Supplemental Digital Content is available in the text
Abstract
Limited data exist regarding the outcomes of patients with nonobstructive coronary artery disease (CAD) detected by computed tomography coronary angiography (CTCA) or invasive coronary angiography (ICA).
Our aim was to compare the prognosis of patients with nonobstructive coronary artery plaques with that of patients with entirely normal arteries.
The MEDLINE, Cochrane Library, and Embase databases were searched. Studies comparing the prognosis of individuals with nonobstructive CAD versus normal coronary arteries detected by CTCA or ICA were included. The primary outcome was major adverse cardiac events (MACE) including cardiac death, nonfatal myocardial infarction, hospitalization due to unstable angina or revascularization. A fixed effects model was chosen to pool the estimates of odds ratios (ORs).
Forty-eight studies with 64,905 individuals met the inclusion criteria. Patients in the nonobstructive CAD arm had a significantly higher risk of MACE compared to their counterparts in the normal artery arm (pooled OR, 3.17, 95% confidence interval, 2.77–3.63). When excluding revascularization as an endpoint, hard cardiac composite outcomes were also more frequent among patients with nonobstructive CAD (pooled OR, 2.10; 95%CI, 1.79–2.45). All subgroups (age, sex, follow-up duration, different outcomes, diagnostic modality, and CAD risk factor) consistently showed a poorer prognosis with nonobstructive CAD than with normal arteries. When dividing the studies into a CTCA and ICA group for further analysis based on the indications for diagnostic tests, we also found nonobstructive CAD to be associated with a higher risk of MACE in both stable and acute chest pain.
Patients with nonobstructive CAD had a poorer prognosis compared with their counterparts with normal arteries.
INTRODUCTION
Approximately 10% to 25% of the patients referred for invasive coronary angiography (ICA) are found to have normal coronary arteries or nonobstructive coronary artery disease (CAD) (vessel diameter narrowed <50%).1,2 Moreover, with the popularity of computed tomography coronary angiography (CTCA), which is a noninvasive and efficient modality for visualizing coronary artery plaques, the proportion of nonobstructive CAD has increased to 15% to 37%.3,4
For years, a reduction in coronary artery diameter of <50% has been considered to be of clinical insignificance.5 However, accumulating evidence has implied that most deaths from acute coronary syndrome (ACS) are ascribed to ruptured coronary plaques rather than progressive stenosis.6 In addition, Park et al found that patients with <50% coronary stenosis had a 17% rate of ischemia.7 In other words, not all nonobstructive plaques may be clinically insignificant. However, it remains to be seen whether these nonobstructive plaques contribute to poor prognosis. In addition, it is important to note that patients with nonobstructive CAD remain undertreated in the current clinical practice.8 Moreover, guidelines have no definite suggestions for the treatment of nonobstructive CAD. Therefore, there is a need to determine the prognostic significance of nonobstructive CAD. In this article, our aim was to compare the prognosis of patients with nonobstructive coronary artery plaques with patients with entirely normal arteries using a meta-analysis of observational studies or RCTs.
METHODS
This systematic review was conducted according to the Preferred Reporting items for Systematic Reviews and Meta-analysis statement. Ethical approval was not necessary for this review study. The MEDLINE (through PubMed), Cochrane Library, and Embase (through Ovid SP) databases were searched systematically from their inception to July 2015 (see eMethods, Supplemental Content 1, for an illustration of the search strategies). The references of relevant studies and review articles were checked for additional studies.
Two reviewers screened the articles according to prespecified inclusion and exclusion criteria. The inclusion criteria included studies comparing the risk of events in individuals with nonobstructive CAD versus those with normal coronary arteries through CTCA or ICA. Reviews, meta-analyses, editorials, abstracts, diagnostic accuracy studies without clinical outcomes, short-term follow-up (<3 months), and small sample studies (n < 50) were excluded. Studies that evaluated the severity of stenosis with intravascular ultrasound (IVUS) or optical coherence tomography (OCT) were not included because of the limited utilization of the 2 modalities.
Two authors independently extracted the data. A standardized data extraction form was used to collect publication year, country, type of report, follow-up duration, total number of patients, participant characteristics, diagnostic methods, and main outcomes. Baseline characteristics including age, sex, and relevant risk factors of CAD were extracted.
