Supplemental Digital Content is available in the text
Abstract
The metabolic hormone leptin has been implicated in the pathogenesis of various malignancies and may contribute to the high rate of cancer in obese individuals. We reported that leptin and its receptor are expressed by melanoma tumors and cell lines, and that leptin stimulates proliferation of cultured melanoma cells. Here, we tested the hypothesis that leptin contributes to early melanoma progression by assessing its association with sentinel node positivity in cutaneous melanoma patients.
The study enrolled 72 patients who were scheduled to undergo lymphatic mapping and sentinel node biopsy. Fasting blood was obtained before surgery, and serum leptin levels were measured by enzyme-linked immunosorbent assay (ELISA) with a “raw” (assay value) and an “adjusted” value (raw value divided by body mass index). Leptin levels and other clinicopathologic parameters were compared between sentinel node positive and negative groups. Logistic regression models were used to predict sentinel node status using leptin and other relevant clinical parameters.
The raw and adjusted leptin levels were significantly higher in the 15 patients with positive sentinel nodes. These findings could not be attributed to differences in body mass indices. Univariate models revealed raw leptin, adjusted leptin, Breslow thickness, and mitotic rate as significant predictors of sentinel node status. Leptin levels and Breslow thickness remained significant in multivariate models. Survival and follow-up analysis revealed more aggressive disease in diabetic patients.
Elevated serum leptin levels predict sentinel node metastasis in melanoma. Validation of this finding in larger cohorts should enable better stratification of early stage melanoma patients.
INTRODUCTION
With the growing epidemic of obesity in developed nations, considerable interest has been generated in the metabolic hormone leptin, a 16-kDa protein encoded by the OB (obese) gene. Upon caloric intake, leptin is secreted by adipocytes and detected as a satiety signal in the hypothalamus. Binding of leptin to its specific hypothalamic receptor in receptors ultimately activates endocrine pathways involved in energy expenditure.1 Circulating leptin levels are positively correlated with body fat volume, and relatively higher levels of leptin are often seen in obese versus lean individuals, a finding attributed to leptin resistance.2 Patients with type 2 diabetes also tend to have inappropriately elevated leptin levels, and women generally have higher levels than men.3
In addition to its effects in feeding behavior and energy homeostasis, leptin regulates numerous other important biological processes, such as angiogenesis, cell proliferation, cell invasion, and inflammation.4–6 Although the leptin receptor is predominantly expressed in hypothalamus, it is also expressed in various other human normal and neoplastic cells. Interaction of leptin with its receptor on endothelial cells or endothelial progenitor cells promotes angiogenesis4,5 through nitric oxide production7–9 and enhanced expression of vascular endothelial growth factor (VEGF), VEGF receptor 2, and fibroblastic growth factor-2.10,11 Leptin has also been shown to promote cell proliferation and cell growth through its intracellular signaling pathways such as Janus kinase 2/signal transducer and activator of transcription 3, Ras/extracellular signal-regulated kinases 1/2, and phosphoinositide 3 kinase/protein kinase B/glycogen synthase kinase 3signaling cascades.6 Leptin receptors have been described in various types of tumor cells, including breast, colon, endometrium, and others, and leptin has been implicated as a growth and invasion factor for these types of cancer.12–15 Leptin has also been shown to enhance tumor cell invasion16,17 and induce epithelial–mesenchymal transition.18
Less well known is leptin's role as a proinflammatory adipokine. Leptin bears structural homology to type I cytokines and the leptin receptor belongs to the class I cytokine receptor family.5,19 A wide range of proinflammatory functions for leptin has been published, including stimulation of prostaglandins and release of reactive oxygen species,20,21 increasing T-cell-mediated immunity and Th1 cytokines but decreasing Th2 cytokines.5 Interestingly, an inflammatory environment can enhance the progression of some tumor types, generating a wide range of studies related to leptin's potential role in obesity-related cancers.22
We have previously reported that both leptin and its receptor are expressed by cutaneous melanoma tumors and cell lines.23 In our study, melanoma cells responded to treatment with leptin by activating the mitogen-activated protein kinase pathway and by proliferating. Another study group reported in vivo findings that leptin treatment in mice-bearing B16F10 melanoma resulted in significantly heavier tumor weight compared with control treatment. They also observed significant increase in plasma concentration of nitric oxide metabolites in the leptin-treated group, and significant decrease in the leptin receptor antibody-treated group.9 Furthermore, another group demonstrated that a low dose administration of neutralizing nanobody targeting leptin receptor led to the tumor size shrinkage in the mouse model.24 The findings above implicate leptin in melanoma growth and progression and raise the possibility of an oncogenic autocrine loop.25,26
Based on the data from our in vitro study and in vivo murine studies by other groups, we hypothesized that higher levels of leptin are associated with an increased risk of melanoma spread to sentinel lymph nodes (SN), the most likely regional nodes to contain metastatic disease if any are involved.27 Several clinicopathological parameters have been shown to predict SN positivity, such as younger age, higher Breslow thickness, higher Clark level, increasing mitotic rate, tumor site (lower extremity and trunk), low tumor-infiltrating lymphocytes, satellitosis, and ulceration.28–33 These parameters are mostly histopathological parameters. To our knowledge, there is no established serological surrogate marker to detect early SN metastasis. It would be beneficial if we could use a serological marker in combination with other histologic markers, such as Breslow thickness, to monitor the SN or regional lymph node metastasis and avoid invasive SN procedures for patients who are not in need. To test this hypothesis, we prospectively examined serum leptin levels in patients with cutaneous melanoma who were scheduled for SN biopsies, and correlated the findings with clinical outcomes. Here we report significantly higher leptin levels for the subset of patients with SN metastases compared to those with negative SNs.
