Abstract
We designed a retrospective cohort study to assess sex-related differences in clinical manifestations, incidence, and outcomes of patients with symptomatic acute aortic dissection (AAD).
We collected clinical data from 2010 to 2015 of 400 patients with AAD. Patients’ clinical characteristics, treatment, and outcomes were analyzed as a function of sex.
Among 400 patients with AAD, the ratio of men to women was 3.18:1; the incidence of atherosclerosis was higher in women (P = 0.02). Dysphoria (P = 0.01), focal neurological deficits (P = 0.04), and pulse deficits (P = 0.03) were more frequent in men. Imaging findings revealed that pleural effusion (P < 0.01), celiac trunk involvement (P < 0.01), and superior mesenteric artery involvement (P = 0.02) were more frequent in men. Dissection-related pneumonia (P = 0.02), pulmonary atelectasis (P = 0.01), aortic intramural hematoma (P < 0.01), ischemic electrocardiographic changes (P = 0.03), and in-hospital complications such as myocardial ischemia (P = 0.03), hypoxemia (P < 0.01), cardiac tamponade (P = 0.01) occurred more frequently in women. Women with type A dissection had higher in-hospital mortality than men (P < 0.01).
The presentation of AAD varies with a patient's sex. Women with AAD had clinical features different from men as follows: higher age of onset, more frequent inpatient complications, and higher in-hospital mortality. These findings may lead to a better understanding of aortic dissection in women that will improve their outcomes.
INTRODUCTION
Acute aortic dissection (AAD) is a life-threatening condition associated with morbidity and mortality. AAD was considered a rare disease (2.9 to 3.5 cases per 100,000 person-years),1 However, according to the United States Centers for Disease Control and Prevention, diseases of the aorta and its branches account for 43,000 to 47,000 deaths annually in the United States,2 indicating that AAD is not “rare.” Most studies of autopsies suggest that the presentation of aortic disease is often death because of AD and rupture.3,4 The overall prognosis of patients with AAD is poor, and ∼40% of patients will die immediately, 1% per hour will die after onset, and 5% and 20% die during or shortly after surgery, respectively.5
The first cases of aortic dissection was reported in the 18th century,6 with the development of the diagnosis and therapeutic method, interest and research in aortic dissection increased gradually.7–9 These advancements facilitate early diagnosis, exquisite treatment, and improved prognosis.6,10 Regardless of mounting research in AAD, still insufficient information is available to determine if there are sex-related differences in clinical manifestations, and prognosis.6 Accordingly, the aim of the present study was to assess differences between male and female patients with AAD in Xin Jiang, China. We expected that our study would provide valuable information regarding sex-related differences in Symptomatic Acute Aortic Dissection.
METHODS
Ethical Approval of the Study Protocol
This study was approved by the Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University (Urumqi, China) and was conducted according to the standards of the Declaration of Helsinki.
Study Population and Data Collection
We collected clinical data on all patients with acute aortic dissection diagnosed and treated at the First Affiliated Hospital of Xinjiang Medical University from 2010 to 2015 (n = 400). This hospital is the first diagnostic and treatment center for cardiovascular disease in the Xinjiang Uygur Autonomous Region of China and is staffed by a highly experienced and successful medical team that provides advanced medical facilities to patients from every region of Xin Jiang (23 cities, 7 regions, 5 autonomous prefectures, and 68 counties). Data were collected using standardized forms that included information about patient demographics, history, clinical presentation, imaging findings, management, clinical events, and mortality. Patients were identified by searching hospital discharge diagnostic record, surgical and echocardiography laboratory databases. Diagnosis was determined using history, findings of imaging studies, and observations during surgery.
Inclusion and Exclusion Criteria
Inclusion criteria were any symptomatic acute aortic dissection (2 weeks from onset of symptoms, including Stanford types A and B). Exclusion criteria were any chronic dissection (duration >2 weeks), any asymptomatic patients, or aortic disruption secondary to trauma.
Anatomical Classification of Aortic Dissection
AAD is classified according to the origin of the intimal tear or whether the dissection involves the ascending aorta (regardless of site of origin). Accurate classification is important, because it influences decisions regarding surgical versus nonsurgical management. In the present study, we used the Stanford classification system that categorizes dissections as those that do or do not involve the ascending aorta as follows: Type A, all dissections involving the ascending aorta regardless of the site of origin; and Type B, all dissections that do not involve the ascending aorta. (Involvement of the aortic arch without involvement of the ascending aorta is designated Type B.)11.
Statistical Analysis
The summary of the statistical analyses includes the values of the mean and standard deviation (SD) of frequencies and percentages. Missing data were not defaulted to negative, and denominators reflect only reported cases. Univariate associations between the 2 groups for nominal variables were compared using the Pearson χ2 test or Fisher's exact test, and the 2-tailed Student t test was used to evaluate continuous variables. Data analysis was performed using Statistical Package for Social Sciences (SPSS) v. 17.0 for Windows (SPSS Institute, Chicago). P < 0.05 was considered statistically significant.
