Abstract
Purpose
Xerostomia is a common radiation sequela, which has a negative impact on the quality of life of patients with head and neck cancer. Current treatment strategies offer only partial relief. Botulinum toxins (BTX) have been successfully used in treating a variety of radiation sequelae such as cystitis, proctitis, fibrosis, and facial pain. The purpose of this study was to evaluate the effect of BTX on radiation-induced salivary gland damage.
Methods and Materials
We used a previously established model for murine salivary gland irradiation (IR). The submandibular glands (SMGs) of C5BL/6 mice (n=6/group) were injected with saline or BTX 72 hours before receiving 15 Gy of focal irradiation. Saliva flow was measured 3, 7, and 28 days after treatment. The SMGs were collected for immunohistochemistry, confocal microscopy, and Western blotting. A cytokine array consisting of 40 different mouse cytokines was used to evaluate cytokine profiles after radiation treatment.
Results
Irradiated mice showed a 50% reduction in saliva flow after 3 days, whereas mice preinjected with BTX had 25% reduction in saliva flow (P<.05). Cell death detected by TUNEL staining was similar in SMG sections of both groups. However, neutrophil infiltrate, detected by myeloperoxidase staining, was 3-fold lower for the BTX treated mice. A cytokine array showed a 2-fold upregulation of LPS-induced chemokine (LIX/CXCL5) 3 days after IR. BTX pretreatment reduced LIX levels by 40%. At 4 weeks after IR, the saline (control) group showed a 40% reduction in basal SMG weight, compared with 20% in the BTX group. Histologically, BTX-pretreated glands showed relative preservation of acinar structures after radiation.
Conclusions
These data suggest that BTX pretreatment ameliorates radiation-induced saliva dysfunction. Moreover, we demonstrate a novel role for CXCL5 in the acute phase of salivary gland damage after radiation. These results carry important clinical implications for the treatment of xerostomia in patients with head and neck cancer.
Introduction
Radiation therapy is an essential part of multimodality treatment for patients with head and neck cancers (1, 2). Radiation-induced xerostomia, or dry mouth, is a common chronic side effect resulting from unavoidable excessive radiation doses to the salivary glands. The common consequences of xerostomia include difficulty chewing and swallowing, dysgeusia, sleep disturbances, impaired phonation, and decline in quality of life (3-5).
Past studies have provided insight into the mechanisms of radiation-induced salivary gland damage. Loss of acinar cells through apoptosis occurs during the acute phase. This process is p53 dependent and can be reversed by immunoglobulin F-1, CDK inhibition, or Wnt/β-catenin activation (6-11). Alternatively, membrane lipid peroxidation is thought to induce damage to the secretory granules and acinar cellular lysis (12, 13). Recent evidence from our laboratory (14, 15) and others (16, 17) indicates that the chronic phase of salivary gland dysfunction is mediated by damage to stem cells and progenitor cells. This damage can be mitigated by activation of aldehyde dehydrogenase-3 and treatment with glial cell line-derived neurotrophic factor (GDNF), which promote survival of salivary stem cells (14, 15, 18). These studies have generated much interest in further understanding the mechanisms of radiation-induced salivary dysfunction and providing tools for radioprotection.
Saliva secretion is highly regulated by the autonomic nervous system. Cholinergic parasympathetic fibers release acetylcholine, which in turn binds the muscarinic receptors and induces saliva secretion (19). Botulinum toxin (BTX) regulates this process through blocking the release of neuropeptides at the nerve terminals. This mechanism constitutes the basis of using BTX in an ever-expanding list of neurologic, muscular, and cosmetic conditions. We hypothesized that placing the salivary gland in a dormant state through BTX preinjection would confer a radioprotective phenotype. The current study investigated the radioprotective effects of BTX by examining the functional, histologic, and biochemical salivary gland changes in a mouse model.
Methods and Materials
Mice
All animal procedures were approved by the institutional animal care and use committee. A total of 50 female C57BL/6 mice (10-12 weeks old; Jackson Laboratory) were used in the study.
Intraglandular injection and irradiation
The mice (both saline and BTX groups) were anesthetized, and the submandibular gland (SMG) was exposed by a small incision. With a 10-μL microinjection syringe (Hamilton Co), 3.75 mU/g of BTX (BOTOX, Allergan, Irvine, CA) was injected into each SMG. An equivalent volume of saline was injected into the SMGs of the control group. The skin incision was closed with surgical suture. Irradiation experiments were conducted 48 to 72 hours later, after appropriate wound healing was confirmed. The C57BL/6 mice were exposed to a single dose of 15-Gy irradiation (7.5 Gy per side, total 15 Gy). The irradiation was performed with a 225-kVp x-ray system filtered with 0.5 mm Cu (COMET, MXR-226). The mice were placed in special jigs, and the SMGs were irradiated from the lateral side, with the rest of the body protected by a lead shield, as previously described (14).