PATIENTS AND METHODS
Patients
The study was approved by The University of Texas MD Anderson Cancer Center (MDACC) Institutional Review Board and conducted in accordance with Health Insurance Portability and Accountability Act guidelines. Informed consent was obtained from all study participants. Eligible patients included those who presented with stage I or II primary cutaneous melanoma, were within 8 weeks of biopsy or resection of the primary lesion, and had a SN biopsy scheduled at MDACC between December 2007 and July 2009. The body mass index (BMI), defined as the weight in kilograms divided by the square of the height in meters, was calculated for each patient upon enrollment. A fasting blood sample for leptin was obtained preoperatively on the morning of SN biopsy. The sample was clotted at room temperature, then centrifuged and separated for serum, which was stored at −80 °C until analysis. Before assay, samples were thawed at 4 °C overnight, and serum leptin levels were then measured in duplicate using the Quantikine Human Leptin ELISA Kit (R&D Systems, Minneapolis, MN) according to the manufacturer's protocol. Based on the data obtained, each patient was assigned a “raw” leptin value which was the mean assay value in units of ng/mL. Additionally, to account for the influence of obesity, an “adjusted” leptin value was calculated by converting the raw value to pg/mL and dividing by the BMI. Clinical information obtained for the study participants included gender, age, height, weight, Clark level, Breslow thickness, mitotic rate, ulceration, and prior history of diabetes.
Statistical Analysis
The association between sentinel lymph node status and leptin levels as well as other clinicopathologic parameters was assessed by Fisher's exact test or Wilcoxon rank sum test as appropriate. Univariate logistic regression models were used to predict SN status using leptin and the other clinical factors. The Cox proportional hazard model was used to analyze survival endpoints. In multivariate analyses, the raw and adjusted leptin level were assessed separately and a backward elimination procedure was used to identify a final model with only significant predictors remaining. Tests were 2-sided and P values of 0.05 or less were considered statistically significant. All statistical analyses were carried out using SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
Patient Characteristics
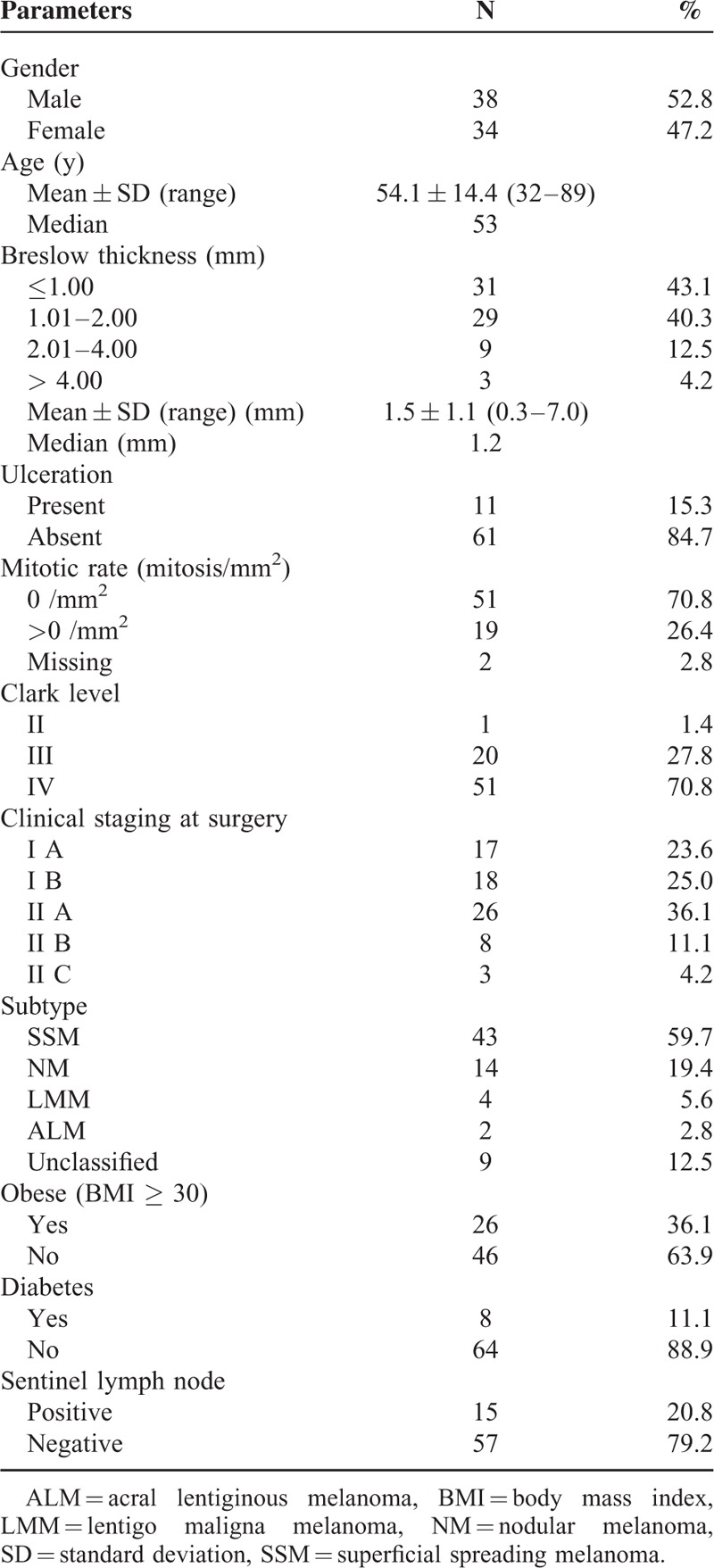
Patient characteristics are summarized in Table 1. A total of 72 patients, 38 men and 34 women, were included in this study. Patient age ranged from 32 to 89 years (median 53 years). Notably, 26 patients (36.1%) were obese, having a BMI 30 or greater, and 8 (11.1%) were diabetic. Of the 72 patients, 15 were SN positive and 57 were SN negative.