The quality of the nonrandomized studies was assessed using the Newcastle–Ottawa quality scale, which mainly includes 3 broad perspectives: the selection of the study groups (4 items), the comparability of the groups (1 item), and the collection of the outcome (3 items).9 See Supplemental Content 2 for further details. Each item can be assigned a maximum of 1 point except for the item in the “comparability” category, which can be given 2 points.
Outcome Measures
The cardiovascular (CV) outcomes included major adverse cardiac events (MACE) (cardiac death, nonfatal myocardial infarction [MI], hospitalization due to unstable angina [UA], or revascularization), hard cardiac outcomes excluding revascularization after ICA or CTCA, and the individual outcomes of cardiac death, nonfatal MI, and hospitalization due to UA. We also evaluated all-cause (AC) composite outcomes comprising all-cause death, MI, UA requiring hospitalization and revascularization.
Statistical Analysis
Due to the small number of events in the normal artery group, we conducted a fixed effects meta-analysis using Mantel–Haenszel methods with Robins–Breslow–Greenland variance for pooling effect sizes.10 Sweeting et al demonstrated that a fixed effects model has a better performance than a random effects model in cases of rare events in which the sizes of the study arms are unequal.11 However, we also conducted a random effects meta-analysis using DerSimonian and Laird methods for sensitivity analyses. For zero cells, we used the “treatment arm” continuity correction recommended by Sweeting et al, which has been indicated to outperform the common use of adding a constant 0.5 to each cell.11 Briefly, this correction was inversely proportional to the relative size of the opposite arm of the study. For example, the continuity correction was 1/(1+R) for the normal arm and R/(1+R) for the nonobstructive arm, where R was equal to the ratio of the size of the nonobstructive group to that of the normal group. According to the recommendations of Sweeting et al, a statistical sensitivity analysis was conducted by repeating the meta-analysis using logistic regression, Mantel–Haenszel with an added correction constant of 0.5 and 0.05 and with inverse-variance methods. The heterogeneity was detected by calculating the I2 statistic and its P value. I2 < 25% was considered low heterogeneity, 25% to 50% moderate heterogeneity, and >50% substantial heterogeneity. To explore the potential sources of heterogeneity, a meta-regression analysis and subgroup analyses were performed by prespecified variables: the proportion of male participants, the average age, the diagnostic methods used, the different endpoints (hard endpoints vs endpoints including revascularization), follow-up duration and the proportion of risk factors of CAD such as diabetes, hypertension, hyperlipidemia, family history of premature CAD, and pretest symptomatic patients. As patients referred for different diagnostic tests may have different risk profiles, we divided these studies into a CTCA and an ICA group for subgroup analyses. All analyses were calculated using STATA/SE 12.1 (STATA Corp LP, TX) except for logistic regression, which was calculated with SPSS software (version 19). Finally, a P value of 0.05 was considered to indicate statistical significance.
RESULTS
The search identified 9929 publications in the 3 databases. A total of 48 studies enrolling 64,905 patients were eligible for our inclusion criteria. The selection process is summarized in Figure 1.
FIGURE 1.
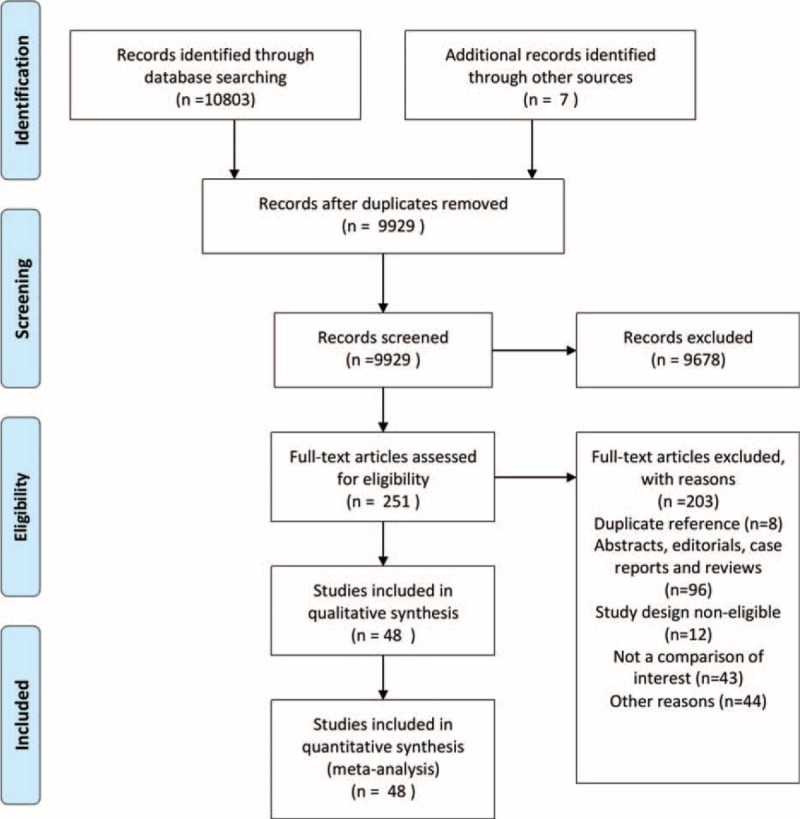
Flow diagram of the study selection.
