Supplemental Digital Content is available in the text
Abstract
The risk factors influencing the natural course of chronic kidney disease (CKD) are complex and heterogeneous, and few systematic reviews to date have focused on this issue. The aim of the study is to identify the risk factors for disease development and progression in each stage of CKD. We conducted electronic literature searches of PubMed, MEDLINE, Scopus, and the Cochrane Library up to October 15, 2012, for observational studies evaluating the risk factors on the development or progression of CKD. Eligible studies should have collected repeated information that could evaluate changes in renal function. Extracted information from all the included studies was synthesized narratively. Quality assessments were performed using the Newcastle–Ottawa Scale. An exploratory random-effects meta-analysis was performed where feasible to pool effect sizes across studies for a specific risk factor in a specific outcome. We identified 38 cohort studies and 2 case-control studies from 40 articles, with a total of 318,898 participants from 14 countries. The follow-up duration ranged from 1.5 to 16 years. The majority of the included studies were of high quality. The baseline CKD stages of the included studies ranged from normal to later stages, and only 19 studies could be classified into a specific range of CKD stages during follow-up. Three risk factors from studies of the same baseline and follow-up CKD stages were eligible for the exploratory meta-analysis, including male sex, substantial proteinuria, and diabetes. The hazard ratios for the progression from CKD stages 3–5 to end-stage renal disease (ESRD) were 1.37 (95% confidence interval 1.17–1.62), 1.64 (1.01–2.66), and 1.16 (0.98–1.38) for male sex, substantial proteinuria, and diabetes, respectively. In conclusion, our analyses comprehensively summarize the initiating and perpetuating factors for CKD. Male sex and substantial proteinuria are significant perpetuating factors for the progression from late stage CKD to ESRD, and diabetes may play a minor role for the outcome of ESRD among patients with later stages of CKD.
INTRODUCTION
Chronic kidney disease (CKD) is a major noncommunicable disease with the prevalence varying between 10.5% and 13.1% around the world.1,2 CKD leads to higher risks of dialysis, hospitalization, cardiovascular morbidity and mortality.3–7 End-stage renal disease (ESRD) is the final stage for CKD, and with the improvement in dialysis techniques and the quality of medical care, dialysis patients have longer lifespans which in turn leads to the increasing prevalence of ESRD.8 According to the latest annual report of United States Renal Data System, the prevalence of ESRD increased across countries from 6 to 135% during the period of 2006 to 2012, thus placing a greater burden on the health insurance system of many countries.9
Previous epidemiological studies have reported many risk factors for CKD and classified them into initiating and perpetuating factors. Initiating factors play a role in starting the cycle of nephron loss, such as older age, male sex, or diabetes, and perpetuating factors drive the disease process onward, such as proteinuria, hypertension, or hyperuricemia.10,11 There are also some emerging biomarkers reported to be associated with the progression of CKD, such as urinary connective tissue growth factor, tumor necrosis factor-α receptor 2, and interleukin-6.12,13 With a greater understanding of the risk factors for CKD, it can be more possible to prevent disease onset or the progression into later stages. Because of the high burden for taking care of the dialysis patients, attention in recent years has been increasingly shifted toward early intervention and risk factor modification for patients with CKD. Due to the complexity and heterogeneity of risk factors influencing the natural course of CKD, we therefore conducted this systematic review to identify the risk factors for disease development and progression in each stage of CKD.
METHODS
Data Sources and Searches
We conducted electronic literature searches of MEDLINE, PubMed, Scopus, and the Cochrane Library from the earliest available date of indexing through October 15, 2012. We also hand-searched additional studies in the reference lists of all identified publications, including relevant meta-analyses and systematic reviews. The detailed study protocol and search strategies are provided as a Supplemental Content.
Study Selection
We followed these predefined inclusion criteria: (1) studies of cohort or case-control design, (2) studies that evaluated the risk factors for development or progression of CKD, and (3) studies that collected repeated information which could evaluate the change in renal function. The stages of CKD were defined by the K/DOQI Clinical Practice Guidelines according to the glomerular filtration rate (GFR) and evidence of kidney damage.14 Participants of GFR ≥ 60 mL/min and without kidney damage were designated as stage 0. The requirement for dialysis therapy or kidney transplantation was designated as ESRD. We included studies of adult participants with stage 0 to 5 CKD at baseline. Eligible studies had to be published as full-length articles in peer-reviewed journals. There was no restriction on language of publication.
