Abstract
Microbial translocation from the gut is associated with immune dysfunction, persistent inflammation, and likely plays a role in the pathogenesis of neurocognitive dysfunction during HIV infection. (1→3)-β-D-Glucan (BDG) is a component of most fungal cell walls and might be a useful indicator of gut mucosal barrier impairment. The objective of this study was to evaluate whether higher blood BDG levels correlate with impaired neurocognitive functioning in a cohort of HIV-infected adults with suppressed levels of HIV RNA in blood plasma.
In this cross-sectional cohort study, we measured levels of BDG in blood plasma and cerebrospinal fluid (CSF) supernatant samples in a cohort of adults with acute/early HIV infection, who initiated antiretroviral therapy (ART) during the earliest phase of infection and achieved suppressed levels of HIV RNA in blood plasma (<50 copies/mL) thereafter. We compared BDG with established biomarkers of microbial translocation, immune activation, and cognitive dysfunction (evaluated by global deficit score).
We found that higher blood BDG levels were significantly related to higher global deficit scores, reflecting worse neurocognitive performance (Spearman r = 0.47; P = 0.042) among HIV-infected adults with suppressed viral loads who initiated ART early in infection. Two CSF samples presented elevated BDG levels. Interestingly, these 2 samples originated from the 2 subjects with the highest global deficit scores of the cohort.
BDG may be a promising independent biomarker associated with neurocognitive functioning in virologically suppressed HIV-infected individuals.
INTRODUCTION
The advent of combination antiretroviral therapy (ART) has increased life expectancy and decreased severe forms of HIV-associated dementia and other co-morbidities in most HIV-infected individuals.1,2 However, milder forms of HIV-associated neurocognitive disorders are still estimated to affect one-third to one-half of successfully treated patients.3 Neurocognitive impairment has been associated with residual immune dysfunction, which persists in some individuals despite long term suppressive ART.4 The exact mechanism of chronic immune dysfunction in these individuals is incompletely understood and most likely multifactorial. Translocation of microbial products from the gastrointestinal tract into the systemic circulation is likely an important driver of immune dysfunction and persistent inflammation during suppressive ART. It may also play a role in the pathogenesis of neurocognitive dysfunction during HIV-infection.5,6
(1→3)-β-D-Glucan (BDG) is a polysaccharide cell wall component of most fungal species including Candida spp.7 Blood and cerebrospinal fluid (CSF) BDG levels are useful for early diagnosis of invasive fungal infections.8,9 In the absence of an active invasive fungal infection, increased blood BDG levels may be an indicator of gut mucosal barrier disruption10,11 and microbial translocation.12 The latter was recently reported also for a cohort of HIV-infected subjects.6
The objective of this study was to evaluate whether higher blood BDG levels correlate with impaired neurocognitive functioning (evaluated by global deficit score [GDS]) in a cohort of adults with acute/early HIV infection, who initiated ART during the earliest phase of infection and achieved suppressed levels of HIV RNA in blood plasma thereafter.
METHODS
In this cross-sectional cohort study, we measured levels of BDG in blood plasma and CSF supernatant samples in a cohort of adults with suppressed levels of HIV RNA in blood plasma (<50 copies/mL), and compared them with established biomarkers of microbial translocation, immune activation, and cognitive dysfunction.
Participants and Samples
All 21 subjects participated in the San Diego Primary HIV Infection Research Consortium (SD PIRC), which comprised of adults with acute or early HIV diagnosis, early ART initiation, and suppressed levels of HIV RNA in blood plasma throughout treatment.13–17 Study samples were collected prospectively as part of the SD PIRC between December 2013 and June 2014 at the University of California, San Diego, and stored at −80 °C on the day of collection.
