Supplemental Digital Content is available in the text
Abstract
To establish the comparative effectiveness of all available biologic therapy regimens for ankylosing spondylitis, we performed a systematic review and a Bayesian network meta-analysis of randomized controlled trials.
PubMed, Medline, Embase, Cochrane library, and ClinicalTrials.gov were searched from the inception of each database to June 2015. Systematic review and network meta-analysis was reported according to the Preferred Reporting Items of Systematic Reviews and Meta-Analyses Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses. The primary outcome was 20% improvement of Assessments in SpondyloArthritis International Society Response Criteria (ASAS20) at Week 12 or 14; secondary outcomes were ASAS40, ASAS5/6, ASAS partial remission and 50% improvement in baseline Bath ankylosing spondylitis (AS) disease activity index. We reported relative risks and 95% confidence intervals from direct meta-analysis and 95% credible intervals from Bayesian network meta-analysis, and ranked the treatment for outcomes. We also used Grading of Recommendations Assessment, Development and Evaluation criteria to appraise quality of evidence.
Fourteen RCTs comprising 2672 active AS patients were included in the network meta-analysis. Most biologic therapy regimens were more effective than placebo regarding all the outcomes assessed, except for secukinumab and tocilizumab. No differences between biologic therapies in the treatment of AS could be found, except for the finding that infliximab 5 mg was superior to tocilizumab. Infliximab 5 mg/kg had the highest probability of being ranked the best for achieving ASAS20, whereas notably, secukinumab had the highest probability of being ranked the second best.
Our study suggests that no differences between biologic therapies in the treatment of AS could be found except that infliximab 5 mg was superior to tocilizumab. Infliximab 5 mg/kg seems to be the better biologic therapy regimen for AS. Secukinumab appears promising, though additional data is warranted. Nevertheless, these interpretations should be accepted very cautiously.
INTRODUCTION
Ankylosing spondylitis (AS) is the prototype of spondyloarthritis, an inter-related group of rheumatic diseases that share common primary clinical features such as inflammatory back pain, peripheral arthritis, and enthesitis.1 Characteristic symptoms of AS are spinal stiffness and loss of spinal mobility, which are explained by spinal inflammation, structural damage due to extensive osteoproliferation, or both.2 AS usually disables a person by severe back pain and remarkable spinal kyphotic deformity in later stage, which finally may necessitate a major corrective procedure. However, the procedure itself can be much challenging and perioperative risks are high. Therefore, it is very meaningful to control the symptoms and progress of AS in early stage by effective medication.
Biologic agents are becoming increasingly welcomed worldwide because of their obvious advantages of acting speed and efficacy over traditional pharmacies in treating AS.3 In the past decade, multiple tumor necrosis factor (TNF) inhibitors had been developed and confirmed effective in clinical randomized controlled trials (RCT)3 which made them mainstream biologic therapies so far. But clinicians and patients were often confused when they tried to select the most appropriate agent due to different drugs and doses introduced by different pharma companies. Even in the latest update of the Assessments in SpondyloArthritis International Society (ASAS) recommendations4 and 2014 update of Canadian Rheumatology Association /Spondyloarthritis Research Consortium of Canada Treatment Recommendations for the use of anti-TNF agents,5,6 no concrete agent and regimen was recommended. Additionally, several new biologic agents have emerged in recent years with the deepening of exploration and understanding of pathogenesis of AS and have been claimed to be effective in RCTs or observational studies.7–9 For instance, anti-IL-23 or anti-IL-17 agents have attracted many interests as they may represent the latest progress on the treatment of AS.10–12 However, most of the RCTs are small and placebo-controlled and may be underpowered to detect intrinsic differences, while the comparative efficacy of available biologic therapy regimens is largely unknown, mainly because of the absence of head-to-head trials. Conventional meta-analysis method is not able to resolve the issue owing to its incapability of comparing 3 or more treatments. But a network meta-analysis can summarize a comprehensive and coherent set of comparisons based on all of the available evidence.13 Hereby, the aim of our study was to assess the comparative efficacy of all available biologic therapy regimens in adults with AS using the technique of network meta-analysis and thus provide meaningful information in the hope of establishing the optimal treatment regimen for the treatment of AS.
METHODS
A Bayesian model was used to accomplish our network meta-analysis. We reported the systematic review and meta-analysis following the Preferred Reporting Items of Systematic Reviews and Meta-Analyses (PRISMA) Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses.14 Ethical approval and informed consent were not required because what we studied neither collected patients’ information nor influenced the patient care.
Eligibility Criteria
We included the literatures and reports according to the following criteria: participants aged 18 years or older who had AS defined by 1984 modified New York criteria15 and had responded inadequately to nonsteroidal anti-inflammatory drugs (NSAIDs); interventions included all available biologic agents for AS at present such as anti-TNF-α agent, anti-IL-23 or anti-IL-17 or anti-IL-6 agents, and placebo; comparison focused on the efficacy discrepancy between biologic agents or placebo; primary or secondary outcomes included the proportion of patients achieving 20% improvement in the ASAS Response Criteria (ASAS20) at Week 12 or 14; study design was randomized controlled trial. All eligible treatment regimens with different doses and dosing intervals were included in the treatment network and each treatment constituted 1 node. No treatment had been merged into the same node.
We excluded the literatures if they recruited patients diagnosed as spondyloarthritis or AS with total spine ankyloses. Studies with overlapping data or about the maintaining of response of biologic therapies with different doses, observational study, systematic review, and meta-analysis were excluded as well. We also excluded uncompleted trials or completed trials without results provided.
Search Strategy and Study Selection
PubMed, Medline, Embase, Cochrane library, and ClinicalTrials.gov were searched from the inception of each database to June 2015 using the following retrieval language: (“ankylosing spondylitis” OR MeSH term “spondylitis, ankylosing”) AND (“biologic agent” OR “biologic therapy”) AND (“randomized controlled trial”). Publication language was restricted to English. Two reviewers selected studies independently based on prespecified inclusion and exclusion criteria. They identify trials through reviewing the titles and abstracts of studies that met the eligibility criteria. Conflicts in opinion between investigators were resolved by consensus and consultation with the principle author. We also performed a recursive search of the bibliographies of published systematic reviews and/or meta-analyses on this topic to identify any additional studies that were not found by searching of above databases.
