Supplemental Digital Content is available in the text
Abstract
Aberrant glycosylated IgA1 molecules, mainly galactose-deficient IgA1 (Gd-IgA1), are important causal factors in IgA nephropathy; however, the underlying mechanism for the production of aberrantly glycosylated IgA1 is unknown. A recent genome-wide association study identified a novel IgAN susceptibility gene, TNFSF13, which encoded a proliferation-inducing ligand (APRIL) that promotes lymphocyte proliferation and IgA class switching. We aimed to explore the mechanism of APRIL's involvement in IgAN.
We enrolled 166 patients with IgAN and 77 healthy controls and detected the plasma APRIL levels by the ELISA method, identified the mRNA expression of APRIL and its receptors by relative quantitative PCR, and confirmed by in vitro experiment.
We identified increased plasma APRIL levels in IgAN, which was further proved by upregulated mRNA expression in B-lymphocytes from 27 IgAN patients. Analysis of the clinical characteristics of patients with IgAN showed that higher plasma APRIL level was associated with more severe clinical presentations (high proteinuria and low eGFR). The plasma APRIL level was positively correlated with Gd-IgA1 levels. Furthermore, exogenous APRIL could induce more production of Gd-IgA1 in cultured lymphocytes from patients with IgAN, compared with that from healthy controls. And, the relative higher expression of receptors of APRIL, that is, BCMA and TACI, in B-lymphocytes from IgAN patients were observed.
Our findings implied that in patients with IgAN, increased APRIL is accompanied elevated expression of its receptors in B-lymphocytes, which induces overproduction of Gd-IgA1, ultimately contributing to the pathogenesis of IgAN.
INTRODUCTION
Primary IgA nephropathy (IgAN) is the most common primary glomerulonephritis worldwide. In China, it accounts for 58.2% of biopsy proved primary glommerulonephriti.1 Approximately 30% to 40% of the patients with IgAN will progress to renal replacement therapy within 20 years after presentation.2,3 The pathogenesis of the initiation and progression for IgAN remains obscure. Recently, increasing evidence has indicated that aberrantly glycosylated IgA1 molecules, mainly circulating galactose-deficient IgA1, are the trigger factors for mesangial deposition and subsequent kidney injury in IgAN. However, regarding the factors and mechanisms that induce the production of galactose-deficient IgA1, limited scientific data are available.
A genome-wide association study in IgAN revealed a locus containing the TNFSF13 gene,4 which encodes a proliferation-inducing ligand (APRIL) that shows high homology (48%) with B-cell activation factor (BAFF),5 to be a genetic susceptible locus for IgAN. In addition, a genetic variant of TNFSF13 was reported to be associated with serum IgA levels in both patients with IgAN and in the general population.4,6 Recently, McCarthy et al reported a transgenic mouse overexpressing BAFF, which showed high similarity to human IgA nephropathy in the presence of commensal flora. Upon overexpression of BAFF, the mice showed high circulating levels of polymeric aberrantly glycosylated IgA, mesangial deposition of IgA, and findings of hematuria and proteinuria.7 These studies suggested the potential involvement of APRIL in IgA production and disease pathogenesis of IgAN.
APRIL is a member of tumor necrosis factor super family (TNF). In addition to influencing the survival and proliferation of human B-cells, it is an important factor that drives human B-cells to induce Ig heavy chain class switch to IgA.8–11 After binding to their shared receptors, including TACI (transmembrane activator and calcium modulator and cyclophilin ligand interactor) and BCMA (B-cell maturation antigen), APRIL activates the downstream nuclear factor-κB (NF-κB) and then stimulates immunoglobulin production by peripheral blood B-cells.12 In APRIL-deficient mice, a selective deficiency in IgA was observed,13 which indicated an important role of APRIL in the IgA production process.
In the present study, to explore the underlying mechanism of APRIL in IgA nephropathy, we detected the expression of APRIL in patients with IgAN and further evaluated its effect on glycosylation of IgA1 molecules.
