Supplemental Digital Content is available in the text
Abstract
The purpose of this study was to investigate the efficacy and safety of programmed cell death 1 (PD-1) and programmed cell death 1 ligand (PD-L1) inhibitors using a meta-analysis of present trials for advanced melanoma.
A fully recursive literature search of the primary electronic databases for available trials was performed. The objective response rate (ORR) and the median progression-free survival (PFS) of clinical responses were considered the main endpoints to evaluate the efficacy, whereas Grade 3–4 adverse effects (AEs) were analyzed to evaluate safety.
The ORR of PD-1 and PD-L1 inhibitors was 30% (95% CI: 25–35%). No significant difference in the ORR was observed after the comparisons of low-dose, median-dose, and high-dose cohorts. In addition, the rate of Grade 3–4 AEs was 9% (95% CI: 6–12%). According to the 3 randomized controlled trials that compared PD-1 inhibitors with chemotherapy, the difference between these 2 groups was found to be statistically significant with respect to the ORR, PFS and the incidence of Grade 3–4 AEs; that is, the relative risk (RR) of the ORR was 3.42 (95% CI: 2.49–4.69, P < 0.001), the hazard ratio (HR) of the PFS was 0.50 (95% CI: 0.44–0.58, P < 0.001), and the RR of Grade 3–4 AEs was 0.45 (95% CI: 0.31–0.65, P < 0.001).
According to a meta-analysis of limited concurrent studies, PD-1 and PD-L1 inhibitors appear to be associated with improved response rates, superior response durability and tolerable toxicity in patients with advanced melanoma.
INTRODUCTION
Advanced melanoma, which has a high somatic mutation frequency, is one of the most aggressive and life-threatening solid tumors. Melanoma is associated with a poor prognosis, and therefore, limited treatment options exist,1,2 which is why the 5-year survival of patients with advanced melanoma is less than 15%.3 However, in the past few years, an increase in the understanding of how to subvert antitumor immunity mechanisms and associated molecular pathways with regards to melanoma pathogenesis, have led to new treatments that may carry the potential for improved survival of patients with melanoma.4,5
In recent years, immune checkpoint blockade has undoubtedly become a popular topic, as this mechanism has induced regressions in several types of neoplasms. Cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) inhibitors, such as ipilimumab, block this inhibitory receptor that down-modulates the initial stages of T-cell activation. Treatment with CTLA-4 inhibitors has demonstrated a survival benefit in patients with advanced melanoma in several randomized, controlled phase III clinical trials.6–10 However, distinct immune-related adverse events could not be ignored, and even life-threatening complications were observed.11 Programmed cell death 1 (PD-1) is another checkpoint inhibitor that is expressed on antigen-stimulated T cells. PD-1 interacts with its ligands PD-L1 and PD-L2 to induce downstream signaling that inhibits T-cell activation and proliferation, which then promotes immunological self-tolerance.12–14 Anti-PD-1 and PD-L1 antibodies, such as nivolumab and pembrolizumab, respectively, can reverse this T-cell suppression and induce long-term antitumor responses in patients with advanced solid tumors. This has resulted in relatively higher durable response rates and lower toxicity in some large phase I studies.15,16 As for melanoma, improved survival outcomes after treatment with an immune checkpoint inhibitor antibody have been demonstrated in some clinical trials.17–22 Moreover, a phase III study that compared treatment with nivolumab versus dacarbazine in patients with advanced melanoma was discontinued at an early stage by an independent data monitoring committee because patients who were treated with nivolumab demonstrated an improved overall survival compared with patients who were treated with dacarbazine.23
The objective of this meta-analysis was to systematically combine data from current clinical trials in order to assess the efficacy and safety of anti-PD-1 and PD-L1 antibodies for the treatment of advanced melanoma according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.24
MATERIALS AND METHODS
This systematic review and meta-analysis was registered in http://www.crd.york.ac.uk/PROSPERO, and the registration number is CRD42015024184. All analyses were based on previous published studies, thus no ethical approval and patient consent are required.
Literature Search Strategy and Study Criteria
A systematic literature search of studies published until July 2015 was performed in EMBASE, Medline, Cochrane Controlled Trials Register Databases, and the Chinese Biomedical Literature Database for relevant articles published in any language. The relevant Medical Subject Heading search terms we used were as follows: “anti-PD-1,” “anti-PD-L1,” “pembrolizumab,” “lambrolizumab,” “nivolumab,” “pidilizumab,” “BMS936558,” “BMS935559,” “AMP-224,” “AMP-514” combined with “melanoma.” In addition, the Information Sciences Institute Proceedings database was also searched for meeting abstracts. Two investigators conducted the search independently, and the quality of the studies was also evaluated according to the Cochrane recommendations.25 Any discrepancies during the evaluation were resolved by discussion with a third reviewer.
