Supplemental Digital Content is available in the text
Abstract
We carried out the study to investigate and quantitatively assess the potential association between current level of physical activity and the risk of osteoporosis hip fracture in older women.
Relevant publications before October 2015 were identified using the PubMed and Ovid searching tools. A dose–response meta-analysis was carried out to combine and analysis results. Fourteen prospective studies were included in the meta-analysis. A general analysis of 9 studies showed a significant inverse relationship between increasing level of physical activity and risk of hip fracture in older women [relative risk (RR) = 0.93, 95% confidence interval (95% CI): 0.91–0.96]. The result of a sensitivity analysis was consistent with the general analysis (RR = 0.94, 95% CI: 0.93–0.96). The association between increasing level of physical activity and risk of wrist fracture was not statistically significant in a general analysis of three studies (RR = 1.004, 95% CI: 0.98–1.03). A potential direct association between increasing level of physical activity and risk of wrist fracture was observed after removing 1 study with the greatest weight (RR = 1.01, 95% CI: 1.00–1.03). No significant publication bias was observed in our analysis.
Our results show that increasing level of physical activity within an appropriate range may reduce the risk of hip fracture but not the risk of wrist fracture in older women.
INTRODUCTION
The occurrence of fragility fractures among older adults is an important public health concern because of the associated high morbidity and heavy social burden.1–3 The incidence and medical expense is predicted to increase rapidly over the coming decades.4 Moreover, population aging is likely to further escalate this condition. Women aged 45 years or older are considered more vulnerable to fragility fractures due to the potential osteoporosis and obesity after menopause.5,6 Therefore, the prediction and prevention of fragility fractures is a particular priority for older women.
Physical activity is a simple and inexpensive way to gain health. The American Center for Disease Control and Prevention has recommended that individuals undertake a regime of aerobic and muscle-strengthening physical activity.7,8 Physical activity is also a therapeutic tool against pre- and postmenopausal loss of bone density. In recent decades, the association between physical activity and bone health in older adults has aroused particular attention. It is reported that bone mineral density (BMD) could be improved through an appropriate amount of physical activity in the older population. In the Osteoporosis Risk Factor and Prevention Study, Rikkonen et al9 found that regular physical activity significantly decreased proximal femur bone loss among older women after 15 years of follow-up. In addition, the results of several meta-analyses have also shown that BMD could benefit from physical activity.10 However, long-term weight-bearing or excessive exercise may lead to stress fractures, especially in older women with osteoporosis.5,11 Several prospective studies have investigated the association between physical activity and the risk of fracture in older women. However, the relationship between physical activity and the risk of fragility fracture varies according to level of physical activity and fracture site.12–16
We conducted a dose–response meta-analysis to assess the association between level of physical activity and risk of fracture in older women. A subgroup analysis was also performed to investigate the relationship between level of physical activity and risk of fracture in a predetermined site.
METHODS
Literature Search
We carried out a literature search using the PubMed (MEDLINE) and Ovid (Embase and Cochrane library) searching tools. The search was restricted to prospective cohort studies investigating the association between physical activity and risk of fracture in older women, published between January 1990 and October 2015. The following key words and heading terms were used in the PubMed search: “(bone fracture) AND ((physical activity) OR exercise) AND women.” Similar heading terms were used in the Ovid search, with restrictions to the Embase and Cochrane Library. Furthermore, reference lists of all included studies were scrutinized. We followed the standard criteria for conducting and reporting a meta-analysis of observational studies.17 All analyses were based on previous published studies; thus, patient consent and ethical approval were not required.
Study Selection Criteria
Studies were included if they met the following criteria: prospective cohort study; investigated the association between physical activity and risk of fracture in older women; size effect evaluated using relative risk (RR), odds ratio (OR), or hazard ratio (HR) values with 95% confidence intervals (CIs), or sufficient data provided to calculate these values; and physical activity divided into at least 3 quantitative categories. In addition, studies conducted in a population with exposure to chronic disease (excluding osteoporosis) or drug consumption were excluded. If several studies were conducted in the same population, publications with the most relevant information and longest period of follow-up were included. The final list of included studies was obtained from the combined searches and selections of 2 independent investigators (KR and XFY).