Ethics Statement
The Institutional Review Board of the Far Eastern Memorial Hospital has approved this study and waived the requirement for informed consent, because this study were designed to retrospectively collect available data from articles published in peer-reviewed journals.
Data Extraction and Quality Assessment
Two investigators (W-CT and H-YW) independently extracted the following information and entered it into a database: details of the study design, location and published year of study, patient demographic characteristics (age, sex, and ethnicity), numbers of patients enrolled and excluded, the CKD stages at baseline and follow-up, duration of study, study outcomes, identified risk factors and their adjusted effect size, as well as other covariates adjusted in the regression models. When relevant information regarding design or outcomes was unclear, or when doubt existed for duplicate publications, the original authors were contacted to obtain the necessary information. Two investigators (W-CT and H-YW) independently evaluated the methodological quality and risk of bias of eligible studies by using the Newcastle–Ottawa Scale.15,16 Disagreements between the 2 authors were resolved by discussion. If the disagreement persisted, 2 other senior investigators (Y-SP and K-LC) were consulted to attain consensus.
Data Synthesis and Analysis
All data from each eligible study were extracted and entered into a computer database using a spreadsheet software (Microsoft Excel 2010; Microsoft Corp, Redmond, WA). Categorical variables were presented as frequencies or percentages and continuous variables as mean values, unless stated otherwise. Data from all the included studies were synthesized narratively. An exploratory meta-analysis was performed where feasible to pool hazard ratios (HRs) of a specific risk factor for a specific outcome, from studies with the same range of baseline and follow-up stages of CKD. The pooled estimates of HR and 95% confidence interval (CI) of risk factors for development or progression of CKD were calculated using the method of DerSimonian and Laird random-effects model whereas heterogeneity of treatment effects across studies were assessed using I2, the Cochrane Q-test, and the Galbraith plot method.16,17 Publication bias was examined with the funnel plot method and Egger's regression asymmetry test.18,19 Sensitivity analyses were conducted by the same methods after omission of data from specific studies, such as studies with a different population or large sample size that might dominate the pooled effect sizes. Two-sided P ≤ 0.05 was considered statistically significant. Statistical analyses were performed with Stata software (version 11.1, StataCorp LP, College Station, TX).
RESULTS
Search Results
The flow chart in Figure 1 shows the literature search process. We found 93 articles from MEDLINE, 233 from PubMed, 115 from Scopus, 2 from Cochrane Library, and 7 additional articles from hand searching. After these searches were combined and duplicates were removed, the total number of articles was 308. Of these, 258 were excluded on the basis of their title and abstract. Of the 50 that underwent full-text evaluation, 40 met our inclusion criteria (see Supplementary References).
FIGURE 1.
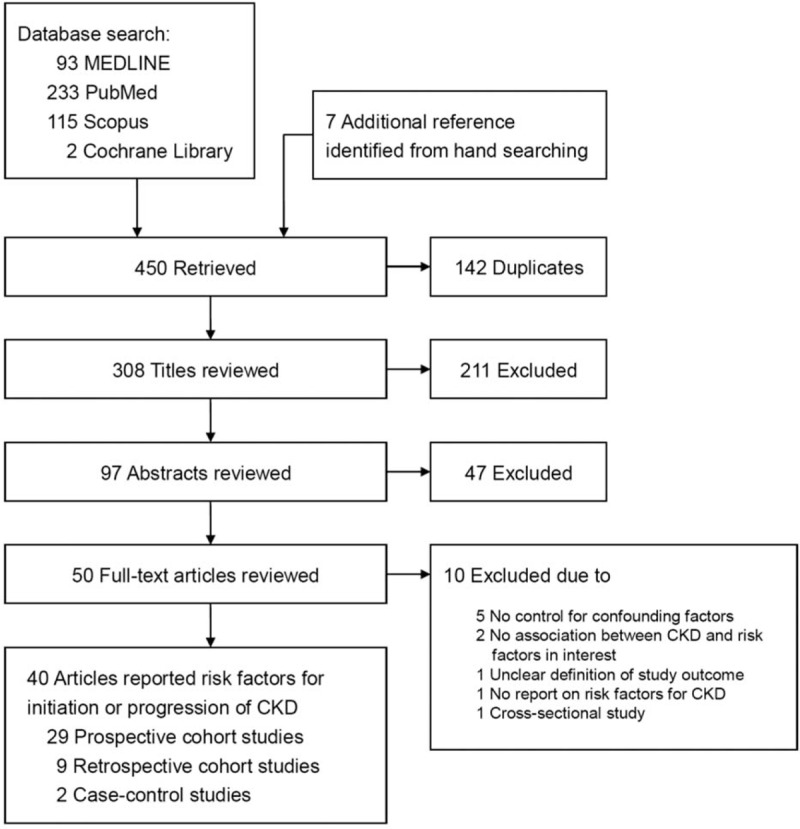
Summary of study identification and selection.