RESULTS
Sex-Specific Differences Among Age Groups
As shown in Figure 1, of 400 patients with ADD, ages ranged from 22 to 91 years. The incidence of AAD was significantly higher in men aged 20 to 39, 40 to 49 years compared with that of women (P < 0.05), the proportion of women with AAD was slightly increased after 60 years of age. As shown in Table 1, there were significant differences in the average age of onset between the men and women (men 49.6 ± 12.6 years; women 54.2 ± 12.4 years, P < 0.01).
FIGURE 1.
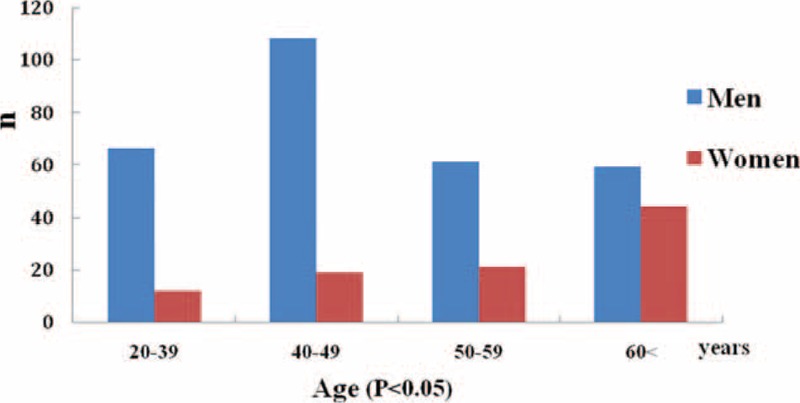
Differences in age of onset between women and men. Total 400 patients, ages ranged from 22 to 91 years, the proportion of men was significantly higher in the 20 to 39, 40 to 49 age groups compared with that of women (P < 0.05), and the proportion of women with AAD was slightly increased after 60 years of age. AAD = acute aortic dissection.
TABLE 1.
Patients’ Histories and Clinical Manifestations Between Women and Men
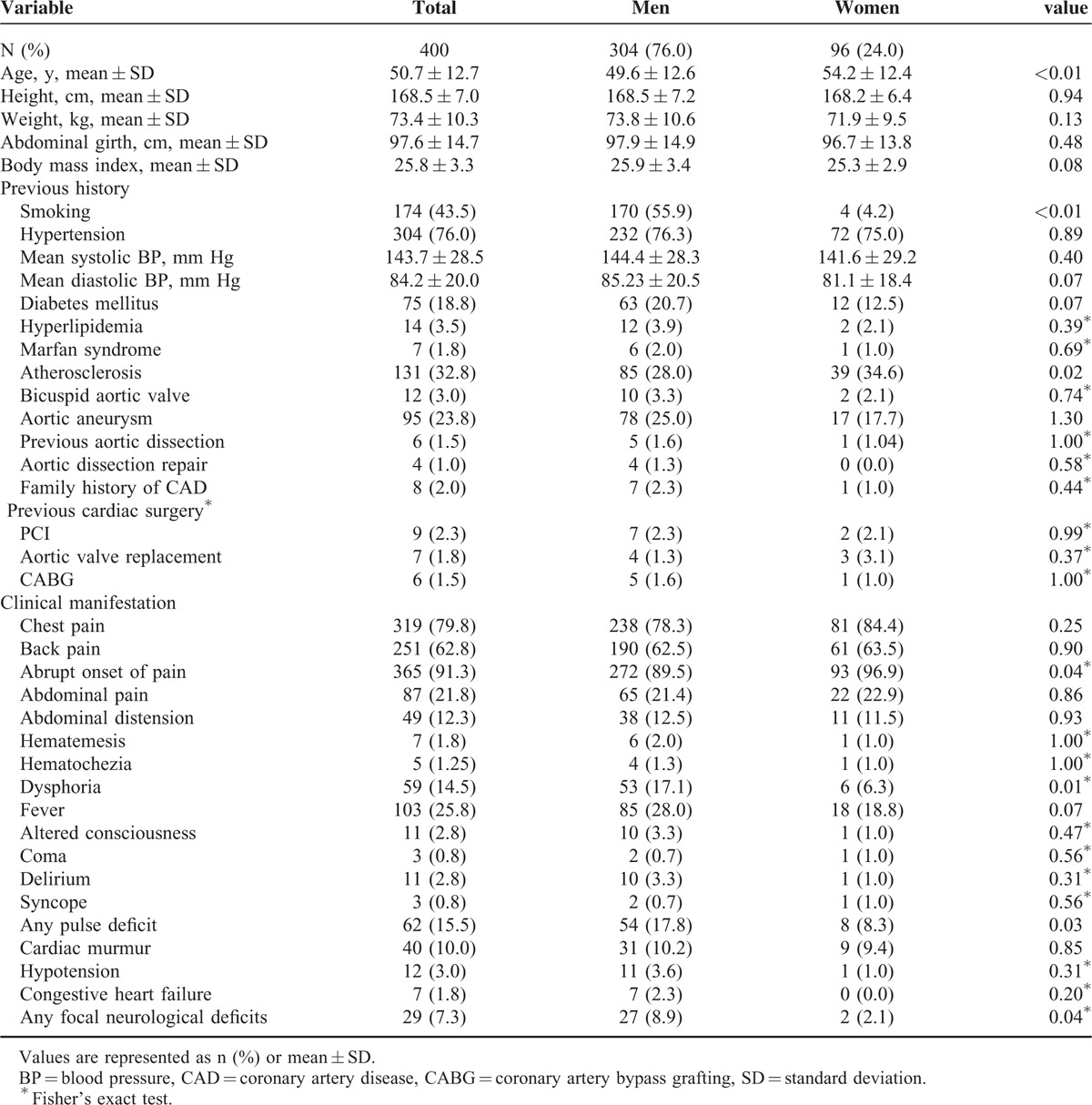
Sex-Related Differences in Patients’ Histories and Clinical Characteristics
As shown in Table 1, of 400 patients with ADD, 76% were men and 24% were women, and the ratio of men to women was ∼3.18:1. According to their previous medical histories, the percentage of men who smoked was significantly higher than that of women (55.9% vs 4.17%, P < 0.01), and the percentage of women with arthrosclerosis12 was higher than that of men (40.6% vs 28.21%, P = 0.02). There were no significant differences in other variables, including Marfan syndrome,13–14 bicuspid aortic valve,15–16 aortic aneurysm,17–18 and cardiac surgeries (percutaneous coronary intervention, coronary artery bypass grafting, and aortic valve replacement, etc).
The mean values of systolic and diastolic blood pressures upon presentation did not differ between groups, whereas the classic presentation of chest or back pain at onset was similar, although women experienced abrupt onset of pain more frequently than men (96.9% vs 89.5%, P = 0.04). Dysphoria (17.1% vs 6.3%, P = 0.01), focal neurological deficits (8.9% vs 2.1%, P = 0.04), and pulse deficit symptoms (17.8% vs 8.3%, P = 0.03) were more frequent in men. There were no significant differences in the frequencies of uncommon symptoms upon onset such as abdominal pain, hematochezia, coma, and congestive heart failure.
Sex-Related Differences in Diagnostic Imaging and Electrocardiographic Data
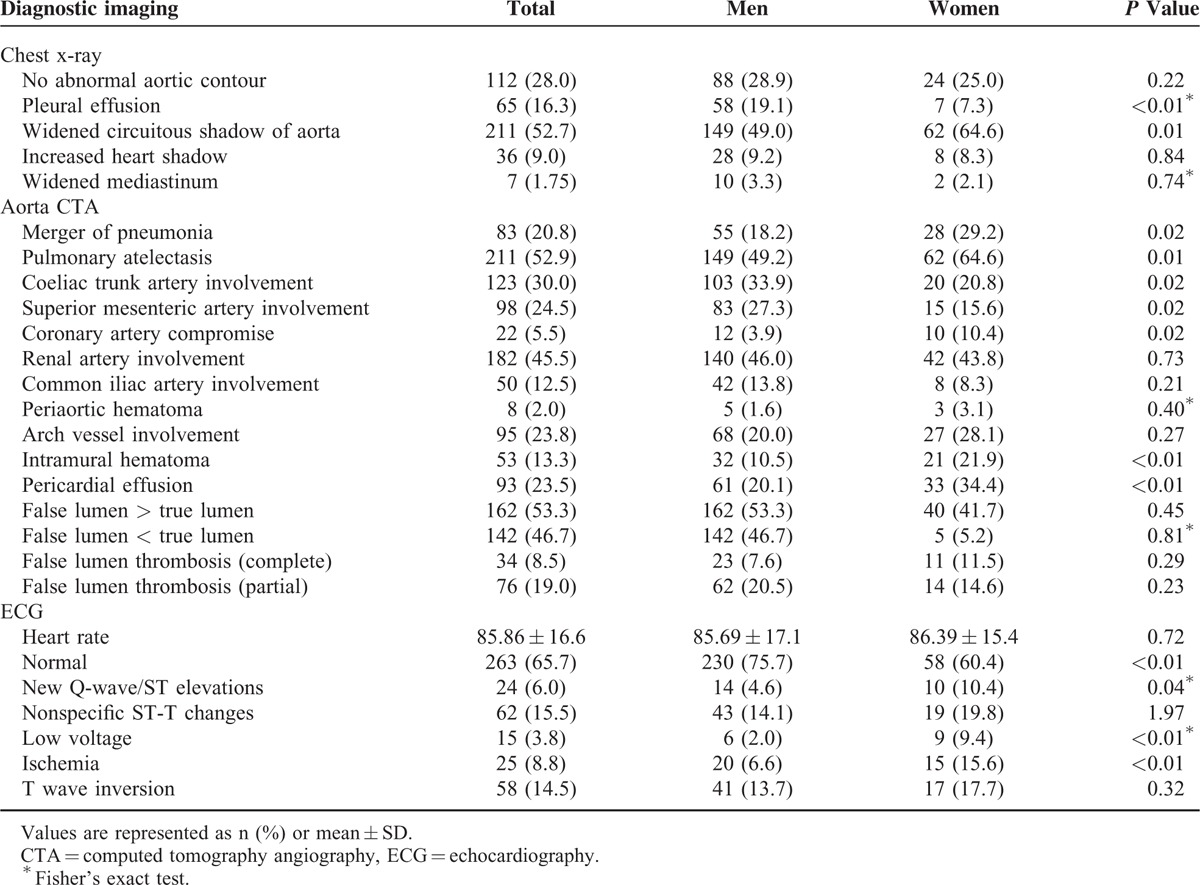
As shown in Table 2, diagnostic imaging studies were used similarly, and the findings suggested that pleural effusion (19.1% vs 7.3%, P < 0.01), celiac trunk involvement (33.9% vs 20.8%, P = 0.02), and superior mesenteric artery involvement (27.3% vs 15.6%, P = 0.02) were more frequent in men. In contrast, widened aortic shadow (49.6% vs 64.6%, P = 0.01), comorbid pneumonia (18.2% vs 29.2%, P = 0.02), pulmonary atelectasis (49.2% vs 64.6%, P = 