Supplemental Digital Content is available in the text
Abstract
Nonapnea sleep disorders (NASDs) and associated problems, which are highly prevalent in patients with kidney diseases, are associated with unfavorable medical sequelae. Nonetheless, whether NASDs are associated with acute kidney injury (AKI) development has not been thoroughly analyzed. We examined the association between NASD and AKI.
We conducted a population-based study by using 1,000,000 representative data from the Taiwan National Health Insurance Research Database for the period from January 1, 2000, to December 31, 2010. We studied the incidence and risk of AKI in 9178 newly diagnosed NASD patients compared with 27,534 people without NASD matched according to age, sex, index year, urbanization level, region of residence, and monthly income at a 1:3 ratio.
The NASD cohort had an adjusted hazard ratio (hazard ratio [HR]; 95% confidence interval [CI] = 1.15–2.63) of subsequent AKI 1.74-fold higher than that of the control cohort. Older age and type 2 diabetes mellitus were significantly associated with an increased risk of AKI (P < 0.05). Among different types of NASDs, patients with insomnia had a 120% increased risk of developing AKI (95% CI = 1.38–3.51; P = 0.001), whereas patients with other sleep disorders had a 127% increased risk of subsequent AKI (95% CI = 1.07–4.80; P = 0.033). Men with NASDs were at a high risk of AKI (P < 0.05).
This nationwide population-based cohort study provides evidence that patients with NASDs are at higher risk of developing AKI than people without NASDs.
INTRODUCTION
Acute kidney injury (AKI) is an immense clinical problem because of its growing incidence and high mortality and morbidity in the United States and worldwide over the past decade.1,2 Although AKI was once considered a reversible disease, a considerable amount of evidence has indicated that AKI may have an unfavorable impact on subsequent renal function and long-term prognosis.3,4 The risk factors for AKI include old age, sepsis, hypovolemia, pre-existing chronic kidney disease (CKD), cardiovascular disease (CVD), hypertension, type 2 diabetes mellitus (DM), dementia, and cancer.1,5 Sleep disorders are changes in sleep patterns and habits accompanied by several symptoms, including irritability and fatigue during wakefulness. The estimated prevalence rate of insomnia and sleep disorder in the general population is ∼20%.6 In 1 study, patients with kidney diseases had a higher prevalence of sleep disorders and sleep-related problems compared with patients without kidney diseases.7 Among sleep disorders, sleep-related breathing disorders, such as obstructive sleep apnea, are a well-established risk factor for hypertension, CVD, stroke, mortality, and CKD.8–10 However, the association between nonapnea sleep disorders (NASDs) and AKI has not been extensively investigated.
The Taiwan National Health Insurance Research Database (NHIRD) is a national medical database containing records on 26 million administered insurants for the period January 2000 to December 2010. The National Health Insurance (NHI) program covers health care for 99% of the population and offers unrestricted access to any health care provider of patients’ choice. The aim of this study was to determine the incidence of AKI in patients with NASDs compared with patients without NASDs.
METHODS
Study Population
The Taiwan NHI program is a nationwide, comprehensive, and compulsory insurance system established by the Bureau of National Health Insurance, Department of Health in 1995. The insurance program has contracts with 97% of Taiwan hospitals and clinics and provides healthcare to 99% of the 23.74 million persons in Taiwan.11 From the NHIRD, one of the largest databases in the world, the claims data of 1 million persons, selected systematically from the population of all insurants, were released for research purposes. The NHIRD comprises encrypted patient identification numbers as well as details on inpatient orders, ambulatory care, dental services, medical facility registries, physicians providing services, and prescribed drugs. Diagnoses are coded according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). This study is based in part on data from the NHIRD provided by the Bureau of NHI (NHIRD-101-552), Department of Health and managed by the National Health Research Institutes. This cohort study was approved by the Ethics Review Board of Kaohsiung Medical University (KMUH-IRB-EXEMPT-20140059).
Study Patients
We executed a retrospective cohort study. The NASD cohort comprised patients with newly identified sleep disorders other than sleep apnea, including sleep disturbances and nonorganic sleep disorders diagnosed by physicians (ICD-9-CM codes 780.5x, 307.4x, 333.94, 347, and 327.3x), as well as sleep promoting medications,12 including benzodiazepine (BZD) derivatives, BZD-related drugs, chlordiazepoxide, eszopiclone, zaleplon, zolpidem, and zopiclone use before bedtime for at least 3 months between January 1, 2000, to December 31, 2010, according to the NHIRD records. The date of the first NASD diagnosis was used as the index date. We excluded patients who were younger than 18 years, had a history of AKI (ICD-9-CM codes 580.X, 584.X, and 586), and had sleep apnea syndrome (ICD-9-CM codes 780.51, 780.53, 780.57, and 327.23) before the index year.13 NASDs were classified as unspecified sleep disturbance (ICD- 9-CM code 780.50), sleep of nonorganic origin (ICD- 9-CM code 307.4), sleep disturbance (ICD-9-CM code 780.5), insomnia (ICD-9-CM code 780.52), unspecified hypersomnia (ICD-9-CM code 780.54), other sleep disturbance (ICD-9-CM code 780.59), restless legs syndrome (ICD-9-CM code 333.94), dysfunctions associated with sleep stages or arousal from sleep (ICD-9-CM code 780.56), circadian rhythm sleep disorder (ICD-9-CM code 327.3x), unspecified disruptions of the 24-h sleep–wake cycle (ICD- 9-CM code 780.55), unspecified sleep-related movement disorder (ICD-9-CM code 780.58), and cataplexy and narcolepsy (ICD-9-CM code 347). The control cohort comprised randomly selected patients without a history of sleep disorders, AKI, or sleep promoting medications use, frequency matched according to age, sex, urbanization level, index year, monthly income, and region of residence. A matching strategy was used to strengthen the comparability between the NASD and control cohorts. The index year was defined as the year of NASD diagnosis for the NASD cohort and was used for randomly matching the NASD patients with control patients who had outpatient visits in the same year. Age was calculated from the date of birth to the date of NASD diagnosis for the NASD cohort and from the date of birth to the date of inclusion for the control cohort. For each NASD patient, 3 patients were included in the control cohort. The 2 cohorts were followed up until the development of AKI, death, or the end of 2010 (Figure 1).