TABLE 1.
Patient Characteristics
Correlation of Leptin Levels With Sentinel Lymph Node Status
As shown in Table 2 and Figure 1, both raw and adjusted leptin levels were significantly higher in the patients with positive SN. For SN-positive patients, the mean raw leptin was 26.6 ng/mL vs 15.3 ng/mL for those with negative nodes (P = 0.041). Similarly, the average adjusted leptin in SN-positive patients was 845.0 compared to 503.6 for node-negative patients (P = 0.029). The mean BMI was virtually identical for the SN-positive and -negative groups (29.9 and 28.5, respectively), eliminating obesity as an explanation for higher leptin values in the SN-positive patients. As expected, Breslow thickness was also greater in patients with a positive SN (P = 0.004).
TABLE 2.
Summary of Clinical Features by Sentinel Lymph Node Status
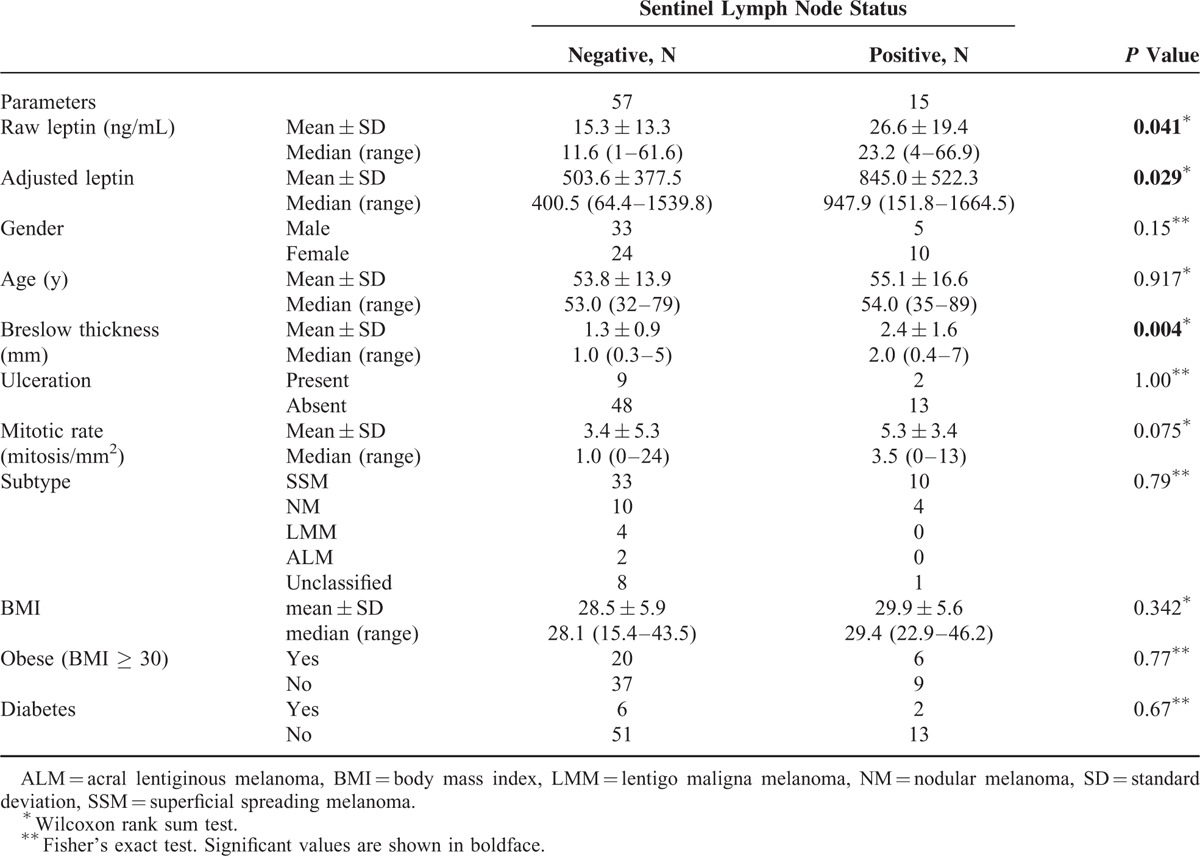
FIGURE 1.