Data Collection Process and Data Items
Data on the study, participant, and intervention characteristics were extracted from selected reports into a standardized form by 2 investigators independently. The extracted data were then double-checked and confirmed by a third investigator. The following data items were documented: study characteristics: primary author and year of publication/ClinicalTrials.gov Identifier (NCT number), geographic location and study design-native/continental/international, single/multiple center(s), funding source(s); participant characteristics: total number, mean age, number of male patients, number of HLA-B27 positive patients, duration of AS, number of extra-articular manifestations, number of concomitant disease-modifying antirheumatic drugs (DMARDs), number of concomitant corticosteroids, number of concomitant NSAIDs, baseline C-reactive protein (CRP) level, baseline Bath AS disease activity index (BASDAI); intervention characteristics: dosing and schedule; outcome assessment: number of patients achieving ASAS20, ASAS40, ASAS5/6, ASAS partial remission, and BASDAI 50 at Week 12 or 14 in intervention and comparator group if available.
Geometry of the Network
We used NetMetaXL16—a Microsoft-Excel-based tool—to present graphs of the networks. Each node in a network symbolizes a treatment and the edges represent randomized comparisons.14 The size of each node is proportional to the total sample size for each treatment and the width of the edge corresponds to the number of RCTs.14 Considerations were made to construct networks according to drug classes based on mechanism of action and drug companies. We provided networks for the treatment of AS based on the splitting and lumping of treatment regimens.14 In the network of lumping treatment wherein a drug is compared with the same agent in different doses or administrating intervals, it is represented by an autoloop. In the networks of companies, nodes represent companies and autoloops stand for trials utilizing agents from a same pharma. Edges between different nodes indicate trials comparing agents from different pharmas.
Risk of Bias Within Individual Studies
We utilized the revised Cochrane Collaboration's Tool for Assessing Risk of Bias17 to assess the risk of bias within individual studies. This tool addresses 7 specific domains: sequence generation, allocation concealment, blinding of participants, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and “other issues.” With this tool, the quality of each RCT could be comprehensively evaluated which might be helpful when interpreting the results.
Outcome Assessed
The primary outcome was ASAS20 which was defined as the percentage of patients with at least 20% response and absolute improvement of at least 1 unit as compared with baseline in at least 3 of the following 4 domains18 at Week 12 or 14: patient's global assessment of disease activity during the previous week18 (0–10 cm Visual Analog Scale [VAS]); patient's assessment of pain during the previous week, represented by the total back pain score18 (0–10 cm VAS); function, represented by BASFI score18 (0–10 cm VAS); and inflammation, represented by the mean of the severity and morning stiffness18 (questions 5 and 6 of the BASDAI) (0–10 cm VAS), without deterioration in the remaining domain.
Secondary outcomes included ASAS40 (40% response), ASAS5/6 (improvement in 5 of 6 domains: pain, patient global assessment, function, inflammation, spinal mobility, C-reactive protein without deterioration in the 6th domain), ASAS partial remission (a value of <2 on a 0–10 scale in each of the 4 domains of the ASAS20), and BASDAI50 (number of patients achieving 50% improvement in baseline BASDAI).19
Statistical Analysis
Two types of meta-analysis were conducted. We first did direct pairwise meta-analyses with Review Manager v5.3.5 (http://tech.cochrane.org/revman/download) using random-effect model to evaluate pooled relative risk and 95% confidence intervals. Heterogeneity was assessed with I2 statistic. Whereafter, we performed random-effect Bayesian network meta-analyses using Markov chain Monte Carlo methods with Aggregate Data Drug Information System20 v1.16.6 (http://drugis.org/software/addis1/addis1.16) and presented the estimates as odd ratios and the corresponding 95% credible intervals (95% CrI). Aggregate Data Drug Information System software is programmed using the prior distribution of all parameters in Bayesian analyses. We updated Markov chain Monte Carlo model with 100,000 simulation iterations. Convergence was assessed using the Brooks–Gelman–Rubin method.21 This method compares within-chain and between-chain variance to calculate the Potential Scale Reduction Factor (PSRF). Convergence was deemed to be reached if a PSRF was close to 1.21
We assumed that all participants included in our study could be randomly allocated to any treatment group being compared. Transitivity might not hold when direct-comparison evidences differ with indirect evidences for the same comparison, which is also termed “inconsistency.” Inconsistency occurs only when there are closed loops in the network structure. We assessed inconsistency in 2 ways. First, we estimated the inconsistency factors with a loop-specific approach within each closed triangular or quadratic loop.22 Inconsistent loops would be identified if they yielded a 95% Crl excluding 0. Second, we used node-splitting technique to assess whether direct and indirect evidences on a specific node (the split node) are in agreement.23 A large P value indicates no significant inconsistency was found.
We ranked the treatments for outcomes with use of rank probability plots which presented the rankings by different color columns. The plots showed rankings as the best, the second best, and so on.
Quality of Evidence
To appraise the quality of evidences of current direct and network meta-analysis for the primary outcome, we adopted the Grading of Recommendations Assessment, Development and Evaluation (GRADE) method24–31 and summarized and presented the information with the online application of Guideline Developing Tool (GDT, previously known as GRADEpro) (http://www.guidelinedevelopment.org/). When the ratings of direct and indirect evidence are semblable, we used the higher one as the grading of our network meta-analysis estimates. When the direct evidence had higher quality, we selected this over the network evidence.
RESULTS
Two hundred ninety-seven studies were screened after removing duplications from a total of 316 studies identified using the searching strategy. Two hundred sixty-seven studies were excluded due to ineligibility after reviewing the titles and abstracts. Finally, 14 RCTs (13 published18,19,32–42 and NCT00195819) were included in this network meta-analysis after assessing for eligibility of full text. Figure 1 shows the PRISMA flow diagram of study selection process.
FIGURE 1.