In terms of inclusion criteria, trials that involved treatment with an anti-PD-1 antibody or an anti-PD-L1 antibody for the treatment of melanoma were included. Studies without raw data available for further analysis were excluded. If there was any doubt whether the trials shared the same participants, either completely or partially (through the identification of common authors and centers), the authors of the trials were contacted to clarify whether the trial was duplicated and to provide more details on the clinical trials if possible.
Outcome and Endpoints
The primary outcomes were the objective response rate (ORR) and the median progression-free survival (PFS).The secondary outcome was the occurrence of Grade 3–4 adverse effects (AEs).
Data Extraction and Quality Control
Two researchers evaluated the titles and abstracts of all references retrieved by the search strategies according to the back-to-back principle. Each reference that appeared to fulfill the inclusion criteria was listed as “preselected.” Complete articles that corresponded to all of the preselected references were then retrieved. Next, the preselected references were analyzed by 2 different researchers, who decided to include or exclude studies according to previously reported criteria. Additionally, excluded studies and the reasons for their exclusion were listed and verified by a third reviewer. Upon inclusion of the studies, all data of interest were extracted by 2 reviewers according to the established protocol. If some descriptions related to the analysis were not reported in the original paper, additional details sought from many different sources, such as meeting publications, secondary publications, and direct contact with authors, among others.
Statistical Analysis
All meta-analyses were performed using Stata/SE 12.0 software (Stata, College Station, TX). Statistical heterogeneity among the selected studies was verified through the Chi-square test and the I2 statistic.26 If no statistically significant heterogeneity (P > 0.05 or I2 < 50%) was shown among the results of the included trials, a fixed effects model was used to calculate the synthesized results. If significant heterogeneity (P < 0.05 or I2 > 50%) was observed in the analyses, a random effects model was used for the meta-analysis. Subgroup analyses were also performed to explore potential causes of heterogeneity. The relative risk (RR) and hazard ratio (HR) were designed to be calculated for dichotomous data and PFS events, respectively, with 95% confidence intervals (CIs) for all analyses.25,27 All P values complied with 2-sided tests and were considered to be statistically significant if the P-value was <0.05 except in the tests for heterogeneity.
The funnel plot test described by Egger et al.28 was performed to evaluate the potential of publication bias among the included trials.
RESULTS
Eligible Studies
Under the predefined search strategy, 923 records were found through initial searches of the electronic databases. First, after the exclusion of 129 duplicated records, we verified the titles and abstracts of the remaining 794 records based upon the inclusion and exclusion criteria listed above. In all, 732 records were then removed for the following reasons: 139 studies did not involve melanoma, 255 studies were not based on anti-PD-1 or anti-PD-L1 agents, 180 were studies were conducted in vivo and in vitro, and 158 were reviews. Then, among the 62 articles that remained for further full-text review, only 12 clinical trials provided sufficient data that satisfied the inclusion criteria for this meta-analysis. The reference flow chart is shown in Figure 1, and the main characteristics of the included studies are summarized in Table 1.
FIGURE 1.
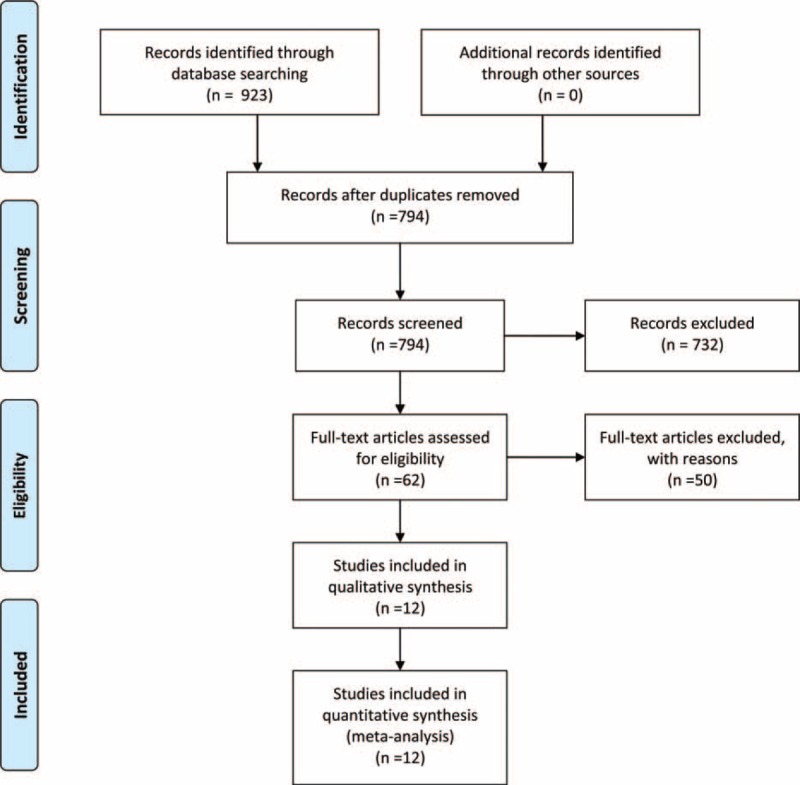
Selection of publications included in the meta-analysis.