Data Extraction and Quality Assessment
Data were extracted independently by 2 investigators (KR and XYL using a standard-format table). The following information was extracted from each study: author's name, publication year, published journal, study design, geographic region, age at baseline, level of physical activity, sample size (women only), number of years of follow-up, adjusted covariates, endpoint outcomes, endpoint case ascertainment, and RR, HR, or OR with 95% CIs for each physical-activity level. The number of person-years was extracted directly or calculated using the follow-up time provided and the number of participants quoted in the publication.
According to the Newcastle–Ottawa Scale,18 a 12-score grading system was used in the quality assessment, principally across the following 4 domains: selection of participants; exposure measurement; study design and control of potential bias; and comprehensive assessment of outcomes and follow-up. Studies scored greater than 9 were considered to be of “good” quality in our meta-analysis. RK and XFY evaluated the quality score independently, and the mean value was used as the final score.
Statistical Analysis
RRs of the included studies were used as the effect size in the meta-analysis. Given that the absolute risk of fragility fracture is relatively low in older women,12,19,20 HRs and ORs were deemed equivalent to RRs in the analysis. RRs, HRs, or ORs were standardized using the effective count method proposed by Hamling et al21 to make the group with the lowest physical-activity level the reference group in each study. Women-specific RRs were extracted from general searches.12,16,22–25 For publications that did not present stratified RRs in the female population, a binary logistic model was used to estimate stratified RRs using the fracture case and person-years.
For each study included, we calculated the study-specific slopes across increasing physical-activity level with 95% CIs using a liner regression model proposed by Greenland and Longnecker.26 We derived the RR value for every 3 units of increased physical activity for risk of fracture based on the liner model. The forest plot and analysis of publication bias was based on the derived RRs.
Potential heterogeneity was estimated using the Cochran Q and I2 statistics. A P value ≤0.1 was considered to indicate significant heterogeneity in the Q-test. The I2 statistics, which represent the total variation contributed by included studies, was used to assist the heterogeneity estimation. I2 > 75% was considered to indicate significant heterogeneity. A fixed-effect model (Mantel–Haenszel method) was used when the heterogeneity was not considered to be significant; otherwise, the random-effect model (DerSimonian–Laird method) was used in the analysis.
Publication bias was evaluated using the Begg test and Egger linear regression.27 A 2-sided P-value <0.05 was considered to be statistically significant. A funnel plot was presented in the final analysis.
RESULTS
Literature Search
Figure 1 shows the process of literature search and selection using a flow chart. We identified 1273 potential articles in PubMed and 3180 articles in Ovid (Embase and Cochran library) published between January 1990 and October 2015. After removal of duplicates and screening of the general criteria, 352 articles remained for further review. A total of 321 papers were excluded after a review of the abstract due to a small sample size, retrospective study design, or exposure to chronic disease and medicine, leaving 31 potential articles for full-text review. After a full-text evaluation of the 31 articles, 14 papers were finally included in our meta-analysis. Six papers were excluded as repeated reports of the same population and 11 papers were excluded due to lack of sufficient data or for not reporting the RR for men and women separately.
FIGURE 1.
