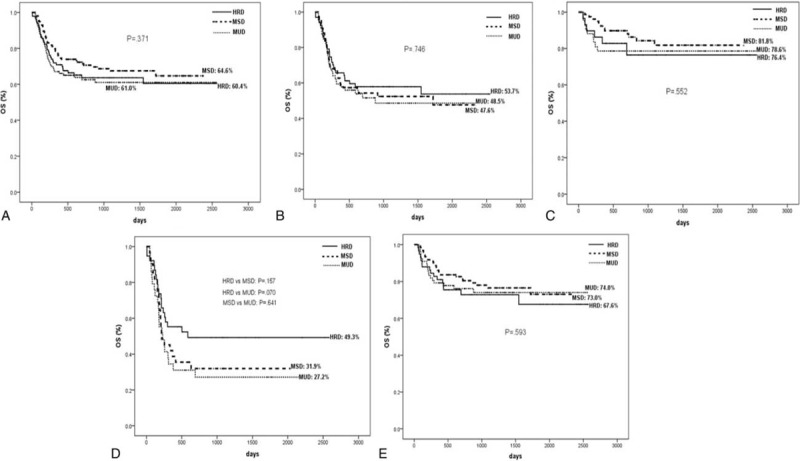
OS at 5 years after transplantation stratified according to the donor types: (A) all patients, (B) high-risk patients, (C) standard-risk patients, (D) patients in NR, (E) patients in CR. CR = complete remission, NR = nonremission.
FIGURE 4.
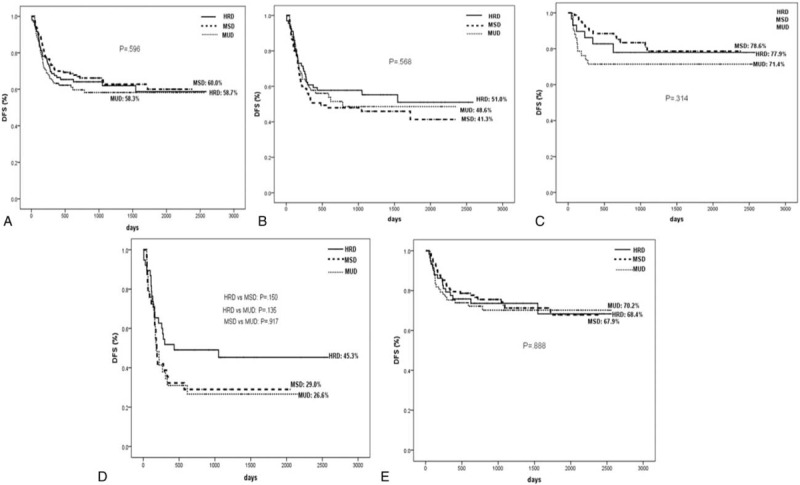
DFS at 5 years after transplantation stratified according to the donor types: (A) all patients, (B) high-risk patients, (C) standard-risk patients, (D) patients in NR, (E) patients in CR. CR = complete remission, DFS = disease-free survival, NR = nonremission.
Risk factors for survival are presented in Table 2. In multivariate analysis for OS, NR at transplantation (P < 0.001, RR = 3.473), high-risk (P = 0.038, RR = 1.709), non-AML (P < 0.001, RR = 2.220), grade II to IV aGVHD (P = 0.025, RR = 1.518), and older age (P = 0.006, RR = 1.696) were independently risk factors, cGVHD (P < 0.001, RR = 0.340) and DLI (P < 0.001, RR = 0.337) were beneficial factors.
DISCUSSION
With technical advances in HLA typing, GVHD prophylaxis and supportive care, alternative donors such as MUD and HRD have been widely used. Many studies had demonstrated that outcomes using MUD approached those using MSD,19–22 but it remains controversial whether HRD should be used on an equal basis to MUD or MSD.10–12,23 The recent reports from Bashey et al and Luo et al suggested that HRD transplant performed using TCR grafts result in outcomes equivalent to MUD and MSD transplants for hematologic malignancies.8,9 In this report, we performed HRD transplant by using G-CSF primed T-cell-replete peripheral blood stem cells and bone marrow mixed grafts in combination with ATG to deplete T lymphocytes in vivo.24 The results also suggested that HRD transplant achieved comparable OS and DFS compared with MUD and MSD transplants.