TABLE 1.
Characteristics of the Included Studies
Objective Response Rate
Because significant heterogeneity was observed in the included studies (I2 = 83.1%, P < 0.001), a random effects model was used to calculate the ORR of treatment with PD-1 and PD-L1 inhibitors, which was 30% (95% CI: 25–35%, P < 0.001) (Figure 2A).
FIGURE 2.
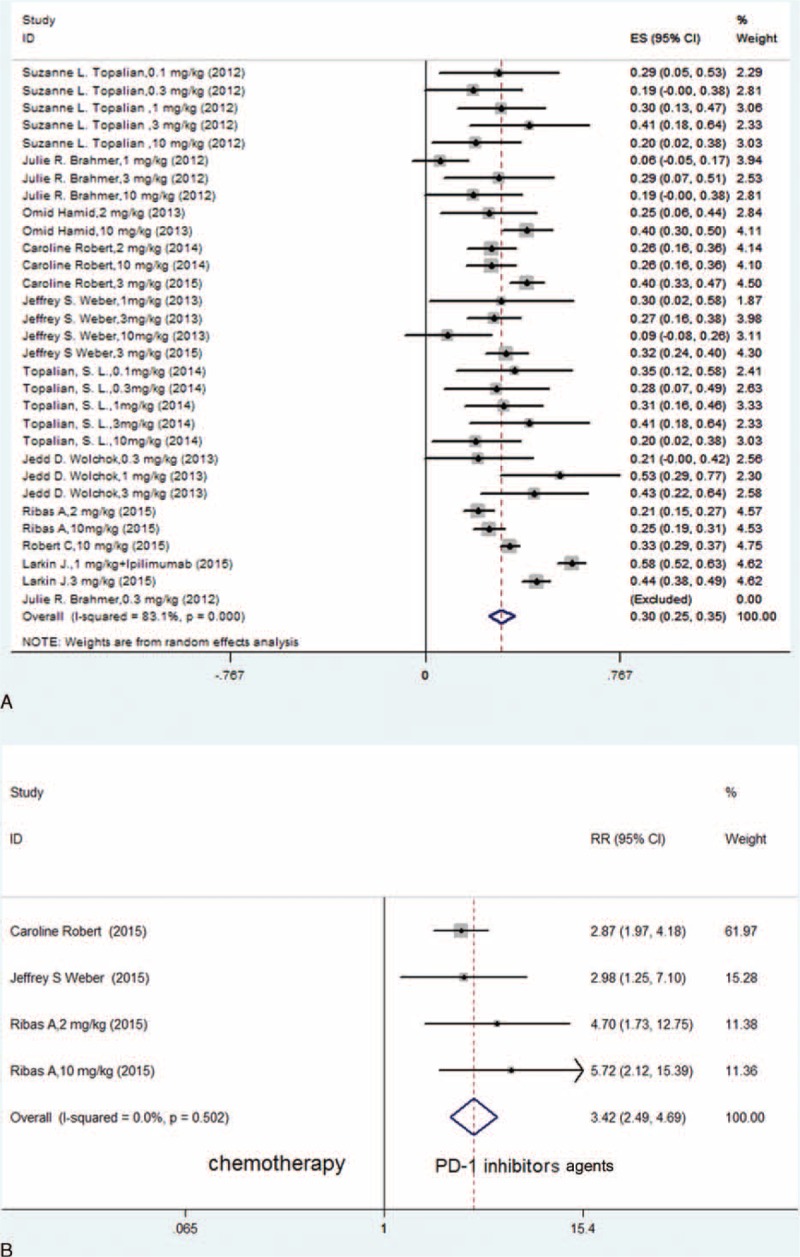
(A) Meta-analysis of included studies with an analysis of the ORR of PD-1 and PD-L1 inhibitors for patients with advanced melanoma (random effects model). (B) Meta-analysis of included RCTs with a comparison of the ORR between PD-1 inhibitors and chemotherapy in patients with advanced melanoma (fixed-effects model).
As no significant heterogeneity was shown (I2 = 0.0%, P = 0.502), we performed the meta-analysis based on the 3 randomized controlled trials (RCTs) and compared the PD-1 inhibitor group and the chemotherapy group using a fixed effects model. We found that the difference between these 2 groups was statistically significant (RR = 3.42, 95% CI: 2.49–4.69, P < 0.001) (Figure 2B).
Subgroup analyses were also conducted according to the dose of the PD-1 and PD-L1 inhibitors. The difference in homogeneity within these subgroups was not found to be statistically significant, and thus, a fixed effects model was used to analyze the differences between the subgroups. No significant difference was observed in the ORR upon comparisons among a low-dose cohort (≤1 mg/kg), a median-dose cohort (2 or 3 mg/kg) and a high-dose cohort (10 mg/kg) (Figure 3A–C).