Stimulated saliva collection
Saliva was collected for 15 minutes from the oral cavity of individual mice with 75-mm hematocrit tubes (Drummond Scientific, Broomall, PA) after the subcutaneous injection of 2 mg/kg pilocarpine (Sigma-Aldrich, St. Louis, MO) subcutaneous injection. The saliva flow rates were determined at basal conditions and 3, 7, and 28 days after irradiation. The measured saliva secretion was normalized to the mouse body weight, assuming 1 g/mL density for saliva.
Immunohistochemistry and immunofluorescence
Frozen tissue sections were warmed to room temperature and fixed on ice in acetone for 20 minutes. Paraffin sections were deparaffinized, rehydrated, and antigen retrieved (Vector laboratories). Slides were washed in phosphate-buffered saline (PBS) solution, incubated overnight at 4°C with different primary antibodies: rabbit anti myeloperoxidase (Santa Cruz Biotechnology, Dallas, TX), mouse anti-CK 7 (Abcam, Burlingame, CA), rinsed with PBS, and incubated with antimouse and antirabbit fluorescent secondary antibodies (1:200 dilution; Invitrogen, Carlsbad, CA) at room temperature for 1 hour. The sections were then mounted with 4′, 6-diamidino-2-phenylindole (DAPI; Vector Laboratories). Immunofluorescence images were acquired with a Zeiss LSM 510 confocal microscope (Zeiss, Germany).
Quantification of acinar surfaces in the salivary gland was done as previously described (17). Briefly, sections of glands stained with hematoxylin and eosin were studied under bright field microscopy (Olympus, Germany) under 400× magnification using 100 squares of 0.25 mm2 each. The percentage of surface area occupied by acinar cells was determined from 2 different sections of each gland, and the results were reported as averages of triplicate per experimental condition.
Analysis of neutrophil infiltration was performed with immunofluorescence staining for myeloperoxidase (20). Four randomly selected fields of stained sections were photographed with a Zeiss LSM 510 confocal microscope (Zeiss, Germany). Myeloperoxidase-positive cell numbers and the number of DAPI-stained nuclei per field were recorded and used to evaluate the ratio of neutrophils to total cells.
Immunoblotting and cytokine array
Proteins were probed by use of mouse cytokine antibody arrays (Mouse Cytokine Antibody Array 3, Raybiotech, Norcross, GA) using homogenates from irradiated and control SMGs. All steps were performed according to the manufacturer's protocols. For protein detection, SMG ly-sates were used to load 20 μg protein on 18% SDS-PAGE gel (BioRad, Hercules, CA) and further detected with an anti-CXCL5 antibody (Abcam).
Statistical analysis
Statistical analysis was performed with GraphPad Prism software (GraphPad, San Diego, CA). The data were expressed as mean ± standard equivalent of the mean. Statistical analysis of variance and Student t tests (2-tailed) were used to compare the data. P<.05 was considered to be significant.
Results
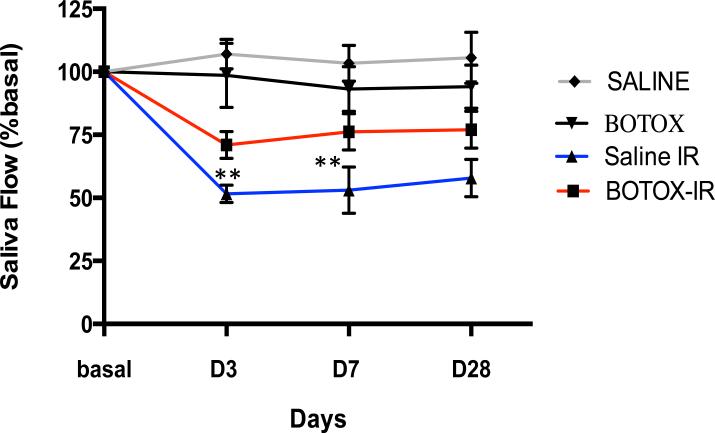
First, we examined the effect of BTX pretreatment on saliva flow measured from control and focally irradiated animals. Radiation caused a sharp decrease in saliva flow at 3 days without significant recovery of function after 28 days. By contrast, mice pretreated with BTX retained 75% of basal saliva flow during the same time frame (Fig. 1).