The characteristics and quality assessment of the included studies are shown in Table 1 and in the Supplemental Table (see Table, Supplemental Content 2, which illustrates the quality of the included studies). The median follow-up duration was 27 months (ranging from 6 to 120 months). Twelve studies were retrospective, whereas 36 studies were prospective. Most of the studies used CTCA as the diagnostic tool, and 12 studies used ICA. Twenty-nine studies reported MACE, whereas 23 reported AC composite endpoints. Most studies were from Europe and North America. The indications for a diagnostic test in most studies were chest pain, abnormal functional test, or elevated risk profile. The characteristics of the patients in the included studies are shown in the Supplemental Table, (see Table, Supplemental Content 3, which illustrates the characteristics of the patients in the included studies and reference of those included studies). In brief, of the 64,905 total subjects (mean age was 58.2 ± 3 years; 63.7% ± 23.7% male), 57.1% had hypertension, 21.8% had diabetes mellitus, 56.7% had dyslipidemia, 34.1% smoked, and 24.5% had a family history of premature CAD. The median proportion of individuals with an indication of symptoms for ICA or CTCA was 71.9%.
TABLE 1.
Overview of the Design of Studies Included

Cardiovascular Outcomes
Twenty-nine studies including 25,669 patients reported MACE between a nonobstructive CAD (n = 9683) and a normal artery group (n = 15,986). Patients in the nonobstructive CAD arm had a significantly higher occurrence of MACE compared to those with normal arteries (number of events: 622 vs 377, respectively; fixed effects model: pooled odd ratio [OR], 3.17, 95% confidence interval [CI], 2.77–3.63; Random effects model: OR, 2.39, 95%CI 2.06–2.77; Figure 2A). In 12 studies with a total of 11,499 patients (4971 cases vs. 6528 controls), hard CV composite endpoints occurred in 388 patients (7.8%) who had nonobstructive CAD, and 345 subjects (5.3%) with normal arteries (pooled OR, 2.10, 95%CI, 1.79–2.45; random effects model: OR, 1.86, 95%CI 1.58–2.19; Figure 2B). There was a significantly higher incidence of the other individual outcomes in the nonobstructive CAD group than in the normal artery group (see Table, Supplemental Content 4 which demonstrates the poorer prognosis of patients with nonobstructive CAD for all outcomes). The statistical sensitivity analysis obtained similar results (see Figure, Supplemental Content 5, which shows the similar results obtained for each main outcome when statistical sensitivity analyses were performed).
FIGURE 2.
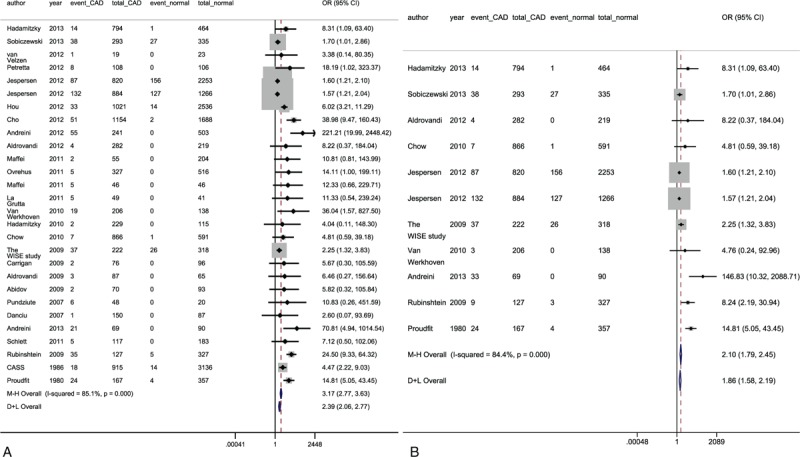
Forest plots of major adverse cardiac endpoints (A) and hard cardiac composite outcomes (B). CAD = coronary artery disease, CI = confidence interval, OR = odds ratio; other abbreviations as in Table 1 .