FIGURE 1.
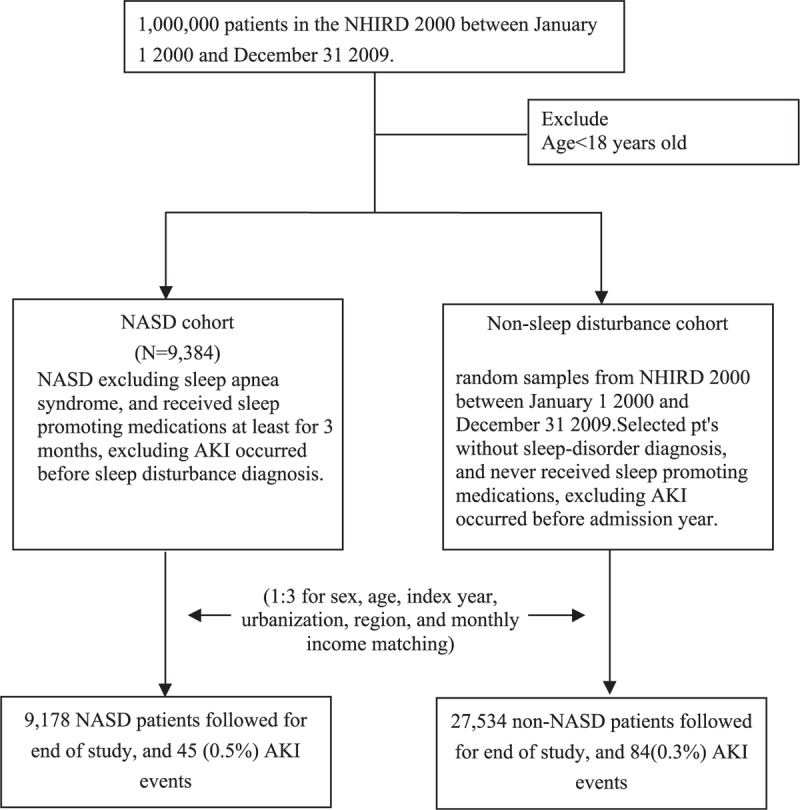
Flow diagram of patient selection.
Outcome Measures
Patients with AKI were defined as those who received a diagnosis coded as ICD-9-CM 580.X, 584.X, or 586 during hospitalization or an outpatient visit at least once. The person-years of follow-up were estimated from the index date to AKI diagnosis; censoring because of withdrawal from the insurance system, death during hospitalization, or loss to follow-up; or December 31, 2010. Comorbidities examined in our study were DM (ICD-9-CM code 250), hypertension (ICD-9-CM codes 401–405), CVD (ICD-9-CM codes 410, 412, and 428), hyperlipidemia (ICD-9-CM code 272), cerebrovascular diseases (ICD-9-CM codes 430–438), liver disease (ICD-9-CM codes 571–572 and 456.0–456.2), gout (ICD-9-CM code 274.x), CKD (ICD-9-CM codes 585–589, 250.4, 274.1, 283.11, 403.x1, 404.x2, 404.x3, 440.1, 442.1, 447.3, 572.4, 642.1x, 646.2x, and 794.4), advanced CKD (ICD-9-CM codes for CKD with erythropoietin-stimulating agent treatment, indicating that serum creatinine levels were greater than 6 mg/dL and hematocrit levels were <28%),14 peptic ulcer disease (ICD-9-CM codes 531, 532, and 533), peripheral vascular disease (ICD-9-CM codes 443.9, 441.x, 785.4, and V43.4), and depression (ICD-9-CM codes 296.2 and 296.3).
VALIDATION
We validated the ICD-9-CM codes for identifying NASDs and AKI by analyzing the medical records (charts) of 200 patients who had 1 or more of the NASD ICD-9-CM codes 780.52, 780.5, 780.50, 780.54, 780.55, 780.56, 780.58, and 780.59 and AKI ICD-9-CM code 580.X, 584.X, and 586 in the inpatient and outpatient claims database of Kaohsiung Municipal Ta-Tung Hospital, which is a regional teaching hospital in Taiwan, between January 2008 and December 2010. The contents of this database were identical to those of the NHIRD. The clinical diagnoses of NASDs were confirmed by psychiatrists and neurologists. Clinical diagnosis of AKI was determined according to the Acute Kidney Injury Network criteria.15 Positive predictive values (PPVs) of both diseases were estimated. In total, 184 confirmed cases of NASDs and 192 confirmed cases of AKI were identified. The PPVs of NASDs and AKI were 0.92 and 0.96, respectively.