Fig. 1.
Botulinum toxin (Botox) injection rescues saliva flow after irradiation (IR). Stimulated saliva secretion measured before irradiation (basal) and 3, 7, and 28 days after irradiation of submandibular glands with 15 Gy: n=6/group (6 animals per time point). **P=.02.
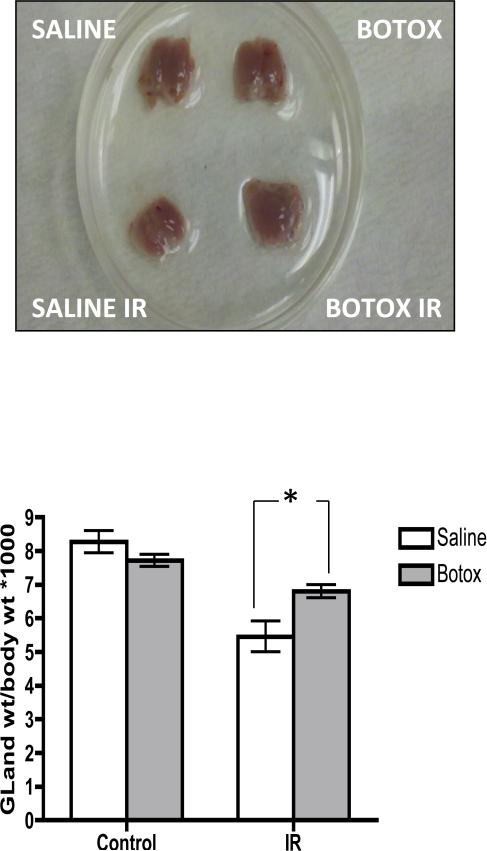
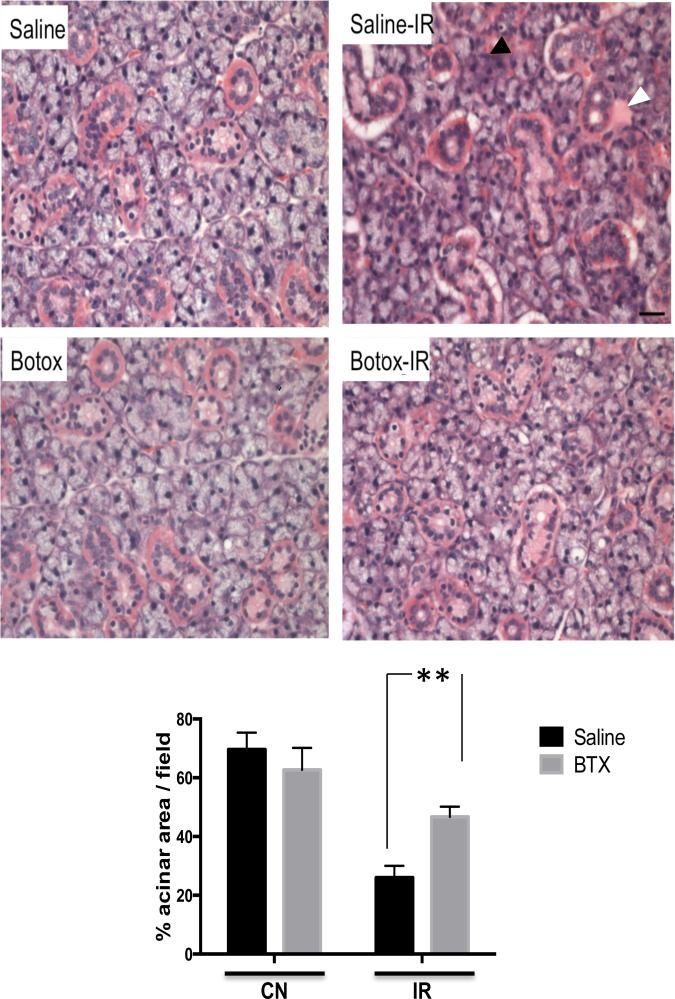
Irradiated salivary glands appeared atrophic and lost 30% of baseline weight. Interestingly, glands preinjected with BTX lost only 15% of basal weight (Fig. 2). Histologic changes were studied from each of the treatment groups 4 weeks after radiation injury by use of hematoxylin and eosin staining. As shown in Figure 3, irradiated SMGs in the control group showed marked acinar atrophy and periductal changes. Quantification of relative acinar surface area revealed a drop from 69% to 28% after irradiation. These histologic changes were less pronounced in the BTX preinjected glands, which showed 46% of relative acinar surface along with an interesting emergence of acinar vacuoles/autophagic vesicles (Fig. 3).