FIGURE 3.
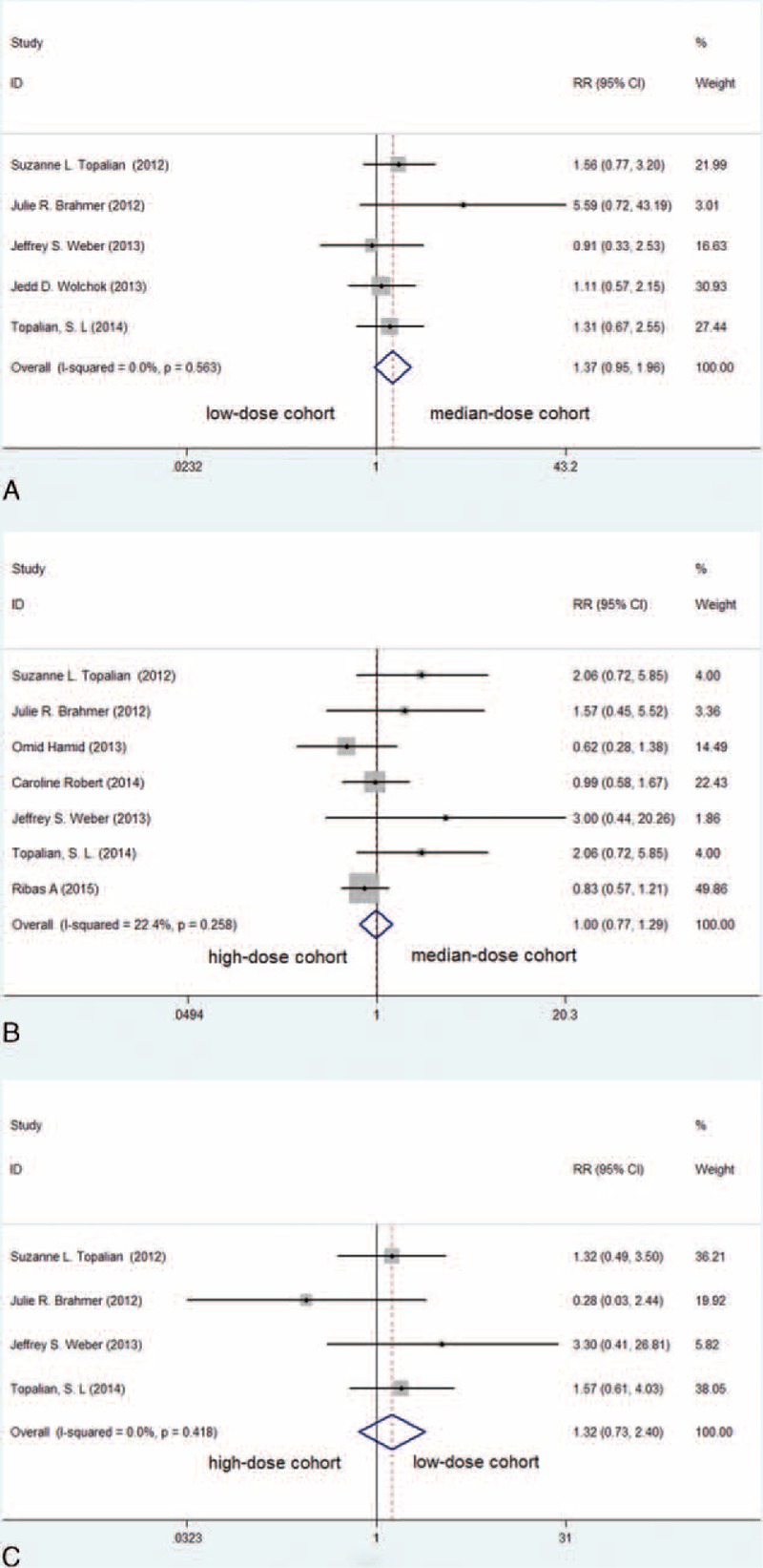
Meta-analysis of included clinical trials with an analysis of the ORR of PD-1 and PD-L1 inhibitors among different dose groups in patients with advanced melanoma (fixed-effects model). (A) The comparison between the median-dose cohort and the low-dose cohort (RR = 1.37, P = 0.089); (B) the comparison between the median-dose cohort and the high-dose cohort (RR = 1.00, P = 0.990); (C) the comparison between the low-dose cohort and the high-dose cohort (RR = 1.32, P = 0.357).