0.01), compromised coronary artery (3.9% vs 10.4%, P = 0.02), intramural hematoma (10.5% vs 21.9%, P < 0.01), and pericardial effusion (20.2% vs 33%, P < 0.01) were more frequent in women. The ECG findings showed new Q waves or elevation of the ST-segment (4.6% vs 10.4%, P = 0.04), low voltage (2.0% vs 9.4%, P < 0.01), and ischemic changes (6.6% vs 15.6%, P < 0.01) were more frequent among women (Table 2).
TABLE 2.
Imaging Findings for Men and Women
Sex-Related Differences Among in-Hospital Complications and Mortality
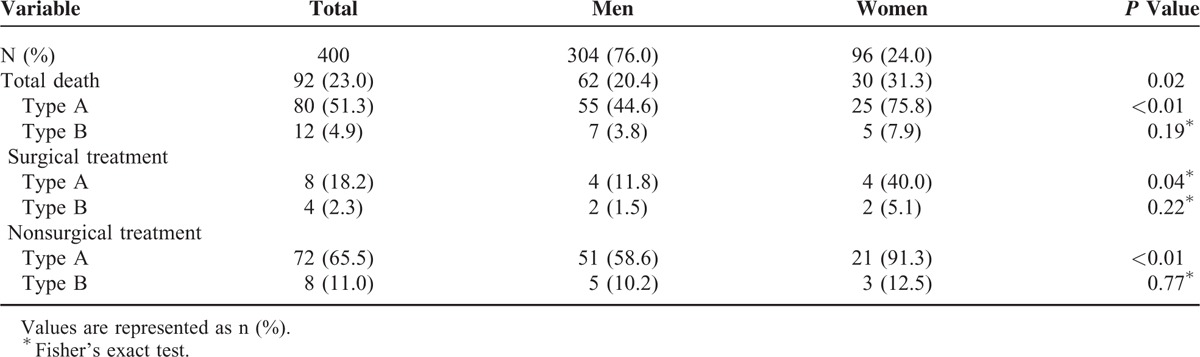
There were no significant differences in the classifications of dissection, hours from onset symptoms to diagnosis. The frequencies of in-hospital treatments such as surgery and endovascular treatment were not significantly different between men and women (Table 3). Severe in-hospital complications such as myocardial ischemia/infarction, hypoxemia, and cardiac tamponade occurred more frequently among women (all P < 0.05). In contrast, men experienced mesenteric ischemia/infarction and acute renal damage more frequently (Table 4). As shown in Table 5, the overall in-hospital mortality was 23%. The survival rate of women was lower than men with type A dissection (P < 0.01). The surgical and nonsurgical outcomes were less favorable for women. In particular, type A dissection in women was associated with increased surgical mortality (P = 0.04) and nonsurgical mortality (P < 0.01).
TABLE 3.