All-Cause Outcomes
The AC composite outcomes were evaluated in 48 studies with 64,905 patients (28,046 cases vs 36,859 controls). The pooled OR was 2.51 (95%CI, 2.27–2.78) for patients with nonobstructive CAD versus normal arteries when a fixed effects model was used (number of events: 1137 vs 649, respectively; random effects model: OR, 2.11, 95%CI 18.9–2.35; Figure 3A). If revascularization was excluded from the combined endpoint, the pooled OR was 1.93 (95%CI, 1.72–2.16) in the fixed effects model and 1.88 (95% 1.67–2.11) in the random effects model. The statistical sensitivity analysis obtained similar results (see Figure, Supplemental Content 5, which shows the similar results obtained in the statistical sensitivity analyses performed for each main outcome).
FIGURE 3.

Forest plots of AC composite endpoints (A) and AC composite outcomes excluding revascularization (B). AC = all-cause, CI = confidence interval, OR = odds ratio; other abbreviations as in Table 1 .
Meta-Regression and Subgroup Analyses
Significant heterogeneity in MACE and AC composite outcomes based on the I2 values (78.9% and 70.3%, respectively) was observed. Thus, we conducted a metaregression and subgroup analyses to explore the potential sources of heterogeneity. As Table 2 shows, the differences in diagnostic tools and endpoints between studies were responsible for 64.77% and 61.27% of the heterogeneity, respectively (P < 0.001 and P = 0.001). In contrast, no significant heterogeneity was observed in the duration of follow-up, proportion of men, risk factors of CAD, and proportion of patients who were symptomatic pretest. All of these subsets consistently showed that patients with nonobstructive CAD had a higher incidence of MACE than patients with normal arteries.
TABLE 1 (Continued).
Overview of the Design of Studies Included
Study Characteristics and Pooled Outcomes Grouped by Diagnostic Instrument
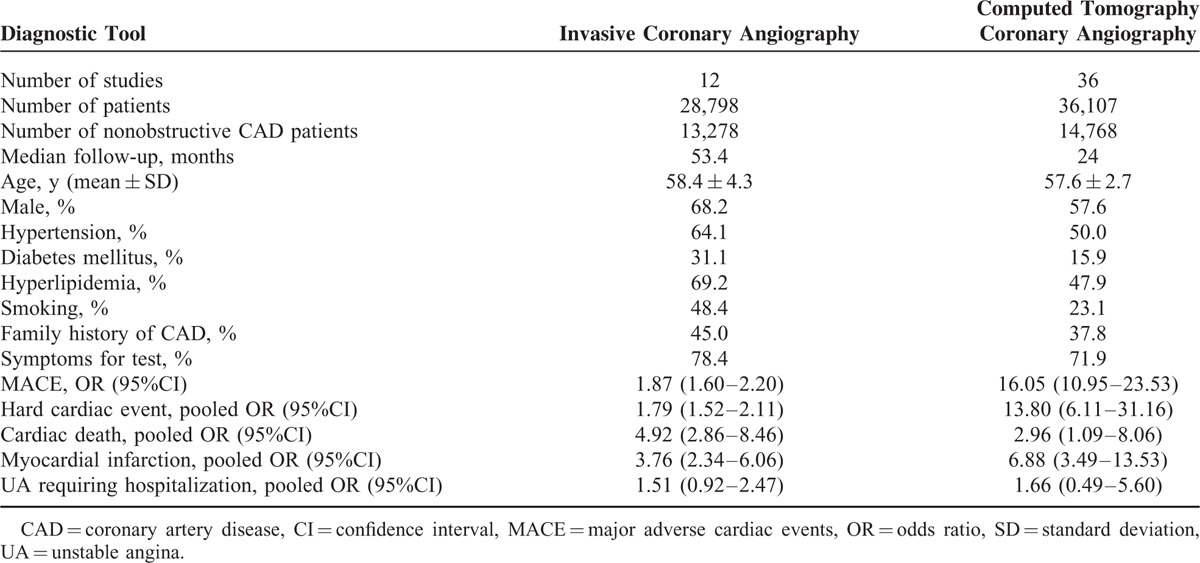