MATERIALS AND METHODS
Study Population
One hundred and sixty-six IgAN patients diagnosed in Peking University First Hospital between January 2014 and August 2014, and who gave their consent to donate 10 mL of venous blood, were enrolled in this study. At the same time, 77 healthy volunteers whose age and gender matched with patients were also recruited. Plasma (EDTA anticoagulated) samples were collected from all individuals in this study, for patients on the morning of renal biopsy and for controls on the day of recruitment. The plasma samples were divided into aliquots and stored in −80°C for the subsequent measurement of APRIL protein levels. In addition, peripheral blood mononuclear cells (PBMCs) isolation and RNA extraction were performed in 27 IgAN patients and 21 healthy controls, who were also enrolled in July and August 2014, to determine the expression of APRIL mRNA, as well as the expression of BCMA, TACI mRNA.
For patients with IgAN, diagnosis was based on the granular deposition of IgA in the glomerular mesangium by immunofluorescence detection, and the deposition of electron-dense material in the mesangium using the ultrastructural examination. Patients with Henoch Schonlein Purpura, systemic lupus erythematosus and chronic hepatic diseases were excluded by detailed clinical and laboratory examinations. Clinical information, including 24-hour urine protein excretion and blood pressure, were collected from medical records. The estimated glomerular filtration rate (eGFR) was evaluated using the Modified Glomerular Filtration Rate Estimating Equation for Chinese patients.14 For the evaluation of pathological lesions, Oxford classifications,15,16 were scored by 1 pathologist and independently checked by another pathologist, both of them blind to the clinical data.
The Medical Ethics Committee of Peking University First Hospital approved the study protocol and informed written consent was obtained from all individuals.
Plasma APRIL Detection
Plasma APRIL levels were detected using a commercial enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer's specifications (eBioscience, San Diego, CA).
Assay for IgA1 and Gd-IgA1
Total IgA1 and total Gd-IgA1 levels in plasma and in cell culture supernatant were determined by ELISA, as previously reported;17 however, there was a minor change in Gd-IgA1 standard compared with the previous report.18 In IgA1 detection, native IgA1 purified by normal human plasma (EMD Chemicals, Gibbstown, NJ) was used as standard for quantification of total IgA1. While for Gd-IgA1 detection, initially, the IgA1 protein was purified from plasma from a patient with multiple myeloma using an agarose-bound jacalin affinity chromatography column (Pierce Chemical Company, State of Illinois). The residual IgG was removed by a protein G column (GE, State of Connecticut). Finally, the terminal sialic acid from O-linked GalNAc was removed by neuraminidase (Roche Diagnostic Corp, Basel, CH), and galactose from O-linked GalNAc was removed by galactosidase (Sigma, State of Missouri). As the standard IgA1 myeloma protein is not entirely devoid of galactose, we expressed the results as U/mL, in which 1 unit of Gd-IgA1 was defined as 1 ng of this standard Gd-IgA1 myeloma protein. The abnormality of the glycosylation status of IgA1 molecules were expressed as adjusted Gd-IgA1 levels and calculated as total Gd-IgA1/total IgA1 levels.
B- and T-lymphocytes Isolation
Peripheral blood mononuclear cells (PBMCs) were prepared by density gradient centrifugation using Ficoll-Paque plus (GE, State of Connecticut, America). Then, B- and T-lymphocytes were isolated using CD19+ and CD3+ magnetic beads (Invitrogen, State of California), respectively, according to the manufacturer's instructions.
Total RNA Extraction and Reverse Transcription PCR (RT-PCR)
After cell isolation, total RNA was extracted from T-lymphocytes and B-lymphocytes using the commercial TRIZOL® Reagent (Invitrogen, Carlsbad, CA). cDNA was got from 1 μg total RNA using Reverse Transcription System (Promega, State of Wisconsin) and stored at −20°C for the next amplification. The mRNA levels of TNFSF13, BCMA, TACI, and GAPDH were measured by semiquantitative reverse transcriptase–PCR (the primer pairs were listed in Table 1), using SYBR® Premix Ex Taq™ (Takara, Shiga, JP) and performed on an Applied Biosystem 7500 Real-Time PCR System. The fold change between patients and controls was expressed by the 2-ΔΔCT method.
TABLE 1.
Primers Used to Amplify the TNFSF13, TACI, BCMA, and GAPDH Genes

Lymphocyte Culture and Treatment
Lymphocytes were isolated from venous blood as previously reported.19 Briefly, peripheral blood mononuclear cells (PBMCs) were first isolated by density gradient centrifugation. Monocytes were then depleted by the wall-sticking method from PBMCs. Thereafter, most of the remaining cells were lymphocytes, which were cultured in the RPMI-1640 medium supplemented with 10% fetal calf serum, 2 mM L-glutamine, 100 U/mL penicillin, and 100 mg/mL streptomycin at 37°C in a humidified 5% CO2 incubator and used for subsequent experiments.
