Abstract
The present study was carried out to investigate the prognostic factors in patients who received intra-arterial infusion for advanced pancreatic cancer. In addition, the detailed procedure of intra-arterial infusion chemotherapy was described.
A total of 354 patients with advanced unresectable pancreatic adenocarcinoma were recruited from January 2012, to April 2015, at Zhongshan Hospital Fudan University, Shanghai, China. Demographic and clinic characteristics of the patients were extracted from electronic medical records. Restricted cubic spline was used to assess the nonliner regression between baseline CA19-9 value and overall survival. Kaplan–Meier analysis and Cox proportional hazard models were used to estimate the association between overall survival and clinical characteristics.
Of all 354 included patients, 230 (65%) were male (male/female ratio = 1.8), and 72 (20%) patients were diagnosed with detectable distant metastases. Pretreatment CA19-9 value of patients with metastases was significantly higher as compared to those with locally advanced cancer (median: 922.30 vs 357.00 U/mL, P = 0.0090). Totally 274 patients completed 1 cycle of intra-arterial infusion, whereas 80 patients received 2 or more cycles of the chemotherapy. For all the 354 patients, median OS was 7.0 months (95% CI: 6.0, 8.0 months) with a 6-, 12-, and 18-month survival rate of 0.48, 0.28, and 0.18, respectively. The median OS of patients, who received 1 cycle of intra-arterial infusion therapy, was 6.0 months (95% CI: 5.0, 8.0 months), which was similar to 7.0 months (95% CI: 6.0, 9.0 months) in patients who received 2 or more cycles. Restricted cubic spline revealed the nonline association between baseline CA19-9 and prognosis. The Cox proportional hazard model showed that age, CA19-9 baseline, CA19-9 value, and tumor location were significantly associated with the OS.
In conclusion, the gemcitabine-based RIAC presented a potential treatment method for advanced pancreatic adenocarcinoma. Young age, pretreatment CA19-9 value <1000 U/mL, and tumor located at the head of pancreas indicated better response to the regional intra-arterial chemotherapy and better overall survival.
INTRODUCTION
Pancreatic adenocarcinoma is one of the most lethal malignancies, with a 5-year survival rate of <7%.1,2 China accounts for 15.68% of patients with pancreatic cancer in the world.3 However, early detection of this disease still remains a challenge.4,5 At the time of diagnosis, ∼85% of the patients are estimated to have progressed to an advanced stage.6,7 Gemcitabine-based systemic chemotherapy is one of the most efficient treatments for advanced pancreatic adenocarcinoma.2,8,9 Nevertheless, systemic use of gemcitabine may increase the risk of severe side effects, such as cytopenia, nausea, and bone marrow suppression.2,10
Regional intra-arterial infusion chemotherapy helps to deliver anticancer drugs into the carcinoma tissue more selectively and is considered to be a potential alternative treatment for advanced pancreatic adenocarcinoma with less systemic adverse effects.11,12 Ohigashi et al13 first reported that intra-arterial chemotherapy using 5-fluorouracil improved the prognosis and quality of life in Asian patients with advanced pancreatic carcinoma. In a phase II trial, Cantore et al observed that 5-fluorouracil-based intra-arterial infusion was well tolerated and active in Western patients with unresectable pancreatic cancer.14 In another report, Tanaka et al15 described that intra-arterial administration of full-dose gemcitabine and 5-fluorouracil improved the overall survival (OS) without significant toxicity. The study was well designed, except for the small simple size of 20 patients. A recent meta-analysis, comprising of 6 randomized controlled trials with a total of 298 patients, was performed to investigate the effect of intra-arterial infusion versus systemic chemotherapy for advanced pancreatic cancer.11 Results showed that the intra-arterial method was significantly superior to traditional oral or intravenous chemotherapy with less complications and myelosuppression.11 However, the clinical procedure of the intra-arterial infusion was not detailed in the study. Furthermore, heterogeneity between different trials might have a significant influence on the combined effects of the meta-analysis. Studies with a larger simple size are still essential to illuminate the effects of intra-arterial infusion in advanced pancreatic adenocarcinoma, along with more detailed clinical procedures.