As patients referred for different diagnostic tests may have different risk profiles, we divided these studies into a CTCA and an ICA group for further analysis. As displayed in Table 3, ICA was used in 12 studies of 28,798 subjects, and CTCA was applied in 36 studies of 36,107 individuals. Nonobstructive CAD was determined in 13,278 (46.1%) and 14,768 (40.9%) patients upon ICA and CTCA, respectively. A longer duration of follow-up was observed in the ICA group (median, 53.4 months) compared with the CTCA group (median, 24 months). The average age did not differ between the 2 arms (58.4 years for the ICA and 57.6 years for the CTCA group). Regardless of the diagnostic tool used, there was a significantly higher risk of cardiovascular events in patients with nonobstructive CAD than those with normal arteries (Table 3). As individuals in the ICA arm had more CAD risk factors compared with those in the CTCA group, we performed further subgroup analyses to balance the impact of risk profiles on outcomes based on the indications (ACS or Stable CAD) for both diagnostic tests. Figure 4 shows that nonobstructive CAD resulted in a high risk of MACE in both stable and acute chest pain. In addition, the subgroups decreased the interstudy heterogeneity.
TABLE 2.
Meta-Regression and Subgroup Analyses of Major Adverse Cardiac Events

FIGURE 4.
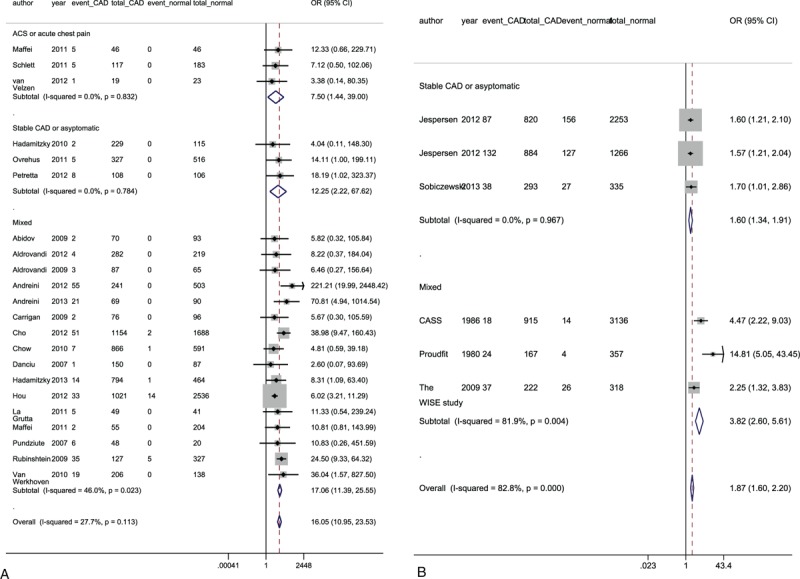
Subgroup analyses of major adverse cardiac endpoints according to the indications for CTCA (A) and ICA (B). “Mixed” indicates that the indications for diagnostic tests could not be clearly divided into ACS and stable CAD. ACS = acute coronary syndrome, CAD = coronary artery disease, CTCA = computed tomography coronary angiography, ICA = invasive coronary angiography; other abbreviations as in Table 1 and Figure 2.
TABLE 3.
Overview of the Characteristics and Pooled Odds Ratios of Studies Grouped by Diagnostic Instrument Used
DISCUSSION
To the best of our knowledge, this is the first meta-analysis to explore the prognosis of nonobstructive CAD versus normal coronary artery determined by ICA or CTCA. We found that for both MACE and cardiac hard events, patients with nonobstructive CAD had a worse mid- and long-term prognosis than those with completely normal arteries. Furthermore, subgroup analyses as well as statistical sensitivity analyses confirmed the above results.