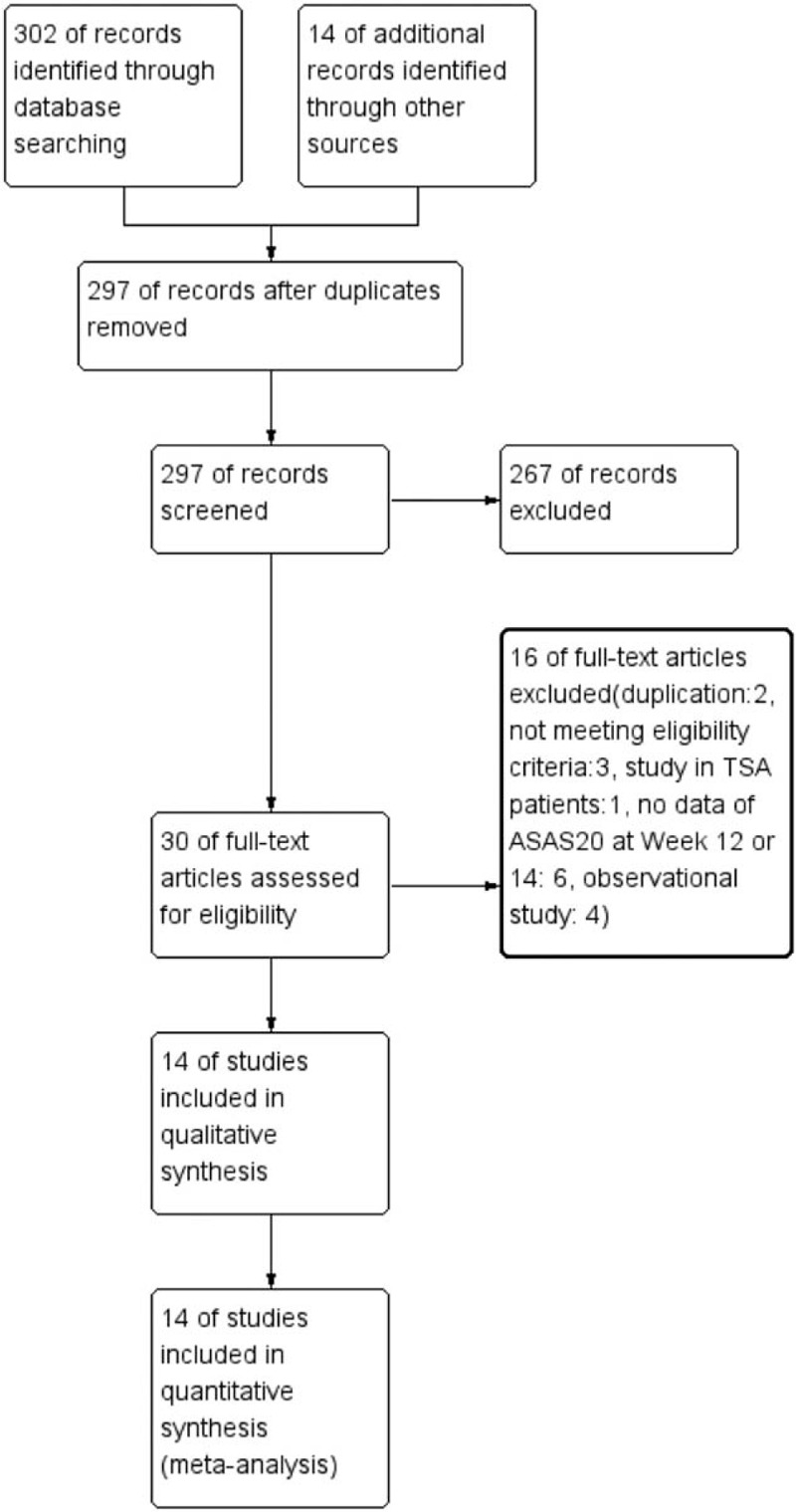
The PRISMA flow diagram of study selection process.
Baseline characteristics of studies included in the network meta-analysis are summarized in Table 1. A total of 2672 active AS patients in 14 trials received biologic therapies or placebo. DMARDs and NSAIDs were permitted to continue in most studies. In general, patients were similar in terms of baseline data such as age, sex, HLA-B27-positive proportion, duration of AS, concomitant drugs, CRP, and BASDAI.
TABLE 1.
Baseline Characteristics of Studies Included in the Network Meta-Analysis
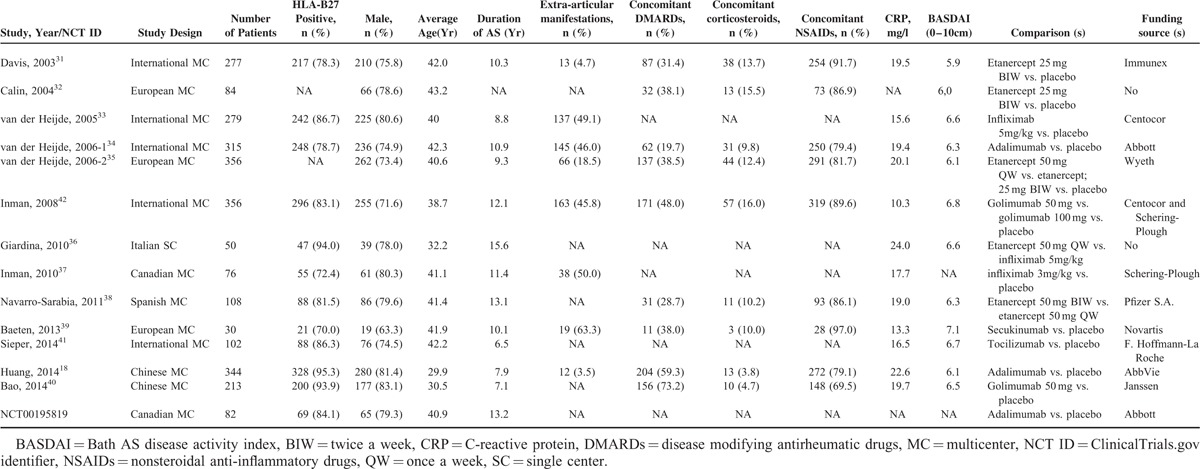
Thirteen of 14 (92.9%) trials were multicenter design (5 native, 3 European, 5 international). Only 1 trial was carried out in a single center. Twelve of 14 (85.7%) studies were funded by pharmaceutical companies. Biologic agents studied in all trials included adalimumab, etanercept, golimumab, infliximab, secukinumab, and tocilizumab. Treatment regimens investigated included adalimumab 40 mg every other week for 12 weeks or 24 weeks (“adalimumab” for short), etanercept 25 mg or 50 mg twice a week for 12 or 24 weeks (“etanercept 25 mg BIW” or “etanercept 50 mg BIW”), etanercept 50 mg once a week for 12 weeks or 102 weeks (“etanercept 50 mg QW”), golimumab 50 mg or 100 mg every 4 weeks for 24 weeks (“golimumab 50 mg” or “golimumab 100 mg”), infliximab 5 mg/kg at weeks 0, 2, 6 and every 6 weeks for 18 weeks or 102 weeks (“infliximab 5 mg”), infliximab 3 mg/kg at weeks 0, 2, 6 and every 6 weeks for 12 weeks (“infliximab 3 mg”), secukinumab 2 × 10 mg/kg given 3 weeks apart for 28 weeks (“secukinumab”) and tocilizumab 8 mg/kg every 4 weeks for 12 weeks (“tocilizumab”). Twelve of 14 studies were 2-arm trials comparing biologic agent with placebo, 2 of 14 studies were 3-arm trials comparing 2 dose and/or dosing interval of etanercept, golimumab, and placebo, respectively.