A major obstacle to success for HRD transplant is high incidence and mortality of GVHD.25,26 But growing improvement has been made in the past few years in the prophylaxis of GVHD, particularly the use of ex vivo4,27 and in vivo T-cell-depletion (TCD).8,24 In the mode of ex vivo TCD, investigators in Perugia, Italy achieved the best aGVHD and cGVHD rates of around 7.8% and 5.2% by using “megadoses” of CD34+ stem cells. But delayed immune reconstruction, high relapse and TRM in this technique remain major problems.4,5,28 To overcome the above weaknesses, they recently developed a novel strategy using ex vivo T-cell-depleted stem cells coinfused with donor-derived T regulatory cells and conventional T-cells, which protected recipients against GVHD and made relapse rate post-transplantation decrease remarkably.29 There are 2 major methods to in vivo TCD by using CY or ATG. Investigators at Johns Hopkins University developed an approach using TCR bone marrow grafts in combination with post-transplantation high-dose CY to deplete selectively alloreactive T-cells in vivo.30 They and other researchers reported acceptable grade II to IV aGVHD of around 30%, III to IV aGVHD of 10% and cGVHD of 35%,8,30–32 which were not inferior to MSD and MUD transplants.8,32 Investigators in China mainly focused on TCR by using ATG to deplete T lymphocytes in vivo. Huang et al at Peking university reported that 756 leukemia patients underwent HRD transplant achieved 14% of grades III to IV aGVHD and 23% of extensive cGVHD by using G-CSF primed PBSC and BM mixed grafts combined with ATG.24 Despite higher incidences of grades II to IV aGVHD in patients undergoing HRD transplant, grade III to IV aGVHD and cGVHD rates were equivalent to those undergoing MSD or MUD transplants.13,23 Luo et al at Zhejiang university recently reported that HRD transplant performed using G-CSF primed PBSC grafts and low-dose ATG achieved 17.2% of grade III to IV aGVHD and 41.4% of cGVHD, comparable to those in MUD transplant.9 In this study, a similar GVHD rate was obtained to those of TCR transplant using ATG9,13,23 and comparable incidences of grade III to IV aGVHD and cGVHD were observed among 3 donors.
Leukemia relapse remains the major cause of transplant failure. Many factors are correlated with relapse, such as donor sources, underlying diseases, disease status at transplantation, genetics, and conditioning regimens. Some studies had suggested that MUD had lower relapse than MSD because of stronger GVL effect.20–22 However, a recent report33 from Center for International Blood and Marrow Transplant Research (CIBMTR), which assessed 4099 leukemia patients undergoing a myeloablative transplant from MUD or MSD, demonstrated that MUD transplant did not have a more potent GVL effect than MSD transplant. It is also debatable whether HRD might have a stronger GVL effect than MUD or MSD.8,9,10,23,32,34 Burroughs et al and Bashey et al suggested that relapse rates were not significantly different among 3 donors for relapsed/refractory Hodgkin lymphoma and hematologic malignancies, respectively.8,32 On the other hand, Wang et al and Luo et al demonstrated that HRD can achieve a stronger GVL effect than MSD or MUD for high-risk acute leukemia.9,34 Ottinger et al and Huang et al reported a lower relapse rate in HRD compared to MSD and MUD, respectively, for early stage and standard-risk hematologic malignancies.10,23 In this article, relapse rates were not significantly different among 3 donors for standard-risk acute leukemia. For high-risk leukemia, the relapse rate was not significantly different between MUD and MSD, but was lower in HRD compared to MSD, especially for patients in NR. Furthermore, multivariate analysis showed that HRD had beneficial impact on relapse.