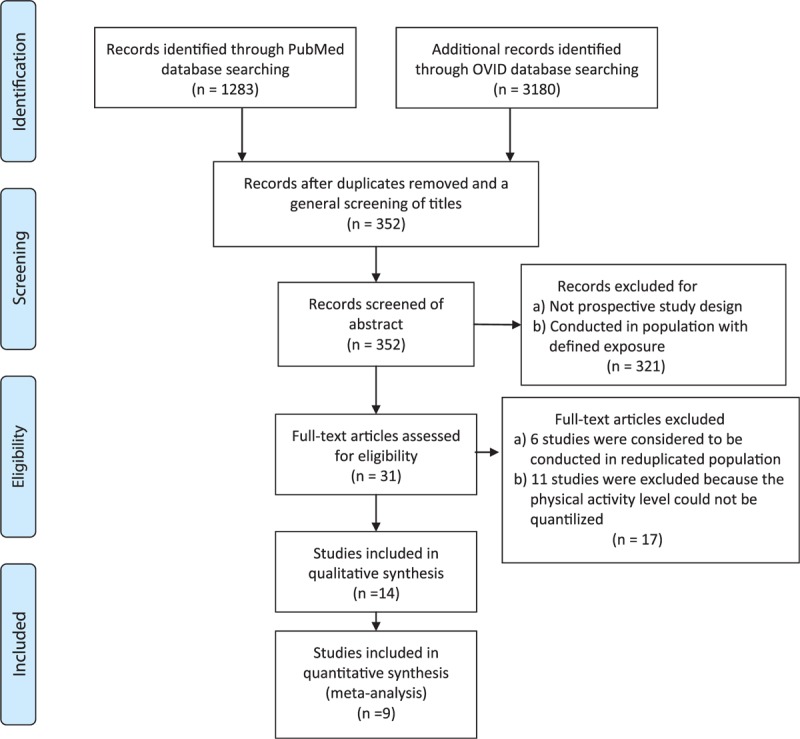
Selection of studies for inclusion in the dose–response meta-analysis.
Study Characteristics and Quality Assessment
Tables 1 and 2 present the characteristics and data extracted from the 14 included studies, 13 of which were of prospective cohort design and 1 that was considered to be a prospective nested case–control study. Data from 9 studies were finally analyzed in the dose–response meta-analysis. Data from the rest 5 papers are presented in Tables 1 and 2 but not included in the final analyses, because the investigated fracture sites were rarely reported in other literature and the information was insufficient for combined analysis. All included articles were published from 2002 to 2012, including 28,871 fracture cases and 1,380,285 participants (women only). The total observation time was estimated to be 12,127,159 person-years. Hip fracture and wrist fracture were 2 most common fractures in the cohort follow-up. Nine thousand four hundred one hip fractures and 10,960 cases were observed in the general studies. Physical activity was predominantly measured using a self-administered questionnaire, and the endpoint of fractures was confirmed by self-report diagnosis or medical record. Of the 14 included prospective studies, 3 were conducted in the United States and the other 11 were conducted among European countries (4 in the UK, 2 in Finland, 1 in Sweden, 1 in Norway, and 1 in Denmark and 1 in France). Quality assessments (score 1–12) are listed in Table 1. Twelve included studies were considered to be of “good” quality (Quality Score> = 9) and 2 were considered to be of “intermediate” quality (Quality Score <9 and ≥6) for our meta-analysis.
TABLE 1.
Characteristics of the Included Studies of Physical-activity Level in Relation to Risk of Fracture in Older Women

TABLE 2.