In-Hospital Treatment of Women and Men
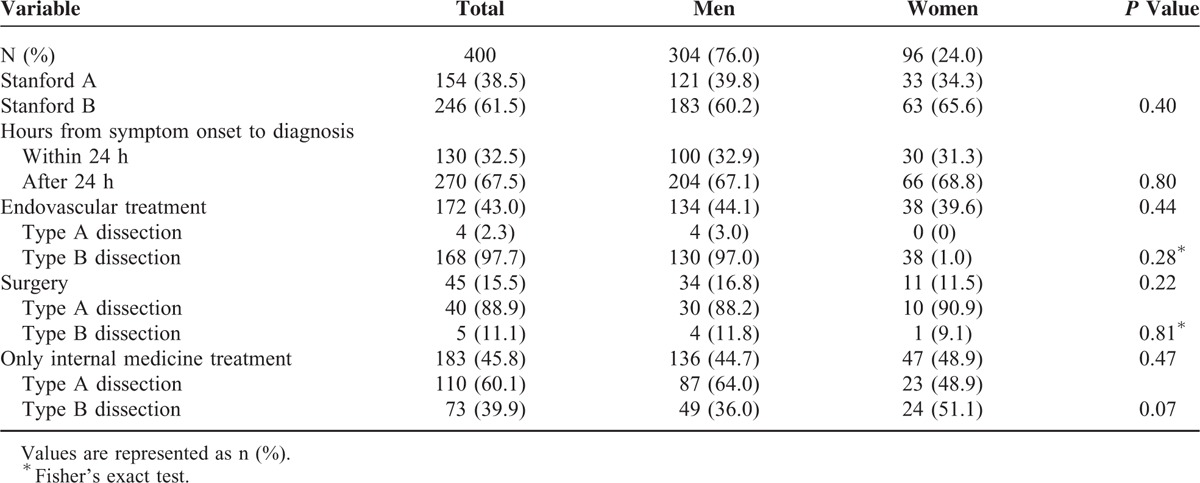
TABLE 4.
In-Hospital Complications of Women and Men
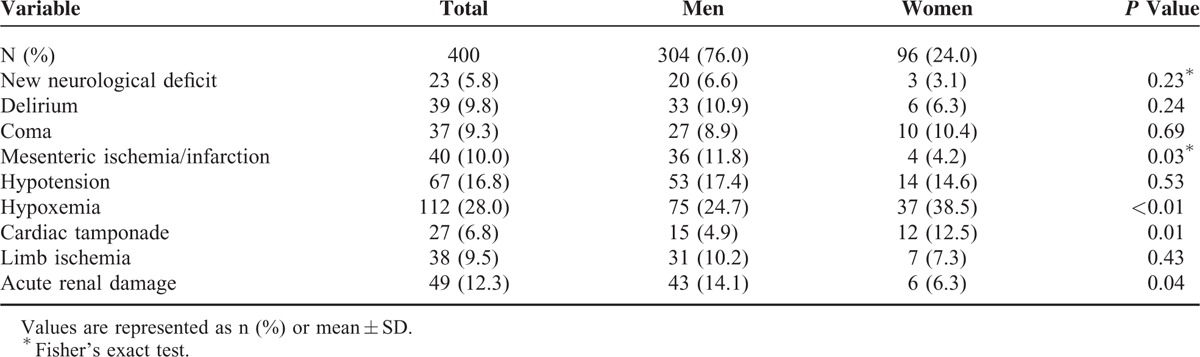
TABLE 5.
In-Hospital Mortality of Women and Men
DISCUSSION
With the recognition of cardiovascular disease as the leading cause of global mortality among women, researchers focused on sex-related differences in AAD.19–20 The International Registry of Acute Aortic Dissection (IRAD) study6 evaluated differences in clinical features, outcomes of male and female with AD. The IRAD study reports that the ages of onset are 60.3 ± 13.7 and 66.7 ± 13.9 years for men and women, respectively. In contrast, in this study, we showed that the mean age of onset for men was earlier compared with women (49.6 ± 12.6 years and 54.2 ± 12.4 years, respectively), indicating that in the Xin Jiang region of China, the onset age of AAD is earlier than that in western countries. This difference related to a lower standard of healthcare, and unbalanced distribution of medical conditions between different regions in China.