Neurocognitive Assessment
Degree of neurocognitive impairment at the time of sample collection was measured using the Global Deficit Score (GDS), which is an established and sensitive method to determine neurocognitive functioning among individuals living with HIV.18–20 The GDS has been shown to detect mild, HIV-associated cognitive impairment based on assessment of multiple cognitive domains.19 Briefly, individuals completed a comprehensive neuropsychological test battery consistent with Frascati recommendations for neuroAIDS research.19 Raw scores were converted to demographically adjusted T-scores, which, in turn, were scaled to deficit scores ranging from 0 (normal, T > 39) to 5 (severely impaired, T < 20). Individual tests scores were summarized using the GDS.18,19
Testing of BDG and Established Biomarkers of Microbial Translocation and Immune Activation
Nineteen plasma and 16 CSF supernatant samples (paired same-day plasma and CSF samples were available from 14/21 subjects, plasma samples only from 5/21 subjects, and CSF samples only from 2/21 subjects) were retrospectively evaluated for BDG levels and established biomarkers of microbial translocation and immune activation. BDG levels were measured using the Fungitell assay in June 2015 at the Associates of Cape Cod, Inc, research laboratories (Associates of Cape Cod, Inc, East Falmouth, MA). Soluble cluster of differentiation 14 (sCD14) levels were measured by an enzyme-linked immunosorbent assay (ELISA; R&D Systems Inc, Minneapolis, MN). Interleukin (IL) 8 levels were measured by an electrochemiluminescence multiplex assay (Meso Scale Diagnostics, Rockville, MD), each according to the manufacturer's procedures.
Statistical Analysis/Ethical Approval
For statistical analysis, SPSS 21 (SPSS Inc, Chicago, IL) was used. Correlation between levels of BDG, GDS, and levels of other biomarkers were calculated using Spearman correlation analysis because of the non-normal distributions of GDS scores and other biomarkers. GDS scores were also squareroot-transformed to achieve a distribution close to normal to allow for additional Pearson correlation analysis between normally distributed biomarkers and squareroot-transformed GDS. Power analysis revealed that a sample size of 19 plasma samples provides at least 80% power (with alpha = 0.05) to detect a correlation of r2 = 0.351 or higher for correlations of 2 variables. The UCSD Human Research Protections Program approved the study protocol, consent, and all study-related procedures. All study participants provided voluntary, written informed consent before any study procedures were undertaken.
RESULTS
The study cohort was composed of 21 HIV-infected participants without symptoms of opportunistic infections. Demographics and clinical characteristics at the time of sample collection are depicted in Table 1. All participants were virologically suppressed at the time of sampling in both compartments (blood and CSF). The median CNS Penetration Effectiveness index was 6 (IQR 4–7) for their current ART regimens. No correlations were found between blood BDG levels and age, sex, and estimated duration of infection.
TABLE 1.
Demographics and clinical characteristics of the study population

Median GDS was 0.39 (range 0–2.67; IQR 0.11–1.11; 10 subjects [including one subject with CSF sample only] had GDS >0.5, which is considered at least mild cognitive impairment). Median BDG level in blood plasma was 66 pg/mL (range: 20–101 pg/mL), whereas median BDG level in CSF supernatant was 5 pg/mL (range: 0–53 pg/mL). Higher levels of plasma BDG were associated with more severe cognitive impairment as measured by the GDS (Spearman r = 0.47, P = 0.042; Pearson [correlation with squareroot-transformed GDS] r = 0.46, P = 0.049; Figure 1 and Table 2]. Of the other biomarkers, a significant correlation with GDS was found for IL-8 (Spearman r = 0.55; P = 0.014), whereas no significant correlations were found for sCD14 (Spearman r = 0.4, n.s.), and nadir CD4+ count (Spearman r = 0.01, n.s.). Also, we found no significant correlation between plasma BDG and both IL-8 (Spearman r = 0.12, n.s.) and sCD14 (Spearman r = 0.38, n.s.).
FIGURE 1.
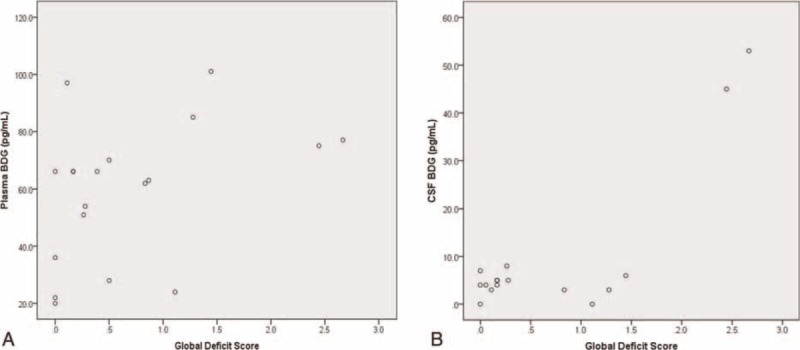
Scatterplots of correlation of blood beta-d-glucan levels (A) and cerebrospinal fluid beta-d-glucan levels (B) with global deficit Scores.
TABLE 2.