Figure 2 demonstrates the network graph of all eligible comparisons for the primary outcome. The node(s) with the same color indicate(s) that the intervention(s) belong(s) to the same drug class. Pairwise comparisons for the primary outcome were more commonly seen of TNF inhibitors against placebo (11/17 direct comparisons). Most biologic treatment regimens were investigated by only 1 RCT, except for adalimumab, etanercept 25 mg BIW and golimumab 50 mg, which was studied by 3, 3, and 2 RCTs respectively. Network graphs of lumping treatments and sponsored drug companies were shown in Supplementary Digital Content, Figures 1.1 and 1.2. Autoloops were drawn for agents such as etanercept, golimumab as well as companies such as Centocor and Pfizer. No comparison but 1 was conducted between biologic agents from different companies.
FIGURE 2.
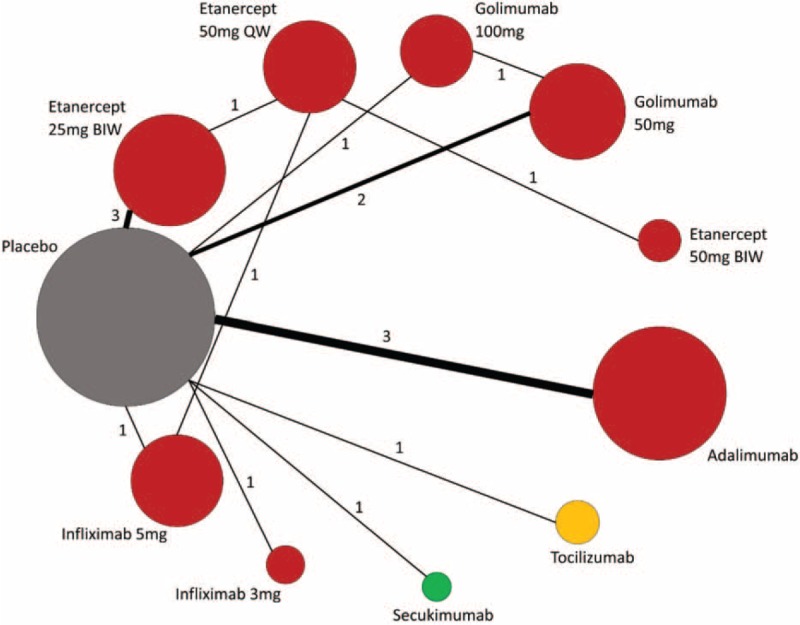
Network of all eligible comparisons for the primary outcome of biologic therapies in patients with AS. AS = ankylosing spondylitis.
Risk of bias assessments of overall and study level are summarized in Figure 3 and Supplementary Digital Content, Figure 2, respectively. In general, the studies were considered to be at low risk of bias regarding selection, performance, detection, attrition, and reporting bias.
FIGURE 3.
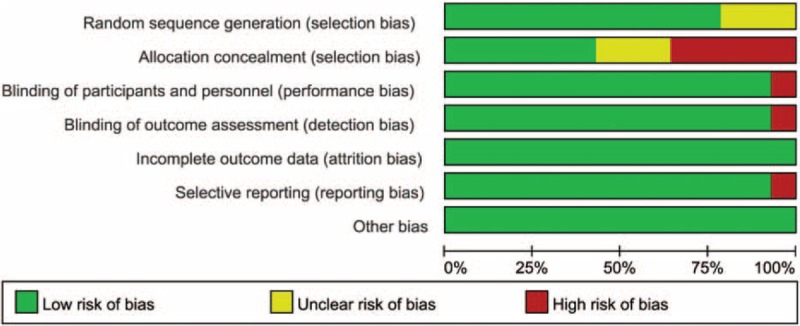
Risk of bias graph of included studies on overall level.
Data for direct pairwise comparisons for primary outcome are shown as forest plots in Figure 4 and, for secondary outcomes, Supplementary Digital Content, Figures 3.1–3.4. Compared with placebo, most biologic therapies were associated with significantly higher proportions of patients achieved ASAS20, ASAS40, ASAS5/6, ASAS partial remission and BASDAI50, except for secukinumab and tocilizumab. In the head-to-head trials, etanercept 50 mg QW was comparable to infliximab 5 mg, the effects of etanercept 50 mg QW were substantially equal to that of etanercept 50 mg BIW. The direct pairwise meta-analyses were highly heterogeneous in general for all outcomes assessed.
FIGURE 4.
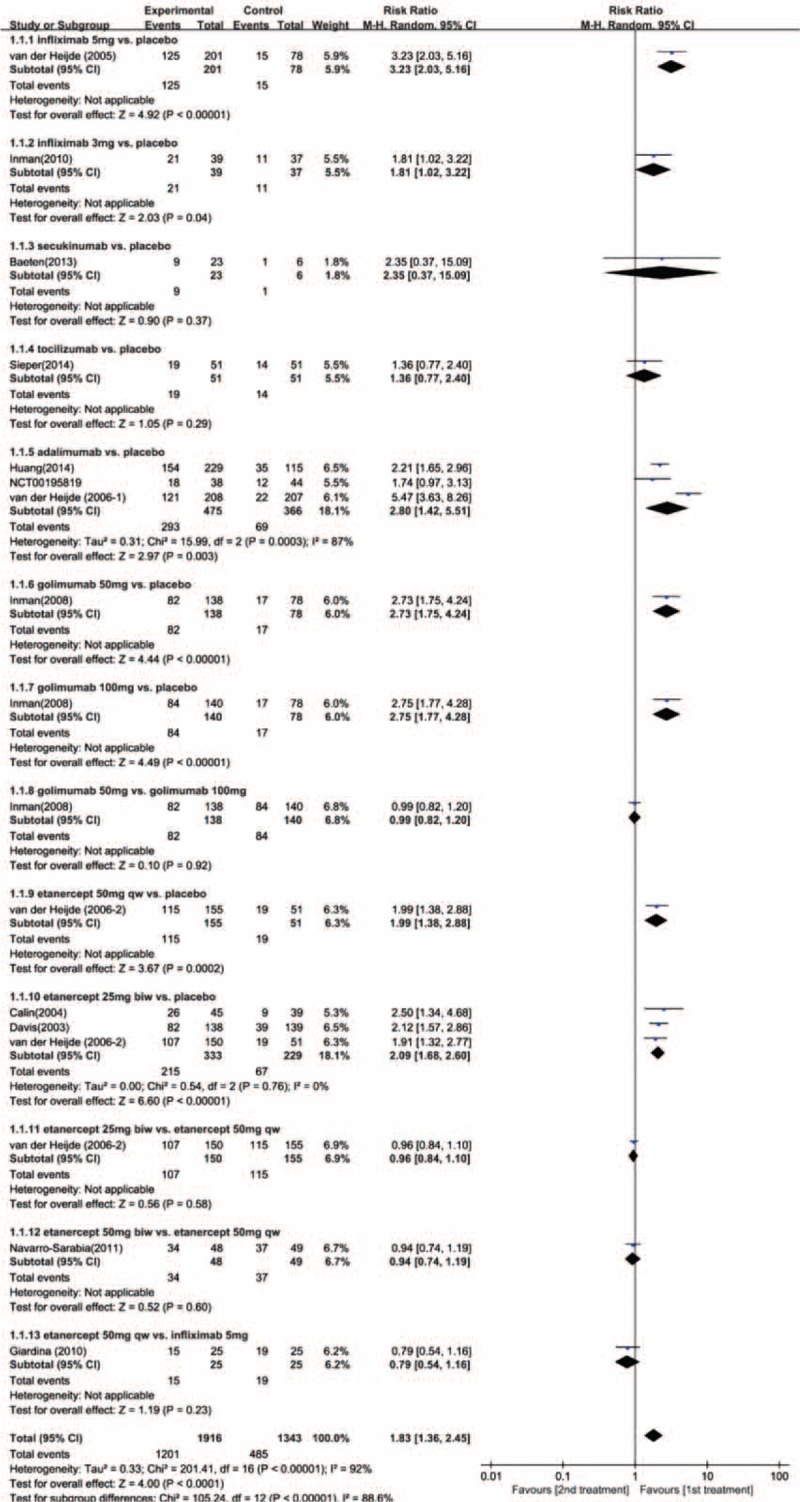
Forest plot of direct pairwise comparisons for ASAS20.