Qualitative Summary and Data Synthesis
The clinical and methodological characteristics, as well as the main results of each study, are summarized in Tables 1 and 2, and Supplementary Table 1. We retrieved 40 studies that enrolled a total of 318,898 participants from 14 countries. There were 162,261 participants from 29 prospective cohort studies, 156,023 participants from 9 retrospective cohort studies, and 614 participants from 2 case-control studies. Table 1 and Supplementary Table 2, summarize the Newcastle–Ottawa Scales of included studies, and the majority of those studies were of high quality. Only 2 prospective and 2 retrospective cohort studies did not follow up the cohort adequately, and only 1 retrospective cohort study did not show acceptable comparability between groups in the cohort.
TABLE 1.
Characteristics of Studies Included in the Systematic Review

TABLE 2.
The Study Endpoints and Identified Risk Factors of Studies Included in the Systematic Review

The included studies were conducted in countries from Europe, Asia, North America, or South America. The ethnicity of the study population included Black, White, Asian, Hispanic, and Native American. The follow-up duration of the studies ranged from 1.5 to 16 years. The mean age of the study participants ranged from 37 to 76 years, and the female percentage ranged from 0 to 73%. The baseline CKD stages of the included studies ranged from normal to later stages, with 18 studies using ESRD as the outcome, 9 studies identifying incident CKD as the outcome, and 1 study adopting stage 4 to 5 CKD as the study outcome. Eighteen studies used the decline or change in renal function as the outcome, and 2 studies used the increase in proteinuria as the outcome.
Supplementary Table 3, summarizes the adjusted effect sizes and results of statistical test of major risk factors, including male sex, baseline proteinuria, diabetes, age, and blood pressure, from studies included in this systematic review. In 10 studies analyzing the influence of male sex, 4 studies showed that male sex was a significant risk factor for ESRD. There were 21 studies adjusting baseline proteinuria in the multivariate models, of which 20 studies showed significant associations between baseline proteinuria and the progression of CKD. Among 12 studies assessing diabetes, 7 studies reported a significant association between diabetes and the deterioration of renal function. For the 16 studies evaluating the influence of age, 5 studies reported that younger age is associated with ESRD, and 7 studies reported that older age is associated with the decline in renal function. In 15 studies evaluating blood pressure, 12 studies reported that higher blood pressure was a significant risk factor for CKD.
Among the various study outcomes, only 19 studies could be classified into a specific range of CKD stages during follow-up (Supplementary Table 4). Three studies assessed initiating factors for CKD, 6 studies analyzed perpetuating factors from early stage CKD to late stage CKD, and 6 studies evaluated perpetuating factors from late stage CKD to ESRD. Another 4 studies examined risk factors for ESRD among participants with wide range of CKD at baseline. Among the various risk factors, only 3 risk factors from a total of 5 studies were eligible for the exploratory meta-analysis, including male sex, baseline proteinuria >1 g/day, and diabetes.
Effects of Male Sex on Progression of CKD
The effects for male sex were assessed in 4 studies with patients of CKD stages 3 to 5 or 4 to 5 at baseline, and ESRD as the study outcome (Supplementary Table 4).20–23 A total of 7724 patients with a male prevalence of 59.4% were assessed in the exploratory meta-analysis. There were 2882 patients who developed ESRD during follow-up. Compared with female patients, male patients showed a significantly higher hazard for the progression from late stage CKD to ESRD (HR 1.37, 95% CI 1.17–1.62; P < 0.001) (Figure 2A). There was moderate heterogeneity (I2 = 56.8%; P = 0.07) but no publication bias (P = 0.12) among the studies (Supplementary Figures 1A and 2A).
FIGURE 2.