Statistical Analysis
We used the independent t test, the chi-square test, or fisher's exact test to compare the distribution of risk factors between the NASD and control cohorts. Crude and adjusted hazard ratios (HRs) were calculated using Cox proportional hazard regression models for the risk of AKI or prognosis events. In multiple Cox proportional hazard regression, age, sex, and past medical histories of DM, hypertension, hyperlipidemia, CVD, cerebrovascular disease, liver disease, gout, obesity, and depression were adjusted. We used the Kaplan–Meier curve to estimate the probability of AKI onset events and used the log-rank test or Gehan–Breslow–Wilcoxon test to identify differences among 2 or more groups. All statistics were analyzed using SAS 9.3 software (SAS Institute, Inc, Cary, NC). Statistical significance was set at P < 0.05.
RESULTS
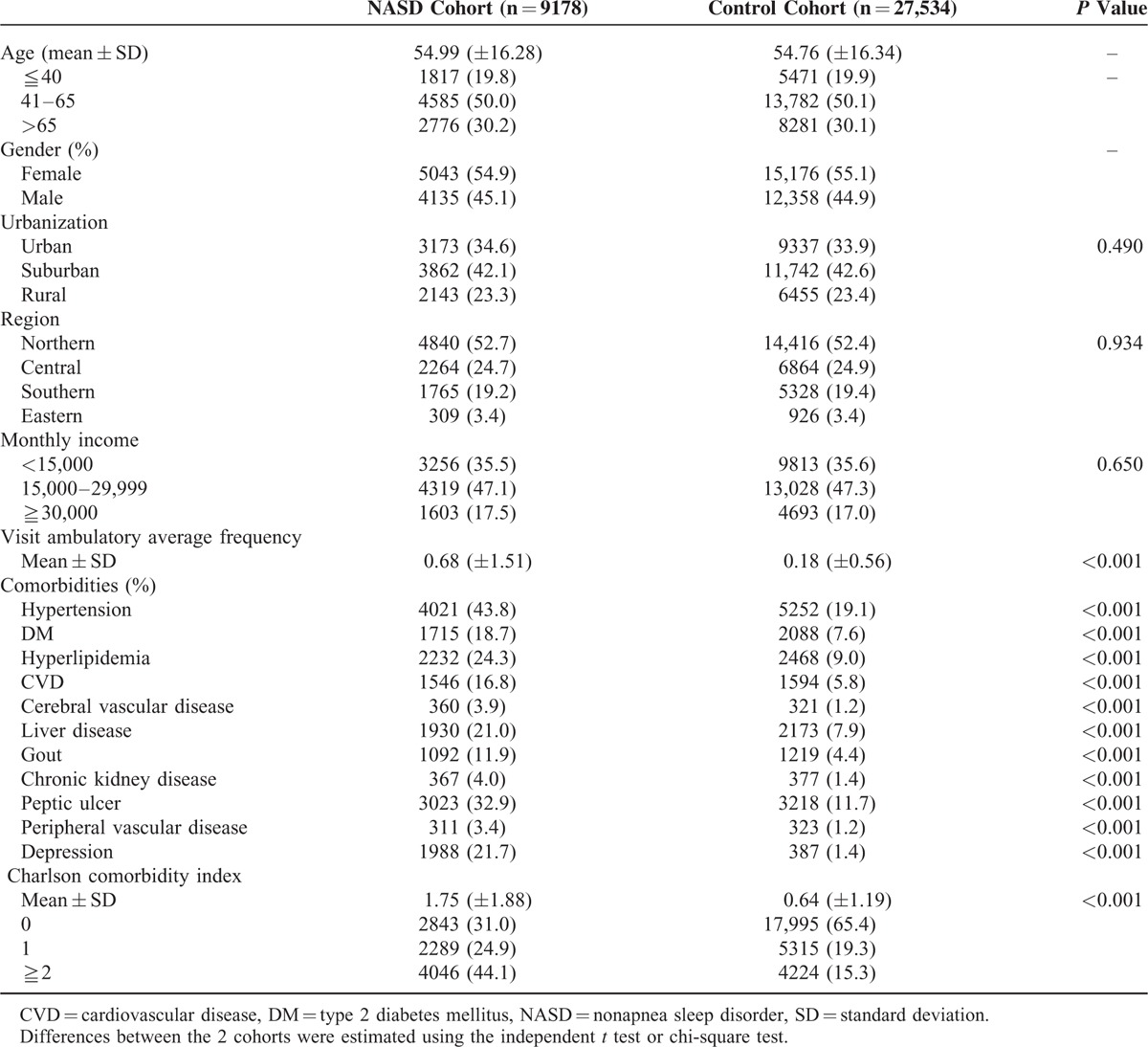
Baseline Characteristics of the Study Cohorts
During 2000 to 2010, we identified 9178 patients for the NASD cohort and 27,534 patients for the control cohort. The mean age was 54.99 ± 16.28 years, and 45.1% of the patients in the NASD cohort were men (Table 1). The distributions of age, sex, index year, urbanization level, region of residence, and monthly income were similar between the 2 cohorts. The patients in the NASD cohort had a higher frequency of medical visits during the year before the index date (P < 0.001) and were more likely to have comorbidities including hypertension, DM, hyperlipidemia, CVD, cerebrovascular disease, liver disease, gout, CKD, peptic ulcer disease, peripheral vascular disease, and depression as well as a higher Charlson comorbidity index before the index date (P < 0.001).