The present study was carried out to investigate the prognostic factors in patients who received gemcitabine-base intra-arterial infusion for advanced pancreatic cancer. In addition, the detailed procedure for intra-arterial infusion chemotherapy was described in this manuscript.
PATIENTS AND METHODS
Patient Recruitment and Clinical Procedure
A total of 354 patients were included in the study from January 2012 to April 2015 at Zhongshan Hospital Fudan University, Shanghai, China. All the patients met the following criteria: (1) >18 years of age; (2) presented with advanced pancreatic adenocarcinoma; (3) received intra-arterial infusion at Zhongshan Hospital; and (4) did not undergo any previous treatment for pancreatic cancer. Patients who received ERBD or PTCD without infusion chemotherapy were excluded from this study. Patients with locally advanced disease were all considered to be not suitable for resection because of bad lung function, cardiac function, or cachexia, and were transferred from the surgery department.
Gemcitabine-based RIAC is a kind of recognized therapeutic strategy by Chinese Society of Clinical Oncology-Pancreatic cancer Professional Committee. RIAC is a routine treatment for advanced pancreatic cancer patients in China currently.3 Informed Consent of the RIAC procedure was signed by the patients or their family members. The RIAC chemotherapy was carried out under local anesthesia in the clinical practice. Right femoral artery puncture and intubation was performed with 5-Fr arterial sheath. Meanwhile, celiac artery and superior mesenteric artery angiography was carried out with 4- or 5-Fr catheter. Gemcitabine was given at a dose of 1000 mg/m2, followed by oxaliplatin 100 mg/m2. In patients with lesions in head of pancreas, one-third of the drug was infused via super-mesenteric artery, whereas the other two-thirds was infused via gastroduodenal artery. In patients with lesions in pancreatic body or tail, great pancreatic artery, caudal pancreatic artery, and the dorsal pancreatic artery were all contributed to the blood supply of tumor. The potentially infused artery was evaluated carefully before the clinical procedure. Alternative procedures were considered if necessary.
A full dose of chemotherapeutic drug was infused via splenic artery if the above mentioned arteries were originated from the splenic artery (Figure 1A). Chemotherapeutic drug was infused via the celiac artery if the tumor blood-supplying arteries were originated from the common hepatic artery or the celiac artery (Figure 1B). One-third of the drug was given via the super-mesenteric artery (Figure 1C) and two-third via the splenic artery if the super-mesenteric artery contributed to the tumor blood supply. In the last cases, if the tumor blood-supplying arteries could be directly super-selected, drug will be infused via the blood-supplying arteries using 3-Fr catheters (Figure 1D). All patients received supportive care, including antiemetics, antacid agents, liver protection, and anti-inflammatory treatment after the chemotherapeutic infusion.
FIGURE 1.
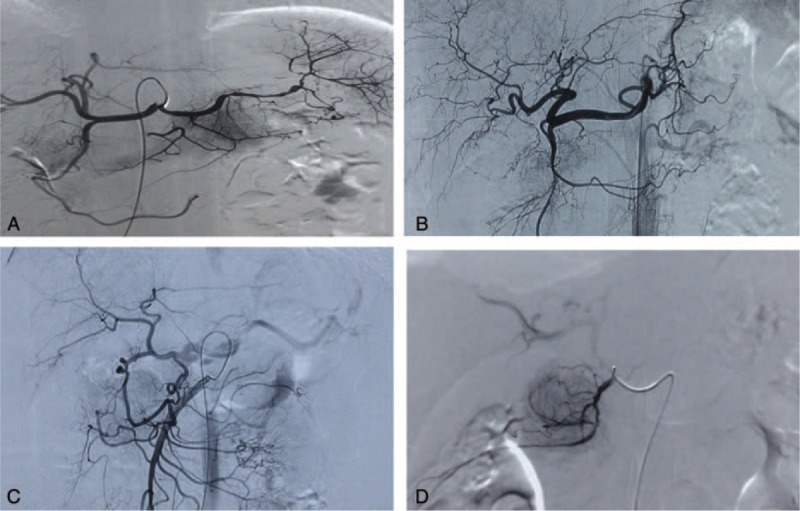
(A) The tumor feeding vessel was the splenic artery. (B) The tumor-supplying arteries were arising from the celiac artery. (C) Supermesenteric artery participated in the blood supply. (D) Blood-supplying artery was catheterized super selectively.