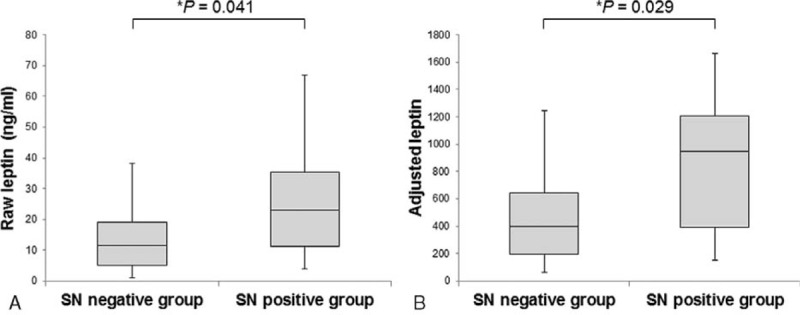
Box-plot analysis of raw and adjusted leptin levels in SN-negative and SN-positive melanoma patients. Data are presented as median values (solid horizontal lines within box-plots), 25th to 75th percentiles (lower and upper column borders), and maximum and minimum range (vertical lines)∗ Wilcoxon rank sum test.
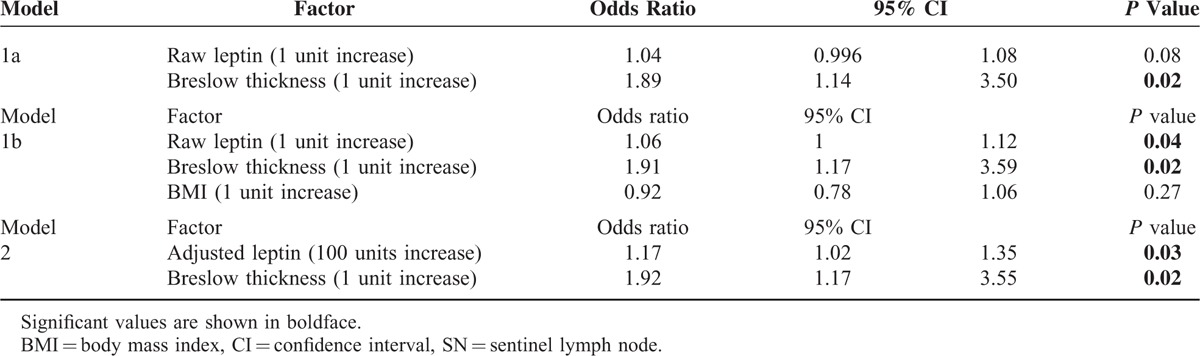
In a univariate logistic regression model, Breslow thickness, raw and adjusted leptin levels, and mitotic rate were significantly associated with positive sentinel lymph nodes (Table 3). The odds ratio for raw leptin was modest (1.04) but was considerably more robust for adjusted leptin (1.19). In multivariate logistic regression analysis, 2 methods were available for adjustment of leptin for BMI, the first being inclusion of raw leptin and BMI in the model (Table 4, Model 1b) and the second being the use of adjusted leptin without BMI (Table 4, Model 2). Multivariate analysis otherwise included all cataloged variables. Upon first analysis using Model 1, raw leptin alone was not found to predict SN status (Table 4, Model 1a). However, if BMI was returned to the model, the findings for raw leptin became significant and the odds ratio improved slightly from 1.04 to 1.06, suggesting that leptin predicted SN positivity only after adjustment for obesity. This was confirmed by the multivariate logistic regression Model 2 using adjusted leptin values, which were already corrected for BMI. In this case serum adjusted leptin levels independently predicted SN positivity. As in the univariate analysis, the odds ratio for adjusted leptin was superior to that of raw leptin (1.17 vs 1.04 or 1.06).
TABLE 3.
Univariate Logistic Regression Model Predicting Probability of Positive SN
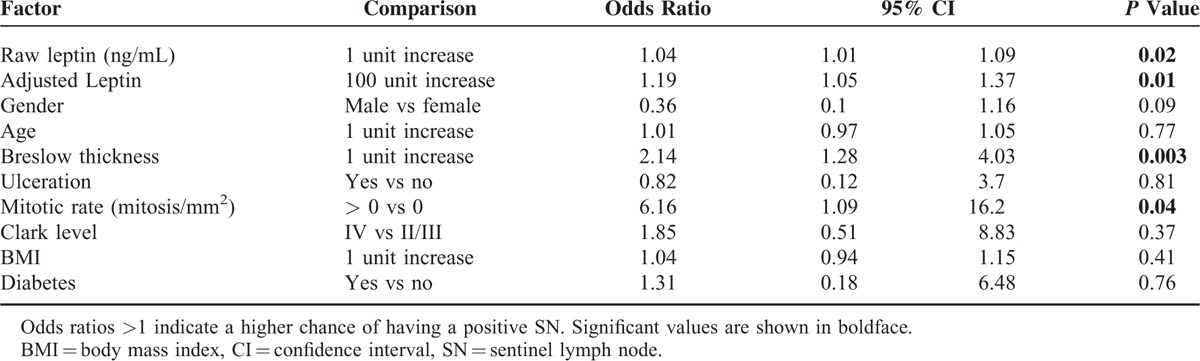
TABLE 4.