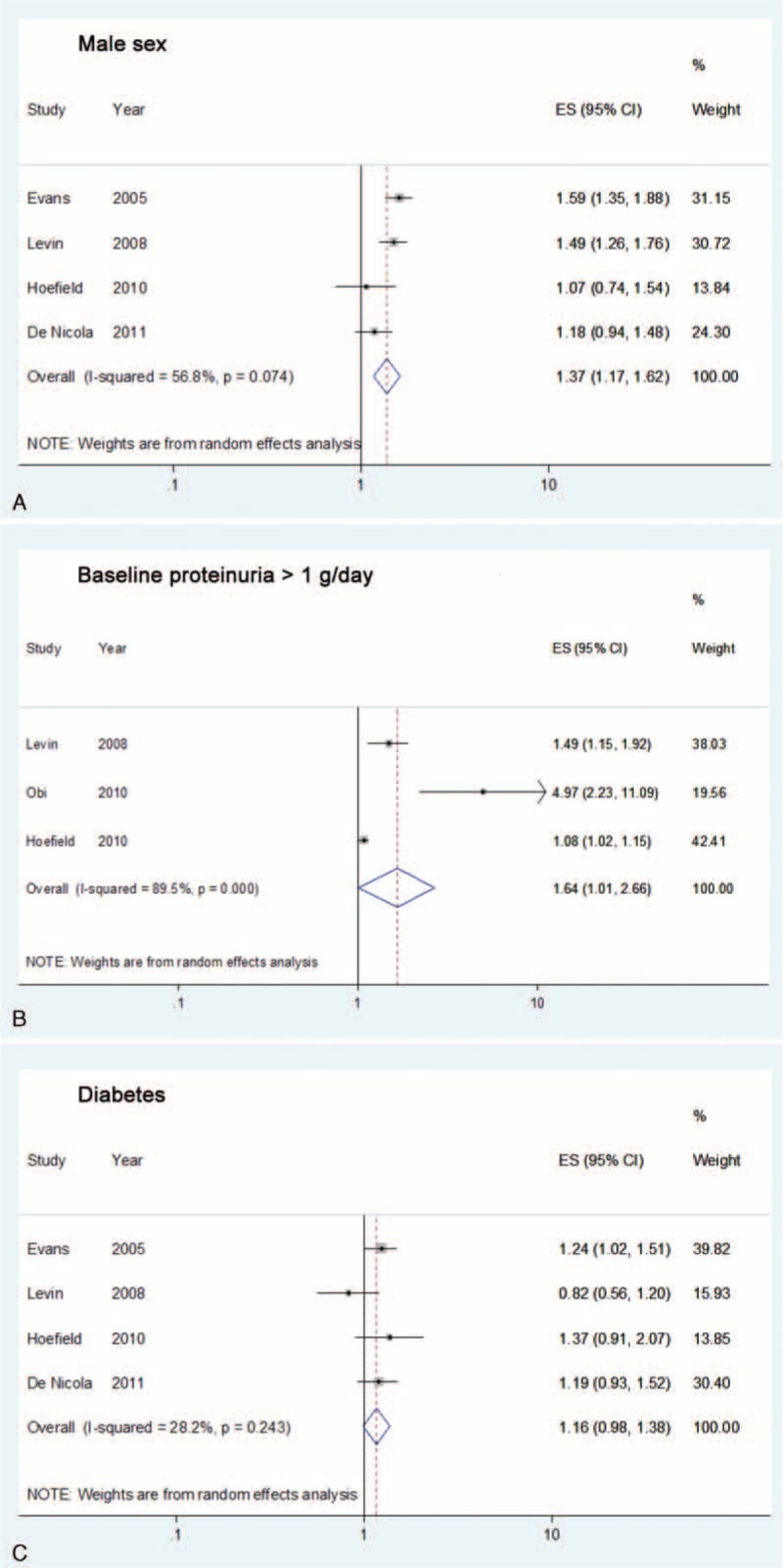
Forrest plots of the hazard ratios among studies included in the exploratory meta-analysis: (A) male sex, (B) proteinuria > 1 g/day, and (C) diabetes, for the outcome of progression from chronic kidney disease stage 3 to 5 to end-stage renal disease.