Estimated effects of biologic agent regimens in network meta-analysis on ASAS20 are shown as league table in Figure 5 and Supplementary Digital Content, Figures 4.1 to 4.4. Convergence was reached in all analyses (data not shown). With regard to ASAS20, adalimumab, etanercept 25 mg BIW or 50 mg QW, golimumab 100 mg or 50 mg, and infliximab 5 mg were associated with better therapeutic effect when compared with placebo. None of the other agents including etanercept 50 mg BIW, infliximab 3 mg, secukinumab, and tocilizumab was superior to placebo. On comparative effectiveness of all biologic interventions of network meta-analysis, only infliximab 5 mg was seen to be superior to tocilizumab (OR, 4.81; 95% Crl, 1.43–17.04). No significant superiority was found among the other regimens. With reference to ASAS40 and ASAS partial remission, as compared with placebo, only adalimumab was associated with clearly better effect (OR, 5.89, 95% Crl, 1.17–31.12; OR, 8.00, 95% Crl, 1.65–42.19; respectively). With reference to ASAS5/6, only infliximab 3 mg was superior to placebo (OR, 60.93; 95% Crl, 1.79–3930.24). Regarding BASDAI50, no biologic interventions were better than placebo. On comparative effectiveness of all biologic interventions of Bayesian network meta-analysis for all the secondary outcomes, no regimen was significantly superior to others, with high degree of imprecision. Statistical inconsistency was not found between estimates from direct and indirect evidences where both were available (only on the estimate of ASAS20, loop-specific approach: 95% Crl was −0.92 to 1.14 and −0.86 to 1.29 for the 2 closed loops, respectively; node-splitting technique: P values ranged from 0.66 to 0.88).
FIGURE 5.
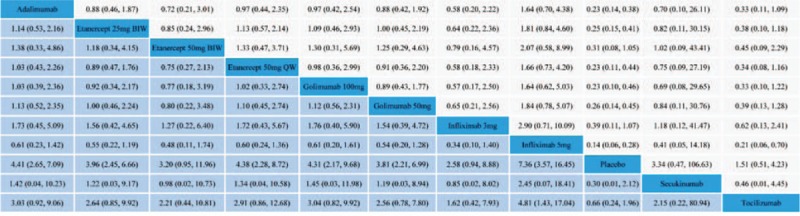
League table of estimated effects of biologic therapy regimens in network meta-analysis on ASAS20.
Figure 6 and Supplementary Digital Content, Figures 5.1 to 5.4 showed the rank probability plots for primary outcome and secondary outcomes, respectively. Infliximab 5 mg had the highest probability of being ranked the best for achieving ASAS20, whereas notably secukinumab had the highest probability of being ranked the second best. For secondary outcomes such as ASAS40 and ASAS5/6, infliximab 3 mg had the highest probability of being ranked the best. While for ASAS partial remission and BASDAI50, etanercept 50 mg BIW had the highest probability of being ranked the best.
FIGURE 6.
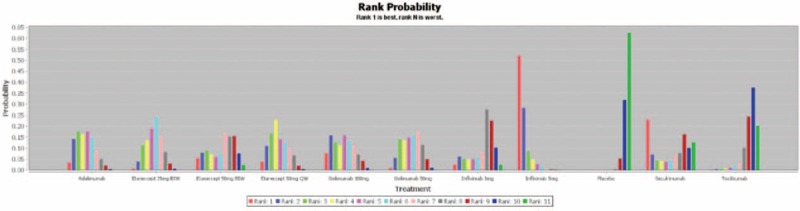
Rank probability plot of network meta-analysis for ASAS20.