Multivariate Logistic Regression Models Predicting Probability of Positive SN
Survival and Clinical Course of Patients With Diabetes
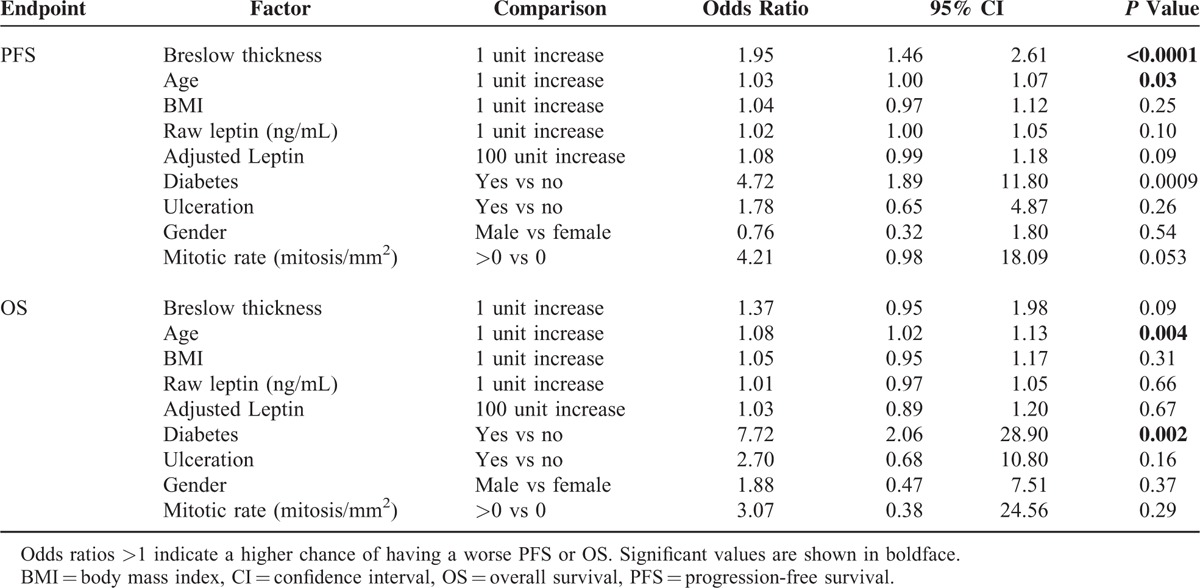
Although survival was not an endpoint in this study, the data were examined for an association of raw or adjusted leptin with progression-free (PFS) or overall survival (OS). Median follow-up periods were 55.5 and 60.5 months for PFS and OS, respectively. No correlation was found between leptin and PFS or OS, likely due in part to the relatively small study population and the paucity of events (Table 6). Significant factors for PFS and OS were Breslow thickness, age, and diabetes (Table 6). Having higher Breslow thickness (P < 0.0001), being older (P = 0.03), or being diabetic (P = 0.0009) was significantly associated with shorter PFS; being older (P = 0.004) or diabetic (P = 0.002) was significantly associated with worse OS.
TABLE 6.
Univariate Cox Proportional Hazard Model Results for PFS and OS by Patient Characteristics
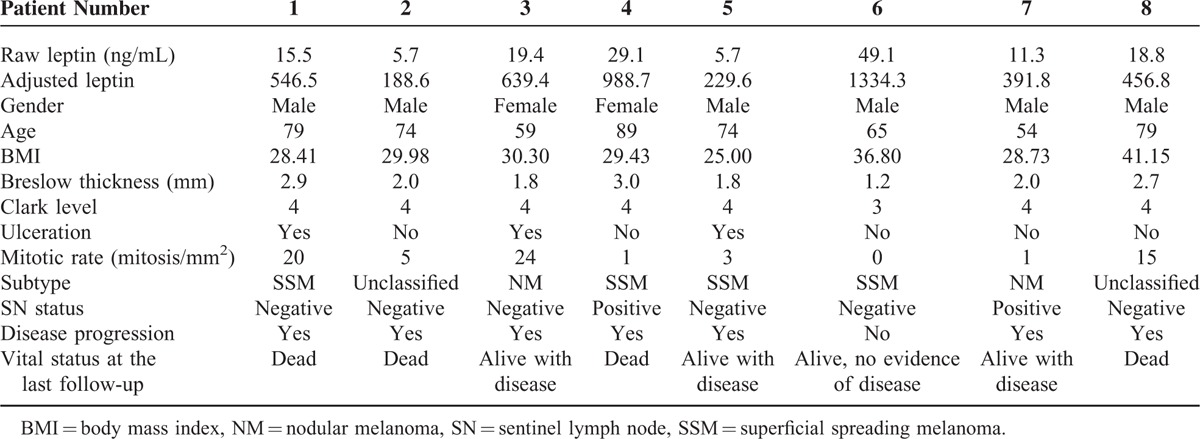
As we observed this poor prognostic trend for diabetic patients, we further looked at each patient's clinical course (Table 5). All 8 diabetic patients had tumor thickness >1.0 mm, but only 2 of these patients had positive SNs, and both developed disease progression. Disease progression, defined as development of local recurrence, in-transit, regional, or distant metastasis, was observed in 7 out of the 8 patients, and 4 of them have died of distant metastasis (Table 5). Of the 4 diabetic patients that remain alive, 3 have progressed to stage IV disease. As diabetes can cause increased levels of leptin and increased BMI, and become a confounding factor, we examined if there is any difference in leptin levels or BMI between the nondiabetic and diabetic patients, but there was no significant difference in these levels between the 2 groups (Supplementary Table).