In the present study, patients’ medical histories show that the number of men who smoked was significantly higher than that of women. Heavy smoking increases oxidative stress, induces endothelial injury, and eventually leads to expansion of aortic aneurysms.21
Pain is the most frequently reported presenting symptom of patients with AAD, regardless of age, sex, or other associated clinical complaints.22–23 Analysis of pooled data of >1000 patients in 8 studies showed that the pain of acute dissection is perceived as abrupt in onset and severe in 84% and 90% of patients, respectively.24 In our study, 79.75% patients presented with the classic onset symptoms of chest or back pain. Women more frequently described this pain as abrupt tearing or ripping, and men more frequently described the pain as sharp or stabbing. Migrating pain was described by 30% of patients, and 21.75% of patients presented with abdominal pain in the absence of chest pain, or with only abdominal distension (12.3%) accompanied by other uncommon symptoms such as hematemesis (1.8%), hematochezia (1.3%), coma/altered consciousness (2.8%), and delirium (2.8%). Dysphoria (17.1%), focal neurological deficits (17.8%), and pulse deficits (8.9%) were present more frequently in men. The cause of most episodes of pain was obstruction by the dissection flap, which can either prolapse across a vessel's origin without entering it or directly extend into a vessel.25,26
Imaging studies showed that pleural effusion, which was the most frequent (16.3%) pulmonary complication of AAD, was more frequent in men. Small effusions result from a nonhemorrhagic exudate, likely caused by inflammation. Large effusions mostly caused by blood leaking from the aorta into the pleural space.27,28 Other pulmonary complications of AAD include dissection-related atelectasis of pulmonary tissue and hypoxemia that were observed more frequently in women and may present with dyspnea as a prominent symptom.29,30
The present data show that mesenteric ischemia and acute renal damage were more frequent in men (Table 4). These results are consistent with the findings of computed tomography angiography (CTA), which showed involvement of the coeliac trunk and superior mesenteric arteries more frequently in men. Mesenteric ischemia is the most frequent gastrointestinal complication of AAD. However, by the time values of serum markers of bowel ischemia or infarction become positive, it is often too late to salvage the bowel or the patient. Therefore, it is essential to improve vigilance for mesenteric ischemia in every AAD patient with abdominal pain. Renal complications of AAD may be acute, or chronic.31 But physical examination was insensitive to renal ischemia early in the course of AAD.32
Heart is the most commonly implicated organ in progression of AAD. The heart-related complications mostly originated by disruption of dissection or aorta.2 For AAD, cardiac ischemia/infarction is an uncommon but critical complication.2,33 In our study, the frequencies of patients with myocardial ischemia and infarction were 8.3% and 4.3%, respectively, and women were more frequently affected by cardiac complications than men. Although the cardiac malperfusion combined with ECG changes in AAD patients, it is hard to distinguish from that of primary cardiac ischemia and infarction, which increase the possibility of misdiagnosis and improper treatment.34,35
Moreover, CTA and echocardiography results showed that pericardial effusion (34%) and tamponade (12.5%) were more frequent in women. Transudation of fluid across the thin wall of an adjacent false lumen into the pericardial space frequently causes pericardial effusion.36 Cardiac tamponade was diagnosed in 6.7% of our patients, mostly those presenting with acute Type A dissection, which is an ominous clinical predictor of poor outcomes as well as the leading cause of mortality in these patients.37
Among 1076 AAD patients in the IRAD study, nearly 50% were diagnosed and received treatment within 24 hours. In contrast, only 32% of patients were diagnosed within 24 hours in our study. Delays in diagnosis directly affect the prognosis of AAD patients.
The overall outcome of women was worse than that of men, and in particular, women with type A dissection had a higher death rate, including 30% higher in-hospital mortality than men. These results may explained, in part, for the reasons as follows: AAD occurs in women an average of 10 to 14 years later than men, advanced age is a risk factor for increased early or late death after surgery.38,39 Diagnostic imaging findings suggest that impending rupture occurs more often in women. In-hospital complications such as a higher incidence of myocardial ischemia/infarction, hypoxemia, and cardiac tamponade occur more frequently among women that characterizes their critically worse preoperative condition at an older age than that of men. In addition, most surgeons recommend immediate surgical repair for AAD patients.40,41 However, we found that women refused emergency surgery more often than men. There was no difference in the proportion of patients with type A or B dissection in the present study, and we found no significant variation between sexes in surgical technique, delay of surgery, or hemodynamics at surgery. The differences in postsurgical outcomes of women appear real, considering the possible association with advanced age and more frequent surgeries performed on patients at high risk.
CONCLUSION
In the present study, we highlighted important differences in clinical characteristics, management, and outcomes between women and men with AAD. The average onset age of women was older than that of men; women had severer in-hospital complications, and higher in-hospital mortality. Therefore, physicians should heighten their suspicion of acute aortic dissection in women and its associated morbidity and mortality. The improvements in regional healthcare systems, early diagnosis, stringent control of hypertension, lipid profile optimization, smoking cessation, and other measures that reduce the risk of AAD. Further, the development of strategies to identify and treat female patients at high-risk may improve their clinical outcomes and reduce the overall mortality of AAD.
LIMITATIONS
This study has some limitations. First, classification of patients as chronic or acute was not random, leading to a relatively small patient-sample size in the acute group. Further, its retrospective nature and small sample size account for the study's insufficient statistical power. Second, we used the Stanford, but not the Debakey classification method, the differences in clinical outcomes between Debakey Types I and II were not analyzed. Third, we lacked long-term follow-up of patients after discharge, and therefore, the prognosis of patients was limited to the duration of their hospitalization.