Fig. 2.
Botulinum toxin (Botox) injection protects from irradiation (IR)-induced submandibular gland (SMG) weight loss. SMG weights and total body weights were recorded at baseline and 4 weeks after irradiation. Representative images of gross SMG morphology are shown, and normalized SMG weights for all 4 treatment groups are plotted; n=4/group, *P<.05.
Fig. 3.
Botulinum toxin (Botox) injection mitigates irradiation (IR)-induced histologic changes. Tissue sections from submandibular glands were stained with hematoxylin and eosin at baseline and 4 weeks after radiation exposure. Degenerative changes with acinar atrophy (black arrowhead), periductal fibrosis (white arrowhead), and lymphocytic infiltrate are seen in the control (saline-IR) group. Botox-pretreated glands are markedly protected from acinar atrophy and periductal reaction (magnification ×40). Acinar area quantification revealed that botox preinjected glands contained significantly larger acinar areas compared with control (CN-saline) conditions; n=4/group, **P<.05.
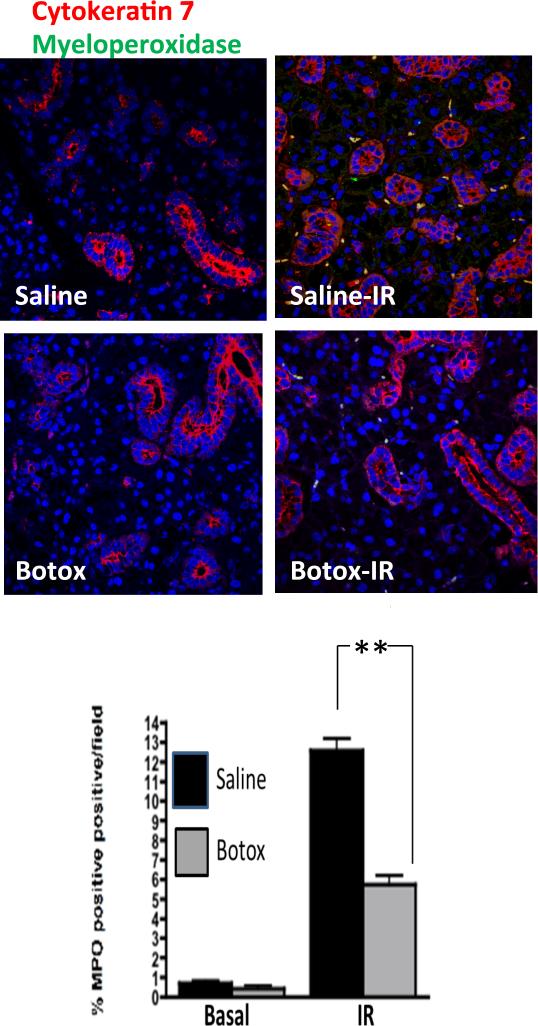
Prior studies have demonstrated acinar apoptosis as a key event during radiation-induced salivary gland damage (6, 8). To further investigate the protective role of BTX injection, we used TUNEL staining to evaluate apoptosis. There were 10% TUNEL positive cells detected after 15 Gy irradiation. No significant differences were observed after BTX pre-injection (data not shown). In search of an alternative mechanism, we evaluated early inflammatory responses after radiation injury. Irradiated glands demonstrated 12% to 13% myeloperoxidase-positive neutrophil infiltrate within 3 days of irradiation (Fig. 4). By contrast, BTX preinjected glands demonstrated significantly less neutrophil infiltrate (5%).
Fig. 4.
Attenuation of irradiation (IR)-induced neutrophil infiltration by botulinum toxin (Botox) injection. After focal irradiation (15 Gy), submandibular glands (SMGs) were removed after 3 days. SMG sections from glands preinjected with botox or saline were stained with cytokeratin 7 (red), myeloperoxidase (green), and DAPI (blue) and imaged by confocal microscopy. Percentage of myeloperoxidase positive cells was averaged over 3 experiments with 4 fields per slide analyzed (**P<.01). A color version of this figure is available at www.redjournal.org.