TABLE 1.
Demographic Characteristics of the NASD and Control Cohorts
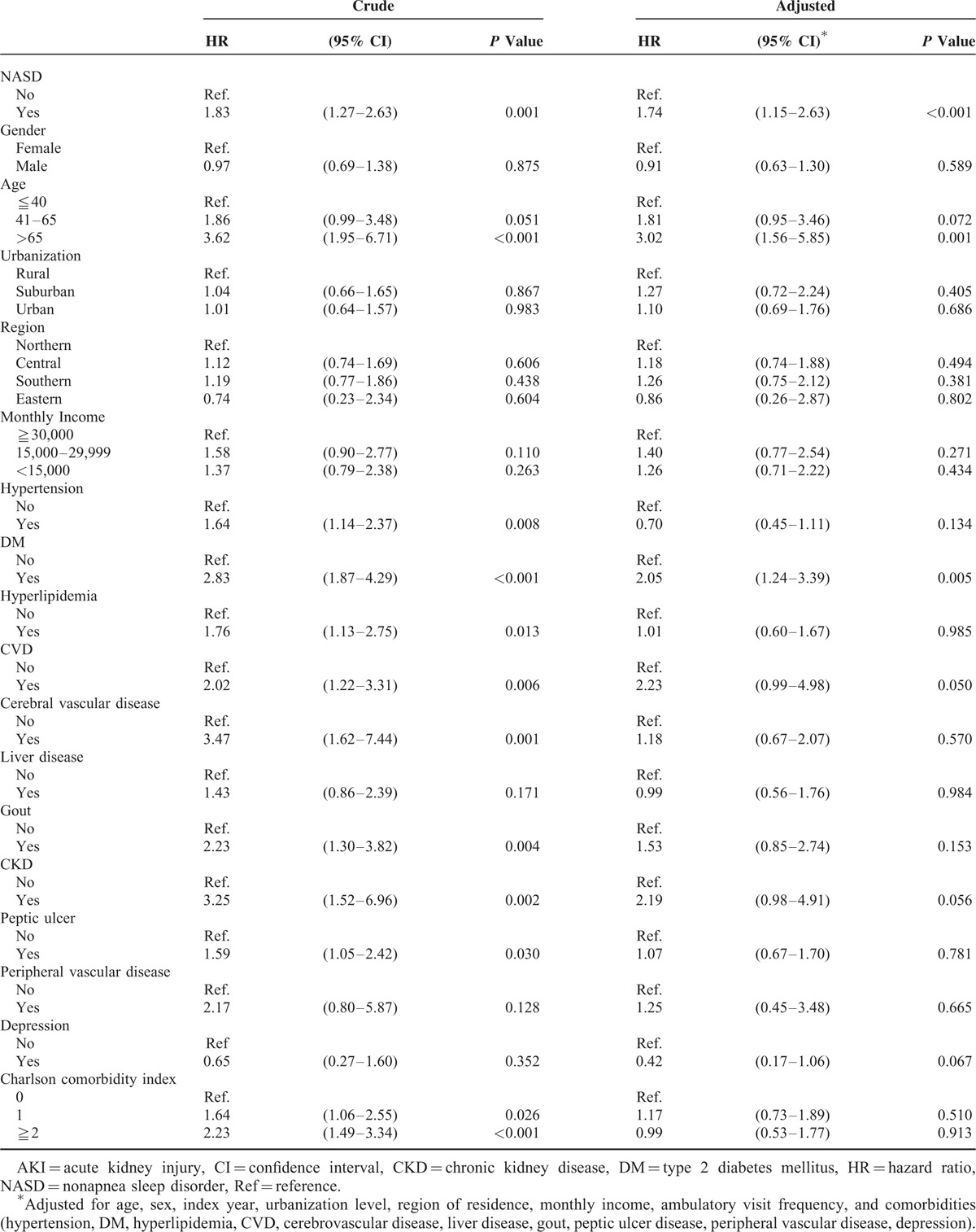
Association of AKI With NASDs According to Age, Sex, and Comorbidities
During the follow-up period, the overall incidence of AKI was higher in the NASD cohort than in the control cohort (0.83 vs 0.43 per 10,000 person-y). After adjustment for baseline characteristics and comorbidities, the risk of AKI was significantly increased in the patients with NASDs (HR = 1.74; 95% confidence interval [CI] = 1.15–2.63, P < 0.001) (Table 2). The incidence of AKI increased with age (P < 0.001). The age-specific relative risks of AKI were higher in older adults than in young adults (HR = 3.02, 95% CI = 1.56–5.85, P = 0.001). Patients with comorbid DM had a higher risk of AKI (HR = 2.05, 95% CI = 1.24–3.39, P = 0.005).