In HRD transplant mode of TCR grafts combined with T-cell depletion in vivo, the lower TRM rate was observed compared to TCD transplant.35 And several reports showed that the TRM rate of HRD transplant was not higher than conventional MSD or MUD transplants by using CY post-transplantation or ATG.8,13,23,32 On the contrary, Luo et al reported that TRM in HRD and MUD transplants was comparable, but higher than MSD transplant by using ATG for GVHD prophylaxis.9 In this report, MSD transplant had lower TRM compared with HRD and MUD transplant, whereas TRM was comparable in HRD and MUD. TRM was affected by complex factors such as donors, grafts, conditioning, GVHD prophylaxis, and transplantation experience. Our higher TRM was mainly associated with higher infection-related mortality in HRD and MUD compared to MSD. A reasonable interpretation of our result might be the use of ATG, which can lead to delay in immune recovery and increase the risk of infections, especially viral infections.15,36,37
Prophylactic DLI is proved to be effective in reducing leukemia relapse, especially in the low residual tumor burden.38,39 In this study, for preventing relapse, early G-CSF mobilized DLI were administered in patients who were in NR pretransplantation or whose MRD was positive post-transplantation. Multivariate analysis revealed that DLI was a favorable factor for reducing relapse and improving survival. Meanwhile, we observed that DLI had acceptable incidence and lethality of GVHD, which were consistent with our previous multicenter study reported.40 Our results suggested that early prophylactic DLI was safe and effective for preventing relapse from these 3 donors.
In conclusion, our results suggest that HRD is an alternative choice for acute leukemia undergoing transplant when an MSD is not available and results in outcomes equivalent to those of MSD and MUD transplants by the strategy of using T-cell-replete grafts combined with ATG to deplete T-cell in vivo. HRD might carry a superior GVL effect compared to MSD for high-risk acute leukemia.
Footnotes
Abbreviations: ABL = acute biphenotypic leukaemia, ALL = acute lymphoblastic leukaemia, allo-HSCT = allogeneic hematopoietic stem cell transplantation, AML = acute myelogenous leukaemia, BM = bone marrow, CML-BC = chronic myelogenous leukemia with blast crisis, CR = complete remission, DFS = disease-free survival, DLI = Donor lymphocyte infusion, GVL = graft versus leukaemia, HRD = haploidentical-related donor, MNC = mononuclear cell, MRD = minimal residual disease, MSD = matched sibling donor, MUD = matched unrelated donor, NR = nonremission, OS = overall survival, PBSC = peripheral blood stem cell, TCR = T-cell-replete
Authorship: SJY performed investigations, analyzed data, and wrote the paper; QF performed investigations, analyzed data; JS, FH, ZPF, HSZ, and XL analyzed data; YZ, MD, HL, and QLJ performed investigations; QFL designed the study and wrote the paper. All authors read and approved the final manuscript.
Funding: the study was supported by grants from the National Natural Science Foundation of China (No.U1401221; 81470349; 81401315; 81300445; 81270647) and Science and Technology Planning Project of Guangdong Province (No. 2014B020226004).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Karanes C, Nelson GO, Chitphakdithai P, et al. Twenty years of unrelated donor hematopoietic cell transplantation for adult recipients facilitated by the National Marrow Donor Program. Biol Blood Marrow Transplant 2008; 14 (9 Suppl):8–15. [DOI] [PubMed] [Google Scholar]
- 2.Schetelig J, Bornhauser M, Schmid C, et al. Matched unrelated or matched sibling donors result in comparable survival after allogeneic stem-cell transplantation in elderly patients with acute myeloid leukemia: a report from the cooperative German Transplant Study Group. J Clin Oncol 2008; 26:5183–5191. [DOI] [PubMed] [Google Scholar]
- 3.Yakoub-Agha I, Mesnil F, Kuentz M, et al. Allogeneic marrow stem-cell transplantation from human leukocyte antigen-identical siblings versus human leukocyte antigen-allelic-matched unrelated donors (10/10) in patients with standard-risk hematologic malignancy: a prospective study from the French Society of Bone Marrow Transplantation and Cell Therapy. J Clin Oncol 2006; 24:5695–5702. [DOI] [PubMed] [Google Scholar]
- 4.Aversa F, Berneman ZN, Locatelli F, et al. Fourth International Workshop on Haploidentical Transplants, Naples, Italy, July 8–10, 2004. Blood Cells Mol Dis 2004; 33:159–175. [DOI] [PubMed] [Google Scholar]