Levels of Investigated Plasma Biomarkers (Median and IQR are Displayed) and Correlations With GDS and Plasma BDG Levels
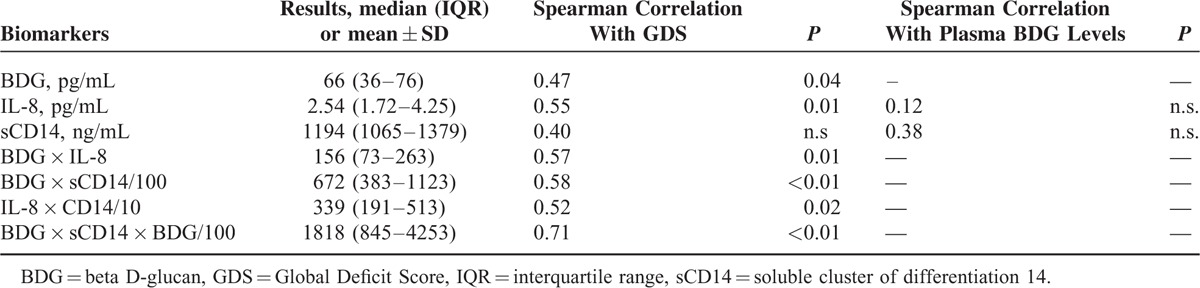
With regard to combinations of BDG, IL-8, and sCD14 and correlation with GDS, BDG + IL-8 (Spearman r = 0.57; P = 0.01) and BDG + sCD14 (Spearman r = 0.58, P < 0.01) were the most promising combinations of 2 biomarkers, whereas the combination of all 3 biomarkers (BDG + IL-8 + sCD14) had the highest correlation with GDS (Spearman r = 0.71; P < 0.01). Plasma BDG levels, levels of other biomarkers including combinations, as well as Spearman correlations with blood BDG and GDS are displayed in Table 2. Using the plasma BDG cut-off recommended by the manufacturer for differentiating negative from intermediate and positive results (ie of 60 pg/mL), the sensitivity for detecting neurocognitive impairment was 78% and the specificity 50%. If BDG cut-off was increased to 70 pg/mL, the sensitivity dropped to 44%, but specificity increased to 90%.
Two of 16 CSF samples presented elevated BDG levels (45 and 53 pg/mL; Figure 1), whereas all other samples had BDG levels <10 pg/mL. Interestingly, these 2 samples originated for the 2 subjects with the highest GDS scores of the cohort. Both of these subjects had also elevated serum BDG levels (4th and 5th highest of the study cohort) and no signs or symptoms of opportunistic infections.
DISCUSSION
This study correlated blood levels of the fungal polysaccharide cell wall component BDG, with cognition among HIV-infected patients. Identifying biomarkers associated with worse neurocognitive functioning among successfully treated HIV-infected individual is important for the development of prophylactic and therapeutic strategies. Overall, our study cohort of HIV-infected individuals with suppressed HIV RNA viral loads presented with markedly higher BDG levels when compared to previously published levels from healthy patients undergoing elective plastic surgery procedures.21
Our study also showed, for the first time, that elevated BDG levels in the absence of fungal infections were associated with negative neurocognitive outcomes (as measured by increased GDS) in virologically suppressed HIV-infected persons who started ART during early HIV infection. Interestingly, GDS was also correlated with IL-8, but BDG and IL-8 were not correlated. Importantly, combining BDG with IL-8 and especially the combination of all 3 plasma biomarkers (BDG, IL-8, and sCD 14) further improved the correlation with GDS.
We hypothesize that elevated plasma BDG may primarily reflect translocation of products from natural fungal flora from the gastrointestinal tract into systemic circulation. Evidence of peripheral fungal cell wall polysaccharides in the systemic circulation has also been reported previously in an HIV-infected outpatient cohort, the majority of whom had measurable HIV RNA in their blood.6 In that study, high serum BDG was associated with HIV-associated immunosuppression (ie, CD4+ cell counts <200 cells/μL), inflammation (correlation with plasma IL-8 and other inflammation markers), and cardiopulmonary comorbidity.6 Together with these previous findings, our results support a theory that BDG translocation occurs in virologically suppressed HIV+ individuals and may relate to important outcomes such as NC impairment.