TABLE 2.
Risk of AKI in the NASD Cohort Versus the Control Cohort
Cumulative Incidences of AKI in the NASD and Control Cohorts
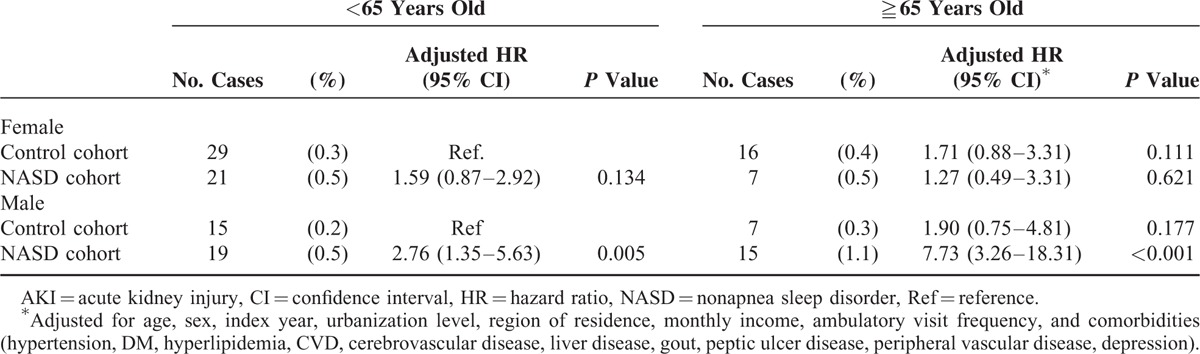
We evaluated the cumulative incidence of AKI and determined that it was significantly higher in the NASD cohort (10-year cumulative incidence, 0.5%; 95% CI = 0.36–0.64) than in the control cohort (0.3%; 95% CI = 0.24–0.36) (log-rank test, P < 0.001) (Figure 2). For men aged ≧65 years, the 10-year cumulative incidence of AKI was 1.1% (HR = 7.73, 95% CI, 3.26–18.31, P < 0.001); for men aged <65 years, the cumulative incidence of AKI was 0.5% (HR = 2.76, 95% CI, 1.35–5.63, P = 0.005) (Table 3).
FIGURE 2.
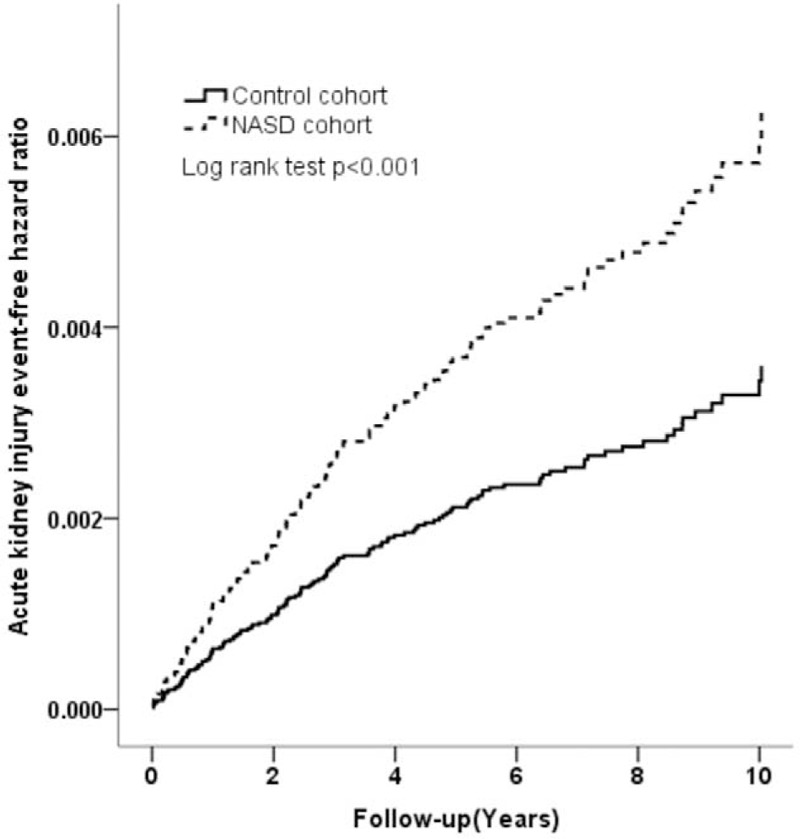
Cumulative incidence of AKI in the NASD (dash line) and control (solid line) cohorts. AKI = acute kidney injury, NASDs = nonapnea sleep disorders.
TABLE 3.
Risk of AKI in the Control Cohort Versus the NASD Cohort Stratified by Sex and Age
Subgroup Analysis
We also examined the association between the risk of AKI and NASD subgroups. The risks of AKI were significantly increased in NASD subgroups of insomnia (HR = 2.20, 95% CI, 1.38–3.51, P = 0.001) and other sleep disorders (HR = 2.27, 95% CI, 1.07–4.80, P = 0.033) (Table 4). The risk of AKI in different patient subgroups is shown in Figure 3A and B. After adjustment for variables, the risk of AKI was more prominent in male NASD patients; aged >65 years; those living in rural area; those living in Central part of Taiwan; those with a monthly income between NT$15,000 and NT$30,000; and those without comorbidities, including hypertension, DM, hyperlipidemia, CVD, cerebrovascular disease, liver disease, gout, CKD, advanced CKD, peripheral vascular disease, obesity, and depression (P < 0.05) (Figure 3A and B).