Effects of Baseline Proteinuria on Progression of CKD
The influence of baseline proteinuria >1 g/day on the outcome of ESRD was evaluated in 3 studies with patients of CKD stages 3 to 5 or 4 to 5 at baseline (Supplementary Table 4).21,22,24 A total of 6017 patients with 1948 ESRD events were evaluated in the meta-analysis. Compared with patients of baseline proteinuria ≤1 g/day, those with baseline proteinuria >1 g/day showed a significantly increased hazard for the progression from late stage CKD to ESRD (HR 1.64, 95% CI 1.01–2.66; P = 0.04) (Figure 2B). There was substantial heterogeneity (I2 = 89.5%; P < 0.001) among the studies, but no publication bias (P = 0.08) was found (Supplementary Figures 1B and 2B).
Effects of Diabetes on Progression of CKD
The impacts of diabetes on the outcome of ESRD were analyzed in 4 studies with patients of CKD stages 3 to 5 or 4 to 5 at baseline (Supplementary Table 4).20–23 A total of 7724 patients with a diabetes prevalence of 32% were assessed in the meta-analysis. There were 2882 patients who developed ESRD during follow-up. Compared to the nondiabetic participants, those with diabetes showed a borderline increased hazard for the progression from late-stage CKD to ESRD (HR 1.16, 95% CI 0.98–1.38; P = 0.08) (Figure 2C). There was low heterogeneity (I2 = 28.2%; P = 0.24) and no evidence of publication bias (P = 0.59) among the studies (Supplementary Figures 1C and 2C). The study by Levin and colleagues had the largest study population and enrolled stage 4 to 5 CKD patients, for whom diabetes was reported as a nonsignificant factor (HR 0.82, 95% CI 0.56–1.20; P = 0.30).21 A sensitivity analysis by omitting Levin's study21 revealed a significantly harmful effect for diabetes on the progression of CKD (HR 1.24, 95% CI 1.07–1.43; P = 0.004), with a lower heterogeneity among the remaining studies (I2 = 0%; P = 0.85).
DISCUSSION
To the best of our knowledge, this study is the first systematic review for the risk factors determining the clinical course of CKD. We have summarized the risk factors for the development and progression of CKD from available observational studies and have provided comprehensive information for clinicians as well as the nephrologists. We have also conducted exploratory meta-analyses for the risk factors of the progression from late stage CKD to ESRD, which show that male sex and substantial proteinuria are significant factors whereas diabetes demonstrates a borderline significance. The strengths of our study are that we followed a standard protocol and used a comprehensive search strategy. We have rigorously performed a narrative data synthesis for 318,898 participants from 40 studies with an observational time of up to 16 years, and also assessed the influence of specific risk factors using the exploratory meta-analytic method.
Results in Relation to Other Studies and Reviews
Animal studies have shown that age-dependent glomerular damage was more prominent in male rodents and castrated males were protected from such injury, which suggests the detrimental effect of androgen.25 Potential mechanisms for this type of damage include genetically determined differences between the sexes in renal structure and function, receptor-mediated effects of sex hormones on glomerular structure, as well as effects on the synthesis and release of cytokines, vasoactive agents, and growth factors.26 Previous meta-analyses have evaluated the effect of sex on the progression of nondiabetic CKD, but with conflicting results. Neugarten performed a meta-analysis of a total of 68 studies and 11,345 patients, showing that male sex was associated with a more rapid progression to ESRD in patients with nondiabetic chronic renal disease, IgA nephropathy, membranous nephropathy, or autosomal dominant polycystic kidney disease.27 In a patient-level meta-analysis, Jafar pooled the data from a total of 1860 nondiabetic CKD patients from 11 randomized clinical trials and demonstrated a higher risk of progression to ESRD for women compared with men (risk ratio 1.68, 95% CI 1.20–2.37), though it should be noted that most of the female participants were postmenopausal and thus lacked the potentially protective effect from estrogen.28
Differing effects regarding sex on the decline in renal function have also been demonstrated in studies among type 1 or type 2 diabetic patients. Some studies have indicated that there were no sex differences in the progression of nephropathy among patients with type 1 or type 2 diabetes.29–32 On the other hand, Jacobsen et al and Gall et al showed that male sex predicted progression of renal disease in patients with diabetes,33,34 and Holl et al reported a detrimental effect of female sex on the progression of diabetic nephropathy.35 As the enrolled study participants were mainly elderly and one-third had diabetes, our exploratory meta-analysis still evidenced a worse renal outcome among male patients. A meta-regression would be helpful in further exploration of the relationship between male sex and diabetes for the influence on CKD, but the small number of included studies here limits the statistical power for a meta-regression.