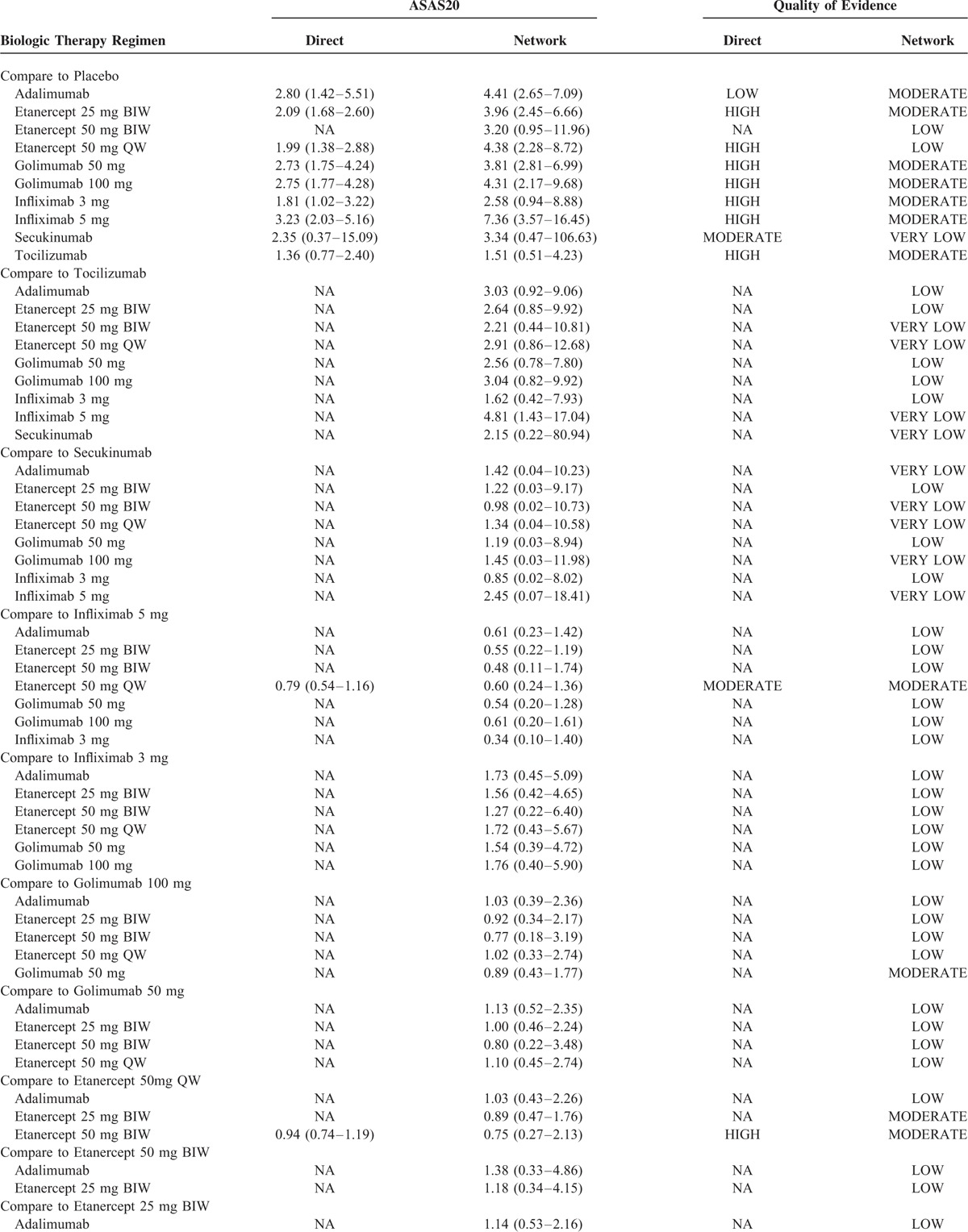
Table 2 reveals the quality of evidence for direct and network meta-analysis for the primary outcome appraised by GRADE approach. Generally, the quality of direct evidence was high, except for the comparison of adalimumab versus placebo, secukinumab versus Placebo, and etanercept 50 mg QW versus infliximab 5 mg, which was respectively rated down for serious inconsistency, imprecision, and risk of bias. The quality of all indirect evidences without head-to-head trials was low or very low due to serious indirectness and/or imprecision. When comparing with placebo, most biologic therapy regimens were supported by at least moderate quality evidence. The only exception was etanercept 50 mg BIW, whose effect was supported by low-quality evidence. No comparison was rated down for the risk of bias and publication bias.
TABLE 2.
Quality of Evidence for Direct and Network Metaanalysis for the Primary Outcome by GRADE
DISCUSSION
This is the first systematic review and network meta-analysis comparing all available biologic therapy regimens for the treatment of AS, with no restriction to anti-TNF agents. This is also the first report rigorously conducted in accordance with the PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-Analyses.14 We provided estimated data of comparative effectiveness of these regimens as well as the GRADE rating of quality of evidence, remedying the deficiency of comparative data in head-to-head trials, and whereupon offering clinicians and policymakers beneficial reference information when selecting a biologic therapy for treating active AS. The following main findings were obtained from our analysis: no differences between biologics in the treatment of AS could be found, except that infliximab 5 mg was superior to tocilizumab; infliximab 5 mg seems to be a better choice among all available biologic therapies for treating AS, as it ranked the first to achieve ASAS20; secukinumab, as a newly introduced biologic agent for treating AS targeting IL-17, was ranked as the second effective regimen to achieve ASAS20, although it was not significantly better than placebo, with moderate confidence in estimates; tocilizumab has no superiority to placebo, with high confidence.
TNF inhibitor is still the prevailing biologic agent to treat AS after failure of conventional therapeutic approaches with NSAIDs, DMARDs, oral corticosteroids, or local corticosteroid injections.3 However, varied products had been developed by diverse pharmaceutical companies, and many of which were devoted to report outcomes of different doses or administering intervals by funding researchers. Under this circumstance, clinicians or health care providers tend to be perplexed by choosing optimal TNF inhibitor regimen for an individual patient. No recommendation or guideline for using specific TNF inhibitor can be found in the latest update of recommendations for management of AS from authoritative associations,4,5 who only suggests that the choice of TNF inhibitor should be determined by consultation between the physician and the patient, and dosing and monitoring of these drugs should be tailored to the individual patient and follow usual standard of care.5 Luckily, 3 previously published network meta-analyses43–45 had concentrated efforts on determining the relative efficacy of TNF inhibitors. Two analyses comparing all TNF inhibitors concluded that infliximab 5 mg/kg seemed to be the best anti-TNF therapy regimen,43,44 which was consistent with our findings. Another analysis that compared subcutaneous TNF inhibitors suggested that golimumab may provide the highest probability of achieving ASAS20 response in AS patients.45 However, these data should be interpreted cautiously, because methodologic defects, such as irrational eligibility criteria, lack of assessment of risk of bias, and appraisal of the quality of evidence, existed commonly in above network meta-analyses.
In addition, as the efficacy of TNF inhibitors was virtually not satisfied yet, researchers have kept working industriously to develop new biologic agent. Particularly, IL-23/IL-17 axis is implicated in the pathogenesis of AS and is considered a promising new target in the treatment of AS.10–12 Secukinumab, an anti-IL-17A monoclonal antibody, was reported rapidly reducing clinical or biologic signs of active AS and thought to be superior to placebo with regard to ASAS20 response at Week 6 in a European multicenter RCT.39 Nevertheless, time of evaluation for ASAS20 response is recommended after at least 12 weeks by ASAS, although this recommendation is established for the use of anti-TNF agents in patients with spondyloarthritis.4 Both our direct comparison and indirect comparison of secukinumab with placebo for ASAS20 at Week 12 showed no significant difference between the 2 interventions. But meanwhile, our network analysis revealed that secukinumab ranked the second best among all biologic therapy regimens for achieving ASAS20 at Week 12. This is an encouraging observation, together with the above report, indicating the potential value of further investigating the effect of anti-IL-17 in treating AS.