Follow-up Information of the Included Studies of Physical-activity Level in Relation to Risk of Fracture in Older Women
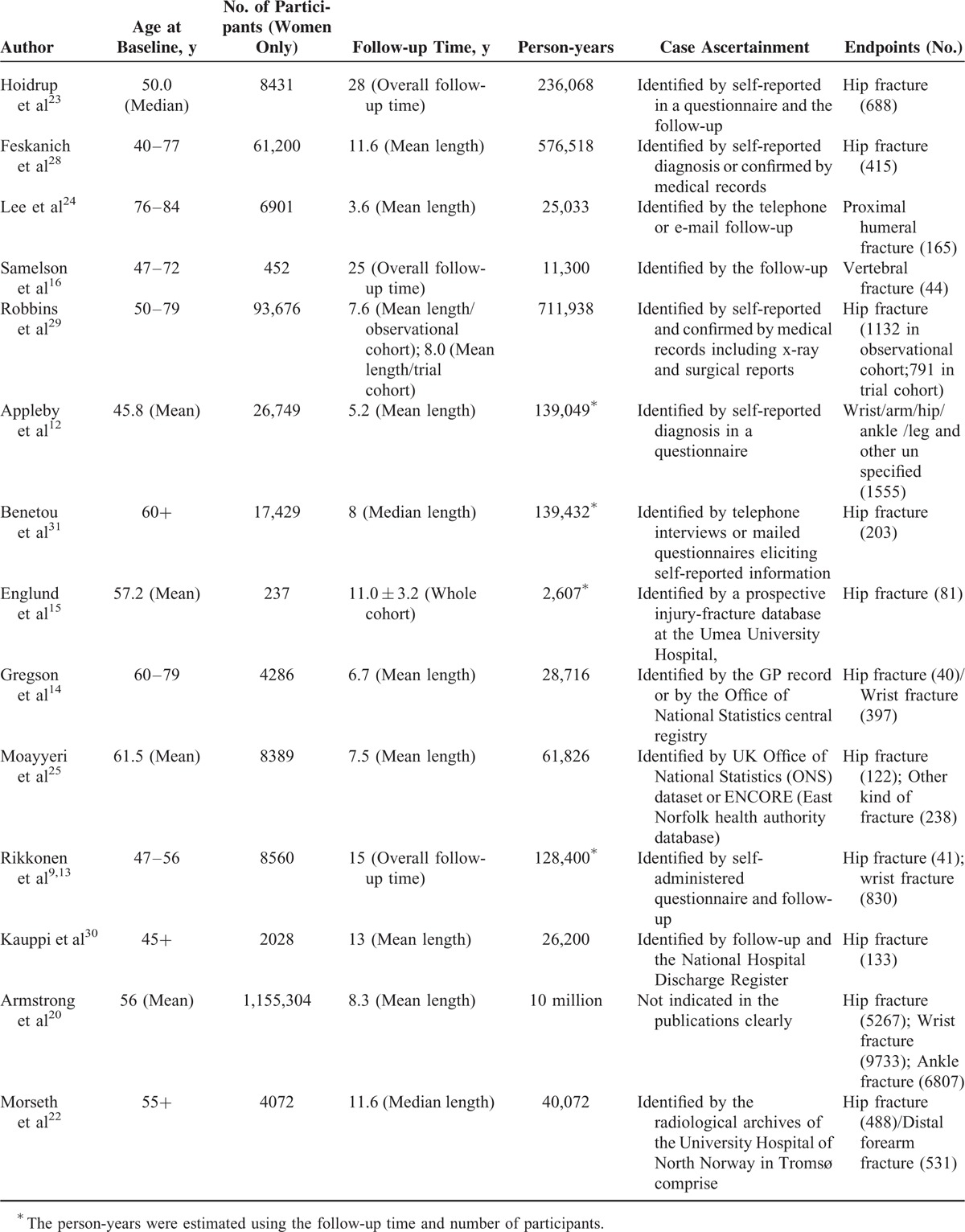
Association Between Physical Activity and the Risk of Hip Fracture in Older Women
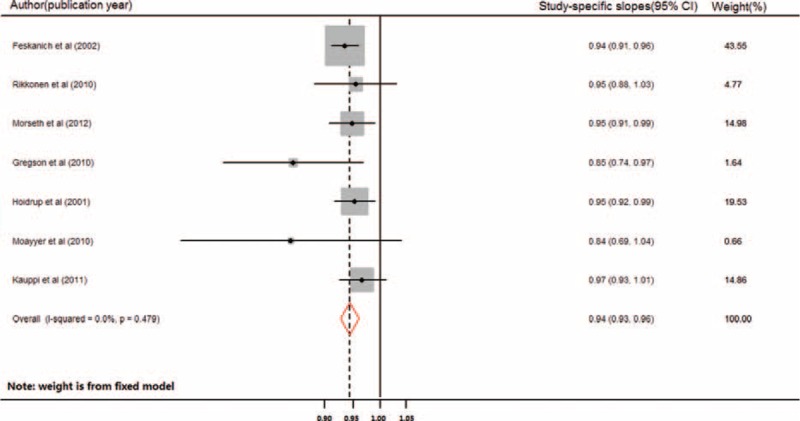
Nine studies were included in the dose–response meta-analysis for hip fracture.13,14,20,22,23,25,28–30 The combined RR for every increase of 3 physical-activity units in each study was 0.93 (95% CI: 0.91–0.96, S1 Table). Significant heterogeneity was observed between these studies (Q = 54.19, P < 0.0001; I2 = 85.2%). In the sensitivity analysis, 2 studies20,29 with the greatest weight were removed (S2 Table). The combined RR of the remaining 7 studies was 0.94 (95% CI: 0.93–0.96, Figure 2), with no significant heterogeneity (Q = 5.52, P < 0.479; I2 = 0%). Furthermore, in an analysis that included these 2 studies, the combined RR was 0.91 (95% CI: 0.86–0.97), showing consistency with the result above. No potential publication bias was found (Begg P = 0.348; Egger P = 0.785, Figure 3) for the 9 included studies.
FIGURE 2.
Forest plot (fixed-effect model) of increasing level of physical activity and risk of hip fracture in older women. The horizontal line indicates the study-specific 95% confidence interval. The square indicates the study-specific weight from the fixed-effect analysis. The diamond indicates the combined relative risk of the 7 included studies after the sensitivity analysis.