Proteinuria has been well established as a marker of kidney damage and widely reported to be an independent perpetuating factor for CKD.5,36–39 Guidelines for the evaluation and management of CKD have emphasized the importance of assessing albuminuria and proteinuria, in addition to the use of estimated GFR, for disease classification and risk stratification.14,40 In consistent with relevant clinical guidelines, our systematic review again demonstrates the strong association between the progression of CKD and various levels of baseline proteinuria, and our exploratory meta-analyses also reveal that substantial proteinuria (>1 g/day) is a significant risk factor for ESRD. Similar associations between proteinuria and ESRD have been reported in other meta-analyses. In a collaborative meta-analysis of 13 studies totaling 21,688 patients with CKD, Astor reported that there was a strong graded association between proteinuria and risk of ESRD, and an 8-fold higher proteinuria was significantly associated with ESRD (HR 3.42, 95% CI 1.84–6.37).41 Furthermore, in an individual meta-analysis using data of 9008 patients from 32 studies, Inker reported that a 50% reduction in proteinuria was associated with a lower risk of composite outcome for kidney disease progression or death (HR 0.74, 95% credible interval 0.67–0.82).42
Diabetes can lead to various macrovascular and microvascular diseases, such as cardiovascular diseases, cerebrovascular diseases, nephropathy, and retinopathy.4,43 Those comorbidities contribute to a large percentage of morbidities and mortality in the diabetic population. Given the high mortality from cardiovascular disease and other comorbidities, many patients with diabetes may develop CKD but not survive long enough to develop ESRD.44 This could partially explain why after omitting Levin's study which enrolled elderly patients with estimated GFR < 30 mL/min,21 our sensitivity analysis revealed a significant harmful effect of diabetes for ESRD.
Limitations of this Study
Our study nonetheless has several limitations. First, retrieved studies were heterogeneous among study outcomes, definitions of risk factors, adjusted covariates, and the stages of CKD at baseline or follow-up. Although we have identified a number of risk factors, only 3 risk factors were feasible for exploratory meta-analyses because pooling of the effect size required the same definition of a risk factor for the same outcome among studies with the same range of baseline and follow-up stages of CKD. However, we have performed a comprehensive systematic search on 4 electronic databases with a predefined protocol and tried to provide the best available evidence through a narrative synthesis. Second, substantial heterogeneity has been found in the pooled estimates of the exploratory meta-analysis for baseline proteinuria, and moderate heterogeneity in the meta-analysis for male sex. However, the small study number limited the power for further exploration of the heterogeneity using methods of meta-regression or subgroup analysis. Finally, included studies were observational in nature because this systematic review was designed to recognize risk factors for the clinical course of CKD. Therefore the identified risk factors from individual studies or exploratory meta-analyses were influenced by the bias of observational studies. Nevertheless, we have assessed the risk of bias using the Newcastle–Ottawa Scale, and the majority of the included studies had high quality in study design and analysis.
CONCLUSIONS
Our analyses comprehensively summarize the initiating and perpetuating factors for CKD. Male sex and substantial proteinuria are significant perpetuating factors for the progression from late stage CKD to ESRD, and diabetes might play a minor role for the outcome of ESRD among patients with later stages of CKD.
Supplementary Material
Acknowledgments
The authors thank Ms Mei-Ru Chen for her assistance in statistical computing.
Footnotes
Abbreviations: CI = confidence interval, CKD = chronic kidney disease, ESRD = end-stage renal disease, GFR = glomerular filtration rate, HR = hazard ratio
H-YW and K-LC contributed equally to this study.
Funding: this study was supported by research grants to Dr. Hon-Yen Wu from the Ministry of Science and Technology, Taiwan (MOST 103-2314-B-418-003-MY2), the National Health Research Institutes, Taiwan (NHRI-EX105-10510PC), and Far Eastern Memorial Hospital, New Taipei City, Taiwan (FEMH 2014-C-041, FEMH 2016-C-027).
The authors have no conflicts of interest to disclose. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplemental Digital Content is available for this article.