TABLE 4.
Comparison of the Risk of AKI Among NASD Subgroups
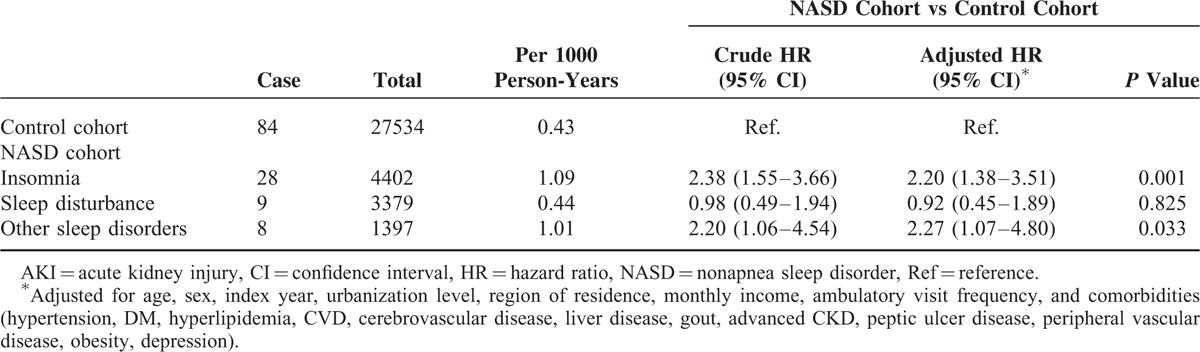
FIGURE 3.
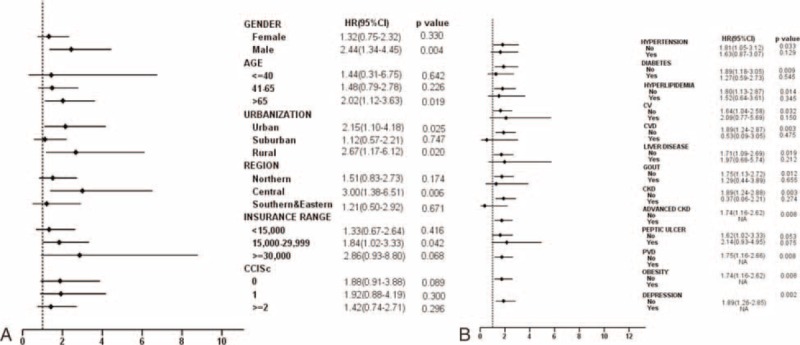
Forest tree plot of increased HRs for AKI for (3A) baseline characteristics and (3B) comorbidities. AKI = acute kidney injury, HR = hazard ratio.
Sensitivity Analysis
When alternative all-comorbidity matching was applied, the association pattern was similar to that obtained in the aforementioned main analyses. As shown in Supplementary Tables 1 and 2, the risk of AKI was significant for patients with NASDs (HR = 1.47; 95% CI = 1.21–1.79, P < 0.001).
DISCUSSION
We investigated the association between NASDs and AKI. During the decade of observation, the patients with NASDs were 1.74-fold more likely to develop AKI than those without NASDs.
Sleep disorders are highly prevalent in patients with kidney diseases.16,17 In previous studies,7,18 sleep quality and related problems were more prevalent in patients with CKD and end-stage renal disease. Increasing evidence indicates that sleep disorders promote the development and exacerbate the severity of several diseases, including DM and hypertension.19–22 Because of the crucial roles of these diseases in kidney disease development, it is rational to evaluate how sleep disorders contribute to kidney disease development is imperative.
In the study by Huang et al23, which evaluated patients from the NHIRD, the risk of developing CKD was significant for patients with NASDs compared with patients without sleep disorders. Our study group confirmed the study results (P < 0.001) by using more strict definitions and validations.24 In the study by Gao et al25 in China, patients sleeping less per night were associated with a higher risk of a lower glomerular filtration rate. Sasaki et al26 observed that a short sleep duration was associated with a significantly higher risk of CKD among shift workers (P < 0.05). Turek et al proposed that sleep disorders may be nontraditional risk factors for the development and progression of CKD.27 Nonetheless, there is no cohort studies exploring the association of NASDs with AKI risk. In this study, we used a large nationwide data set to afford considerable statistical power and to track long-time incident AKI events.