Data Collection
Demographic and clinical data were extracted from electronic medical records using a standard table format. TNM classification was assessed based on the imaging examination (CT scan/MRI) and the criteria set forth by the American Joint Committee on Cancer (AJCC).16 Age, gender, presence of diabetes, serum CA19-9 and CEA values, presence of jaundice, tumor location (head or not), and metastases information were collected. CA19-9 and CEA levels were examined at the clinical laboratory. Detectable upper limit of CA19-9 value was 10000 U/mL. Survival information was obtained via telephone follow-up by a full-time investigator. The follow-up was carried out every 3 months as a routine, with the last follow-up completed in April, 2015. Survival time was defined as the period from first diagnosis of pancreatic cancer to death or the last follow-up date. All patients signed informed consent before the gemcitabine-based intra-arterial infusion chemotherapy. Data collection and the follow-up protocols were reviewed and approved by the Institutional Review Board of Zhongshan Hospital, Fudan University (IRB No.: B2014-098).
Statistical Analysis
Patients with pretreatment serum CEA value ≥5.0 μg/mL were considered to be CEA positive (CEA+).17 Chi-square test (categorical variables) or Wilcoxon rank test (continuous variables) were used to compare patients’ characteristics across subgroups. Nonliner association between pretreatment CA19-9 value and OS was assessed using Cox regression based Restricted Cubic Spline (RCS).18,19 Continuous values of CA19-9 were discretized into categorical levels using determined cut-off points (Table 2). The survival curve was drafted using the Kaplan–Meier method and compared using the log-rank test. The Cox proportional hazard model was used to evaluate prognostic factors in multivariable survival analysis. All 2-sized P values <0.05 were considered to be statistically significant. Only available data were used in the analysis, whereas the missing data were taken as noninformative. All statistical analyses were performed using SAS 9.1.3.
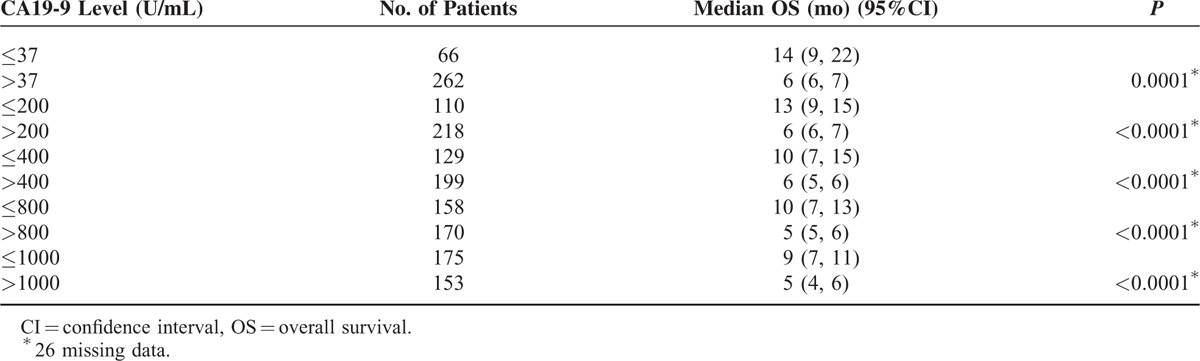
TABLE 2.
Pretreatment CA19-9 Level as a Predictor of Overall Survival∗
RESULTS
Demographic Characteristics and Clinical Parameters of Patients
A total of 354 patients with advanced pancreatic cancer were enrolled in the present study, out of which, 230 (65%) were male (male/female ratio = 1.8), and 72 (20%) patients were diagnosed with detectable distant metastases. Pretreatment CA19-9 value of patients with metastases was significantly higher as compared to those with locally advanced cancer (median: 922.30 vs 357.00 U/mL, P = 0.0090), whereas the pretreatment CEA value was uniform between the 2 subgroups (P = 0.2274). Presence of jaundice was significantly higher in patients with solid tumor located at the head of pancreas (0.28 vs 0.11, χ2 test, P = 0.0003).