REFERENCES
- 1.James MT, Hemmelgarn BR, Tonelli M. Early recognition and prevention of chronic kidney disease. Lancet 2010; 375:1296–1309. [DOI] [PubMed] [Google Scholar]
- 2.Wen CP, Cheng TY, Tsai MK, et al. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462,293 adults in Taiwan. Lancet 2008; 371:2173–2182. [DOI] [PubMed] [Google Scholar]
- 3.Wu HY, Huang JW, Lin HJ, et al. Comparative effectiveness of renin-angiotensin system blockers and other antihypertensive drugs in patients with diabetes: systematic review and Bayesian network meta-analysis. BMJ 2013; 347:f6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu HY, Hung KY, Tu YK, et al. Comparative effectiveness of antihypertensive drugs in patients with diabetes. J Comp Eff Res 2014; 3:213–215. [DOI] [PubMed] [Google Scholar]
- 5.Iseki K, Iseki C, Kinjo K. C-reactive protein is a predictor for developing proteinuria in a screened cohort. Nephron Clin Pract 2011; 117:c51–56. [DOI] [PubMed] [Google Scholar]
- 6.Chien KL, Lin HJ, Lee BC, et al. A prediction model for the risk of incident chronic kidney disease. Am J Med 2010; 123:836–846.e832. [DOI] [PubMed] [Google Scholar]
- 7.Fukuma S, Kurita N, Fukagawa M, et al. Impact of cinacalcet introduction on MBD management: the MBD-5D study in Japan. Kidney Int Suppl 2013; 3:436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu HY, Huang JW, Peng YS, et al. Microalbuminuria screening for detecting chronic kidney disease in the general population: a systematic review. Ren Fail 2013; 35:607–614. [DOI] [PubMed] [Google Scholar]
- 9.Saran R, Li Y, Robinson B, et al. US Renal Data System 2014 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis 2015; 66 (1 suppl 1):S1–S305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taal MW, Brenner BM. Predicting initiation and progression of chronic kidney disease: developing renal risk scores. Kidney Int 2006; 70:1694–1705. [DOI] [PubMed] [Google Scholar]
- 11.McClellan WM, Flanders WD. Risk factors for progressive chronic kidney disease. J Am Soc Nephrol 2003; 14:S65–70. [DOI] [PubMed] [Google Scholar]
- 12.O'Seaghdha CM, Hwang SJ, Bhavsar NA, et al. Lower urinary connective tissue growth factor levels and incident CKD stage 3 in the general population. Am J Kidney Dis 2011; 57:841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shankar A, Sun L, Klein BE, et al. Markers of inflammation predict the long-term risk of developing chronic kidney disease: a population-based cohort study. Kidney Int 2011; 80:1231–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39 (2 suppl 1):S1–266. [PubMed] [Google Scholar]
- 15.Wells GA, Shea B, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf (accessed January 18, 2016). [Google Scholar]
- 16.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration. http://www.cochrane-handbook.org (accessed January 18, 2016). [Google Scholar]
- 17.Galbraith RF. A note on graphical presentation of estimated odds ratios from several clinical trials. Stat Med 1988; 7:889–894. [DOI] [PubMed] [Google Scholar]
- 18.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50:1088–1101. [PubMed] [Google Scholar]
- 19.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans M, Fryzek JP, Elinder CG, et al. The natural history of chronic renal failure: results from an unselected, population-based, inception cohort in Sweden. Am J Kidney Dis 2005; 46:863–870. [DOI] [PubMed] [Google Scholar]
- 21.Levin A, Djurdjev O, Beaulieu M, et al. Variability and risk factors for kidney disease progression and death following attainment of stage 4 CKD in a referred cohort. Am J Kidney Dis 2008; 52:661–671. [DOI] [PubMed] [Google Scholar]
- 22.Hoefield RA, Kalra PA, Baker P, et al. Factors associated with kidney disease progression and mortality in a referred CKD population. Am J Kidney Dis 2010; 56:1072–1081. [DOI] [PubMed] [Google Scholar]
- 23.De Nicola L, Chiodini P, Zoccali C, et al. Prognosis of CKD patients receiving outpatient nephrology care in Italy. Clin J Am Soc Nephrol 2011; 6:2421–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Obi Y, Kimura T, Nagasawa Y, et al. Impact of age and overt proteinuria on outcomes of stage 3 to 5 chronic kidney disease in a referred cohort. Clin J Am Soc Nephrol 2010; 5:1558–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baylis C. Age-dependent glomerular damage in the rat. Dissociation between glomerular injury and both glomerular hypertension and hypertrophy. Male gender as a primary risk factor. J Clin Invest 1994; 94:1823–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silbiger SR, Neugarten J. The impact of gender on the progression of chronic renal disease. Am J Kidney Dis 1995; 25:515–533. [DOI] [PubMed] [Google Scholar]