BDG levels were markedly higher (mean 142 pg/mL) in a HIV-infected cohort with lower median CD4 counts (26, IQR 10–53, all without opportunistic infections), when compared with the cohort studied here (with a median CD4 count >700 pg/mL).22 In contrast, lower BDG levels (median 15 pg/mL) were found in another study that evaluated chronically HIV-infected individuals with a median duration of HIV infection of 15 years and high CD4+ counts (643, range 196–1740).23 Although elevated blood BDG levels may be associated with microbial translocation in all HIV-infected individuals (ie, independent of CD4+ cell counts), interpretation of elevated blood BDG levels in patients with CD4+ cell counts below 200 to 300 cells/μL may be more complicated. Although it seems intuitive that deteriorating CD4+ counts are associated with worse mucosal barrier function,12 other reasons for elevated BDG may include potential colonization or subclinical infection with Candida spp or Pneumocystis that may occur more frequently in individuals with lower CD4 counts.7,22
Other studies suggested that BDG may also be elevated in bloodstream infections caused by primarily enteric bacteria, such as Enterococcus spp, whereas in a recent study, BDG was not elevated (i.e. > 80 pg/mL) in patients with mild-to-moderate mucositis.12,24,25 Whether this increase in BDG levels reflects concomitant translocation of fungal elements, still needs to be determined.
Major limitations of this pilot study include the small sample size, that it is single-site, cross-sectional, and restricted to patients treated in early stages of HIV infection. To further examine the role of BDG as a potential biomarker for translocation of gut luminal contents and its correlation with neurocognitive impairment, more comprehensive studies will be necessary. BDG levels in the intestinal luminal contents are also likely to be highly variable on an individual basis, and a standardized oral BDG challenge approach may be more suitable for assessing gut leakiness. Longitudinal studies are also needed to investigate the mechanisms of blood BDG increase in individuals with translocation of gut microbes or their components.
In conclusion, BDG may be an indicator of gut mucosal barrier disruption and an independent biomarker associated with neurocognitive functioning in virologically suppressed HIV-infected individuals with high CD4+ counts. In particular, when BDG is combined with established markers of immune activation, diagnostic potential for neurocognitive functioning may be promising.
Acknowledgements
None.
Footnotes
Abbreviations: ART = antiretroviral therapy, BDG = (1→3)-β-D-Glucan, CSF = cerebrospinal fluid, ELISA = enzyme-linked immunosorbent assay, GDS = global deficit score, IL = interleukin, IQR = inter-quartile range, sCD14 = Soluble cluster of differentiation 14, SD PIRC = San Diego Primary HIV Infection Research Consortium
MH and MO contributed equally to this work.
Original data of this manuscript have been presented in part at CROI 2016 in Boston, USA (Abstract Nr 410).
MH served on the speakers’ bureau of Merck. YZ and MF are employees of Associates of Cape Cod.
This work was supported by funds from the following: the Max Kade Foundation, New York (Max Kade Postdoctoral Research grant), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-Brazil), Interdisciplicinary Research Fellowship in NeuroAIDS (R25-MH081482), HNRP developmental grant PST-HN39, and grants from the National Institutes of Health: AI106039, MH100974, MH101012, MH62512, AI108351, AI100665, MH097520, DA034978, AI036214, AI007384, AI027763, AI043638, AI074621. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors report no conflicts of interest.
REFERENCES
- 1.Hunt PW. HIV and inflammation: mechanisms and consequences. Curr HIV/AIDS Rep 2012; 9:139–147. [DOI] [PubMed] [Google Scholar]
- 2.Palella FJ, Jr, Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr 2006; 43:27–34. [DOI] [PubMed] [Google Scholar]
- 3.Heaton RK, Clifford DB, Franklin DR, Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology 2010; 75:2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deeks SG, Verdin E, McCune JM. Immunosenescence and HIV. Curr Opin Immunol 2012; 24:501–506. [DOI] [PubMed] [Google Scholar]