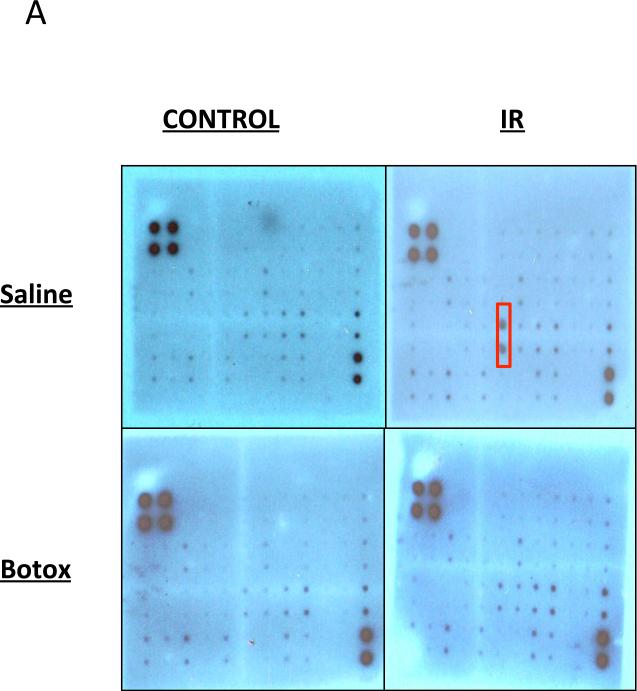
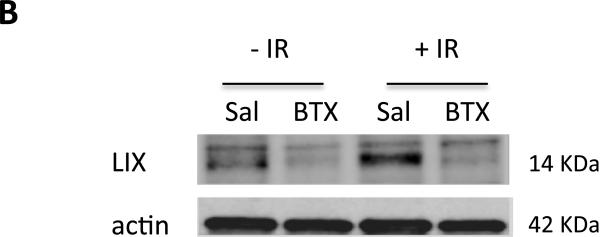
These results prompted us to further evaluate the differential cytokine responses. Using an array of 40 different inflammatory cytokines, we detected increased levels of the cytokines IL-3, IL-4, IL-6, RANTES, and LIX (CXCL5) in irradiated salivary glands homogenates (Fig. 5A and Fig. E1; available online at www.redjournal.org). Specifically, there was a 2-fold elevation in the chemokine LIX (CXCL5). This response was markedly attenuated in SMGs preinjected with BTX (Fig. 5A). Consistent findings were also obtained by immunoblotting using LIX-specific antibody (Fig. 5B). These results suggest that BTX suppresses chemokine levels and subsequent neutrophil infiltration after radiation injury.
Fig. 5.
Regulation of radiation-induced cytokine response by botulinum toxin (Botox). (A) Membranes from cytokine arrays of submandibular gland (SMG) specimens from glands preinjected with saline (Sal) or Botox. (B) Western blot for LIX/CXCL5 and actin (control) from SMG lysates. Abbreviation: IR = irradiation.
Discussion
Salivary glands are often damaged during definitive radiation therapy for head and neck cancers. This results in both acute and chronic side effects, including substantial reduction in saliva flow leading to xerostomia, a condition that negatively impacts patients’ quality of life (21). Over the past decade, work from our group and others started to uncover mechanisms of radiation induced salivary gland damage and potential mitigating therapies (15, 22, 23). In this study we identify a novel intervention by which pre-radiation injection of BTX reduces damage to murine salivary glands resulting in higher saliva flow compared to control conditions. Pretreatment with BTX was specifically associated with a lower level of the cytokine CXCL5 and a lower percentage of neutrophil infiltration after focal radiation.
The impact of BTX on salivary function has been reported in different clinical settings. Several studies have reported the successful use of BTX in the management of hypersalivation in specific neurologic and otolaryngologic disorders (24, 25). Furthermore, the injection of BTX into rat SMGs has been recently shown to attenuate radiation-induced glandular atrophy and periductal fibrosis (26). Therefore, we speculated that BTX pretreatment induces a dormant salivary state, which in turn could protect from radiation damage. Consistent with prior reports (10, 27), we detected a 50% decline in saliva flow within 3 days of radiation exposure. However, BTX-pretreated animals showed a reduction of only 25% in saliva flow rate compared with saline control. These results demonstrate a novel modulatory aspect of BTX on salivary function associated with radiation stress.
In recent years we have begun to further understand the mechanisms of salivary gland radiosensitivity. Epithelial apoptosis, stem cell injury, and lipid peroxidation are all potential mechanisms with supporting evidence (6, 12, 13, 16). Several studies support a role for cholinergic agents in modulating the radiation response of salivary glands. For instance, pilocarpine was shown to enhance the proliferation of acinar cells and in a phase 3 study improved parotid salivary flow in a subset of patients with head and neck cancer (28, 29). The current work shows an association of BTX treatment with a lower level of CXLC5 and intra-glandular neutrophils after radiation, suggesting an anti-inflammatory effect of BTX treatment. Indeed, BTX is well known to block the release of secretory neurovesicles at synapses. As such, BTX could be indirectly modulating CXCL5 by blocking the release of inflammatory neuropeptides. Interestingly, other investigators have reported on the anti-inflammatory role of BTX and inhibition of COX-2 (30).