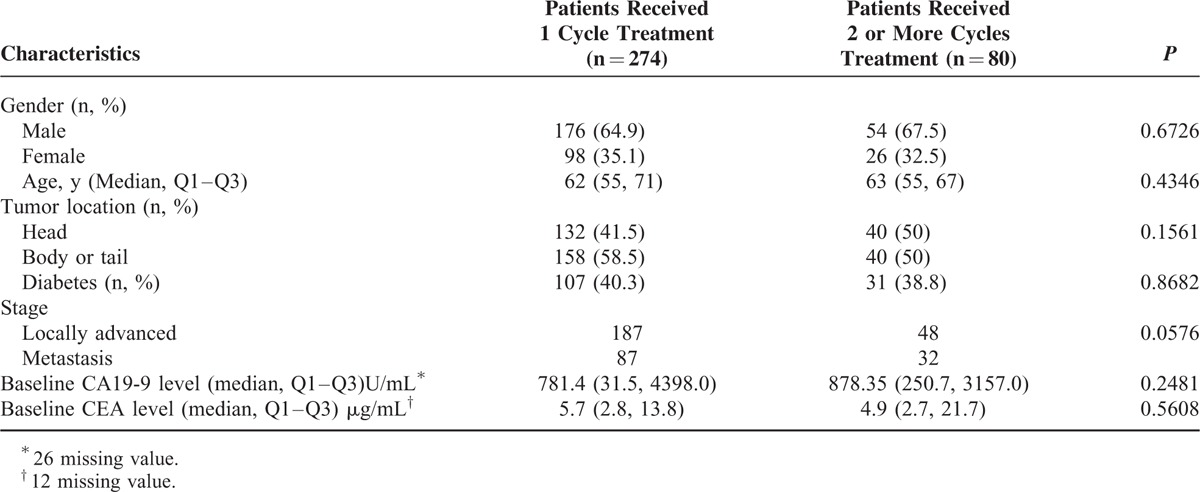
Of all the included patients, 274 completed 1 cycle of intra-arterial infusion, whereas 80 patients received 2 or more cycles of the chemotherapy. As shown in Table 1, no significant heterogeneity of characteristics or clinical parameters was found between the 2 subgroups. Approximately one-third of the patients presented a record of treatment with Chinese herbal medicines during the cycle of intra-arterial infusion. However, this factor was not included in the analysis, because the record of herbal medicines was ambiguous and the curative effect of traditional Chinese medicine (TCM) was difficult to assess.
TABLE 1.
Demographic and Clinical Characteristics of All Included Patients (n = 354)
Survival Analysis
For all the 354 patients, median OS was 7.0 months (95% CI: 6.0, 8.0 months) with a 6-, 12-, and 18-month survival rate of 0.48, 0.28, and 0.18, respectively. The median OS of patients, who received 1 cycle of intra-arterial infusion therapy, was 6.0 months (95% CI: 5.0, 8.0 months), which was similar to 7.0 months (95% CI: 6.0, 9.0 months) in patients who received 2 or more cycles.
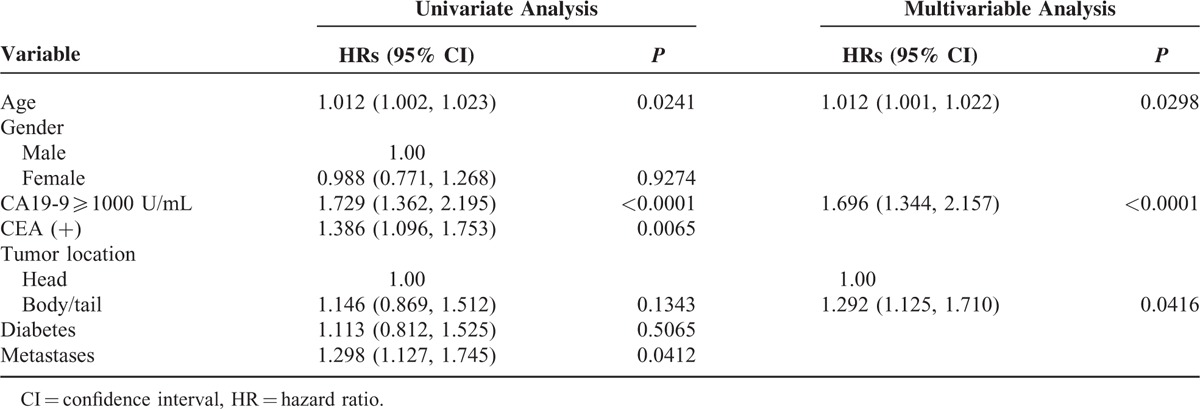