TABLE 5.
Characteristics of the 8 Diabetic Patients in this Study
DISCUSSION
The role of leptin and other metabolic hormones in the promotion of cancer growth and metastasis has become an important topic of research over the past several decades. In the present study, we provide evidence to support a role for leptin in the progression of cutaneous melanoma from its primary site to the regional draining lymph nodes.
SN biopsy is now a widely used and standardized technique for patients with melanoma. Although the long-term clinical significance of SN metastasis in melanoma has been debated,34–36 recent multi-institutional studies investigating >2000 patients with melanoma have revealed that SN status can be an independent predictor of survival,34 and early surgical intervention for patients with positive SNs may improve disease-free and melanoma-specific survival in a subgroup of patients.37 Interestingly, a study comparing the gene expression levels of immunoregulatory cytokines between tumor-negative vs tumor-positive SNs showed that tumor-positive SNs had significantly higher gene expression level of leptin compared with tumor-negative SNs.38 Our study coincided with their observation, in that the serum level of leptin was significantly higher in the SN-positive group compared to the SN-negative group. Taken together, the SN-positive patient group has a higher serum protein and tumor gene expression level of leptin compared to the SN-negative group. These findings suggest that leptin has a potential as a tumor progression marker. Future studies will need to investigate whether tissue and serum expression levels are associated with each other.
An interesting finding in our study is the fact that BMI was identical in SN-positive and -negative patients. We were thus unable to reconcile the differences in leptin levels based upon adipose volume. Furthermore, it was necessary to include BMI in the multivariate regression models in order for leptin to predict the SN status. Previous studies have demonstrated that circulating leptin levels exhibit a strong correlation with total body fat, but to a lesser degree with BMI.39,40 These findings point to a secondary source of leptin that remains after accounting for adipose leptin. That source could well be the tumor cells themselves acting in an autocrine manner. In this regard, it would be interesting to immunostain the metastatic tumor cells for leptin to determine a potential correlation with circulating levels.
Another interesting finding was that although the number of patients was small, patients with diabetes tended to have a worse prognosis, as 7 of 8 had disease progression. Four of these patients have already died of metastatic disease and 3 of the 4 that remain alive have progressed to distant metastasis. Univariate analysis for PFS and OS revealed diabetes to be significantly associated both with worse survival (Table 6). There are several reports addressing the higher risk of melanoma among individuals with diabetes,41,42 but to our knowledge there has been no study comparing the aggressiveness of melanoma between diabetic and nondiabetic patients. Possible mechanisms include insulin resistance, hyperinsulinemia which might stimulate tumor growth by increasing insulin-like growth factors- I, obesity, and leptin.42 As this study tested the serum levels at only 1 timepoint before surgery, we cannot exclude the possibility of greater variations in leptin levels over the clinical course of each patient. A prospective study with a larger cohort examining serum leptin levels at multiple follow-ups would enable us to determine if there is any increase or decrease in the level before or after disease progression, and if leptin can be used as a predictive marker.
Although tumor-derived leptin cannot currently be distinguished from adipose leptin, the measurement of total leptin levels adjusted for BMI might be a valuable surrogate for regional metastases and may be a biomarker for disease progression. Whereas opinions vary as to whether melanoma is an obesity-related cancer, these data support that maintenance of a normal body weight would be advisable for these patients.43,44 Additionally, this study does not address the potential role of leptin in the pathogenesis of mucosal melanoma, a particularly aggressive form of this disease. As we have previously reported expression of leptin by spontaneous canine oral melanoma, the dominant form of this malignancy in dogs, examination of the human counterpart is warranted.45 Finally, due to the relatively small number of study subjects and events, these data should be interpreted with caution, as odds ratios and P values, while positive, are modest. Future studies of the association of leptin, adjusted for BMI, with melanoma progression, and the role of diabetes in the clinical behavior of melanoma, will require larger patient numbers to confirm our findings and address leptin's role in distant metastasis and survival.