Progression-Free Survival
Since no significant heterogeneity was found (I2 = 16.9%, P = 0.307), in the current meta-analysis, a fixed effects model was used to calculate and evaluate the HR of the PFS in the 3 RCTs for the PD-1 inhibitor group and the chemotherapy group. A significantly prolonged PFS was observed in the PD-1 inhibition group (HR = 0.50, 95% CI: 0.44–0.58, P < 0.001) (Figure 4).
FIGURE 4.
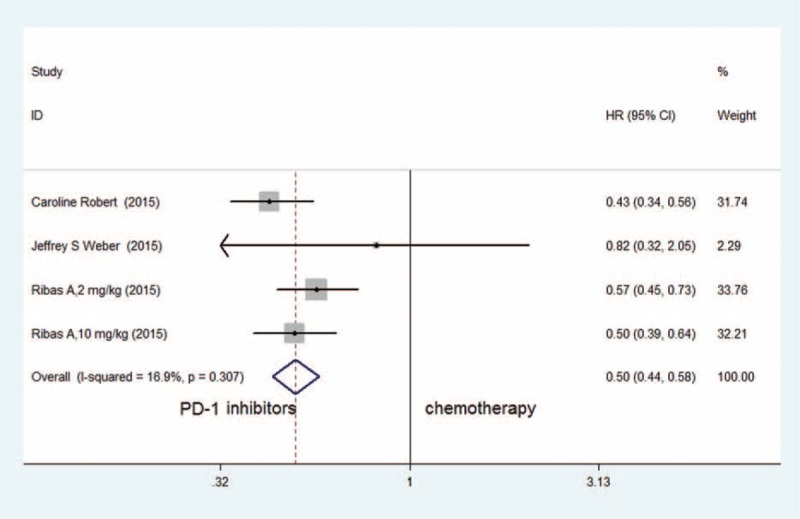
Meta-analysis of included randomized controlled trials with the HR of the PFS between PD-1 inhibitors and chemotherapy in patients with advanced melanoma (fixed-effects model).
The Rate of Grade 3–4 Adverse Effects
Because significant heterogeneity was demonstrated (I2 = 72.5%, P < 0.001), a random effects model was used to synthesize the rate of Grade 3–4 AEs, which was 9% (95% CI: 6–12%, P < 0.001) (Figure 5A). According to the included clinical trials, the most common AEs of PD-1 and PD-L1 inhibitors included fatigue, decreased appetite, diarrhea, nausea, cough, dyspnea, constipation, vomiting, rash, pyrexia, and headache.
FIGURE 5.
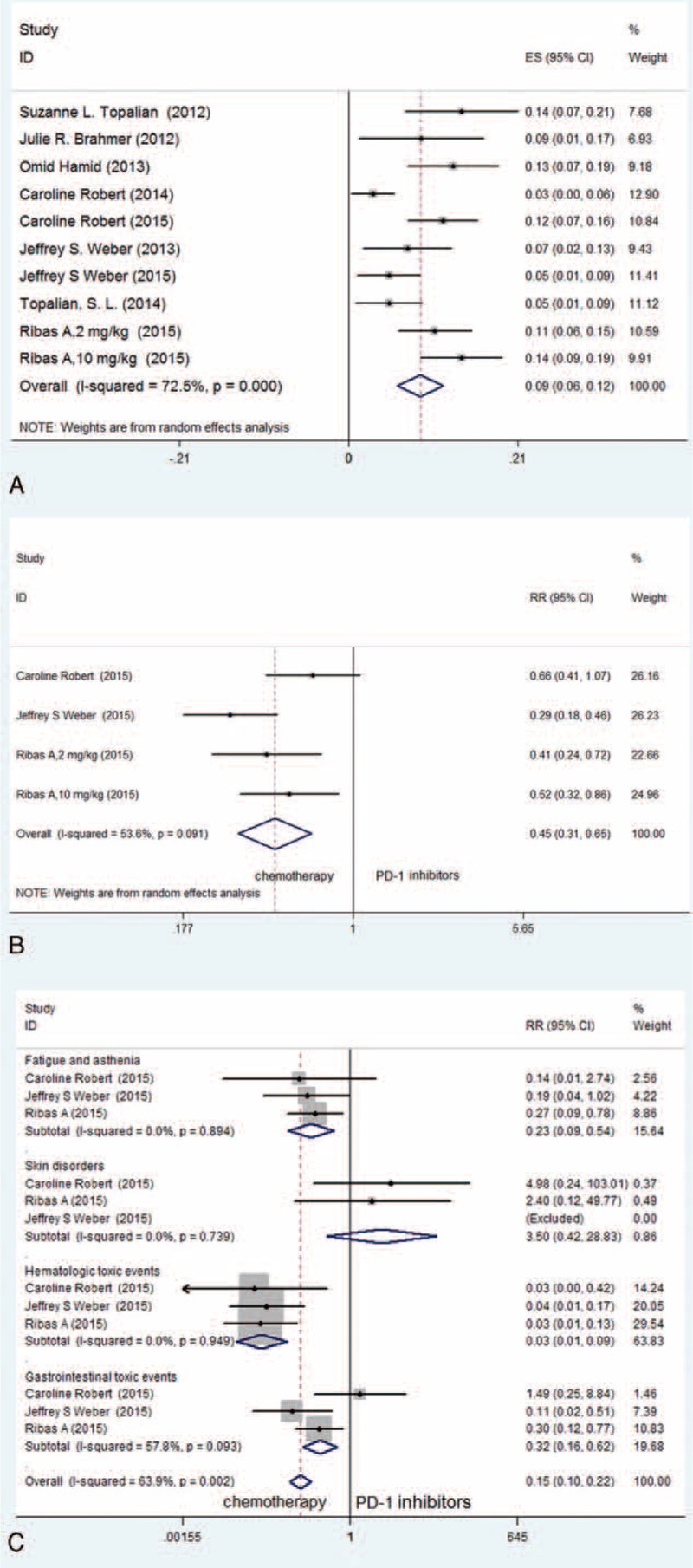
(A) Meta-analysis of included studies in terms of Grade 3–4 AEs of PD-1 and PD-L1 inhibitors in patients with advanced melanoma (random effects model). (B) Meta-analysis of included RCTs with an analysis of the rate of Grade 3–4 AEs between PD-1 inhibitors and chemotherapy for patients with advanced melanoma (fixed-effects model). (C) Meta-analysis of included RCTs with an analysis of the most common treatment-related Grade 3–4 AE profiles between PD-1 inhibitors and chemotherapy in patients with advanced melanoma (fixed-effects model).
Based on the included RCTs that focus on the comparison of PD-1 inhibitors and chemotherapy, we conducted a meta-analysis using a random effects model because of the significant heterogeneity that was present (I2 = 53.6%, P = 0.091). The outcome was that the PD-1 inhibitor group demonstrated a significantly lower rate of Grade 3–4 AEs compared with the chemotherapy group (RR = 0.45, 95% CI: 0.31–0.65, P < 0.001) (Figure 5B). An additional subgroup analysis was conducted for the most common treatment-related Grade 3–4 AE profiles in these 2 groups. We found that the group treated with PD-1 inhibitors experienced significantly lower frequencies of fatigue and asthenia (RR = 0.23, 95% CI: 0.09–0.54, P = 0.001), hematologic toxicity events (RR = 0.03, 95% CI: 0.01–0.09, P < 0.001) and gastrointestinal toxicity events (RR = 0.32, 95% CI: 0.16–0.62, P = 0.001) than the group treated with chemotherapy. However, no statistical significance was observed in the frequency of skin disorders between these 2 groups (RR = 3.50, 95% CI: 0.42–28.83, P = 0.245) (Figure 5C).
Funnel plots were generated, and Egger test was conducted to assess the potential publication bias of the included literature. According to this test, no significant publication bias (P > 0.05) existed in any of the studies (Supplementary Figure 1).
DISCUSSION
Immunotherapy has become the last frontier and a popular topic in research that concerns the treatment of various types of cancers, especially melanoma. Recently, many experts have considered immunotherapy to be the fourth treatment modality for cancer, along with surgery, radiation, and chemotherapy.29 In regards to the progress achieved in immunotherapy during the past few years, antibodies to the checkpoint inhibitor CTLA-4 and inhibitors of PD-1/PD-L1 for the treatment of advanced melanoma are the focus of most discussions.