FIGURE 3.
Funnel plot (Begg test) of 9 studies included in the analysis of association between increasing level of physical activity and hip fracture in older women (SE: standard error).
Association Between Physical Activity and the Risk of Wrist Fracture in Older Women
Three studies were included in the dose–response meta-analysis for wrist fracture,13,14,20 with a combined RR of 1.004 (95% CI: 0.98–1.03) for every increase of 3 physical-activity units. Heterogeneity was significant between the 3 studies (Q = 10.53, P < 0.005; I2 = 81.0%). The combined RR of the studies remaining after omitting the study by Armstrong et al20 was 1.01 (95% CI: 1.00–1.03, Figure 4), with no significant heterogeneity (Q = 0.460, P < 0.500; I2 = 0%). Egger test and Begg test indicated no significant publication bias between the 3 studies (P = 0.296 and P = 0.107, respectively).
FIGURE 4.
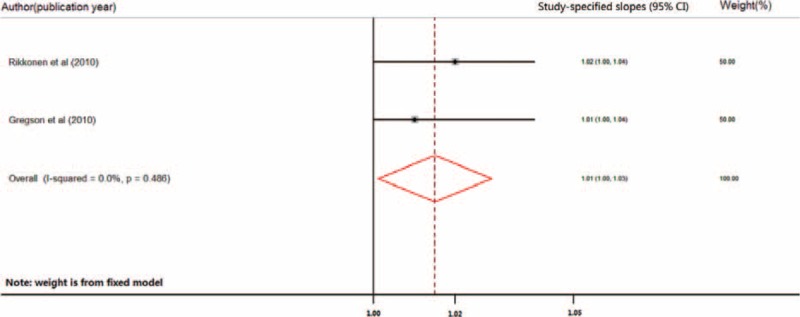
Forest plot (fixed-effect model) of increasing level of physical activity and the risk of wrist fracture of older women. The horizontal line indicates the study-specific 95% confidence interval. The square indicates the study-specific weight from fixed-effect analysis. The diamond indicates the combined relative risk of the 2 included studies after the sensitivity analysis.