During the experiment, lymphocytes were seeded into 96-well plates at density of 3 × 105/well and incubated with 25 ng/mL APRIL (Peprotech, NJ) for 48 hours. After the centrifugation of 800 g, 10 min, the supernatants were collected and used for detection of IgA1 and Gd-IgA1 levels.
Statistical Analysis
Statistical analyses were performed using SPSS software (version 16.0; SPSS, Chicago, IL). For continuous variables, data with a normal distribution was expressed as the mean ± SD and compared by an independent-samples t test, whereas other data were expressed as the median (first quartile and third quartile) and analyzed by the Mann–Whitney U test. Categorical variables were summarized as absolute frequencies or percentages and were compared using a χ2 test. A 2-tailed P-value <0.05 was considered statistically significant.
RESULTS
Clinical and Pathological Manifestations of Patients With IgAN
The clinical and pathological feature of IgAN patients in the present study (Table 2) was in accordance with previously reported IgAN cohorts. The average 24 hours proteinuria and eGFR in our patients and the grading of the pathological lesions by Oxford classification are also shown in Table 2.
TABLE 2.
The Baseline Data for Patients With IgAN and Healthy Controls
Patients With IgAN Had High Levels of APRIL
To evaluate whether APRIL is involved in IgA nephropathy, we at first compared the plasma levels of APRIL in patients with IgAN and healthy controls. We found that plasma levels of APRIL were significantly higher in patients with IgAN than in the healthy controls (APRIL: 0.15 [0.00, 2.41] vs 0.00 [0.00, 0.82] ng/mL, P = 0.009 Figure 1). Indeed, plasma APRIL levels were very low in some individuals. In our study population, 41.57% (69/166) IgAN patients and 57.14% (44/77) healthy controls had undetectable (below the limit of quantification) plasma APRIL levels when detected by ELISA. More individuals with undetectable plasma APRIL levels were in the control group than in the IgAN group, which was in accordance with our finding that patients with IgAN had higher APRIL levels in their plasma, suggesting APRIL's involvement in IgAN.
FIGURE 1.
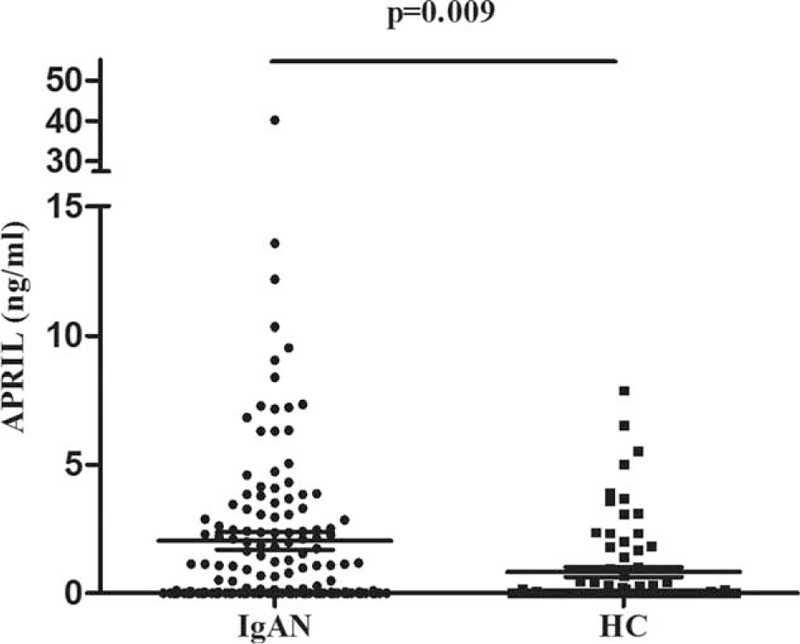
Plasma APRIL levels in IgAN patients and healthy controls. In patients with IgAN, APRIL levels were significantly higher than in healthy controls. APRIL = a proliferation-inducing ligand, IgAN = IgA nephropathy.