- 5.Aversa F, Terenzi A, Tabilio A, et al. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol 2005; 23:3447–3454. [DOI] [PubMed] [Google Scholar]
- 6.Lv M, Huang XJ. Allogeneic hematopoietic stem cell transplantation in China: where we are and where to go. J Hematol Oncol 2012; 5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luznik L, O’Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant 2008; 14:641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bashey A, Zhang X, Sizemore CA, et al. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol 2013; 31:1310–1316. [DOI] [PubMed] [Google Scholar]
- 9.Luo Y, Xiao H, Lai X, et al. T-cell-replete haploidentical HSCT with low-dose anti-T-lymphocyte globulin compared with matched sibling HSCT and unrelated HSCT. Blood 2014; 124:2735–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ottinger HD, Ferencik S, Beelen DW, et al. Hematopoietic stem cell transplantation: contrasting the outcome of transplantations from HLA-identical siblings, partially HLA-mismatched related donors, and HLA-matched unrelated donors. Blood 2003; 102:1131–1137. [DOI] [PubMed] [Google Scholar]
- 11.Kanda Y, Chiba S, Hirai H, et al. Allogeneic hematopoietic stem cell transplantation from family members other than HLA-identical siblings over the last decade (1991–2000). Blood 2003; 102:1541–1547. [DOI] [PubMed] [Google Scholar]
- 12.Drobyski WR, Klein J, Flomenberg N, et al. Superior survival associated with transplantation of matched unrelated versus one-antigen-mismatched unrelated or highly human leukocyte antigen-disparate haploidentical family donor marrow grafts for the treatment of hematologic malignancies: establishing a treatment algorithm for recipients of alternative donor grafts. Blood 2002; 99:806–814. [DOI] [PubMed] [Google Scholar]
- 13.Lu DP, Dong LJ, Wu T, et al. Conditioning including antithymocyte globulin followed by unmanipulated HLA-mismatched/haploidentical blood and marrow transplantation can achieve comparable outcomes with HLA-identical sibling transplantation. Blood 2006; 107:3065–3073. [DOI] [PubMed] [Google Scholar]
- 14.Liu QF, Fan ZP, Wu MQ, et al. Allo-HSCT for acute leukemia of ambiguous lineage in adults: the comparison between standard conditioning and intensified conditioning regimens. Ann Hematol 2013; 679–687. [DOI] [PubMed] [Google Scholar]
- 15.Xuan L, Huang F, Fan ZP, et al. Effects of intensified conditioning on Epstein–Barr virus and cytomegalovirus infections in allogeneic hematopoietic stem cell transplantation for hematological malignancies. J Hematol Oncol 2012; 5:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu QF, Liu C, Zhang Y, et al. Peripheral blood stem cell transplantation compared with bone marrow transplantation from unrelated donors in patients with leukemia: a single institutional experience. Blood Cells Mol Dis 2010; 45:75–81. [DOI] [PubMed] [Google Scholar]
- 17.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995; 15:825–828. [PubMed] [Google Scholar]
- 18.Horwitz ME, Sullivan KM. Chronic graft-versus-host disease. Blood Rev 2006; 20:15–27. [DOI] [PubMed] [Google Scholar]
- 19.Woolfrey A, Lee SJ, Gooley TA, et al. HLA-allele matched unrelated donors compared to HLA-matched sibling donors: role of cell source and disease risk category. Biol Blood Marrow Transplant 2010; 16:1382–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horowitz MM. Does matched unrelated donor transplantation have the same outcome as matched sibling transplantation in unselected patients? Best Pract Res Clin Haematol 2012; 25:483–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho VT, Kim HT, Aldridge J, et al. Use of matched unrelated donors compared with matched related donors is associated with lower relapse and superior progression-free survival after reduced-intensity conditioning hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2011; 17:1196–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saber W, Opie S, Rizzo JD, et al. Outcomes after matched unrelated donor versus identical sibling hematopoietic cell transplantation in adults with acute myelogenous leukemia. Blood 2012; 119:3908–3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao-Jun X, Lan-Ping X, Kai-Yan L, et al. Partially matched related donor transplantation can achieve outcomes comparable with unrelated donor transplantation for patients with hematologic malignancies. Clin Cancer Res 2009; 15:4777–4783. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Liu DH, Liu KY, et al. Long-term follow-up of haploidentical hematopoietic stem cell transplantation without in vitro T cell depletion for the treatment of leukemia: nine years of experience at a single center. Cancer 2013; 119:978–985. [DOI] [PubMed] [Google Scholar]