The RCS model presented a nonliner association between baseline CA19-9 value and prognosis in all the advanced patients (Figure 2). The increasing rate of hazard ratio was observed to slope down at the inflexion point at ∼1000 U/mL. Furthermore, the increase in the level of pretreatment CA19-9, as revealed by the univariate analysis, indicated worse OS after the gemcitabine-based intra-arterial infusion (Table 2). Taken together, 1000 U/mL was determined as the cut-off point for prognostic prediction. Patients with the pretreatment CA19-9 value of <1000 U/mL showed a significantly prolonged median OS compared with those with pretreatment CA19-9 ≥1000 U/mL (9.0 vs 5.0 months, log-rank P < 0.0001, Figure 3A). The 6-, 12-, 18-month survival rate of patients with baseline CA19-9 value <1000 U/mL was 0.58, 0.40, and 0.25, respectively. Only an estimated 35% of the patients with baseline CA19-9 value ≥1000 U/mL survived >6 months. Age, CEA+, and tumor metastases were revealed as strong prognostic predictors by univariate analysis (Table 3). The Cox proportional hazard model showed that age, CA19-9 baseline, CA19-9 value, and tumor location were significantly associated with the OS (Table 3).
FIGURE 2.

Nonliner association between pretreatment CA19-9 value and prognosis of all patients.
FIGURE 3.
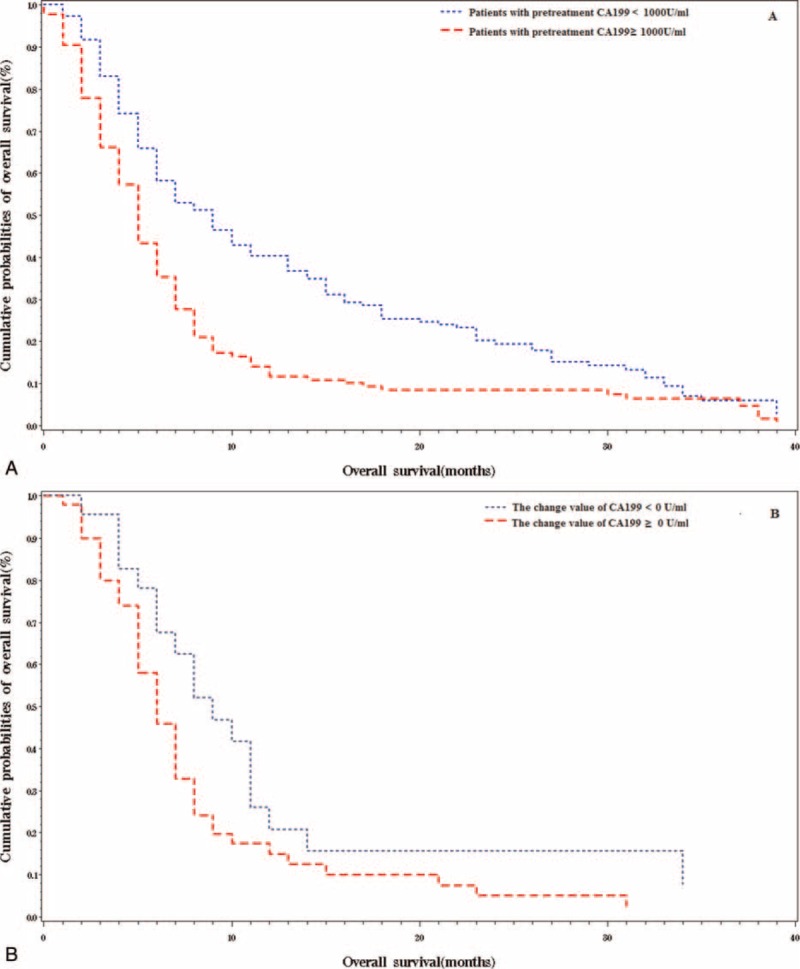
(A) Association between the pretreatment CA19-9 level and overall survival. (B) Association between post-treatment change of CA19-9 value and overall survival.
