Abstract
AV7909 vaccine being developed for post-exposure prophylaxis of anthrax disease may require fewer vaccinations and reduced amount of antigen to achieve an accelerated immune response over BioThrax® (Anthrax Vaccine Adsorbed).
A phase 2, randomized, double-blind, BioThrax vacccine-controlled study was conducted to evaluate the safety and immunogenicity of three intramuscular vaccination schedules and two dose levels of AV7909 in 168 healthy adults. Subjects were randomized at a 4:3:2:4:2 ratio to 5 groups: 1) AV7909 on Days 0/14; 2) AV7909 on Days 0/28; 3) AV7909 on Days 0/14/28; 4) half dose AV7909 on Days 0/14/28; and 5) BioThrax vaccine on Days 0/14/28.
Vaccinations in all groups were well tolerated. The incidences of adverse events (AEs) were 79% for AV7909 subjects and 65% for BioThrax subjects; 92% of AV7909 subjects and 87% of BioThrax subjects having AEs reported Grade 1-2 AEs. No serious AEs were assessed as potentially vaccine-related, and no AEs of potential autoimmune etiology were reported. There was no discernible pattern indicative of a safety concern across groups in the incidence or severity of reactogenicity events.
Groups 2, 3, and 4 achieved success for the primary endpoint, demonstrated by a lower 95% confidence limit of the percentage of subjects with protective toxin neutralizing antibody NF50 values (≥ 0.56) to be ≥ 40% at Day 63. Group 1 marginally missed the criterion (lower bound 95% confidence limit of 39.5%). Immune responses were above this threshold for Groups 1, 3 and 4 at Day 28 and all groups at Day 42.
Further study of an AV7909 two-dose schedule given 2 weeks apart is warranted in light of the favorable tolerability profile and immunogenicity response relative to three doses of BioThrax vaccine, as well as preliminary data from nonclinical studies indicating similar immune responses correlate with higher survival for AV7909 than BioThrax vaccine.
Keywords: BioThrax® (Anthrax Vaccine Adsorbed), vaccine, anthrax, CPG 7909, post-exposure prophylaxis
1. INTRODUCTION
The combination of BioThrax® (Anthrax Vaccine Adsorbed [AVA]) and CPG 7909 (a synthetic immunostimulatory oligonucleotide), known as AV7909, is being clinically investigated for its potential to achieve an accelerated immune response for protection against anthrax disease in a post-exposure prophylaxis (PEP) setting. A 3-dose series of BioThrax vaccine administered subcutaneously (SC) at 0, 2, and 4 weeks alongside a 60-day antimicrobial regimen is approved by the United States (US) Food and Drug Administration (FDA) and recommended by the Advisory Committee on Immunization Practices for PEP of anthrax [1]. Compliance with the antimicrobial regimen has been problematic: less than 50% of exposed individuals were compliant following the 2001 anthrax attacks in the US [2]. An enhanced anthrax vaccine including CPG 7909 may require fewer vaccine doses and reduced amount of antigen, potentially promoting compliance with the full vaccine schedule while providing a greater probability of survival. Earlier protection may reduce the duration of antimicrobials required.
Two phase 1 trials exploring the AV7909 vaccine have been completed in 174 healthy adults. In the initial trial, an admixture prior to injection of 0.5 mL BioThrax vaccine + 1 mg CPG 7909 significantly increased serum levels of anti-protective antigen (PA) antibody and toxin neutralizing antibody (TNA) compared with BioThrax vaccine alone, including at a much earlier time point, by at least 3 weeks [3]. Vaccination was well tolerated with a trend toward increased frequency and severity of local and systemic reactions in the BioThrax vaccine + CPG 7909 group. The second trial identified a formulation of AV7909 for further clinical development (0.5 mL BioThrax vaccine + 0.25 mg CPG 7909) that produced an enhanced immune response without increased reactogenicity [4]. This formulation was evaluated in the phase 2 trial described here.
The primary objectives of the current phase 2 trial were to assess safety of the selected AV7909 formulation and assess immunogenicity measured as TNA 50% neutralization factor (NF50) at Day 63. As a secondary objective, immunogenicity at earlier time points was also assessed (Days 28, 42).