Sleep and the circadian rhythm are biologically essential for animals and humans. Healthy sleep is optimal in quality and quantity. A satisfactory sleep duration can improve the general condition including mood, daily performance, alertness, and long-term health outcomes. However, patients with deficient sleep quality and quantity may experience increasing glucose intolerance and inflammation as well as an increasing blood pressure (BP) and heart rate.19–21 Our study is consistent with previous studies reporting that patients with NASDs and more comorbidities, including hypertension, DM, and CKD, had a higher risk of AKI development than that of comparison groups. In the course of sleep, a decrease in sympathetic tone and an increase in vagal tone cause nocturnal reduction of the BP. When people are in a sleep-deficient condition, the activity of the sympathetic nervous system is increased.21 Activation of the sympathetic nervous system may be the pathogenesis of renal hypertension and is proposed to be a risk factor for renal function progression.28 The circadian rhythm is vital to human homeostasis. The oscillation of the rennin–angiotensin–aldosterone system is inflected by the rapid eye movement (REM)–non-REM cycle and circadian rhythm.29,30 Patients with depression who were deprived of sleep exhibited increasing renin secretion and a concomitant trend of decreasing hypothalamic-pituitary-adrenal axis activity during the recovery night.31 Activating rennin–angiotensin–aldosterone system could be another pathological mechanism of kidney disease development. Diminishing sleep duration and quality increased the levels of high-sensitivity C-reactive protein, white blood cells, and proinflammatory cytokines.20,32 Ohkuma et al33 determined that the urinary albumin–creatinine ratio was associated with sleep duration in patients with type 2 diabetes. Inflammation causes glomerular endothelial dysfunction, which may lead to renal function decrease. Sauvet et al34 observed vascular dysfunction before an increase in sympathetic activity and systolic BP under sleep deprivation.
The strength of our study is that it was designed to reduce environmental effects, selection, and detection bias. Moreover, the study population was well-defined and complete follow-up because our design relied on computerized registries that provide complete nationwide coverage. Therefore, our finding of an increased risk of AKI in patients with NASDs is robust.
LIMITATIONS
This study has several limitations. First, data on objective sleep quality and other mental health conditions highly comorbid with NASDs were lacking. Second, diseases may be misclassified when an administrative database is used. To mitigate this problem, we identified NASD diagnoses according to ICD-9-CM codes and sleep promoting medications use. Moreover, the NHI Administration of Taiwan reviews charts, audits medical charges, and imposes heavy penalties for inappropriate charges or malpractice to ensure the accuracy of claims. Third, the NASD cohort had significantly more comorbidities than did the control cohort. Hence, we performed a sensitivity test to match the comorbidities of the 2 cohorts and still obtained a positive result. Fourth, because of the definition of NASDs in our cohort, the effect of sleep promoting medications use on AKI development requires investigation. We analyzed the dose–response relationship between sleep promoting medications use and AKI development and the result was negative. Fifth, the NHIRD lacks information on variables that may contribute to the risk of AKI development, namely a family history of kidney disease, lifestyle, body weight, and laboratory data. Thus, we could not adjust for and include these variables in the propensity analysis, leading to a difference in the propensity score between cohorts. Therefore, we added the Charlson comorbidity index score to the propensity score in multivariable and stratified analyses to control for confounders. Finally, the NHIRD is a disconnected research database. The unknown symptom period of NASDs may cause underestimation of the incidence of CKD in patients with NASDs. Despite these limitations, this nationwide population-based longitudinal cohort study provides a valuable contribution by clarifying the relationship between NASDs and the risk of subsequent AKI events in an Asian population. Our findings may benefit from further analysis regarding specific sleep disorders contributing to AKI incidence in future studies.
CONCLUSION
In a Taiwanese nationwide population cohort, NASDs were significantly associated with an increased risk of AKI, especially in men. Because the number of patients with NASDs is increasing, enhancing sleep disorder management may be vital for AKI prevention.
Supplementary Material
Acknowledgments
The authors appreciate the help from the Statistical Analysis Laboratory, Department of Internal Medicine, Kaohsiung Municipal Ta-Tung Hospital.
Footnotes
Abbreviations: AKI = acute kidney injury, BP = blood pressure, BZD = benzodiazepine, CI = confidence interval, CKD = chronic kidney disease, CVD = cardiovascular disease, DM = type 2 diabetes mellitus, HR = hazard ratio [HR], NASDs = nonapnea sleep disorders, NHI = National Health Insurance, NHIRD = The Taiwan National Health Insurance Research Database (NHIRD), PPVs = positive predictive values
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
REFERENCES
- 1.Ali T, Khan I, Simpson W, et al. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol 2007; 18:1292–1298. [DOI] [PubMed] [Google Scholar]
- 2.Liangos O, Wald R, O’Bell JW, et al. Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol 2006; 1:43–51. [DOI] [PubMed] [Google Scholar]