APRIL is widely produced by many cell types, such as T-lymphocytes, B-lymphocytes, and dendritic cells. Therefore, we isolated T- and B-lymphocytes and detected the expression of TNFSF13 mRNA and APRIL protein in them. Our results showed that compared with healthy controls, upregulated mRNA expression of TNFSF13 in IgAN patients was only observed in B-lymphocytes (2.56 ± 2.84 vs 1.06 ± 0.72, P = 0.003, Figure 2A), but not in T-lymphocytes (4.30 ± 7.41 vs 1.92 ± 1.50, P = 0.371, Figure 2B). Thus, in IgAN, B-lymphocytes may contribute to elevated plasma APRIL levels.
FIGURE 2.
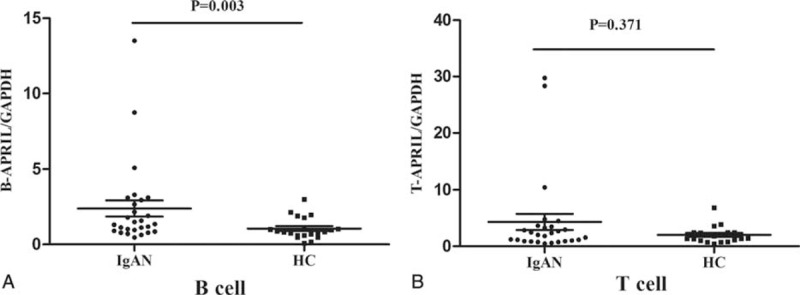
The mRNA expression of TNFSF13 in T- and B-lymphocytes. The expression of TNFSF13 in B-lymphocytes was significantly upregulated in patients with IgAN (A). However, in T-lymphocytes from patients with IgAN, the expression of TNFSF13 was comparable to those from healthy controls (B). IgAN = IgA nephropathy.
Plasma APRIL Levels Correlated With Severity of IgAN
After the identification of increased APRIL levels in patients with IgAN, we further explored its association with clinical findings and pathological lesions in patients with IgAN.
As some patients with IgAN presented with undetectable plasma APRIL levels, we initially classified them into the low value group (n = 69). We classified the other patients into 2 groups according to the median value of their plasma APRIL levels (median value: 2.02 ng/mL): middle value group (below median, n = 48) and high value group (above median, n = 49). We found that patients with high APRIL levels had significantly higher levels of proteinuria and lower levels of eGFR compared with patients with lower APRIL levels (Table 3). The results indicated that increased plasma APRIL levels correlated with more severe clinical manifestations in IgAN.
TABLE 3.
Clinical and Pathological Manifestations of IgAN Patients Grouped According to Plasma APRIL Levels
Plasma APRIL Levels Correlated With Adjusted Gd-IgA1 Levels in IgAN
APRIL contributes to the production of IgA, which is the characteristic molecule of mesangial deposition in IgAN. Currently, increased Gd-IgA1 molecules are widely accepted as the initial factor for IgAN. Therefore, after the identification that patients with IgAN had increased plasma APRIL levels, we investigated whether plasma APRIL correlated with Gd-IgA1 levels in IgAN. In patients with detectable APRIL, the plasma APRIL levels showed a strong positive correlation with adjusted Gd-IgA1 levels (r = 0.570, P = 0.021, Figure 3A), whereas for those with undetectable APRIL, their adjusted Gd-IgA1 levels were significantly lower than others (undetectable APRIL group vs detectable APRIL group: 0.36 ± 0.10 vs 0.53 ± 0.16, P = 0.005, Figure 3B). The correlation between APRIL and Gd-IgA1 suggested that APRIL might be involved in the mechanism of increased Gd-IgA1 production in IgAN.
FIGURE 3.
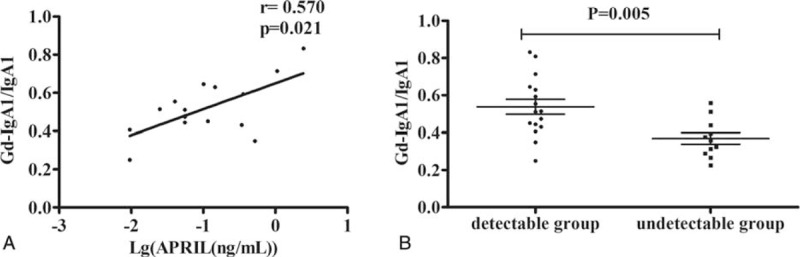
The correlation between APRIL and adjusted Gd-IgA1 levels. In patients with detectable APRIL levels, their APRIL levels showed significant positive correlation with adjusted Gd-IgA1 levels (A), whereas for patients with undetectable APRIL, their adjusted Gd-IgA1 levels were significantly lower compared with those with detectable APRIL (B). APRIL = a proliferation-inducing ligand, Gd-IgA1 = galactose-deficient IgA1.