Ipilimumab has achieved an improvement in overall survival in 2 randomized, controlled phase III clinical trials9,10 and was approved for the treatment of advanced melanoma by the FDA in March 2011.29 In the phase III study CA184-024 for untreated unresectable stage III or IV melanoma, the survival rates at 1, 2, 3, and 5 years were higher in patients who were treated with ipilimumab plus dacarbazine compared with the survival rates of those who were treated with placebo plus dacarbazine.7 However, the rate of Grade 3 or 4 AEs was 56.3% in patients who were treated with ipilimumab plus dacarbazine compared with 27.5% in the control group (P < 0.001).10 It was revealed in current clinical trials that immune-related Grade 3 or 4 AEs are not uncommon during treatment with ipilimumab (the rate is approximately 10–15%) and that they can be severe and life-threatening.29
Checkpoint inhibitor antibodies to PD-1/PD-L1 have demonstrated promising improved ORR and prolonged PFS associated with fewer AEs in melanoma, as shown in this meta-analysis. As it is presented, a maximum tolerated dose was not defined at the doses tested in these included phase I studies. Upon the subgroup analyses of different agent dosages, we failed to find a significant difference in the ORR in comparisons of low-dose, median-dose, and high-dose cohorts, which may be due to the limited sample size. In the included clinical trials, no clinically meaningful difference was reported after a comparison of different doses.17,30 Some phase II and III RCTs have demonstrated that nivolumab and pembrolizumab led to improved ORR, prolonged PFS and a decrease in the rate of Grade 3 or 4 AEs compared with chemotherapy based on dacarbazine, paclitaxel, or carboplatin.19,21,30 For patients with previously untreated metastatic melanoma who do not have a BRAF mutation, the study by Robert et al19 demonstrated that the 1-year overall survival rate was significantly higher in the nivolumab group compared with the dacarbazine group (72.9% vs 42.1%) and that the occurrence of Grade 3 or 4 AEs appeared to be less frequent in the nivolumab group compared with the dacarbazine group (11.7% vs 17.6%). Apart from this, for patients with advanced melanoma who have progressed after treatment with ipilimumab and BRAF inhibitors, Weber et al21 directed a trial that showed that the ORR of the nivolumab group was clearly higher than that of the chemotherapy group (31.7% vs 10.6%). Moreover, Grade 3–4 AEs occurred in 24 (9%) of the 268 patients in the nivolumab group versus 32 (31%) of the 102 patients in the chemotherapy group. In the phase II trial KEYNOTE-002, which focused on patients with ipilimumab-refractory melanoma,30 pembrolizumab resulted in a statistically significant improved progression-free and overall survival compared with chemotherapy; in addition, the ORR was 21% in the pembrolizumab 2 mg/kg group and 25% in the 10 mg/kg group compared with 4% in the chemotherapy group. Moreover, the incidence of Grade 3–4 AEs was higher in the chemotherapy group (45 [26%] of 171 patients) than in the 2 pembrolizumab groups (20 [11%] of 178 patients in the pembrolizumab 2 mg/kg group and 25 [14%] of 179 patients in the 10 mg/kg group); a lower frequency of gastrointestinal and hematologic toxicity events was also observed in the pembrolizumab group compared with the chemotherapy group. With pembrolizumab treatment, potentially immune-mediated adverse events were observed infrequently and were primarily Grade 1 or 2 in severity, such as hypothyroidism, hypophysitis, colitis, pneumonitis, hepatitis, and nephritis.