- 27.Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. J Am Soc Nephrol 2000; 11:319–329. [DOI] [PubMed] [Google Scholar]
- 28.Jafar TH, Schmid CH, Stark PC, et al. The rate of progression of renal disease may not be slower in women compared with men: a patient-level meta-analysis. Nephrol Dial Transplant 2003; 18:2047–2053. [DOI] [PubMed] [Google Scholar]
- 29.Breyer JA, Bain RP, Evans JK, et al. Predictors of the progression of renal insufficiency in patients with insulin-dependent diabetes and overt diabetic nephropathy. The Collaborative Study Group. Kidney Int 1996; 50:1651–1658. [DOI] [PubMed] [Google Scholar]
- 30.Monti MC, Lonsdale JT, Montomoli C, et al. Familial risk factors for microvascular complications and differential male-female risk in a large cohort of American families with type 1 diabetes. J Clin Endocrinol Metab 2007; 92:4650–4655. [DOI] [PubMed] [Google Scholar]
- 31.Rossing P, Hougaard P, Parving HH. Risk factors for development of incipient and overt diabetic nephropathy in type 1 diabetic patients: a 10-year prospective observational study. Diabetes Care 2002; 25:859–864. [DOI] [PubMed] [Google Scholar]
- 32.Ruggenenti P, Gambara V, Perna A, et al. The nephropathy of non-insulin-dependent diabetes: predictors of outcome relative to diverse patterns of renal injury. J Am Soc Nephrol 1998; 9:2336–2343. [DOI] [PubMed] [Google Scholar]
- 33.Jacobsen P, Rossing K, Tarnow L, et al. Progression of diabetic nephropathy in normotensive type 1 diabetic patients. Kidney Int Suppl 1999; 71:S101–105. [DOI] [PubMed] [Google Scholar]
- 34.Gall MA, Hougaard P, Borch-Johnsen K, et al. Risk factors for development of incipient and overt diabetic nephropathy in patients with non-insulin dependent diabetes mellitus: prospective, observational study. BMJ 1997; 314:783–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holl RW, Grabert M, Thon A, et al. Urinary excretion of albumin in adolescents with type 1 diabetes: persistent versus intermittent microalbuminuria and relationship to duration of diabetes, sex, and metabolic control. Diabetes Care 1999; 22:1555–1560. [DOI] [PubMed] [Google Scholar]
- 36.Hunsicker LG, Adler S, Caggiula A, et al. Predictors of the progression of renal disease in the modification of diet in Renal Disease Study. Kidney Int 1997; 51:1908–1919. [DOI] [PubMed] [Google Scholar]
- 37.Ruggenenti P, Perna A, Mosconi L, et al. Urinary protein excretion rate is the best independent predictor of ESRF in non-diabetic proteinuric chronic nephropathies. “Gruppo Italiano di Studi Epidemiologici in Nefrologia” (GISEN). Kidney Int 1998; 53:1209–1216. [DOI] [PubMed] [Google Scholar]
- 38.Remuzzi G, Bertani T. Pathophysiology of progressive nephropathies. N Engl J Med 1998; 339:1448–1456. [DOI] [PubMed] [Google Scholar]
- 39.Wu HY, Peng YS, Chiang CK, et al. Diagnostic performance of random urine samples using albumin concentration vs ratio of albumin to creatinine for microalbuminuria screening in patients with diabetes mellitus: a systematic review and meta-analysis. JAMA Intern Med 2014; 174:1108–1115. [DOI] [PubMed] [Google Scholar]
- 40.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 2013; 3:1–150. [Google Scholar]
- 41.Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int 2011; 79:1331–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inker LA, Levey AS, Pandya K, et al. Early change in proteinuria as a surrogate end point for kidney disease progression: an individual patient meta-analysis. Am J Kidney Dis 2014; 64:74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayashino Y, Fukuhara S, Akizawa T, et al. Cost-effectiveness of administering oral adsorbent AST-120 to patients with diabetes and advance-stage chronic kidney disease. Diabetes Res Clin Pract 2010; 90:154–159. [DOI] [PubMed] [Google Scholar]
- 44.Hsu CY, Bates DW, Kuperman GJ, et al. Diabetes, hemoglobin A(1c), cholesterol, and the risk of moderate chronic renal insufficiency in an ambulatory population. Am J Kidney Dis 2000; 36:272–281. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