The current study expands our understanding of the role of BTX in radiation mitigation. Encouraging results were reported by Stubblefield et al (31) using BTX in radiation fibrosis. Additional applications for BTX injections include management of genitourinary toxicities such as radiation cystitis (32) and proctitis (33). Furthermore, BTX is known to potentiate radiation therapy (34), which is desirable in a location such as the salivary glands, especially when nearby pathologic nodes are suspected.
Physicians often face cases in which excessive radiation doses are delivered to the parotid and SMG glands to target pathologic levels I and II lymph nodes. Our data provide preclinical support for a phase 1 trial using BTX injections in high-risk patients. In fact, head and neck surgeons have been injecting BTX successfully in even more difficult anatomic sites, such as the vocal cords (35). Favorable enrollment in such a study is anticipated, especially if dosimetric salvage of a specific salivary gland is not feasible, given its proximity to a high radiation dose region.
Although our data support a role for BTX in radioprotection of salivary glands, several questions remain unanswered. In the current study a single BTX injection was noted to be radioprotective; however, the optimal schedule and frequency of BTX injections remain to be elucidated. Another question relates to the mechanism of BTX action in this model. Our results suggest a relationship between BTX injection, CXCL5, and neutrophil infiltration. However, it remains to be elucidated whether these are direct or indirect consequences. Furthermore, it is unknown whether BTX modulates the survival of salivary stem cells, which have evolved as key targets for radiation injury. Our study also has its limitations and weaknesses. First, the radiation dose per fraction used (15 Gy in a single fraction), although experimentally convenient, is quite different from common clinical scenarios using 2 Gy per fraction. Assuming an α/β of 10, the isoeffective dose would be 32 Gy in 16 fractions. Second, our mouse model did not test for differential changes in parotid gland versus SMGs. This would be ideally investigated in a rat model, as previously reported (36). Third, the array used is limited in the number of cytokines detected, warranting further investigation of the significance of CXCL5. We hope to further test this finding using the CXCL5 knockout mouse model (37) and specific pharmacologic inhibitors against its receptor CXCR2 (38).
In summary, we successfully show that the direct injection of BTX into SMGs mitigates radiation-induced histologic changes and neutrophil infiltration. The current study is the first to show that BTX preinjection protects live animals from radiation-induced decline in stimulated saliva flow. A novel mechanism is proposed whereby BTX injection prevents intraglandular neutrophil infiltration through modulating the chemokine CXCL5. These pre-clinical results support the initiation of a phase 1 trial for head and neck cancer patients who are at high risk for the development of radiation-induced xerostomia.
Supplementary Material
Summary.
Injection of botulinum toxin mitigates several histologic and physiologic attributes of radiation damage of murine salivary glands. Mechanistically, botulinum toxin is shown to modulate levels of the cytokine LIX after radiation injury. These results have major implications for the management of xerostomia in patients with head and neck cancer.
Footnotes
Presented at the 55th annual meeting of the American Society for Radiation Oncology, Atlanta, Georgia, Sept 22-25, 2013.
Conflict of interest: none.
Supplementary material for this article can be found online at www.redjournal.org.