DISCUSSION
Physical activity has been proofed to benefit bone health in elder population.32 However, exercise may also cause accident fall, sports injury, and even fracture in elder population. Results of several previous studies were inconsistent,12–17 and at what level of physical activity would influence the risk of fracture in older woman was uncertain. Thus, we carried out a meta-analysis and found a dose–response relationship between physical activity and risk of hip fracture in elder women. The risk of hip fractures showed an inverse association with increasing levels of physical activity.
As a persistent cause of excessive morbidity, fracture of elder population continues as a serious public health problem and is of high concern. Osteoporosis is considered to be the most common reason for fracture among old people.33,34 Older women are more vulnerable to osteoporosis fracture due to increased bone loss after menopause. Bone loss is inevitable due to lower levels of estrogen.20 Although increasing physical activity levels resulting in better muscle strength and balance could decrease the risk of osteoporosis fracture, the procedure can delay bone demineralization,35,36 and reinforce bone architecture and strength.37 Accident fall is another important cause of fracture, especially hip and wrist fracture.19,38 Although strenuous activity may result in accident fall and fracture, leisure physical activity is usually hypothesized to reduce the risk of fall and fall-related fracture in multiple ways.32On one hand, physical activity participation was related with health status of older people. Lower level of physical activities usually indicates worse health status and susceptibility to fall and fracture.39 On the other hand, higher participation of exercise helps to maintain joint flexibility, muscle strength, and balance, connected with decreased risk of accident fall and risk of fracture.40 In our study, increasing levels of physical activity reduced the risk of hip fracture. The result is highly consistent with the theoretical hypothesis and studies.
There are several limitations of the study. First, we tried to uniform the dose of physical activity of different studies using standard criteria; however, the assessment was not precise. And only common leisure activity was reported in the included studies. Thus, strenuous exercise was not recommended for elder people. Second, the baseline characteristics of each study were not the same. Although increasing age, lifestyle, and economic conditions are known to be associated with osteoporosis and fractures, whether those factors have similar effects in different cohorts could not be well estimated. Especially, the heterogeneity of baseline age may lead to bias in the study. Third, calcium, Vitamin-D3, and other anti-osteoporotic drugs were popularized for long in elder women; it was not considered in several included studies. Thus, even smoking, diabetes, and other covariates were adjusted; the effect of anti-osteoporotic treatment may be an important confounder of the study. The last, the study has a significant ethnic background. The included cohorts were mainly from Europe and the USA, and generalization of the conclusion should be cautious. Further studies are still needed to investigate what kind of physical activity and how best physical activities could reduce the risk of fracture in elder population.
CONCLUSIONS
Our results showed that increasing level of leisure physical activity reduced the risk of hip fracture, but not the risk of wrist fracture in older women.
Supplementary Material
Supplementary Material
Acknowledgment
The authors are grateful to all the staff who have contributed to this study.
Footnotes
Abbreviations: BMD = bone mineral density, CI = confidence interval, HR = hazard ratio, OR = odds ratio, RR = relative risk
The authors declare no conflicts of interest.
Supplemental Digital Content is available for this article.