APRIL Upregulates Gd-IgA1 Secretion From Lymphocytes
To evaluate whether APRIL served as the causal factor for increased Gd-IgA1 in IgAN, we further investigated the influence of APRIL on the production of Gd-IgA1 in an in vitro assay. Interestingly, we found that the responses to APRIL in lymphocytes were different between those derived from patients with IgAN and those from healthy controls. After APRIL treatment, in the supernatant of lymphocytes isolated from patients with IgAN, the adjusted Gd-IgA1 levels increased about 1.20 ± 0.15 fold, which was significantly higher than those from healthy controls (IgAN vs controls: 1.20 ± 0.15 fold vs 1.00 ± 0.17 fold, P = 0.014, Figure 4A). Our in vitro assay findings implied a role for APRIL in Gd-IgA1 production in patients with IgAN. More importantly, the findings suggested that not only APRIL, but also lymphocyte abnormality, might also contribute to elevated Gd-IgA1 in IgAN, because APRIL increased Gd-IgA1 production in lymphocytes from IgAN patients, but not from healthy controls.
FIGURE 4.
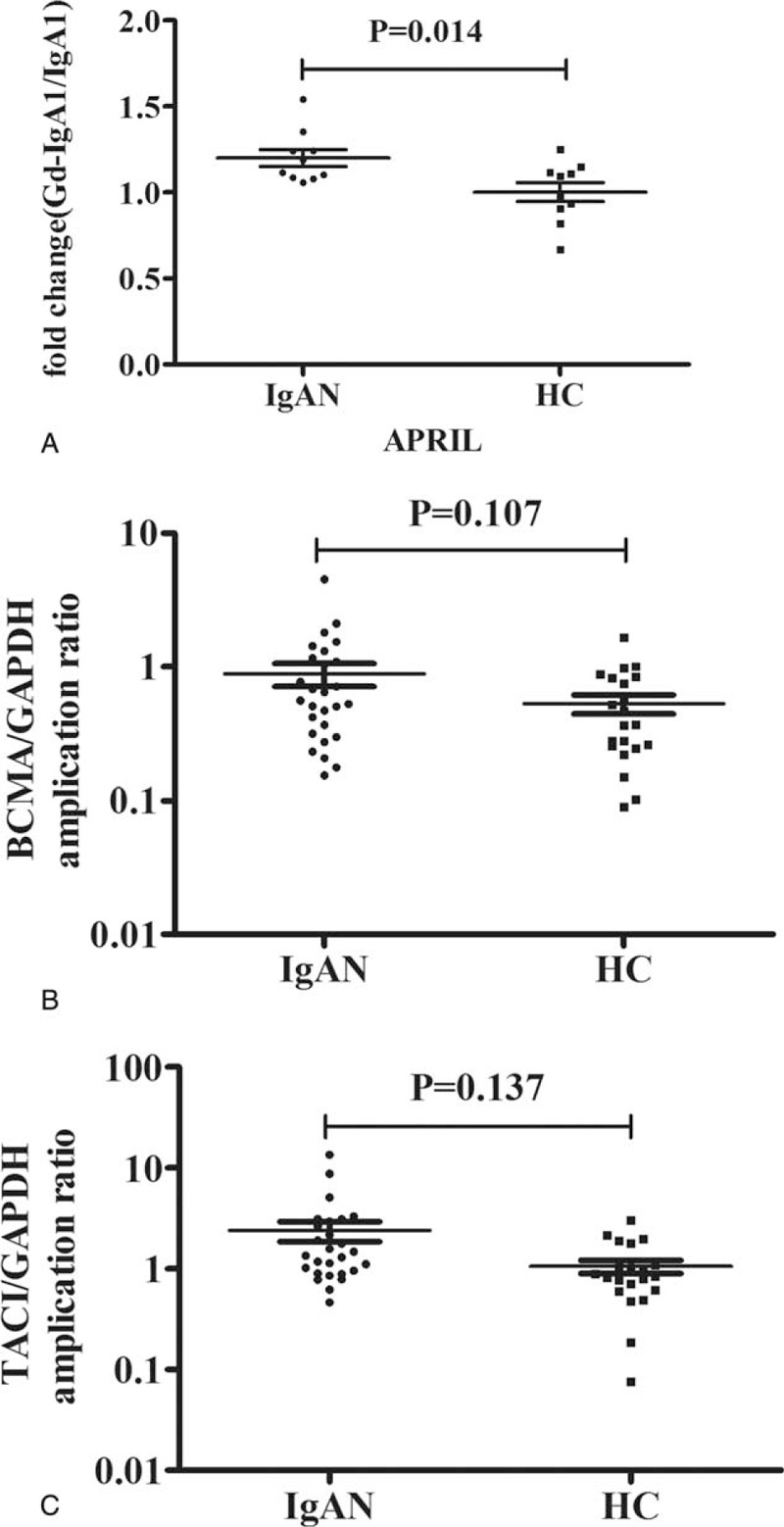
Gd-IgA1 production after APRIL stimulation and the expression of BCMA and TACI in B-lymphocytes. A scatter plot showing that adjusted the Gd-IgA production was significantly higher in patients with IgAN compared with healthy controls after APRIL stimulation (A); compared with healthy controls, the expression of BCMA (B) and TACI (C) in B-lymphocytes of IgAN patients showed a trend of upregulation, although statistical significance was not reached. APRIL = a proliferation-inducing ligand, Gd-IgA1 = galactose-deficient IgA1, IgAN = IgA nephropathy.
mRNA Levels of TACI and BCMA, the Receptors for APRIL
In circulation, CD19+B lymphocytes are the major cells for IgA1 production. To explore the causal factor of the lymphocytes abnormality in patients with IgAN, in terms of APRIL induced Gd-IgA1 production, we detected the mRNA expression of TACI and BCMA, encoding the receptors for APRIL, in CD19+B lymphocytes. In patients with IgAN, the mRNA expression of TACI and BCMA in B-lymphocytes showed a trend of upregulation, although statistical significance was not reached (BCMA: 0.88 ± 0.90 vs 0.53 ± 0.39, P = 0.107, Figure 4B; TACI: 1.70 ± 1.20 vs 1.26 ± 0.78, P = 0.137, Figure 4C).
DISCUSSION