Unfortunately, we did not find any RCT for comparison of anti-IL-23 agent with placebo for the treatment of AS, although ustekinumab—a fully human IgG1κ monoclonal antibody that binds with the p40 protein subunit of IL-12 and IL-23—has been shown to be effective in the treatment of psoriasis46,47 and psoriatic arthritis.48,49 IL-23 is largely involved in the onset and progress of AS10 and plays a key role in inducing CD4 memory T cells to produce IL-17.12 A recent prospective, open-label, proof-of-concept trial discovered that ustekinumab in a dose of 90 mg administered subcutaneously at baseline, week 4 and week 16 was associated with a reduction of signs and symptoms in active AS and was well tolerated.50 Future RCTs are expected to confirm the results.
Up to date, our network meta-analysis provides the most comprehensive information of comparative effectiveness of almost all available biologic therapy regimens with different mechanism of actions for AS. Infliximab 5 mg may be the better biologic therapy regimen, although it was associated with higher costs compared with other major TNF inhibitors.51 Tocilizumab should no longer be considered for the treatment of AS due to its ineffectivity.
However, our study has potential limitations. First, although the overall risk of bias of studies included was considered to be low, there was 1 unblinded study36 which might introduce bias. Second, considering the relatively small amount of studies contributing to this comparative analysis, the results of the rank probability plots should be very cautiously interpreted due to insufficient stability. Third, because of scant original data for the secondary outcomes, the significances of estimated effects for ASAS40, ASAS5/6, ASAS partial remission and BASDAI50 were discounted. Fourth, we did not compare the safety of biologic therapy regimens for AS, because most regimens were proved to be safe with scarce severe adverse event occurred. Finally, language is restricted to English for included RCTs; therefore, trials reported by other languages may be missed.
CONCLUSION
Our analysis shows that except for the finding that infliximab 5 mg was superior to tocilizumab, no differences between biologic therapies in the treatment of AS could be found. Infliximab 5 mg/kg may be a better biologic therapy regimen for AS, but this interpretation should be accepted very cautiously. Secukinumab also appears promising, though additional data is warranted. This systematic review and network meta-analysis would be helpful to inform the development of future practice recommendations in the treatment of AS.
Supplementary Material
Footnotes
Abbreviations: AS = ankylosing spondylitis, ASAS = the Assessments in SpondyloArthritis international Society, BASDAI = bath AS disease activity index, BIW = twice a week, DMARDs = disease modifying antirheumatic drugs, GRADE = the Grading of Recommendations Assessment, Development and Evaluation, IL = interleukin, NSAIDs = nonsteroidal anti-inflammatory drugs, OR = odd ratio, QW = once a week, RCT = randomized controlled trials, RR = relative risk, SpA = spondyloarthritis, TNF = tumor necrosis factor, VAS = Visual Analog Scale
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
REFERENCES
- 1.Braun J, Sieper J. Ankylosing spondylitis. Lancet 2007; 369:1379–1390. [DOI] [PubMed] [Google Scholar]
- 2.Wanders A, Landewe R, Dougados M, et al. Association between radiographic damage of the spine and spinal mobility for individual patients with ankylosing spondylitis: can assessment of spinal mobility be a proxy for radiographic evaluation. Ann Rheum Dis 2005; 64:988–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun J, Baraliakos X, Heldmann F, et al. Tumor necrosis factor alpha antagonists in the treatment of axial spondyloarthritis. Expert Opin Investig Drugs 2014; 23:647–659. [DOI] [PubMed] [Google Scholar]
- 4.van der Heijde D, Sieper J, Maksymowych WP, et al. 2010 Update of the international ASAS recommendations for the use of anti-TNF agents in patients with axial spondyloarthritis. Ann Rheum Dis 2011; 70:905–908. [DOI] [PubMed] [Google Scholar]
- 5.Rohekar S, Chan J, Tse SM, et al. 2014 update of the Canadian Rheumatology Association/Spondyloarthritis Research Consortium of Canada treatment recommendations for the management of spondyloarthritis. Part II: specific management recommendations. J Rheumatol 2015; 42:665–681. [DOI] [PubMed] [Google Scholar]
- 6.Rohekar S, Chan J, Tse SM, et al. 2014 update of the Canadian Rheumatology Association/Spondyloarthritis Research Consortium of Canada treatment recommendations for the management of spondyloarthritis. Part I: principles of the management of spondyloarthritis in Canada. J Rheumatol 2015; 42:654–664. [DOI] [PubMed] [Google Scholar]
- 7.Van den Bosch F, Deodhar A. Treatment of spondyloarthritis beyond TNF-alpha blockade. Best Pract Res Clin Rheumatol 2014; 28:819–827. [DOI] [PubMed] [Google Scholar]
- 8.Toussirot E. Biologics in spondyloarthritis: TNFalpha inhibitors and other agents. Immunotherapy 2015; 7:669–681. [DOI] [PubMed] [Google Scholar]
- 9.Wendling D. Treating to target in axial spondyloarthritis: defining the target and the arrow. Expert Rev Clin Immunol 2015; 11:691–693. [DOI] [PubMed] [Google Scholar]
- 10.Yeremenko N, Paramarta JE, Baeten D. The interleukin-23/interleukin-17 immune axis as a promising new target in the treatment of spondyloarthritis. Curr Opin Rheumatol 2014; 26:361–370. [DOI] [PubMed] [Google Scholar]
- 11.Ebihara S, Date F, Dong Y, et al. Interleukin-17 is a critical target for the treatment of ankylosing enthesitis and psoriasis-like dermatitis in mice. Autoimmunity 2015; 48:259–266. [DOI] [PubMed] [Google Scholar]
- 12.Jethwa H, Bowness P. The IL23/IL17 axis in Ankylosing Spondylitis: new advances and potentials for treatment. Clin Exp Immunol 2016; 183:30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh S, Khera R, Allen AM, et al. Comparative effectiveness of pharmacological interventions for non-alcoholic steatohepatitis: a systematic review and network meta-analysis. Hepatology 2015; 62:1417–1432. [DOI] [PubMed] [Google Scholar]
- 14.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015; 162:777–784. [DOI] [PubMed] [Google Scholar]
- 15.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984; 27:361–368. [DOI] [PubMed] [Google Scholar]
- 16.Brown S, Hutton B, Clifford T, et al. A Microsoft-Excel-based tool for running and critically appraising network meta-analyses: an overview and application of NetMetaXL. Syst Rev 2014; 3:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins J, Green S. Cochrane Handbook for Systematic Reviews ofInterventions Version 5.1.0. The Cochrane Collaboration, 2011. Available at: www.cochrane-handbook.org Accessed March, 2011. [Google Scholar]