TABLE 3.
Predictors of Overall Survival in Patients Received Intra-Artery Infusion
For the 80 patients who received 2 or more cycles of intra-arterial infusion chemotherapy, the post-treatment CA19-9 value was measured as 40 (q1–q3: 28, 46) days after the initial infusion cycle. The median value of post-treatment CA19-9 was 1185.0 U/mL (q1–q3: 242.5, 4692.0 U/mL). Fifty (63%) patients were found with a declined CA19-9 value, whereas 30 patients presented with increased CA19-9 value. Univariate analysis showed that patients with declined CA19-9 after the first treatment cycle may show prolonged OS as compared to those with increased CA19-9 values (11.0 vs 7.0 months, P = 0.0479; Figure 3B). Nevertheless, the decline of CA19-9 was not a strong indicator of prolonged OS in the multivariable analysis (P = 0.1376).
DISCUSSION
Herein, we have described the procedure for gemcitabine-based regional intra-arterial infusion for advanced pancreatic adenocarcinoma. Our results showed that young age, pretreatment CA19-9 value of <1000 U/mL, and tumor located at the head of pancreas resulted in better OS in the Chinese patients who received gemcitabine-based regional intra-arterial chemotherapy (RIAC). A decline in the value of CA19-9 following the intra-arterial infusion was a strong predictor of better OS in univariate analysis.
In the present study, the median OS was 7.0 months in patients who received gemcitabine-based RIAC, which apparently was not worse than 6.7 months, as reported in advanced patients who underwent systemic gemcitabine chemotherapy.2 Although RIAC is considered to be more effective for tumor with rich blood supply, especially for hepatic carcinomas, rather than pancreatic adenocarcinoma,20,21 anatomical analysis has shown that gemcitabine infused through the celiac artery or superior mesenteric artery can cover the whole pancreas, and hepatic artery perfusion may also be effective for hepatic metastases.22 In addition to the anatomical evidence, our results corroborated the findings reported by other researchers.11,14,23,24 With drugs concentrating in the cancer tissue, RIAC was observed to be superior to systematic chemotherapy with less adverse effects, and no disadvantage with respect to the OS in relevant studies.24,25
More importantly, tumor location was found to be significantly associated with the prognosis. As mentioned above, tumor located at the head of pancreas indicated better OS in patients who received RIAC. This could be attributed to abundant blood vessels present at the pancreatic head, which implies greater concentration of gemcitabine and inhibition of tumor progression.21 Thus, patients with tumor located at pancreatic head may benefit more from anticancer drugs. Furthermore, this hypothesis was partly supported by the observations of Arredondo et al form animal experiment.26 The clinical significance of our results relates to assessing the benefits of RIAC, and making a clinical decision. Pretreatment CA19-9 level was also noted as a strong predictor of OS in the present study. This can be explained by the tight association of the biomarker of CA19-9 with cancer cell proliferation and metabolism.27 Higher CA19-9 values usually reflect aggravated tumor burden and worse prognosis.28,29 Several studies have demonstrated that the pretreatment CA19-9 value could be an indicator of OS in localized or advanced pancreatic adenocarcinomas.30–32 We found an optimized cut-off point for CA19-9 using a nonliner regression model, which was also confirmed by previous studies.
The strengths of this study included the detailed description of the clinical procedure of RIAC for advanced pancreatic adenocarcinoma, and the large cohort for survival analysis. However, there were certain limitations associated with the present research. First, only patients who received RIAC were included in the retrospective analysis. And the major side effects of RIAC were considered to be RIAC-induced pain or fever. However, the analgesic medicines were used to release severe pain of PDAC in our clinical center. This may covered up the potential side effect caused by RIAC. Furthermore, patients receiving RIAC chemotherapy usually stay 1 to 2 days in hospital in the presented study, trichomadesis and skin rashes were rarely observed. Rigorously designed clinical trials are further needed to assess the effects of RIAC in comparison to the systemic chemotherapy, and quantified adverse effect should also be seriously assessed. Second, as previously mentioned, some patients also followed TCM as an adjuvant therapy. Yang et al,33 in their retrospective analysis, reported that TCM benefited patients with advanced pancreatic cancer. However, the curative effect of this therapy for pancreatic cancer has still not been recognized and is controversial. Thus, we did not include TCM in our analysis. Third, this study was limited to the ethnic background, and only the available parameters were included in the analysis. Therefore, further studies are required to validate the findings of the present research.