IgA molecules, as the predominant deposits in glomeruli of patients of IgAN, have attracted the most attention in research into the pathogenesis of the disease.20 APRIL is a cytokine that stimulates B-lymphocyte proliferation and IgA production.11,21 Following previous report that patients with IgAN had elevated APRIL levels,7 in the present study, we at first validated the increased APRIL expression in IgAN, and further found that in patients with IgAN, the elevated APRIL levels together with marginal increases in the expressions of its receptors, BCMA and TACI, in B lymphocytes, inducing increased production of aberrantly glycosylated IgA1 molecules, which ultimately contributed to IgAN pathogenesis.
Consistent with a previous report,7 we also observed elevated plasma APRIL levels in our patients with IgAN. We then investigated which kind of cells contributed to this significant increase. APRIL is expressed and secreted by multiple cells, including monocytes, macrophages, and lymphocytes;22 therefore, we investigated its mRNA expression in T- and B-lymphocytes, because these 2 cells account for a large proportion of peripheral circulating cells and are involved frequently in autoimmune diseases. We observed upregulated APRIL expression in B-lymphocytes, but not in T-lymphocytes. The sample exhibited highest APRIL protein level (Figure 1) was the same as the one showed highest mRNA expression level in B-APRIL (Figure 2), but it was not comparable in T-APRIL in mRNA and protein levels. It might imply that B -lymphocytes play more important role in deciding the plasma APRIL level. We also detected the expression of BAFF, which is a highly homologous member with APRIL in TNFSF family, in T- and B-lymphocytes, and we found that the upregulated BAFF expression was also observed in B-lymphocytes, but not in T-lymphocytes (Supplementary Figure 1), which was consistent with the previous study that the expression of APRIL always positively correlated with that of BAFF. Although no information was available regarding other cells contributing to the elevated plasma APRIL levels, our results implied that, at least in part, B-lymphocytes contribute to the elevated plasma APRIL levels in IgAN, which suggested an abnormality of B-lymphocytes in IgAN. In the B-lymphocytes of IgAN, in addition to the significantly upregulated APRIL expression, the expressions of the receptors for APRIL also showed marginal elevation. Therefore, we hypothesized that APRIL and its downstream signaling should be activated in B-lymphocytes of IgAN patients.