REFERENCES
- 1.Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet 2002; 359:1761–1767. [DOI] [PubMed] [Google Scholar]
- 2.Sale J, Gignac M, Hawker G, et al. Non-pharmacological strategies used by patients at high risk for future fracture to manage fracture risk—a qualitative study. Osteoporos Int 2014; 25:281–288. [DOI] [PubMed] [Google Scholar]
- 3.Kannus P, Parkkari J, Niemi S, et al. Prevention of hip fracture in elderly people with use of a hip protector. N Engl J Med 2000; 343:1506–1513. [DOI] [PubMed] [Google Scholar]
- 4.Health UDo, Services H. Bone Health and Osteoporosis: a Report of the Surgeon General. Rockville, MD: US Department of Health and Human Services, Office of the Surgeon General; 2004. [PubMed] [Google Scholar]
- 5.Yang S, Nguyen ND, Center JR, et al. Association between abdominal obesity and fracture risk: a prospective study. J Clin Endocrinol Metab 2013; 98:2478–2483. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen ND, Ahlborg HG, Center JR, et al. Residual lifetime risk of fractures in women and men. J Bone Miner Res 2007; 22:781–788. [DOI] [PubMed] [Google Scholar]
- 7.American College of Sports Medicine. ACSM's guidelines for exercise testing and prescription [M]. Lippincott Williams & Wilkins, 2013. [DOI] [PubMed] [Google Scholar]
- 8.Tucker JM, Welk GJ, Beyler NK. Physical activity in US adults: compliance with the physical activity guidelines for Americans. Am J Prevent Med 2011; 40:454–461. [DOI] [PubMed] [Google Scholar]
- 9.Rikkonen T, Salovaara K, Sirola J, et al. Physical activity slows femoral bone loss but promotes wrist fractures in postmenopausal women: a 15-year follow-up of the OSTPRE study. J Bone Miner Res 2010;25:2332–2340. [DOI] [PubMed] [Google Scholar]
- 10.Kelley GA. Aerobic exercise and bone density at the hip in postmenopausal women: a meta-analysis. Prevent Med 1998; 27:798–807. [DOI] [PubMed] [Google Scholar]
- 11.Fyhrie D, Milgrom C, Hoshaw S, et al. Effect of fatiguing exercise on longitudinal bone strain as related to stress fracture in humans. Ann Biomed Eng 1998; 26:660–665. [DOI] [PubMed] [Google Scholar]
- 12.Appleby PN, Allen NE, Roddam AW, et al. Physical activity and fracture risk: a prospective study of 1898 incident fractures among 34,696 British men and women. J Bone Miner Metab 2008; 26:191–198. [DOI] [PubMed] [Google Scholar]
- 13.Rikkonen T, Salovaara K, Sirola J, et al. Physical activity slows femoral bone loss but promotes wrist fractures in postmenopausal women: a 15-year follow-up of the OSTPRE study. J Bone Miner Res 2010; 25:2332–2340. [DOI] [PubMed] [Google Scholar]
- 14.Gregson CL, Carson C, Amuzu A, et al. The association between graded physical activity in postmenopausal British women, and the prevalence and incidence of hip and wrist fractures. Age Ageing 2010; 39:565–574. [DOI] [PubMed] [Google Scholar]
- 15.Englund U, Nordstrom P, Nilsson J, et al. Physical activity in middle-aged women and hip fracture risk: the UFO study. Osteoporos Int 2011; 22:499–505. [DOI] [PubMed] [Google Scholar]
- 16.Samelson EJ, Hannan MT, Zhang Y, et al. Incidence and risk factors for vertebral fracture in women and men: 25-year follow-up results from the population-based Framingham study. J Bone Miner Res 2006; 21:1207–1214. [DOI] [PubMed] [Google Scholar]
- 17.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 2000; 283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 18.Wells G A, Shea B, O’connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2011[J]. URL: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp, 2014. [Google Scholar]
- 19.Armstrong AL, Wallace WA. The epidemiology of hip fractures and methods of prevention. Acta Orthop Belg 1994; 60 Suppl 1:85–101. [PubMed] [Google Scholar]
- 20.Armstrong ME, Cairns BJ, Banks E, et al. Different effects of age, adiposity and physical activity on the risk of ankle, wrist and hip fractures in postmenopausal women. Bone 2012; 50:1394–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamling J, Lee P, Weitkunat R, et al. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med 2008; 27:954–970. [DOI] [PubMed] [Google Scholar]