- 25.Beatty PG, Clift RA, Mickelson EM, et al. Marrow transplantation from related donors other than HLA-identical siblings. N Engl J Med 1985; 313:765–771. [DOI] [PubMed] [Google Scholar]
- 26.Szydlo R, Goldman JM, Klein JP, et al. Results of allogeneic bone marrow transplants for leukemia using donors other than HLA-identical siblings. J Clin Oncol 1997; 15:1767–1777. [DOI] [PubMed] [Google Scholar]
- 27.Di Ianni M, Falzetti F, Carotti A, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood 2011; 117:3921–3928. [DOI] [PubMed] [Google Scholar]
- 28.Aversa F. Haploidentical haematopoietic stem cell transplantation for acute leukaemia in adults: experience in Europe and the United States. Bone Marrow Transplant 2008; 41:473–481. [DOI] [PubMed] [Google Scholar]
- 29.Martelli MF, Di Ianni M, Ruggeri L, et al. HLA-haploidentical transplantation with regulatory and conventional T-cell adoptive immunotherapy prevents acute leukemia relapse. Blood 2014; 124:638–644. [DOI] [PubMed] [Google Scholar]
- 30.O’Donnell PV, Luznik L, Jones RJ, et al. Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using posttransplantation cyclophosphamide. Biol Blood Marrow Transplant 2002; 8:377–386. [DOI] [PubMed] [Google Scholar]
- 31.Solomon SR, Sizemore CA, Sanacore M, et al. Haploidentical transplantation using T cell replete peripheral blood stem cells and myeloablative conditioning in patients with high-risk hematologic malignancies who lack conventional donors is well tolerated and produces excellent relapse-free survival: results of a prospective phase II trial. Biol Blood Marrow Transplant 2012; 18:1859–1866. [DOI] [PubMed] [Google Scholar]
- 32.Burroughs LM, O’Donnell PV, Sandmaier BM, et al. Comparison of outcomes of HLA-matched related, unrelated, or HLA-haploidentical related hematopoietic cell transplantation following nonmyeloablative conditioning for relapsed or refractory Hodgkin lymphoma. Biol Blood Marrow Transplant 2008; 14:1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ringdén O, Pavletic SZ, Anasetti C, et al. The graft-versus-leukemia effect using matched unrelated donors is not superior to HLA-identical sibings for hematopoietic stem cell transplantation. Blood 2009; 113:3110–3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Liu DH, Xu LP, et al. Superior graft-versus-leukemia effect associated with transplantation of haploidentical compared with HLA-identical sibling donor grafts for high-risk acute leukemia: an historic comparison. Bone Marrow Transplant 2011; 821–830. [DOI] [PubMed] [Google Scholar]
- 35.Ciurea SO, Mulanovich V, Saliba RM, et al. Improved early outcomes using a T cell replete graft compared with T cell depleted haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2012; 18:1835–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Esser JW, van der Holt B, Meijer E, et al. Epstein–Barr virus (EBV) reactivation is a frequent event after allogeneic stem cell transplantation (SCT) and quantitatively predicts EBV-lymphoproliferative disease following T-cell—depleted SCT. Blood 2001; 98:972–978. [DOI] [PubMed] [Google Scholar]
- 37.Wu XL, Liu QF. Epstein–Barr virus-associated diseases in allogeneic hematopoietic stem cell transplantation. J Hematol Oncol 2012; 5 Suppl 1:A8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan CH, Liu DH, Liu KY, et al. Risk stratification-directed donor lymphocyte infusion could reduce relapse of standard-risk acute leukemia patients after allogeneic hematopoietic stem cell transplantation. Blood 2012; 119:3256–3262. [DOI] [PubMed] [Google Scholar]
- 39.Huang XJ, Wang Y, Liu DH, et al. Modified donor lymphocyte infusion (DLI) for the prophylaxis of leukemia relapse after hematopoietic stem cell transplantation in patients with advanced leukemia—feasibility and safety study. J Clin Immunol 2008; 28:390–397. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Liu DH, Fan ZP, et al. Prevention of relapse using DLI can increase survival following HLA-identical transplantation in patients with advanced-stage acute leukemia: a multicenter study. Clin Transplant 2012; 26:635–643. [DOI] [PubMed] [Google Scholar]