Although abnormal APRIL expression in B-lymphocytes had not been reported before in IgAN, studies on BAFF, which has similar functions to APRIL (e.g., induction of germline Cα gene expression, AID expression, and IgA class switching in a CD40-independent manner23), could be used as a reference. Recent studies reported the upregulated expression of BAFF in tonsillar mononuclear cells (TMC) in patients with IgAN after capsaicin or CpG-ODN stimulation.24,25 Moreover, they found that upregulation of BAFF caused aberrant IgA1 O-glycosylation by suppressing C1GALT1 and Cosmc expression. Similarly, in our study, we proved that APRIL could also induce the production of Gd-IgA1. Taken together, these findings indicated that activation of the APRIL/BAFF system in B-lymphocytes might play an important role in the overproduction of pathogenic aberrant glycosylated IgA1 molecules in IgAN. However, the underlying mechanism of how APRIL induces Gd-IgA1 production remains unknown here. Future studies will focus on the signaling action and effector gene expression (such as C1GALT1 and Cosmc) downstream of APRIL-TACI/BCMA. In addition, the expression of BCMA and TACI in IgAN patients was needed to verify in more patients, and to be validated in an independent cohort.
In our patients with IgAN, we observed a positive correlation between plasma APRIL levels and Gd-IgA1 levels, which validated our finding that APRIL could induce production of aberrant glycosylated IgA1 molecules in an in vitro cell culture model. In addition, we found that patients with higher levels of APRIL presented with more severe clinical manifestations of IgAN. In the pathogenesis of IgAN, increasing evidence suggests that aberrant O-glycosylation of IgA1 acts as a “trigger.” Moreover, our previous study attributed O-glycosylation abnormality of IgA1 as an independent predictor for long-term renal outcome in IgAN.17 Therefore, the association between APRIL and IgAN severity observed in the present study suggested the important role of APRIL in IgAN and supported APRIL as a causal factor for promoting the production of Gd-IgA1 in IgAN.
In summary, the present study revealed increased APRIL levels and the association of APRIL with disease severity of IgAN, which suggested the involvement of APRIL in IgAN. Furthermore, we proved that APRIL, in cooperation with its receptors in B-lymphocytes, could induce the production of Gd-IgA1, which represent, at least in part, the mechanism of APRIL's action in the pathogenesis of IgAN.
Supplementary Material
Footnotes
Abbreviations: APRIL = a proliferation-inducing ligand, BAFF = B-cell activation factor, BCMA = B-cell maturation antigen, eGFR = estimated glomerular filtration rate, Gd-IgA1 = galactose-deficient IgA1, IgAN = IgA nephropathy, PBMCs = peripheral blood mononuclear cells, TACI = transmembrane activator and CAML interactor
Funding: this work was supported by grants from the Natural Science Foundation for Innovation Research Group of China (81321064); the National Key Technology R&D Program (2013BAI09B14); the Capital of Clinical Characteristics and the Applied Research Fund (Z141107002514037); National Science Foundation of China (Grant No. 81470945) and the Beijing Natural Science Foundation (Grant No. 7131016).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
REFERENCES
- 1.Zhou FD, Zhao MH, Zou WZ, et al. The changing spectrum of primary glomerular diseases within 15 years: a survey of 3331 patients in a single Chinese centre. Nephrol Dial Transplant. [DOI] [PubMed] [Google Scholar]
- 2.D’Amico G. The commonest glomerulonephritis in the world: IgA nephropathy. Q J Med 1987; 64:709–727. [PubMed] [Google Scholar]
- 3.Galla JH. IgA nephropathy. Kidney Int 1995; 47:377–387. [DOI] [PubMed] [Google Scholar]
- 4.Yu XQ, Li M, Zhang H, et al. A genome-wide association study in Han Chinese identifies multiple susceptibility loci for IgA nephropathy. Nat Genet 2012; 44:178–182. [DOI] [PubMed] [Google Scholar]