References
- 1.Ang KK, Chen A, Curran WJ, Jr, et al. Head and neck carcinoma in the United States: First comprehensive report of the longitudinal oncology registry of head and neck carcinoma (LORHAN). Cancer. 2012;118:5783–5792. doi: 10.1002/cncr.27609. [DOI] [PubMed] [Google Scholar]
- 2.Harari PM. Promising new advances in head and neck radiotherapy. Ann Oncol. 2005;16:vi13–vi19. doi: 10.1093/annonc/mdi453. [DOI] [PubMed] [Google Scholar]
- 3.Eisbruch A. Reducing xerostomia by IMRT: What may, and may not, be achieved. J Clin Oncol. 2007;25:4863–4864. doi: 10.1200/JCO.2007.13.4874. [DOI] [PubMed] [Google Scholar]
- 4.Eisbruch A, Rhodus N, Rosenthal D, et al. The prevention and treatment of radiotherapy-induced xerostomia. Semin Radiat Oncol. 2003;13:302–308. doi: 10.1016/S1053-4296(03)00027-4. [DOI] [PubMed] [Google Scholar]
- 5.Eisbruch A, Terrell JE. The relationships between xerostomia and dysphagia after chemoradiation of head and neck cancer. Head Neck. 2003;25:1082. doi: 10.1002/hed.10375. author reply 1082-1083. [DOI] [PubMed] [Google Scholar]
- 6.Avila JL, Grundmann O, Burd R, et al. Radiation-induced salivary gland dysfunction results from p53-dependent apoptosis. Int J Radiat Oncol Biol Phys. 2009;73:523–529. doi: 10.1016/j.ijrobp.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grundmann O, Fillinger JL, Victory KR, et al. Restoration of radiation therapy-induced salivary gland dysfunction in mice by post therapy IgF-1 administration. BMC Cancer. 2010;10:417. doi: 10.1186/1471-2407-10-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Limesand KH, Avila JL, Victory K, et al. Insulin-like growth factor-1 preserves salivary gland function after fractionated radiation. Int J Radiat Oncol Biol Phys. 2010;78:579–586. doi: 10.1016/j.ijrobp.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Limesand KH, Said S, Anderson SM. Suppression of radiation-induced salivary gland dysfunction by IgF-1. PLoS One. 2009;4:e4663. doi: 10.1371/journal.pone.0004663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin KL, Hill GA, Klein RR, et al. Prevention of radiation-induced salivary gland dysfunction utilizing a CDK inhibitor in a mouse model. PLoS One. 2012;7:e51363. doi: 10.1371/journal.pone.0051363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hai B, Yang Z, Shangguan L, et al. Concurrent transient activation of Wnt/beta-catenin pathway prevents radiation damage to salivary glands. Int J Radiat Oncol Biol Phys. 2012;83:e109–e116. doi: 10.1016/j.ijrobp.2011.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de la Cal C, Lomniczi A, Mohn CE, et al. Decrease in salivary secretion by radiation mediated by nitric oxide and prostaglandins. Neuroimmunomodulation. 2006;13:19–27. doi: 10.1159/000093194. [DOI] [PubMed] [Google Scholar]
- 13.Konings AW, Coppes RP, Vissink A. On the mechanism of salivary gland radiosensitivity. Int J Radiat Oncol Biol Phys. 2005;62:1187–1194. doi: 10.1016/j.ijrobp.2004.12.051. [DOI] [PubMed] [Google Scholar]
- 14.Banh A, Xiao N, Cao H, et al. A novel aldehyde dehydrogenase-3 activator leads to adult salivary stem cell enrichment in vivo. Clin Cancer Res. 2011;17:7265–7272. doi: 10.1158/1078-0432.CCR-11-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao N, Cao H, Chen CH, et al. A novel aldehyde dehydrogenase-3 activator (alda-89) protects submandibular gland function from irradiation without accelerating tumor growth. Clin Cancer Res. 2013;19:4455–4464. doi: 10.1158/1078-0432.CCR-13-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coppes RP, Stokman MA. Stem cells and the repair of radiation-induced salivary gland damage. Oral Dis. 2011;17:143–153. doi: 10.1111/j.1601-0825.2010.01723.x. [DOI] [PubMed] [Google Scholar]
- 17.Lombaert IM, Brunsting JF, Wierenga PK, et al. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS One. 2008;3:e2063. doi: 10.1371/journal.pone.0002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao N, Lin Y, Cao H, et al. Neurotrophic factor GDNF promotes survival of salivary stem cells. J Clin Invest. 2014;124:3364–3377. doi: 10.1172/JCI74096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knox SM, Lombaert IM, Haddox CL, et al. Parasympathetic stimulation improves epithelial organ regeneration. Nat Commun. 2013;4:1494. doi: 10.1038/ncomms2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pardo A, Barrios R, Gaxiola M, et al. Increase of lung neutrophils in hypersensitivity pneumonitis is associated with lung fibrosis. Am J Respir Crit Care Med. 2000;161:1698–1704. doi: 10.1164/ajrccm.161.5.9907065. [DOI] [PubMed] [Google Scholar]