As Gould et al originally proposed the concept of “critical” coronary artery stenosis 40 years ago,5 the cut-off, that is, a reduction in coronary artery diameter >50% at which point the cardiac flow reserve begins to diminish, has been rooted in clinicians’ minds. Unlike in obstructive CAD, many people are simply discharged without further evaluation if they have normal or near-normal coronary arteries on angiography. Furthermore, they are less likely to be treated with guideline-recommended therapy, including aspirin and statins.8 However, mounting evidence has shown that obstructive CAD is not the only cause of angina pectoris. Early studies have demonstrated that cardiac events still occur in patients with “normal” arteries on angiogram.12,13 Several studies have aimed to explore the prognosis of patients with nonobstructive CAD.14 However, individual studies have typically been underpowered to detect differences in sparse cardiac events. To increase test power, some of these studies incorporated revascularizations into the composite outcomes. However, including coronary revascularization as a primary endpoint may result in ascertainment bias.15,16 In contrast, for these types of sparse events, meta-analysis may be an efficient method of obtaining reliable evidence.17 More accurate and comprehensive cardiovascular results can be displayed in meta-analyses.
A previous meta-analysis similar to this study investigated the prognosis of patients with a different degree of coronary artery stenosis determined by 64-slice CTCA. They suggested that nonobstructive CAD confers a greater incidence of MACE.18 However, there are several aspects of our study that differ from the prior paper. First, the present meta-analysis covered a more comprehensive population of patients who were referred for either CTCA or ICA. Indeed, in real practice, invasive coronary angiography is more often conducted on patients with high-risk factors including those with arrhythmia. In addition, patients with nonobstructive CAD determined by ICA should require more attention. Second, with regard to the outcome, Abdulla et al primarily focused on composite endpoints including revascularization. As discussed above, this may have introduced ascertainment bias. In regard to individual endpoints, the previous study did not show a significant difference in cardiac death between arms. This finding may be attributed to the insufficient sample size. In our work, we first investigated hard outcomes by separating revascularization from the composite outcomes. Additionally, we found a significant difference in cardiac deaths between patients with nonobstructive and with normal arteries.
It is acknowledged that the instability of plaques rather than the occlusion of plaques is the common pathophysiological mechanism underlying ACS.19 Furthermore, it is important to note that vulnerable plaque, such as thin cap fibroatheromas (TCFA), has consistently been found in sites with <50% diameter stenosis because of compensatory positive remodeling.20 Very recently, Tian et al suggested that the absolute number of TCFA was 3 times greater in nonsevere stenosis than in severe stenosis detected by OCT or IVUS.21 Meanwhile, Park et al demonstrated that positive remodeling was a predictor of ischemia using fractional flow reserve for both nonobstructive and obstructive plaques.7 In other words, nonobstructive plaques can also contribute significantly to hemodynamic disturbances in coronary artery and even myocardial infarction, which should be paid a greater amount of attention. This is particularly true in patients presenting with angina-like chest pain.
A patient-level meta-analysis of 8 RCTs that was conducted by De Ferrari et al focused on the comparison between nonobstructive CAD versus obstructive CAD as detected by ICA only; their results showed that 1 in 10 patients with NSTE-ACS had nonobstructive CAD and >2% of these patients suffered from death or MI.22 In the present meta-analysis, we included studies on nonobstructive CAD determined not only by ICA but also by CTCA. On the one hand, CTCA, which is a cost-effective and efficient modality, is recommended as the first imaging test for low-intermediate-risk patients.23 Those patients account for a large proportion of the patients seen in cardiology outpatient or emergency departments. On the other hand, sometimes, due to compensatory outward expansive remodeling of the involved vessel—that is, positive remodeling—the specific discovery of nonobstructive plaques is difficult using conventional angiography because it focuses on the evaluation of luminal obstruction.24,25 Intracoronary invasive imaging has often shown that coronary arteries determined to be entirely normal by conventional coronary angiography are not truly normal when assessed from inside.26 In contrast, CTCA focuses on luminal obstruction as well as vessel walls or plaques, which is regarded as a more precise modality for assessing the severity of coronary stenosis compared to ICA.16 Therefore, including studies on both CTCA and ICA enabled us to investigate nonobstructive CAD in a broader population and extended the generalizations of the findings of De Ferrari et al.
Several functional testing tools such as exercise-ECG or stress echocardiography can also help identify the presence of obstructive CAD at a lower cost.27 Leischik et al suggested that stress echocardiography is of major prognostic significance in outpatients with chest pain.28 Very recently, results from the PROspective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) trial have indicated that symptomatic patients with an initial strategy of anatomical testing have a comparable primary outcome to those receiving functional testing.29 Indeed, CTCA is very good at identifying coronary plaques, but many of the identified plaques are not functionally significant. Therefore, anatomical tests accompanied by functional data may improve the clinical outcomes of select patients with equivocal plaques. When considering treatment strategies, however, visualization of plaques by anatomical testing is more likely to result in initiating primary prevention.
