Abstract
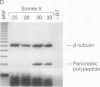
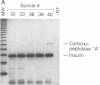
A central question in developmental biology has been the initiation of cell-specific gene expression and its temporal relationship to morphogenesis. We have coupled embryo microdissection with the exquisite sensitivity of the polymerase chain reaction to define the onset of cell-specific gene expression during pancreatic organogenesis. Using the precise assignment of gestational age by the number of somites in each embryo, we determined the onset of transcription of major genes of the endocrine and exocrine pancreas during mouse development to within 2-3 hr. Somatostatin mRNA was detected at the 10-somite stage throughout the foregut, consistent with the presence of somatostatin-producing cells throughout the adult gut. Mature mRNA for insulin and glucagon first appears surprisingly early, at the 20-somite stage in the wall of the embryonic foregut and is restricted to only the area of the duodenum from which the pancreas will arise 10-12 hr later. In contrast, exocrine gene transcription begins 24 hr after formation of the pancreatic diverticulum. Thus cell-specific gene expression in the endocrine pancreas begins in a "pre-morphogenetic phase." This early expression of insulin and glucagon could reflect the initiation of an endocrine cell lineage.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpert S., Hanahan D., Teitelman G. Hybrid insulin genes reveal a developmental lineage for pancreatic endocrine cells and imply a relationship with neurons. Cell. 1988 Apr 22;53(2):295–308. doi: 10.1016/0092-8674(88)90391-1. [DOI] [PubMed] [Google Scholar]
- Alumets J., Sundler F., Håkanson R. Distribution, ontogeny and ultrastructure of somatostatin immunoreactive cells in the pancreas and gut. Cell Tissue Res. 1977 Dec 28;185(4):465–479. doi: 10.1007/BF00220651. [DOI] [PubMed] [Google Scholar]
- Clark W. R., Rutter W. J. Synthesis and accumulation of insulin in the fetal rat pancreas. Dev Biol. 1972 Dec;29(4):468–481. doi: 10.1016/0012-1606(72)90084-x. [DOI] [PubMed] [Google Scholar]
- Han J. H., Rall L., Rutter W. J. Selective expression of rat pancreatic genes during embryonic development. Proc Natl Acad Sci U S A. 1986 Jan;83(1):110–114. doi: 10.1073/pnas.83.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Malaisse-Lagae F., Baetens D., Perrelet A. Pancreatic-polypeptide-rich regions in human pancreas. Lancet. 1978 Dec 2;2(8101):1200–1201. doi: 10.1016/s0140-6736(78)92181-5. [DOI] [PubMed] [Google Scholar]
- Pictet R. L., Clark W. R., Williams R. H., Rutter W. J. An ultrastructural analysis of the developing embryonic pancreas. Dev Biol. 1972 Dec;29(4):436–467. doi: 10.1016/0012-1606(72)90083-8. [DOI] [PubMed] [Google Scholar]
- Rall L. B., Pictet R. L., Williams R. H., Rutter W. J. Early differentiation of glucagon-producing cells in embryonic pancreas: a possible developmental role for glucagon. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3478–3482. doi: 10.1073/pnas.70.12.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappolee D. A., Mark D., Banda M. J., Werb Z. Wound macrophages express TGF-alpha and other growth factors in vivo: analysis by mRNA phenotyping. Science. 1988 Aug 5;241(4866):708–712. doi: 10.1126/science.3041594. [DOI] [PubMed] [Google Scholar]
- Sanders T. G., Rutter W. J. The developmental regulation of amylolytic and proteolytic enzymes in the embryonic rat pancreas. J Biol Chem. 1974 Jun 10;249(11):3500–3509. [PubMed] [Google Scholar]
- Schibler U., Pittet A. C., Young R. A., Hagenbüchle O., Tosi M., Gellman S., Wellauer P. K. The mouse alpha-amylase multigene family. Sequence organization of members expressed in the pancreas, salivary gland and liver. J Mol Biol. 1982 Mar 5;155(3):247–266. doi: 10.1016/0022-2836(82)90004-3. [DOI] [PubMed] [Google Scholar]
- Teitelman G., Joh T. H., Reis D. J. Linkage of the brain-skin-gut axis: islet cells originate from dopaminergic precursors. Peptides. 1981;2 (Suppl 2):157–168. doi: 10.1016/0196-9781(81)90026-7. [DOI] [PubMed] [Google Scholar]
- Wessells N. K., Evans J. Ultrastructural studies of early morphogenesis and cytodifferentiation in the embryonic mammalian pancreas. Dev Biol. 1968 Apr;17(4):413–446. doi: 10.1016/0012-1606(68)90073-0. [DOI] [PubMed] [Google Scholar]
- de Gasparo M., Pictet R. L., Rall L. B., Rutter W. J. Control of insulin secretion in the developing pancreatic rudiment. Dev Biol. 1975 Nov;47(1):106–122. doi: 10.1016/0012-1606(75)90267-5. [DOI] [PubMed] [Google Scholar]