- 3.Wu VC, Wu CH, Huang TM, et al. Long-term risk of coronary events after AKI. J Am Soc Nephrol 2014; 25:595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xue JL, Daniels F, Star RA, et al. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol 2006; 17:1135–1142. [DOI] [PubMed] [Google Scholar]
- 5.Wonnacott A, Meran S, Amphlett B, et al. Epidemiology and outcomes in community-acquired versus hospital-acquired AKI. Clin J Am Soc Nephrol 2014; 9:1007–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buysse DJ. Insomnia. JAMA 2013; 309:706–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plantinga L, Lee K, Inker LA, et al. Association of sleep-related problems with CKD in the United States, 2005–2008. Am J Kidney Dis 2011; 58:554–564. [DOI] [PubMed] [Google Scholar]
- 8.Sakaguchi Y, Shoji T, Kawabata H, et al. High prevalence of obstructive sleep apnea and its association with renal function among nondialysis chronic kidney disease patients in Japan: a cross-sectional study. Clin J Am Soc Nephrol 2011; 6:995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000; 342:1378–1384. [DOI] [PubMed] [Google Scholar]
- 10.Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365:1046–1053. [DOI] [PubMed] [Google Scholar]
- 11.TM Cheng. Taiwan's national health insurance system: high value for the dollar. In: Okma K. G., Crivelli L., editors. Six Countries, Six Reform Models—The Healthcare Reform Experience of Israel, the Netherlands, New Zealand, Singapore, Switzerland and Taiwan. Hackensack, NJ, USA: World Scientific; 2009. pp. 171–204. [Google Scholar]
- 12.Neubauer DN. New and emerging pharmacotherapeutic approaches for insomnia. Int Rev Psychiatry 2014; 26:214–224. [DOI] [PubMed] [Google Scholar]
- 13.Lai TS, Wang CY, Pan SC, et al. Risk of developing severe sepsis after acute kidney injury: a population-based cohort study. Crit Care 2013; 17:R231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu TW, Liu JS, Hung SC, et al. Renoprotective effect of rennin–angiotensin–aldosterone system blockade in patients with predialysis advanced chronic kidney disease, hypertension, and anemia. JAMA Int Med 2014; 174:347–354. [DOI] [PubMed] [Google Scholar]
- 15.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benz RL, Pressman MR, Hovick ET, et al. Potential novel predictors of mortality in end-stage renal disease patients with sleep disorders. Am J Kidney Dis 2000; 35:1052–1060. [DOI] [PubMed] [Google Scholar]
- 17.Hanly P. Sleep apnea and daytime sleepiness in end-stage renal disease. Semin Dial 2004; 17:109–114. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal R, Light RP. Sleep and activity in chronic kidney disease: a longitudinal study. Clin J Am Soc Nephrol 2011; 6:1258–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tochikubo O, Ikeda A, Miyajima E, et al. Effects of insufficient sleep on blood pressure monitored by a new multibiomedical recorder. Hypertension 1996; 27:1318–1324. [DOI] [PubMed] [Google Scholar]
- 20.Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol 2004; 43:678–683. [DOI] [PubMed] [Google Scholar]
- 21.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet 1999; 354:1435–1439. [DOI] [PubMed] [Google Scholar]
- 22.Knutson KL, Van Cauter E, Rathouz PJ, et al. Association between sleep and blood pressure in midlife: the CARDIA sleep study. Arch Int Med 2009; 169:1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang ST, Lin CL, Yu TM, et al. Nonapnea sleep disorders and incident chronic kidney disease: a population-based retrospective cohort study. Medicine 2015; 94:e429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin HY, Hung CC, Chang YH, et al. Nonapnea sleep disorders in patients younger than 65 years are significantly associated with CKD: a nationwide population-based study. PloS One 2015; 10:e0140401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo X, Yu S, Li Z, et al. Self-reported sleep duration is associated with reduced glomerular filtration rate among adults with hypertension: a population-based study from rural northeast China. J Sleep Res 2015; 24:351–358. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki S, Yoshioka E, Saijo Y, et al. Short sleep duration increases the risk of chronic kidney disease in shift workers. J Occup Env Med 2014; 56:1243–1248. [DOI] [PubMed] [Google Scholar]
- 27.Turek NF, Ricardo AC, Lash JP. Sleep disturbances as nontraditional risk factors for development and progression of CKD: review of the evidence. Am J Kidney Dis 2012; 60:823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neumann J, Ligtenberg G, Klein II, et al. Sympathetic hyperactivity in chronic kidney disease: pathogenesis, clinical relevance, and treatment. Kidney Int 2004; 65:1568–1576. [DOI] [PubMed] [Google Scholar]
- 29.Brandenberger G, Follenius M, Goichot B, et al. Twenty-four-hour profiles of plasma renin activity in relation to the sleep–wake cycle. J Hypertens 1994; 12:277–283. [PubMed] [Google Scholar]
- 30.Charloux A, Gronfier C, Lonsdorfer-Wolf E, et al. Aldosterone release during the sleep–wake cycle in humans. Am J Physiol 1999; 276 (1 Pt 1):E43–E49. [DOI] [PubMed] [Google Scholar]
- 31.Murck H, Uhr M, Ziegenbein M, et al. Renin–angiotensin–aldosterone system, HPA-axis and sleep-EEG changes in unmedicated patients with depression after total sleep deprivation. Pharmacopsychiatry 2006; 39:23–29. [DOI] [PubMed] [Google Scholar]
- 32.Vgontzas AN, Zoumakis E, Bixler EO, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab 2004; 89:2119–2126. [DOI] [PubMed] [Google Scholar]
- 33.Ohkuma T, Fujii H, Iwase M, et al. Association between sleep duration and urinary albumin excretion in patients with type 2 diabetes: the Fukuoka diabetes registry. PloS One 2013; 8:e78968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sauvet F, Leftheriotis G, Gomez-Merino D, et al. Effect of acute sleep deprivation on vascular function in healthy subjects. J Appl Physiol 2010; 108:68–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