Footnotes
Abbreviations: AAD = acute aortic dissection, CABG = coronary artery bypass grafting, ECG = echocardiography, IRAD = International Registry of Acute Aortic Dissection, PCI = percutaneous coronary intervention, SD = atandard deviation
Funding: this study was financially supported by grants from the National Natural Science Foundation Joint Research Program (U1403221) and the ministry of education innovation team development Program (IRT13094) of China.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Meszaros I, Morocz J, Szlavi J, et al. Epidemiology and clinicopathology of aortic dissection. Chest 2000; 117:1271–1278. [DOI] [PubMed] [Google Scholar]
- 2.Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010; 55:e27–e129. [DOI] [PubMed] [Google Scholar]
- 3.Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 2000; 283:897–903. [DOI] [PubMed] [Google Scholar]
- 4.Olsson C, Thelin S, Ståhle E, et al. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation 2006; 114:2611–2618. [DOI] [PubMed] [Google Scholar]
- 5.Masuda Y, Yamada Z, Morooka N, et al. Prognosis of patients with medically treated aortic dissections. Circulation 1991; 84:III7–III13. [PubMed] [Google Scholar]
- 6.Nienaber CA, Fattori R, Mehta RH, et al. Gender-related differences in acute aortic dissection. Circulation 2004; 109:3014–3021. [DOI] [PubMed] [Google Scholar]
- 7.Nienaber CA, von Kodolitsch Y, Nicolas V, et al. The diagnosis of thoracic aortic dissection by noninvasive imaging procedures. N Engl J Med 1993; 328:1–9. [DOI] [PubMed] [Google Scholar]
- 8.Kouchoukos NT, Dougenis D. Surgery of thoracic aorta. N Engl J Med 1997; 336:1876–1888. [DOI] [PubMed] [Google Scholar]
- 9.Nienaber CA, Fattori R, Lund G, et al. Nonsurgical reconstruction of thoracic aortic dissection by stent-graft placement. N Engl J Med 1999; 340:1539–1545. [DOI] [PubMed] [Google Scholar]
- 10.Erbel R, Oelert H, Meyer J, et al. Influence of medical and surgical therapy on aortic dissection evaluated by transesophageal echocardiography. Circulation 1993; 87:1604–1615. [DOI] [PubMed] [Google Scholar]
- 11.Chirillo F, Salvador L, Bacchion F, et al. Clinical and anatomical characteristics of subtle-discrete dissection of the ascending aorta. Am J Cardiol 2007; 100:1314–1319. [DOI] [PubMed] [Google Scholar]
- 12.Ross R. “The pathogenesis of atherosclerosis: a perspective for the 1990s”. Nature 1993; 362:801–809. [DOI] [PubMed] [Google Scholar]
- 13.Finkbohner R, Johnston D, Crawford ES, et al. Marfan syndrome. Long-term survival and complications after aortic aneurysm repair. Circulation 1995; 91:728–733. [DOI] [PubMed] [Google Scholar]
- 14.Dietz HC, Loeys B, Carta L, et al. Recent progress towards a molecular understanding of Marfan syndrome”. Am J Med Genet C Semin Med Genet 2005; 139C:4–9. [DOI] [PubMed] [Google Scholar]
- 15.Garg V, Muth AN, Ransom JF, et al. Mutations in NOTCH1 cause aortic valve disease”. Nature 2005; 437:270–274.Bibcode:2005 Natur.437.270G. doi:10.1038/nature03940.PMID 16025100. [DOI] [PubMed] [Google Scholar]
- 16.Cripe L, Andelfinger G, Martin LJ, et al. Bicuspid aortic valveis heritable”. J Am Coll Cardiol 2004; 44:138–143.doi:10.1016/j.jacc.2004.03.050.PMID 15234422. [DOI] [PubMed] [Google Scholar]
- 17.Ab Kent KC. Clinical practice. Abdominal aortic aneurysms”. N Engl J Med 2014; 371:2101–2108.doi:10.1056/NEJMcp1401430. PMID 25427112. [DOI] [PubMed] [Google Scholar]
- 18.Upchurch GR, Schaub TA. Abdominal aortic aneurysm”. Am Fam Physician 2006; 73:1198–1204.PMID 16623206. [PubMed] [Google Scholar]
- 19.Clouse WD, Hallett JW, Jr, Schaff HV, et al. Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc 2004; 79:176–180. [DOI] [PubMed] [Google Scholar]
- 20.Wenger NK, Speroff L, Packard B. Cardiovascular health and disease in women. N Engl J Med 1993; 329:247–256. [DOI] [PubMed] [Google Scholar]
- 21.Piuila M. Cigarette smoking, endothelial injury and cardiovascular disease. J Hat Exp Pathol 2000; 81:219–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Massimo CG, Presenti LF, Marranci P, et al. Extended and total aortic resection in the surgical treatment of acute type A aortic dissection: experience with 54 patients. Ann Thorac Surg 1998; 46:420–424. [DOI] [PubMed] [Google Scholar]