In conclusion, the gemcitabine-based RIAC presented a potential treatment method for advanced pancreatic adenocarcinoma. Young age, pretreatment CA19-9 value <1000 U/mL, and tumor located at the head of pancreas indicated better response to the regional intra-arterial chemotherapy and better overall survival.
Acknowledgments
The authors are grateful to all the staff who have contributed to this study.
Footnotes
Abbreviations: CEA = carcinoembryonic antigen, ERBD = endoscopic retrograde biliary drainage, PTCD = percutaneous trans-hepatic catheter drainage, RCS = restricted cubic spline, RIAC = regional intra-arterial infusion chemotherapy, TCM = traditional Chinese medicine
XYL and XRY contributed equally to this study.
XYL and CYL drafted the manuscript; XRY and XYL performed the statistical analysis; XLW contributed to the revision of manuscript; GFZ, GPL, and CYL contributed to data collection and following up with patients; XLW conceived and designed the study and have primary responsibility for the final content.
This work was supported by the Rongchang Special Fund of Shanghai Charity Foundation and Zhongshan Hospital Youth Fund (2015ZSQN31).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65:5–29. [DOI] [PubMed] [Google Scholar]
- 2.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013; 369:1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Long J, Luo G-p, Xiao Z-w, et al. Cancer statistics: current diagnosis and treatment of pancreatic cancer in Shanghai, China. Cancer Lett 2014; 346:273–277. [DOI] [PubMed] [Google Scholar]
- 4.Sadeghi N, Abbruzzese JL, Yeung S-CJ, et al. Metformin use is associated with better survival of diabetic patients with pancreatic cancer. Clin Cancer Res 2012; 18:2905–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364:1817–1825. [DOI] [PubMed] [Google Scholar]
- 6.Paniccia A, Hosokawa P, Henderson W, et al. Characteristics of 10-year survivors of pancreatic ductal adenocarcinoma. JAMA Surg 2015; 150:701–710. [DOI] [PubMed] [Google Scholar]
- 7.Chen T, Zhang M-G, Xu H-X, et al. Preoperative serum CA125 levels predict the prognosis in hyperbilirubinemia patients with resectable pancreatic ductal adenocarcinoma. Medicine 2015; 94:e751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sultana A, Smith CT, Cunningham D, et al. Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer. J Clin Oncol 2007; 25:2607–2615. [DOI] [PubMed] [Google Scholar]
- 9.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007; 25:1960–1966. [DOI] [PubMed] [Google Scholar]
- 10.Frese KK, Neesse A, Cook N, et al. nab-Paclitaxel potentiates gemcitabine activity by reducing cytidine deaminase levels in a mouse model of pancreatic cancer. Cancer Discov 2012; 2:260–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu F, Tang Y, Sun J, et al. Regional intra-arterial vs. systemic chemotherapy for advanced pancreatic cancer: a systematic review and meta-analysis of randomized controlled trials. PloS One 2012; 7:e40847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi S, Yao W, Xu J, et al. Combinational therapy: new hope for pancreatic cancer? Cancer Lett 2012; 317:127–135. [DOI] [PubMed] [Google Scholar]
- 13.Ohigashi H, Ishikawa O, Imaoka S, et al. A new method of intra-arterial regional chemotherapy with more selective drug delivery for locally advanced pancreatic cancer. Hepatogastroenterology 1995; 43:338–345. [PubMed] [Google Scholar]
- 14.Cantore M, Pederzoli P, Cornalba G, et al. Intra-arterial chemotherapy for unresectable pancreatic cancer. Ann Oncol 2000; 11:569–573. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka T, Sho M, Nishiofuku H, et al. Unresectable pancreatic cancer: arterial embolization to achieve a single blood supply for intraarterial infusion of 5-fluorouracil and full-dose IV gemcitabine. Am J Roentgenol 2012; 198:1445–1452. [DOI] [PubMed] [Google Scholar]