With regard to the comparison between these 2 types of immune checkpoint inhibitors, a preclinical study has demonstrated that CTLA-4-knockout mice experienced fatal lymphocyte hyperproliferation. In contrast, the PD-1 pathway plays more subtle roles in the maintenance of peripheral T-lymphocyte tolerance and the regulation of inflammation.31 Consequently, PD-1/PD-L1 inhibitors may be better tolerated by patients than CTLA-4 inhibitors.32 The multicenter, randomized, phase III study (KEYNOTE-006)33 compared the efficiency and safety of pembrolizumab and ipilimumab in patients with advanced melanoma. It was revealed that treatment with pembrolizumab led to an extended PFS and overall survival and to a reduction in high-grade toxicity compared with ipilimumab. The response rate improved upon administration of pembrolizumab every 2 weeks (33.7%) and every 3 weeks (32.9%) compared with ipilimumab (11.9%) (P < 0.001 for both comparisons). However, the rates of Grade 3 to 5 AEs were also lower in the pembrolizumab groups (13.3% and 10.1%) compared with the ipilimumab group (19.9%). Due to the superior overall survival results, it was recommended by the independent data and safety monitoring committee that the study be discontinued early in order to give patients in the ipilimumab group the option to be treated with pembrolizumab.
In addition to the studies discussed above, the combination of immune checkpoint inhibitors has achieved considerable progress and has garnered attention at the 2015 American Society of Clinical Oncology (ASCO) Annual Meeting. In the phase I study of concurrent treatment with nivolumab and ipilimumab directed by Wolchok et al.,34 it was demonstrated that the ORR of the combination of these 2 types of immune checkpoint blockade agents was 40%, which exceeded the previously reported results with either nivolumab or ipilimumab alone. Moreover, it was observed that the rate of Grade 3 or 4 AEs was 53% in patients who received combination therapy, but this rate was qualitatively similar to that in studies of monotherapy and was manageable and generally reversible.35 At the 2015 ASCO meeting, a randomized, double-blind, phase III study performed by Larkin et al36 presented comparisons among nivolumab plus ipilimumab, nivolumab alone, and ipilimumab alone in previously untreated patients with metastatic melanoma. In PD-L1-positive patients, the ORR was 57.5% in the nivolumab group, 72.1% in the combination group, and 21.3% in the ipilimumab group. In PD-L1-negative patients, the ORRs were 41.3%, 54.8%, and 17.8%, in the nivolumab group, the combination group and the ipilimumab group, respectively. In PD-L1-positive patients, the median PFS was 14.0 months in the combination group and in the nivolumab group, but in PD-L1-negative patients, the PFS was longer with combination therapy than with nivolumab alone (11.2 months vs 5.3 months). Grade 3 or 4 AEs occurred more frequently in the nivolumab-plus-ipilimumab group (55.0%) compared with the monotherapy group (16.3% in the nivolumab group, and 27.3% in the ipilimumab group). Compared with monotherapy, the combination of immune checkpoint inhibitors resulted in numerically higher response rates and longer PFS, especially in patients with PD-L1-negative tumors.37
In conclusion, according to this meta-analysis of limited concurrent studies, PD-1 and PD-L1 inhibitors appear to be associated with improved response rates, superior response durability and tolerable toxicity in patients with advanced melanoma. We may inevitably encounter some limitations because the concurrent studies included in the meta-analysis were mostly phase I trials, and only 3 phase II and III RCTs were included. As a hot issue in the area of cancer treatment, the initiation of a greater number of successive clinical trials associated with immune checkpoint blockade along with a further exploration into the mechanism of tumor immunity would not fail to surprise us.