2. MATERIALS AND METHODS
2.1. Investigational products
All vaccines were administered intramuscularly (IM) in the deltoid muscle using a 25-gauge sterile needle and 1 cc syringe. Alternating arms were used for each successive injection. Refrigeration within the range of 2-8°C was required for the AV7909 and AVA vaccines.
AVA, manufactured by Emergent BioSolutions Inc. (Lansing, Michigan, US) and previously described [4], is a sterile, milky-white suspension made from cell-free filtrates of microaerophilic cultures of an avirulent, nonencapsulated strain of Bacillus anthracis. Commercial lot FAV392A was supplied for the study. A single vaccine dose was 0.5 mL.
AV7909 drug product, a pre-formulated, sterile, milky-white suspension manufactured by Par Pharmaceutical (Rochester, Michigan, US), is composed of AVA bulk drug substance and CPG 7909 adjuvant. Approximately 6.2 mL of AV7909 vaccine was supplied in glass vials as a multidose final drug product. Each full dose contained 0.5 mL AVA + 0.25 mg CPG 7909 and each half-dose contained 0.25 mL AVA + 0.125 mg CPG 7909. Lot TC2994 was used in the study.
Placebo of 0.5 mL of sterile, colorless, preservative-free saline for injection (0.9% sodium chloride) was administered in Groups 1 and 2 to mask the dosing schedule.
2.2. Study design
This was a phase 2, multicenter, randomized, parallel-group, active-controlled, double-blind study evaluating the safety and immunogenicity of AV7909 for PEP of anthrax disease in 168 subjects recruited at four clinical research centers in the US. Eligible subjects were aged 18-50 years and in general good health, having no previous history of exposure to anthrax or anthrax vaccine and no chronic conditions or exposure to products that may have biased an evaluation of the immune response.
Subjects were allocated to treatment groups according to a computer-generated randomization list prepared by an independent, unblinded statistician. An unblinded site staff member accessed the interactive web response system to receive a printout with the treatment assignment, which was subsequently secured in a limited-access area to prevent accidental unblinding. Subjects were randomized using a 4:3:2:4:2 ratio in blocks of 15 to 1 of 5 groups comprising 3 IM vaccination schedules and 2 dose levels: 1) full dose AV7909 on Days 0/14; 2) full dose AV7909 on Days 0/28; 3) full dose AV7909 on Days 0/14/28; 4) half dose AV7909 on Days 0/14/28; and 5) full dose BioThrax vaccine on Days 0/14/28. Randomization was stratified to ensure at least 40% of subjects per group were of each gender and that equal proportions of subjects were between 18-30 or 31-50 years of age.
Procedures were instituted to ensure investigators, all staff performing subject assessments, and all subjects remained blinded to treatment assignment. Vaccine preparation and administration were performed by unblinded site personnel. All syringes were covered during vaccine administration, leaving only the hub exposed.
The BioThrax immunization regimen used in this study has been shown to achieve circulating TNA titers that have conferred at least 70% survival to rabbits and non-human primates after B. anthracis spore challenge [5, 6].
The trial was conducted in accordance with Good Clinical Practice and ethical principles that have their origin in the Declaration of Helsinki. Informed consent was obtained from subjects at Screening after informing them about the study and associated risks. Safety oversight was supplemented by an independent safety monitoring committee (SMC) consisting of 3 physicians, and also independent safety monitors (ISMs). Interim safety data were reviewed by the SMC after 50 subjects and 100 subjects had completed the Day 14 visit. No safety concerns were identified during these reviews.
2.3. Immunogenicity assessment
Serum samples for determination of immunogenicity were collected on Days 0 (before vaccination), 21, 28 (before vaccination), 35, 42, 49, 63, and 84. Samples were tested using a validated, high-throughput, cell-based bioassay by Battelle Memorial Institute (West Jefferson, Ohio, US) to measure neutralization of anthrax lethal toxin (LT) by antibodies generated in response to vaccination. An NF50 value was calculated as the ratio of effective dose that provided 50% neutralization (ED50) of the human test sample to ED50 of a human reference serum from subjects vaccinated with BioThrax vaccine. The reference standard, AVR801, was the same used in the prior clinical studies of AV7909 [3, 4].