- 18.van der Heijde D, Kivitz A, Schiff MH, et al. Efficacy and safety of adalimumab in patients with ankylosing spondylitis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2006; 54:2136–2146. [DOI] [PubMed] [Google Scholar]
- 19.Huang F, Gu J, Zhu P, et al. Efficacy and safety of adalimumab in Chinese adults with active ankylosing spondylitis: results of a randomised, controlled trial. Ann Rheum Dis 2014; 73:587–594. [DOI] [PubMed] [Google Scholar]
- 20.Van Valkenhoef G, Tervonen T, Zwinkels T, et al. ADDIS: a decision support system for evidence-based medicine. Decis Support Syst 2013; 55:459–475. [Google Scholar]
- 21.Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat 1998; 7:434–455. [Google Scholar]
- 22.Lu G, Ades AE. Assessing evidence inconsistency in mixed treatment comparisons. J Am Stat Assoc 2006; 101:447–459. [Google Scholar]
- 23.Dias S, Welton NJ, Caldwell DM. Checking consistency in mixed treatment comparison meta-analysis. Stat Med 2010; 29:932–944. [DOI] [PubMed] [Google Scholar]
- 24.Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011; 64:401–406. [DOI] [PubMed] [Google Scholar]
- 25.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011; 64:383–394. [DOI] [PubMed] [Google Scholar]
- 26.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines 6. Rating the quality of evidence—imprecision. J Clin Epidemiol 2011; 64:1283–1293. [DOI] [PubMed] [Google Scholar]
- 27.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence—inconsistency. J Clin Epidemiol 2011; 64:1294–1302. [DOI] [PubMed] [Google Scholar]
- 28.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 8. Rating the quality of evidence—indirectness. J Clin Epidemiol 2011; 64:1303–1310. [DOI] [PubMed] [Google Scholar]
- 29.Guyatt GH, Oxman AD, Montori V, et al. GRADE guidelines: 5. Rating the quality of evidence—publication bias. J Clin Epidemiol 2011; 64:1277–1282. [DOI] [PubMed] [Google Scholar]
- 30.Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). J Clin Epidemiol 2011; 64:407–415. [DOI] [PubMed] [Google Scholar]
- 31.Guyatt G, Oxman AD, Sultan S, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J Clin Epidemiol 2013; 66:151–157. [DOI] [PubMed] [Google Scholar]
- 32.Davis JC, Jr, Van Der Heijde D, Braun J, et al. Recombinant human tumor necrosis factor receptor (etanercept) for treating ankylosing spondylitis: a randomized, controlled trial. Arthritis Rheum 2003; 48:3230–3236. [DOI] [PubMed] [Google Scholar]
- 33.Calin A, Dijkmans BA, Emery P, et al. Outcomes of a multicentre randomised clinical trial of etanercept to treat ankylosing spondylitis. Ann Rheum Dis 2004; 63:1594–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Heijde D, Dijkmans B, Geusens P, et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT). Arthritis Rheum 2005; 52:582–591. [DOI] [PubMed] [Google Scholar]
- 35.van der Heijde D, Da SJC, Dougados M, et al. Etanercept 50 mg once weekly is as effective as 25 mg twice weekly in patients with ankylosing spondylitis. Ann Rheum Dis 2006; 65:1572–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giardina AR, Ferrante A, Ciccia F, et al. A 2-year comparative open label randomized study of efficacy and safety of etanercept and infliximab in patients with ankylosing spondylitis. Rheumatol Int 2010; 30:1437–1440. [DOI] [PubMed] [Google Scholar]
- 37.Inman RD, Maksymowych WP. A double-blind, placebo-controlled trial of low dose infliximab in ankylosing spondylitis. J Rheumatol 2010; 37:1203–1210. [DOI] [PubMed] [Google Scholar]
- 38.Navarro-Sarabia F, Fernandez-Sueiro JL, Torre-Alonso JC, et al. High-dose etanercept in ankylosing spondylitis: results of a 12-week randomized, double blind, controlled multicentre study (LOADET study). Rheumatology (Oxford) 2011; 50:1828–1837. [DOI] [PubMed] [Google Scholar]
- 39.Baeten D, Baraliakos X, Braun J, et al. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trial. Lancet 2013; 382:1705–1713. [DOI] [PubMed] [Google Scholar]
- 40.Bao C, Huang F, Khan MA, et al. Safety and efficacy of golimumab in Chinese patients with active ankylosing spondylitis: 1-year results of a multicentre, randomized, double-blind, placebo-controlled phase III trial. Rheumatology (Oxford) 2014; 53:1654–1663. [DOI] [PubMed] [Google Scholar]
- 41.Sieper J, Porter-Brown B, Thompson L, et al. Assessment of short-term symptomatic efficacy of tocilizumab in ankylosing spondylitis: results of randomised, placebo-controlled trials. Ann Rheum Dis 2014; 73:95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inman RD, Davis JC, Jr, Dv H, et al. Efficacy and safety of golimumab in patients with ankylosing spondylitis: results of a randomized, double-blind, placebo-controlled, phase III trial. Arthritis Rheum 2008; 58:3402–3412. [DOI] [PubMed] [Google Scholar]
- 43.Migliore A, Broccoli S, Bizzi E, et al. Indirect comparison of the effects of anti-TNF biological agents in patients with ankylosing spondylitis by means of a mixed treatment comparison performed on efficacy data from published randomised, controlled trials. J Med Econ 2012; 15:473–480. [DOI] [PubMed] [Google Scholar]
- 44.Shu T, Chen GH, Rong L, et al. Indirect comparison of anti-TNF-alpha agents for active ankylosing spondylitis: mixed treatment comparison of randomized controlled trials. Clin Exp Rheumatol 2013; 31:717–722. [PubMed] [Google Scholar]
- 45.Migliore A, Bizzi E, Bernardi M, et al. Indirect comparison between subcutaneous biologic agents in ankylosing spondylitis. Clin Drug Investig 2015; 35:23–29. [DOI] [PubMed] [Google Scholar]
- 46.Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet 2008; 371:1675–1684. [DOI] [PubMed] [Google Scholar]
- 47.Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 2008; 371:1665–1674. [DOI] [PubMed] [Google Scholar]
- 48.McInnes IB, Kavanaugh A, Gottlieb AB, et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet 2013; 382:780–789. [DOI] [PubMed] [Google Scholar]
- 49.Ritchlin C, Rahman P, Kavanaugh A, et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis 2014; 73:990–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poddubnyy D, Hermann KG, Callhoff J, et al. Ustekinumab for the treatment of patients with active ankylosing spondylitis: results of a 28-week, prospective, open-label, proof-of-concept study (TOPAS). Ann Rheum Dis 2014; 73:817–823. [DOI] [PubMed] [Google Scholar]
- 51.McLeod C, Bagust A, Boland A, et al. Adalimumab, etanercept and infliximab for the treatment of ankylosing spondylitis: a systematic review and economic evaluation. Health Technol Assess 2007; 11:1–158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