Several potential limitations of this study deserve consideration. (i) Despite the importance of high-risk plaques, there was a lack of suitable studies that directly compared the relative contributions of different types of nonobstructive plaques to future events. Thus, our meta-analysis cannot infer the direct association between prognosis and plaque characteristics among subjects with nonobstructive CAD. (ii) Because all of the data were from observational studies, confounding factors, such as drug administration, are inherent and may have deviated the results from the true condition. The individual patient data from the specific studies cannot be accessed; thus the potential risk factors of CAD could not be precisely corrected. (iii) As is the case for any meta-analysis, composite outcomes were pooled from different studies that had distinct endpoint definitions. However, further analysis of the individual events in the Supplemental Content 4 supported the conclusions reached above. (iv) A very small number of patients who presented with acute coronary syndrome may have had other conditions, such as Takotsubo cardiomyopathy, rather than “true ACS.” As this condition is rather rare compared to “true ACS,” it is unlikely to have deviated the results from the true value. Despite these limitations, our meta-analysis offered a potentially valuable and new insight into recognition of nonobstructive CAD.
CONCLUSION
Patients with nonobstructive CAD experienced a higher risk of major cardiac events compared with patients with normal arteries. These patients should not be neglected in clinical practice.
Supplementary Material
Footnotes
Abbreviations: ACS = acute coronary syndrome, AV = all cause, CAD = coronary artery disease, CI = confidence interval, CTCA = computed tomography coronary angiography, CV = cardiovascular, EBCT = electron beam computed tomography, ICA = invasive coronary angiography, IVUS = intravascular ultrasound, MACE = major adverse cardiac events, MI = myocardial infarction, OCT = optical coherence tomography, OR = odds ratio, TCFA = thin cap fibroatheromas, UA = unstable angina
Funding: this work was granted by the National High-tech Research and Development Program of China (grant number: 2012AA02A510, Beijing, China), the National Natural Science Foundation of China (grant numbers: 81370219, Beijing, China), and the Supporting Project of Sichuan Provincial Department of Science and Technology (grant numbers: 2012FZ0065 and 2014SZ0004, Sichuan, China).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
REFERENCES
- 1.Bugiardini R, Bairey MC. Angina with “normal” coronary arteries: a changing philosophy. JAMA 2005; 293:477–484. [DOI] [PubMed] [Google Scholar]
- 2.Diver DJ, Bier JD, Ferreira PE, et al. Clinical and arteriographic characterization of patients with unstable angina without critical coronary arterial narrowing (from the TIMI-IIIA Trial). Am J Cardiol 1994; 74:531–537. [DOI] [PubMed] [Google Scholar]
- 3.Min JK, Dunning A, Lin FY, et al. Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol 2011; 58:849–860. [DOI] [PubMed] [Google Scholar]
- 4.Jespersen L, Hvelplund A, Abildstrom SZ, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J 2012; 33:734–744. [DOI] [PubMed] [Google Scholar]
- 5.Gould KL, Lipscomb K. Effects of coronary stenoses on coronary flow reserve and resistance. J Am Coll Cardiol 1974; 34:48–55. [DOI] [PubMed] [Google Scholar]
- 6.Marzilli M, Merz CNB, Boden WE, et al. Obstructive coronary atherosclerosis and ischemic heart disease: an elusive link!. J Am Coll Cardiol 2012; 60:951–956. [DOI] [PubMed] [Google Scholar]
- 7.Park H, Heo R, Ó Hartaigh B, et al. Atherosclerotic plaque characteristics by CT angiography identify coronary lesions that cause ischemia: a direct comparison to fractional flow reserve. JACC Cardiovasc Imaging 2015; 8:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Ferrari GM, Leonardi S, Baduena L, et al. Patients with acute coronary syndrome and nonobstructive coronary artery disease in the real world are markedly undertreated. J Cardiovasc Med (Hagerstown) 2011; 12:700–708. [DOI] [PubMed] [Google Scholar]