- 21.Vainshtein JM, Moon DH, Feng FY, et al. Long-term quality of life after swallowing and salivary-sparing chemo-intensity modulated radiation therapy in survivors of human papillomavirus-related oropharyngeal cancer. Int J Radiat Oncol Biol Phys. 2015;91:925–933. doi: 10.1016/j.ijrobp.2014.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vissink A, van Luijk P, Langendijk JA, et al. Current ideas to reduce or salvage radiation damage to salivary glands. Oral Dis. 2015;21:e1–e10. doi: 10.1111/odi.12222. [DOI] [PubMed] [Google Scholar]
- 23.Grundmann O, Mitchell GC, Limesand KH. Sensitivity of salivary glands to radiation: From animal models to therapies. J Dent Res. 2009;88:894–903. doi: 10.1177/0022034509343143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steffen A, Hasselbacher K, Heinrichs S, et al. Botulinum toxin for salivary disorders in the treatment of head and neck cancer. Anticancer Res. 2014;34:6627–6632. [PubMed] [Google Scholar]
- 25.Montgomery J, McCusker S, Hendry J, et al. Botulinum toxin a for children with salivary control problems. Int J Pediatr Otorhinolaryngol. 2014;78:1970–1973. doi: 10.1016/j.ijporl.2014.08.041. [DOI] [PubMed] [Google Scholar]
- 26.Teymoortash A, Muller F, Juricko J, et al. Botulinum toxin prevents radiotherapy-induced salivary gland damage. Oral Oncol. 2009;45:737–739. doi: 10.1016/j.oraloncology.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 27.Coppes RP, Zeilstra LJ, Kampinga HH, et al. Early to late sparing of radiation damage to the parotid gland by adrenergic and muscarinic receptor agonists. Br J Cancer. 2001;85:1055–1063. doi: 10.1054/bjoc.2001.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burlage FR, Faber H, Kampinga HH, et al. Enhanced proliferation of acinar and progenitor cells by prophylactic pilocarpine treatment underlies the observed amelioration of radiation injury to parotid glands. Radiother Oncol. 2009;90:253–256. doi: 10.1016/j.radonc.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 29.Burlage FR, Roesink JM, Kampinga HH, et al. Protection of salivary function by concomitant pilocarpine during radiotherapy: A double-blind, randomized, placebo-controlled study. Int J Radiat Oncol Biol Phys. 2008;70:14–22. doi: 10.1016/j.ijrobp.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 30.Chuang YC, Yoshimura N, Huang CC, et al. Intravesical botulinum toxin a administration inhibits Cox-2 and EP4 expression and suppresses bladder hyperactivity in cyclophosphamide-induced cystitis in rats. Eur Urol. 2009;56:159–166. doi: 10.1016/j.eururo.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Stubblefield MD, Levine A, Custodio CM, et al. The role of botulinum toxin type a in the radiation fibrosis syndrome: A preliminary report. Arch Phys Med Rehabil. 2008;89:417–421. doi: 10.1016/j.apmr.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 32.Chuang YC, Kim DK, Chiang PH, et al. Bladder botulinum toxin a injection can benefit patients with radiation and chemical cystitis. BJU Int. 2008;102:704–706. doi: 10.1111/j.1464-410X.2008.07740.x. [DOI] [PubMed] [Google Scholar]
- 33.Vuong T, Waschke K, Niazi T, et al. Thevalue of botox-a in acute radiation proctitis: Results from a phase I/II study using a three-dimensional scoring system. Int J Radiat Oncol Biol Phys. 2011;80:1505–1511. doi: 10.1016/j.ijrobp.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 34.Ansiaux R, Baudelet C, Cron GO, et al. Botulinum toxin potentiates cancer radiotherapy and chemotherapy. Clin Cancer Res. 2006;12:1276–1283. doi: 10.1158/1078-0432.CCR-05-1222. [DOI] [PubMed] [Google Scholar]
- 35.Morzaria S, Damrose EJ. A comparison of the VHI, VHI-10, and VRQOL for measuring the effect of botox therapy in adductor spasmodic dysphonia. J Voice. 2012;26:378–380. doi: 10.1016/j.jvoice.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 36.Coppes RP, Vissink A, Konings AW. Comparison of radiosensitivity of rat parotid and submandibular glands after different radiation schedules. Radiother Oncol. 2002;63:321–328. doi: 10.1016/s0167-8140(02)00129-9. [DOI] [PubMed] [Google Scholar]
- 37.Mei J, Liu Y, Dai N, et al. Cxcl5 regulates chemokine scavenging and pulmonary host defense to bacterial infection. Immunity. 2010;33:106–117. doi: 10.1016/j.immuni.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Souza DG, Bertini R, Vieira AT, et al. Repertaxin, a novel inhibitor of rat cxcr2 function, inhibits inflammatory responses that follow intestinal ischaemia and reperfusion injury. Br J Pharmacol. 2004;143:132–142. doi: 10.1038/sj.bjp.0705862. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.