- 16.Sobin Leslie H., Gospodarowicz Mary K., Christian Wittekind. TNM classification of malignant tumours[M]. John Wiley & Sons, 2011. [Google Scholar]
- 17.Jalanko H, Kuusela P, Roberts P, et al. Comparison of a new tumour marker, CA 19-9, with alpha-fetoprotein and carcinoembryonic antigen in patients with upper gastrointestinal diseases. J Clin Pathol 1984; 37:218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heinzl H, Kaider A. Gaining more flexibility in Cox proportional hazards regression models with cubic spline functions. Comput Methods Programs Biomed 1997; 54:201–208. [DOI] [PubMed] [Google Scholar]
- 19.Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med 2010; 29:1037–1057. [DOI] [PubMed] [Google Scholar]
- 20.Hong K, Khwaja A, Liapi E, et al. New intra-arterial drug delivery system for the treatment of liver cancer: preclinical assessment in a rabbit model of liver cancer. Clin Cancer Res 2006; 12:2563–2567. [DOI] [PubMed] [Google Scholar]
- 21.Kemeny N, Huang Y, Cohen AM, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med 1999; 341:2039–2048. [DOI] [PubMed] [Google Scholar]
- 22.Bertelli E, Di Gregorio F, Bertelli L, et al. The arterial blood supply of the pancreas: a review. Surg Radiol Anat 1996; 18:1–9. [DOI] [PubMed] [Google Scholar]
- 23.Takamori H, Kanemitsu K, Tsuji T, et al. 5-Fluorouracil intra-arterial infusion combined with systemic gemcitabine for unresectable pancreatic cancer. Pancreas 2005; 30:223–226. [DOI] [PubMed] [Google Scholar]
- 24.Mambrini A, Sanguinetti F, Pacetti P, et al. Intra-arterial infusion of 5-fluorouracil, leucovorin, epirubicin and carboplatin (FLEC regimen) in unresectable pancreatic cancer: results of a ten-year experience. In Vivo 2006; 20 (6A):751–755. [PubMed] [Google Scholar]
- 25.Muchmore JH, Carter RD, Preslan JE, et al. Regional chemotherapy with hemofiltration: a rationale for a different treatment approach to advanced pancreatic cancer. Hepatogastroenterology 1995; 43:346–355. [PubMed] [Google Scholar]
- 26.Arredondo MA, Chaudhuri B, Kar R, et al. Isolated perfusion of pancreas with mitomycin C. Am J Surg 1990; 159:569–574. [DOI] [PubMed] [Google Scholar]
- 27.Partyka K, Maupin KA, Brand RE, et al. Diverse monoclonal antibodies against the CA 19-9 antigen show variation in binding specificity with consequences for clinical interpretation. Proteomics 2012; 12:2212–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlieman MG, Ho HS, Bold RJ. Utility of tumor markers in determining resectability of pancreatic cancer. Arch Surg 2003; 138:951–955.discussion 955–956. [DOI] [PubMed] [Google Scholar]
- 29.Zhang JJ, Zhu Y, Zhu Y, et al. Association of increased DNA methyltransferase expression with carcinogenesis and poor prognosis in pancreatic ductal adenocarcinoma. Clin Translat Oncol 2012; 14:116–124. [DOI] [PubMed] [Google Scholar]
- 30.Micke O, Bruns F, Schafer U, et al. CA 19-9 in the therapy monitoring and follow-up of locally advanced cancer of the exocrine pancreas treated with radiochemotherapy. Anticancer Res 2003; 23 (2a):835–840. [PubMed] [Google Scholar]
- 31.Gogas H, Lofts FJ, Evans TR, et al. Are serial measurements of CA19-9 useful in predicting response to chemotherapy in patients with inoperable adenocarcinoma of the pancreas? Br J Cancer 1998; 77:325–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heinemann V, Schermuly MM, Stieber P, et al. CA19-9: a pedictor of response in pancreatic cancer treated with gemcitabine and cisplatin. Anticancer Res 1999; 19 (4a):2433–2435. [PubMed] [Google Scholar]
- 33.Yang X, Hao J, Zhu C-H, et al. Survival benefits of Western and traditional Chinese medicine treatment for patients with pancreatic cancer. Medicine 2015; 94 26: [DOI] [PMC free article] [PubMed] [Google Scholar]