- 9.2000; Wells GA, Shea B, O Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [Internet]. Ottawa (ON), Canada: Ottawa Hospital Research Institute. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed March 5, 2016). [Google Scholar]
- 10.Robins J, Breslow N, Greenland S. Estimators of the Mantel–Haenszel variance consistent in both sparse data and large-strata limiting models. Biometrics 1986; 42:311–323. [PubMed] [Google Scholar]
- 11.Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med 2004; 23:1351–1375. [DOI] [PubMed] [Google Scholar]
- 12.Kemp HG, Kronmal RA, Vlietstra RE, et al. Seven year survival of patients with normal or near normal coronary arteriograms: A CASS registry study. J Am Coll Cardiol 1986; 7:479–483. [DOI] [PubMed] [Google Scholar]
- 13.Proudfit WL, Bruschke VG, Sones FJ. Clinical course of patients with normal or slightly or moderately abnormal coronary arteriograms: 10-year follow-up of 521 patients. Circulation 1980; 62:712–717. [DOI] [PubMed] [Google Scholar]
- 14.Hulten EA, Carbonaro S, Petrillo SP, et al. Prognostic value of cardiac computed tomography angiography: a systematic review and meta-analysis. J Am Coll Cardiol 2011; 57:1237–1247. [DOI] [PubMed] [Google Scholar]
- 15.Woods KM, Fischer C, Cheezum MK, et al. The prognostic significance of coronary CT angiography. Curr Cardiol Rep 2012; 14:7–16. [DOI] [PubMed] [Google Scholar]
- 16.Rossi A, Dharampal A, de Feyter PJ. Coronary CT angiography for patients with suspected coronary artery disease. Heart 2014; 100:976–984. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration; Copenhagen, DK, 2011. www.cochrane-handbook.org Accessed on March 5, 2016. [Google Scholar]
- 18.Abdulla J, Asferg C, Kofoed KF. Prognostic value of absence or presence of coronary artery disease determined by 64-slice computed tomography coronary angiography a systematic review and meta-analysis. Int J Cardiovasc Imaging 2011; 27:413–420. [DOI] [PubMed] [Google Scholar]
- 19.Libby P. Pathophysiology of coronary artery disease. Circulation 2005; 111:3481–3488. [DOI] [PubMed] [Google Scholar]
- 20.Virmani R, Burke AP, Farb A, et al. Pathology of the vulnerable plaque. J Am Coll Cardiol C13-C18; 2006: 47: [DOI] [PubMed] [Google Scholar]
- 21.Tian J, Dauerman H, Toma C, et al. Prevalence and characteristics of TCFA and degree of coronary artery stenosis: an OCT IVUS, and angiographic study. J Am Coll Cardiol 2014; 64:672–680. [DOI] [PubMed] [Google Scholar]
- 22.De Ferrari GM, Fox KA, White JA, et al. Outcomes among non-ST-segment elevation acute coronary syndromes patients with no angiographically obstructive coronary artery disease: observations from 37,101 patients. Eur Heart J Acute Cardiovasc Care 2014; 3:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Ascenzo F, Cerrato E, Biondi-Zoccai G, et al. Coronary computed tomographic angiography for detection of coronary artery disease in patients presenting to the emergency department with chest pain: a meta-analysis of randomized clinical trials. Eur Heart J Cardiovasc Imaging 2013; 14:782–789. [DOI] [PubMed] [Google Scholar]
- 24.Narula J, Strauss HW. The popcorn plaques. Nat Med 2007; 13:532–534. [DOI] [PubMed] [Google Scholar]
- 25.Mintz GS, Popma JJ, Pichard AD, et al. Limitations of angiography in the assessment of plaque distribution in coronary artery disease: a systematic study of target lesion eccentricity in 1446 lesions. Circulation 1996; 93:924–931. [DOI] [PubMed] [Google Scholar]
- 26.Alfonso F, Macaya C, Goicolea J, et al. Intravascular ultrasound imaging of angiographically normal coronary segments in patients with coronary artery disease. Am Heart J 1994; 127:536–544. [DOI] [PubMed] [Google Scholar]
- 27.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2012; 126:3097–3137. [DOI] [PubMed] [Google Scholar]
- 28.Leischik R, Dworrak B, Littwitz H, et al. Prognostic significance of exercise stress echocardiography in 3329 outpatients (5-year longitudinal study). Int J Cardiol 2007; 119:297–305. [DOI] [PubMed] [Google Scholar]
- 29.Douglas PS, Hoffmann U, Patel MR, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med 2015; 372:1291–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