- 23.Movsowitz HD, Levine RA, Hilgenberg AD, et al. Transesophageal echocardiographic description of the mechanisms of aortic regurgitation in acute type A aortic dissection: implications for aortic valve repair. J Am Coll Cardiol 2000; 36:884–890. [DOI] [PubMed] [Google Scholar]
- 24.Januzzi JL, Eagle KA, Cooper JV, et al. Acute aortic dissection presenting with congestive heart failure: results from the International Registry of Acute Aortic Dissection. J Am Coll Cardiol 2005; 46:733–735. [DOI] [PubMed] [Google Scholar]
- 25.Williams DM, Lee DY, Hamilton BH, et al. The dissected aorta: part III. Anatomy and radiologic diagnosis of branch-vessel compromise. Radiology 1997; 203:37–44. [DOI] [PubMed] [Google Scholar]
- 26.Khan IA, Nair CK. Clinical, diagnostic, and management perspectives of aortic dissection. Chest 2002; 122:311–328. [DOI] [PubMed] [Google Scholar]
- 27.Hata N, Tanaka K, Imaizumi T, et al. Clinical significance of pleural effusion in acute aortic dissection. Chest 2002; 121:825–830. [DOI] [PubMed] [Google Scholar]
- 28.Tristano AG, Tairouz Y. Painless right hemorrhagic pleural effusions as presentation sign of aortic dissecting aneurysm. Am J Med 2005; 118:794–795. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi M, Ikeda U, Shimada K, et al. Unilateral pulmonary edema related to pulmonary artery compression resulting from acute dissecting aortic aneurysm. Am Heart J 1993; 126:1225–1227. [DOI] [PubMed] [Google Scholar]
- 30.Masuo M, Takano H, Takamoto S, et al. Pulmonary artery obstruction caused by thoracic aortic dissection: a case with unique pathological findings. Circ J 2004; 68:392–395. [DOI] [PubMed] [Google Scholar]
- 31.Meyrier A. Cholesterol crystal embolism: diagnosis and treatment. Kidney Int 2006; 69:1308–1312. [DOI] [PubMed] [Google Scholar]
- 32.Hazanov N, Somin M, Attali M, et al. Acute renal embolism. Forty-four cases of renal infarction in patients with atrial fibrillation. Medicine (Baltimore) 2004; 83:292–299. [DOI] [PubMed] [Google Scholar]
- 33.Neri E, Toscano T, Papalia U, et al. Proximal aortic dissection with coronary malperfusion: presentation, management, and outcome. J Thorac Cardiovasc Surg 2001; 121:552–560. [DOI] [PubMed] [Google Scholar]
- 34.Lee SI, Pyun SB, Jang DH. Dysphagia and hoarseness associated with painless aortic dissection: a rare case of cardiovocal syndrome. Dysphagia 2006; 21:129–132. [DOI] [PubMed] [Google Scholar]
- 35.Hansen MS, Nogareda GJ, Hutchison SJ. Frequency of and inappropriate treatment of misdiagnosis of acute aortic dissection. Am J Cardiol 2007; 99:852–856. [DOI] [PubMed] [Google Scholar]
- 36.Armstrong WF, Bach DS, Carey LM, et al. Clinical and echocardiographic findings in patients with suspected acute aortic dissection. Am Heart J 1998; 136:1051–1060. [DOI] [PubMed] [Google Scholar]
- 37.Gilon D, Mehta RH, Oh JK, et al. Characteristics and in-hospital outcomes of patients with cardiac tamponade complicating type A acute aortic dissection. Am J Cardiol 2009; 103:1029–1031. [DOI] [PubMed] [Google Scholar]
- 38.Svensson LG, Crawford ES, Hess KR, et al. Variables predictive of outcome in 832 patients undergoing repairs of the descending thoracic aorta. Chest 1993; 104:1248–1253. [DOI] [PubMed] [Google Scholar]
- 39.Coselli JS, Lemaire SA, Miller CC, III, et al. Mortality and paraplegia after thoracoabdominal aortic aneurysm repair: a risk factor analysis. Ann Thorac Surg 2000; 69:409–414. [DOI] [PubMed] [Google Scholar]
- 40.Xenos ES, Abedi NN, Davenport DL, et al. Meta-analysis of endovascular vs open repair for traumatic descending thoracic aortic rupture. J Vasc Surg 2008; 48:1343–1351. [DOI] [PubMed] [Google Scholar]
- 41.Kouchoukos NT, Masetti P, Rokkas CK, et al. Safety and efficacy of hypothermic cardiopulmonary bypass and circulatory arrest for operations on the descending thoracic and thoracoabdominal aorta. Ann Thorac Surg 2001; 72:699–707. [DOI] [PubMed] [Google Scholar]