- 5.Marchetti G, Tincati C, Silvestri G. Microbial translocation in the pathogenesis of HIV infection and AIDS. Clin Microbiol Rev 2013; 26:2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris A, Hillenbrand M, Finkelman M, et al. Serum (1–>3)-beta-D-glucan levels in HIV-infected individuals are associated with immunosuppression, inflammation, and cardiopulmonary function. J Acquir Immune Defic Syndr 2012; 61:462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reischies FM, Prattes J, Pruller F, et al. Prognostic potential of 1,3-beta-d-glucan levels in bronchoalveolar lavage fluid samples. J Infect 2016; 72:29–35. [DOI] [PubMed] [Google Scholar]
- 8.Prattes J, Hoenigl M, Rabensteiner J, et al. Serum 1,3-beta-d-glucan for antifungal treatment stratification at the intensive care unit and the influence of surgery. Mycoses 2014; 57:679–686. [DOI] [PubMed] [Google Scholar]
- 9.Karageorgopoulos DE, Vouloumanou EK, Ntziora F, et al. Beta-D-glucan assay for the diagnosis of invasive fungal infections: A meta-analysis. Clin Infect Dis 2011; 52:750–770. [DOI] [PubMed] [Google Scholar]
- 10.Shahid Z, Sanathkumar N, Restrepo A, et al. Elevated serum besta-D-glucan (BDG) as A marker for chemotherapy-induced mucosal barrier injury (MBI) in adults with hematologic malignancies: A retrospective ananlysis. ID Week 2011 2011; (Abstract 1334). [Google Scholar]
- 11.Ellis M, Al-Ramadi B, Finkelman M, et al. Assessment of the clinical utility of serial beta-D-glucan concentrations in patients with persistent neutropenic fever. J Med Microbiol 2008; 57 (Pt 3):287–295. [DOI] [PubMed] [Google Scholar]
- 12.Held J, Kohlberger I, Rappold E, et al. Comparison of (1->3)-beta-D-glucan, mannan/anti-mannan antibodies, and cand-tec candida antigen as serum biomarkers for candidemia. J Clin Microbiol 2013; 51:1158–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoenigl M, Anderson CM, Green N, et al. Repeat HIV-testing is associated with an increase in behavioral risk among men who have sex with men: a cohort study. BMC Med 2015; 13:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoenigl M, Weibel N, Mehta SR, et al. Development and validation of the San Diego Early Test (SDET) score to predict acute and early HIV infection risk in men who have sex with men. Clin Infect Dis 2015; 61:468–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoenigl M, Green N, Camacho M, et al. Signs or symptoms of acute HIV infection in a cohort undergoing community-based screening. Emerg Infect Dis 2016; 22:532–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoenigl M, Graff-Zivin J, Little SJ. Costs per diagnosis of acute HIV infection in community-based screening strategies: A comparative analysis of four screening algorithms. Clin Infect Dis 2016; 62:501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoenigl M, Green N, Mehta SR, et al. Risk factors for acute and early HIV infection among men who have sex with men (MSM) in san diego, 2008 to 2014: a cohort study. Medicine (Baltimore) 2015; 94:e1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez R, Heaton RK, Moore DJ, et al. Computerized reaction time battery versus a traditional neuropsychological battery: Detecting HIV-related impairments. J Int Neuropsychol Soc 2003; 9:64–71. [DOI] [PubMed] [Google Scholar]
- 19.Heaton RK, Grant I, Butters N, et al. The HNRC 500–neuropsychology of HIV infection at different disease stages. HIV neurobehavioral research center. J Int Neuropsychol Soc 1995; 1:231–251. [DOI] [PubMed] [Google Scholar]
- 20.Blackstone K, Moore DJ, Franklin DR, et al. Defining neurocognitive impairment in HIV: Deficit scores versus clinical ratings. Clin Neuropsychol 2012; 26:894–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pruller F, Wagner J, Raggam RB, et al. Automation of serum (1–>3)-beta-D-glucan testing allows reliable and rapid discrimination of patients with and without candidemia. Med Mycol 2014; 52:455–461. [DOI] [PubMed] [Google Scholar]
- 22.Sax PE, Komarow L, Finkelman MA, et al. Blood (1->3)-beta-D-glucan as a diagnostic test for HIV-related pneumocystis jirovecii pneumonia. Clin Infect Dis 2011; 53:197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoenigl M, Faria de Oliveira M, Pérez-Santiago J, et al. Correlation of (1→3)-(-D-glucan with other inflammation markers in chronically HIV infected persons on suppressive antiretroviral therapy. GMS Infect Dis 2015; 3:Doc03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez-Jimenez MC, Munoz P, Valerio M, et al. Candida biomarkers in patients with candidaemia and bacteraemia. J Antimicrob Chemother 2015; 70:2354–2361. [DOI] [PubMed] [Google Scholar]
- 25.Prattes J, Raggam RB, Vanstraelen K, et al. Chemotherapy induced intestinal mucosal barrier damage: a cause of falsely elevated serum 1,3-beta-d-glucan levels? J Clin Microbiol 2016; 54:798–801. [DOI] [PMC free article] [PubMed] [Google Scholar]