- 5.Schneider P, MacKay F, Steiner V, et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med 1999; 189:1747–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osman W, Okada Y, Kamatani Y, et al. Association of common variants in TNFRSF13B, TNFSF13, and ANXA3 with serum levels of non-albumin protein and immunoglobulin isotypes in Japanese. PLoS One 2012; 7:e32683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCarthy DD, Kujawa J, Wilson C, et al. Mice overexpressing BAFF develop a commensal flora-dependent, IgA-associated nephropathy. J Clin Invest 2011; 121:3991–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin R, Kaneko H, Suzuki H, et al. Age-related changes in BAFF and APRIL profiles and upregulation of BAFF and APRIL expression in patients with primary antibody deficiency. Int J Mol Med 2008; 21:233–238. [PubMed] [Google Scholar]
- 9.Tangye SG, Bryant VL, Cus AK, et al. BAFF, APRIL and human B cell disorders. Sem Immunol 2006; 18:305–317. [DOI] [PubMed] [Google Scholar]
- 10.Salzer U, Neumann C, Thiel J, et al. Screening of functional and positional candidate genes in families with common variable immunodeficiency. BMC Immunol 2008; 9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castigli E, Wilson SA, Scott S, et al. TACI and BAFF-R mediate isotype switching in B cells. J Exp Med 2005; 201:35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marsters SA, Yan M, Pitti RM, et al. Interaction of the TNF homologues BLyS and APRIL with the TNF receptor homologues BCMA and TACI. Curr Biol 2000; 10:785–788. [DOI] [PubMed] [Google Scholar]
- 13.Castigli E, Scott S, Dedeoglu F, et al. Impaired IgA class switching in APRIL-deficient mice. Proc Natl Acad Sci U S A 2004; 101:3903–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma YC, Zuo L, Chen JH, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 2006; 17:2937–2944. [DOI] [PubMed] [Google Scholar]
- 15.Roberts IS, Cook HT, Troyanov S, et al. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int 2009; 76:546–556. [DOI] [PubMed] [Google Scholar]
- 16.Cattran DC, Coppo R, Cook HT, et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int 2009; 76:534–545. [DOI] [PubMed] [Google Scholar]
- 17.Zhao N, Hou P, Lv J, et al. The level of galactose-deficient IgA1 in the sera of patients with IgA nephropathy is associated with disease progression. Kidney Int 2012; 82:790–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moldoveanu Z, Wyatt RJ, Lee JY, et al. Patients with IgA nephropathy have increased serum galactose-deficient IgA1 levels. Kidney Int 2007; 71:1148–1154. [DOI] [PubMed] [Google Scholar]
- 19.Xie L, Tan C, Fan J, et al. Mycophenolic acid reverses IgA1 aberrant glycosylation through up-regulating Cosmc expression in IgA nephropathy. Int Urol Nephrol 2013; 45:571–579. [DOI] [PubMed] [Google Scholar]
- 20.Floege J, Moura IC, Daha MR. New insights into the pathogenesis of IgA nephropathy. Semin Immunopathol 2014; 36:431–442. [DOI] [PubMed] [Google Scholar]
- 21.Yu G, Boone T, Delaney J, et al. APRIL and TALL-I and receptors BCMA and TACI: system for regulating humoral immunity. Nat Immunol 2000; 1:252–256. [DOI] [PubMed] [Google Scholar]
- 22.Hahne M, Kataoka T, Schroter M, et al. APRIL, a new ligand of the tumor necrosis factor family, stimulates tumor cell growth. J Exp Med 1998; 188:1185–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He B, Raab-Traub N, Casali P, et al. EBV-encoded latent membrane protein 1 cooperates with BAFF/BLyS and APRIL to induce T cell-independent Ig heavy chain class switching. J Immunol 2003; 171:5215–5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goto T, Bandoh N, Yoshizaki T, et al. Increase in B-cell-activation factor (BAFF) and IFN-gamma productions by tonsillar mononuclear cells stimulated with deoxycytidyl-deoxyguanosine oligodeoxynucleotides (CpG-ODN) in patients with IgA nephropathy. Clin Immunol 2008; 126:260–269. [DOI] [PubMed] [Google Scholar]
- 25.Shao J, Peng Y, He L, et al. Capsaicin induces high expression of BAFF and aberrantly glycosylated IgA1 of tonsillar mononuclear cells in IgA nephropathy patients. Hum Immunol 2014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