Supplementary Material
Acknowledgments
The authors would like to thank Dr Chandrani Chattopadhyay for her critical review of the data and helpful comments and Ms. Marilyn K. Johnson for excellent technical work.
Footnotes
Abbreviations: BMI = body mass index, MDACC = MD Anderson Cancer Center, OS = overall survival, PFS = progression-free survival, SN = sentinel lymph node
Financial support: this study was funded by NIH/NCI R21 CA129651 (JAE); the MD Anderson Cancer Center SPORE in Melanoma P50-CA093459 (EAG); The University of Texas MD Anderson Cancer Center Support Grant P30-CA016672; the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation (EAG); and funds from the Polo on the Prairie event (EAG).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
REFERENCES
- 1.Munzberg H, Morrison CD. Structure, production and signaling of leptin. Metabolism 2015; 64:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 1996; 334:292–295. [DOI] [PubMed] [Google Scholar]
- 3.DePaoli AM. 20 years of leptin: leptin in common obesity and associated disorders of metabolism. J Endocrinol 2014; 223:T71–T81. [DOI] [PubMed] [Google Scholar]
- 4.Sierra-Honigmann MR, Nath AK, Murakami C, et al. Biological action of leptin as an angiogenic factor. Science 1998; 281:1683–1686. [DOI] [PubMed] [Google Scholar]
- 5.Huang L, Li C. Leptin: a multifunctional hormone. Cell Res 2000; 10:81–92. [DOI] [PubMed] [Google Scholar]
- 6.Garofalo C, Surmacz E. Leptin and cancer. J Cell Physiol 2006; 207:12–22. [DOI] [PubMed] [Google Scholar]
- 7.Kimura K, Tsuda K, Baba A, et al. Involvement of nitric oxide in endothelium-dependent arterial relaxation by leptin. Biochem Biophys Res Commun 2000; 273:745–749. [DOI] [PubMed] [Google Scholar]
- 8.Vecchione C, Maffei A, Colella S, et al. Leptin effect on endothelial nitric oxide is mediated through Akt-endothelial nitric oxide synthase phosphorylation pathway. Diabetes 2002; 51:168–173. [DOI] [PubMed] [Google Scholar]
- 9.Amjadi F, Javanmard SH, Zarkesh-Esfahani H, et al. Leptin promotes melanoma tumor growth in mice related to increasing circulating endothelial progenitor cells numbers and plasma NO production. J Exp Clin Cancer Res 2011; 30:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez RR, Cherfils S, Escobar M, et al. Leptin signaling promotes the growth of mammary tumors and increases the expression of vascular endothelial growth factor (VEGF) and its receptor type two (VEGF-R2). J Biol Chem 2006; 281:26320–26328. [DOI] [PubMed] [Google Scholar]
- 11.Ribatti D, Nico B, Belloni AS, et al. Angiogenic activity of leptin in the chick embryo chorioallantoic membrane is in part mediated by endogenous fibroblast growth factor-2. Int J Mol Med 2001; 8:265–268. [DOI] [PubMed] [Google Scholar]
- 12.Ando S, Barone I, Giordano C, et al. The multifaceted mechanism of leptin signaling within tumor microenvironment in driving breast cancer growth and progression. Front Oncol 2014; 4:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stattin P, Lukanova A, Biessy C, et al. Obesity and colon cancer: does leptin provide a link? Int J Cancer 2004; 109:149–152. [DOI] [PubMed] [Google Scholar]
- 14.Hardwick JC, Van Den Brink GR, Offerhaus GJ, et al. Leptin is a growth factor for colonic epithelial cells. Gastroenterology 2001; 121:79–90. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Liu L, Li C, et al. Correlation analysis between the expressions of leptin and its receptor (ObR) and clinicopathology in endometrial cancer. Cancer Biomark 2014; 14:353–359. [DOI] [PubMed] [Google Scholar]
- 16.Fan Y, Gan Y, Shen Y, et al. Leptin signaling enhances cell invasion and promotes the metastasis of human pancreatic cancer via increasing MMP-13 production. Oncotarget 2015; 6:16120–16134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Attoub S, Noe V, Pirola L, et al. Leptin promotes invasiveness of kidney and colonic epithelial cells via phosphoinositide 3-kinase-, rho-, and rac-dependent signaling pathways. FASEB J 2000; 14:2329–2338. [DOI] [PubMed] [Google Scholar]
- 18.Yan D, Avtanski D, Saxena NK, et al. Leptin-induced epithelial–mesenchymal transition in breast cancer cells requires beta-catenin activation via Akt/GSK3- and MTA1/Wnt1 protein-dependent pathways. J Biol Chem 2012; 287:8598–8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang F, Chen Y, Heiman M, et al. Leptin: structure, function and biology. Vitam Horm 2005; 71:345–372. [DOI] [PubMed] [Google Scholar]