- 22.Morseth B, Ahmed LA, Bjornerem A, et al. Leisure time physical activity and risk of non-vertebral fracture in men and women aged 55 years and older: the Tromso Study. Eur J Epidemiol 2012; 27:463–471. [DOI] [PubMed] [Google Scholar]
- 23.Hoidrup S, Sorensen TI, Stroger U, et al. Leisure-time physical activity levels and changes in relation to risk of hip fracture in men and women. Am J Epidemiol 2001; 154:60–68. [DOI] [PubMed] [Google Scholar]
- 24.Lee SH, Dargent-Molina P, Breart G. Risk factors for fractures of the proximal humerus: results from the EPIDOS prospective study. J Bone Miner Res 2002; 17:817–825. [DOI] [PubMed] [Google Scholar]
- 25.Moayyeri A, Besson H, Luben RN, et al. The association between physical activity in different domains of life and risk of osteoporotic fractures. Bone 2010; 47:693–700. [DOI] [PubMed] [Google Scholar]
- 26.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 1992; 135:1301–1309. [DOI] [PubMed] [Google Scholar]
- 27.Sterne JA, Egger M, Smith GD. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ 2001; 323:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feskanich D, Willett W, Colditz G. Walking and leisure-time activity and risk of hip fracture in postmenopausal women. JAMA 2002; 288:2300–2306. [DOI] [PubMed] [Google Scholar]
- 29.Robbins J, Aragaki AK, Kooperberg C, et al. Factors associated with 5-year risk of hip fracture in postmenopausal women. JAMA 2007; 298:2389–2398. [DOI] [PubMed] [Google Scholar]
- 30.Kauppi M, Heliovaara M, Impivaara O, et al. Parity and risk of hip fracture in postmenopausal women. Osteoporos Int 2011; 22:1765–1771. [DOI] [PubMed] [Google Scholar]
- 31.Benetou V, Orfanos P, Zylis D, et al. Diet and hip fractures among elderly Europeans in the EPIC cohort[J]. European journal of clinical nutrition 2011; 65:132–139. [DOI] [PubMed] [Google Scholar]
- 32.Marks R. Physical activity and hip fracture disability: a review. J Aging Res 2011; 2011:741918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferrini RL, Barrett-Connor E. Sex hormones and age: a cross-sectional study of testosterone and estradiol and their bioavailable fractions in community-dwelling men. Am J Epidemiol 1998; 147:750–754. [DOI] [PubMed] [Google Scholar]
- 34.Van Caenegem E, Wierckx K, Taes Y, et al. Body composition, bone turnover, and bone mass in trans men during testosterone treatment: 1-year follow-up data from a prospective case–controlled study (ENIGI). Eur J Endocrinol 2015; 172:163–171. [DOI] [PubMed] [Google Scholar]
- 35.Adami S, Passeri M, Ortolani S, et al. Effects of oral alendronate and intranasal salmon calcitonin on bone mass and biochemical markers of bone turnover in postmenopausal women with osteoporosis. Bone 1995; 17:383–390. [DOI] [PubMed] [Google Scholar]
- 36.Armamento-Villareal R, Napoli N, Panwar V, et al. Suppressed bone turnover during alendronate therapy for high-turnover osteoporosis. N Engl J Med 2006; 355:2048–2050. [DOI] [PubMed] [Google Scholar]
- 37.Hartard M, Haber P, Ilieva D, et al. Systematic strength training as a model of therapeutic intervention: a controlled trial in postmenopausal women with osteopenia1. Am J Phys Med Rehab 1996; 75:21–28. [DOI] [PubMed] [Google Scholar]
- 38.Nevitt MC, Cummings SR. Type of fall and risk of hip and wrist fractures: the study of osteoporotic fractures. J Am Geriatr Soc 1993; 41:1226–1234. [DOI] [PubMed] [Google Scholar]
- 39.Michaëlsson K, Olofsson H, Jensevik K, et al. Leisure physical activity and the risk of fracture in men. PLoS Med 2007; 4:e199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robinson-Cohen C, Katz R, Hoofnagle AN, et al. Mineral metabolism markers and the long-term risk of hip fracture: the cardiovascular health study. J Clin Endocrinol Metab 2011; 96:2186–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