Supplementary Material
Acknowledgment
We appreciate the directors of the referred clinical trials and all of the participants in those trials.
Footnotes
Abbreviations: PD-1 = programmed cell death 1, PD-L1 = programmed cell death 1 ligand, ORR = objective response rate, PFS = progression-free survival, AEs = adverse effects, RR = relative risk, HR = hazard ratio, CTLA-4 = cytotoxic T-lymphocyte-associated antigen 4, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, CIs = confidence intervals, RCTs = randomized controlled trials, ASCO = American Society of Clinical Oncology
XG and HW contributed equally to this work.
This study was funded by the National Natural Science Foundation of China (81472453).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
REFERENCES
- 1.Garbe C, Eigentler TK, Keilholz U, et al. Systematic review of medical treatment in melanoma: current status and future prospects. Oncologist 2011; 16:5–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawrence MS, Stojanov P, Polak P, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013; 499:214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975–2010. Bethesda, MD. National Cancer Institute; 2013. http://seer.cancer.gov/csr/1975_2010. [Google Scholar]
- 4.Pardoll DM. Blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12:252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altomonte M, Di Giacomo A, Queirolo P, et al. Clinical experience with ipilimumab 10 mg/kg in patients with melanoma treated at Italian centres as part of a European expanded access programme. J Exp Clin Cancer Res 2013; 32:82.http://www.jeccr.com/content/32/1/82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Margolin K. Moving forward with immunotherapy: the rationale for anti-CTLA-4 therapy in melanoma. Commun Oncol 2008; 5:367–437. [Google Scholar]
- 7.Maio M, Grob JJ, Aamdal S, et al. Five-year survival rates for treatment-naive patients with advanced melanoma who received ipilimumab plusdacarbazine in a phase III trial. J Clin Oncol 2015; 33:1191–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol 2015; 16:522–530. [DOI] [PubMed] [Google Scholar]
- 9.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011; 364:2517–2526. [DOI] [PubMed] [Google Scholar]
- 11.Weber JS, Dummer R, de Pril V, et al. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer 2013; 119:1675–1682. [DOI] [PubMed] [Google Scholar]
- 12.Keir ME, Liang SC, Guleria I, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med 2006; 203:883–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008; 26:677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okazaki T, Chikuma S, Iwai Y, et al. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol 2013; 14:1212–1218. [DOI] [PubMed] [Google Scholar]
- 15.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366:2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366:2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013; 369:134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase1 trial. Lancet 2014; 384:1109–1117. [DOI] [PubMed] [Google Scholar]
- 19.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015; 372:320–330. [DOI] [PubMed] [Google Scholar]
- 20.Weber JS, Kudchadkar RR, Yu B, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol 2013; 31:4311–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (Check Mate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015; 16:375–384. [DOI] [PubMed] [Google Scholar]
- 22.Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014; 32:1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol 2015; pii: JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. http://www.prisma-statement.org/ [Google Scholar]
- 25.Higgins JPT, Green S. Cochrane Handbook for systematic reviews of interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochranehandbook.org [Google Scholar]
- 26.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 27.Petitti DB. Meta-analysis, Decision Analysis, and Cost-effectiveness Analysis. 2000; New York, NY: Oxford University Press, 94–118. [Google Scholar]
- 28.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saraceni MM, Khushalani NI, Jarkowski A. Immunotherapy in melanoma: recent advances and promising new therapies. J Pharm Pract 2015; 28:193–203. [DOI] [PubMed] [Google Scholar]
- 30.Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008; 26:677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahoney KM, Freeman GJ, McDermott DF. The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma. Clin Ther 2015; 37:764–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015; 372:2521–2532. [DOI] [PubMed] [Google Scholar]
- 34.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369:122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brahmer JR, Hammers H, Lipson EJ. Nivolumab: targeting PD-1 to bolster antitumor immunity. Future Oncol 2015; 11:1307–1326. [DOI] [PubMed] [Google Scholar]
- 36.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ascierto PA, Marincola FM, Atkins MB, et al. What's new in melanoma? Combination!. J Transl Med 2015; 13:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