- 20.Gao J, Tian J, Lv Y, et al. Leptin induces functional activation of cyclooxygenase-2 through JAK2/STAT3, MAPK/ERK, and PI3K/AKT pathways in human endometrial cancer cells. Cancer Sci 2009; 100:389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fruhbeck G. Intracellular signalling pathways activated by leptin. Biochem J 2006; 393:7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vansaun MN. Molecular pathways: adiponectin and leptin signaling in cancer. Clin Cancer Res 2013; 19:1926–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellerhorst JA, Diwan AH, Dang SM, et al. Promotion of melanoma growth by the metabolic hormone leptin. Oncol Rep 2010; 23:901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMurphy T, Xiao R, Magee D, et al. The anti-tumor activity of a neutralizing nanobody targeting leptin receptor in a mouse model of melanoma. PLoS One 2014; 9:e89895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salvucci O, Carsana M, Bersani I, et al. Antiapoptotic role of endogenous nitric oxide in human melanoma cells. Cancer Res 2001; 61:318–326. [PubMed] [Google Scholar]
- 26.Ekmekcioglu S, Ellerhorst JA, Prieto VG, et al. Tumor iNOS predicts poor survival for stage III melanoma patients. Int J Cancer 2006; 119:861–866. [DOI] [PubMed] [Google Scholar]
- 27.Gershenwald JE, Ross MI. Sentinel-lymph-node biopsy for cutaneous melanoma. N Engl J Med 2011; 364:1738–1745. [DOI] [PubMed] [Google Scholar]
- 28.Venna SS, Thummala S, Nosrati M, et al. Analysis of sentinel lymph node positivity in patients with thin primary melanoma. J Am Acad Dermatol 2013; 68:560–567. [DOI] [PubMed] [Google Scholar]
- 29.Paek SC, Griffith KA, Johnson TM, et al. The impact of factors beyond Breslow depth on predicting sentinel lymph node positivity in melanoma. Cancer 2007; 109:100–108. [DOI] [PubMed] [Google Scholar]
- 30.Sabel MS, Kozminski D, Griffith K, et al. Sentinel lymph node biopsy use among melanoma patients 75 years of age and older. Ann Surg Oncol 2015; 22:2112–2119. [DOI] [PubMed] [Google Scholar]
- 31.Noveski L, Dzokic G, Dzonov B, et al. Predictors of sentinel lymph node status and onset of regional lymph nodes metastases in melanoma. Prilozi 2012; 33:131–140. [PubMed] [Google Scholar]
- 32.Teixeira V, Vieira R, Coutinho I, et al. Prediction of sentinel node status and clinical outcome in a melanoma centre. J Skin Cancer 2013; 2013:904701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han D, Zager JS, Shyr Y, et al. Clinicopathologic predictors of sentinel lymph node metastasis in thin melanoma. J Clin Oncol 2013; 31:4387–4393. [DOI] [PubMed] [Google Scholar]
- 34.Maurichi A, Miceli R, Camerini T, et al. Prediction of survival in patients with thin melanoma: results from a multi-institution study. J Clin Oncol 2014; 32:2479–2485. [DOI] [PubMed] [Google Scholar]
- 35.Han D, Yu D, Zhao X, et al. Sentinel node biopsy is indicated for thin melanomas ≥ 0.76 mm. Ann Surg Oncol 2012; 19:3335–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Venna SS, Thummala S, Nosrati M, 3rd, et al. Analysis of sentinel lymph node positivity in patients with thin primary melanoma. J Am Acad Dermatol 2013; 68:560–567. [DOI] [PubMed] [Google Scholar]
- 37.Morton DL, Thompson JF, Cochran AJ, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med 2014; 370:599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torisu-Itakura H, Lee JH, Scheri RP, et al. Molecular characterization of inflammatory genes in sentinel and nonsentinel nodes in melanoma. Clin Cancer Res 2007; 13:3125–3132. [DOI] [PubMed] [Google Scholar]
- 39.Ahima RS, Prabakaran D, Mantzoros C, et al. Role of leptin in the neuroendocrine response to fasting. Nature 1996; 382:250–252. [DOI] [PubMed] [Google Scholar]
- 40.Thomas T, Burguera B, Melton LJ, et al. Relationship of serum leptin levels with body composition and sex steroid and insulin levels in men and women. Metabolism 2000; 49:1278–1284. [DOI] [PubMed] [Google Scholar]
- 41.Atchison EA, Gridley G, Carreon JD, et al. Risk of cancer in a large cohort of U.S. veterans with diabetes. Int J Cancer 2011; 128:635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qi L, Qi X, Xiong H, et al. Type 2 diabetes mellitus and risk of malignant melanoma: a systematic review and meta-analysis of cohort studies. Iran J Public Health 2014; 43:857–866. [PMC free article] [PubMed] [Google Scholar]
- 43.Kirkpatrick CS, White E, Lee JA. Case-control study of malignant melanoma in Washington State. II. Diet, alcohol, and obesity. Am J Epidemiol 1994; 139:869–880. [DOI] [PubMed] [Google Scholar]
- 44.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003; 348:1625–1638. [DOI] [PubMed] [Google Scholar]
- 45.Greene VR, Wilson H, Pfent C, et al. Ellerhorst JA. Expression of leptin and iNOS in oral melanomas in dogs. J Vet Intern Med 2013; 27:1278–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