The primary immunogenicity outcome was the lower bound of the 95% conference interval (CI) for the proportion of subjects in each group with Day 63 NF50 values ≥ 0.56, the threshold of 70% protection established for BioThrax vaccine. Secondary outcomes also focused on an evaluation of immunogenicity based on proportion of subjects meeting or exceeding the NF50 threshold of protection at earlier time points (at Day 28 in Groups 1, 3, and 4; at Day 42 in all groups).
2.4. Safety assessment
Subjects were evaluated for safety at clinic visits from Day 0 to Day 84 and also by phone contacts occurring 6 months and 12 months after the last vaccination.
Safety was assessed through Day 84 by clinical laboratory test results graded against a modified version of the FDA’s toxicity grading scale [7], monitoring of adverse events (AEs; including serious AEs [SAEs]; and AEs of special interest [AESI], defined as having a potential autoimmune etiology), vital signs, and physical examinations. Serum samples were collected on Days 0 (before vaccination), 42, and 84 for autoantibody testing (anti-nuclear antibodies and rheumatoid factor) if any subjects reported AESIs during the study. Notably, AEs were recorded for Grade 3 or higher laboratory test abnormalities, any clinical laboratory or vitals sign abnormalities considered clinically significant by the investigator, and any new or changed-in-severity abnormal physical examination finding after the first vaccination.
Reactogenicity (ie, solicited injection site and systemic reactions) was assessed and reported by subjects using e-diaries for 7 days after each vaccination, or longer if reactions persisted beyond 7 days; and assessed and reported by the investigator at clinic visits 7 and 14 days after each vaccination. Events classified as Grade 3 in the e-diary prompted a follow-up telephone call from the physician and decision for an unscheduled visit for assessment of the injection site, if needed. Reactogenicity reactions were not also recorded as AEs.
Any subject who discontinued vaccinations was encouraged to continue safety follow up by attending subsequent visits through Day 84, and also asked to participate in the 6- and 12-month safety follow-up telephone calls.
2.5. Statistical methods and study populations
Descriptive analyses were performed to demonstrate demographic distribution, as well as vaccine immunogenicity and safety. Continuous variables were summarized using number of non-missing observations (n), arithmetic or geometric mean where appropriate, median, standard deviation, minimum, maximum, and 95% confidence interval (CI). Categorical variables were summarized using the frequency count and the percentage of subjects in each category as well as its exact Binomial 95% confidence interval. No imputation was made for missing values. No confidence level was adjusted for multiple 95% CIs. All statistical tabulations and analyses were performed using SAS®, version 9.1.3.
Demographic analyses were conducted for the intent-to-treat (ITT) population, defined as all subjects who were randomized to one of the five groups.
Immunogenicity analyses were conducted on the per protocol (PP) population, defined as subjects who were randomized and did not have any of the following deviations: a) missing or out of window vaccination visit at Day 14 or 28; b) incorrect dose of vaccine at one or more visits; c) vaccine dose associated with a temperature excursion prior to administration at any visit; d) use of prohibited or restricted medications prior to Day 63 which may have impacted immune response to vaccination; and e) missing immunogenicity data at Day 63. For the primary analysis at Day 63 and the secondary analyses at Day 28 and Day 42, it was hypothesized that the proportion of subjects with TNA NF50 above 0.56 in each group was at least 40%. This hypothesis was tested with an exact Binomial two-sided confidence interval approach. Subjects who had missing immunogenicity values were not counted in the denominator.
Safety analyses were conducted on the safety population, defined as all subjects who had received at least one vaccination. Adverse events were coded using the Medical Dictionary for Regulatory Activities®, version 15.1. Missing data were included in the denominator for subjects who missed e-diary or laboratory assessments.
Assuming the true proportion of subjects that reach a TNA NF50 value of at least 0.56 is 80% on Day 63 (supported by unpublished data from Study EBS.AVA.201 [4]) and assuming a 10% dropout rate, a sample size of 44 subjects (Group 1), 34 subjects (Group 2), and 23 subjects (Group 3) provided > 99.0% power (Group 1) and at least 96% power (Groups 2, 3) to obtain a two-sided 95% CI with lower limit ≥ 40%.
3. RESULTS
3.1. Subject disposition and demographics
Trial recruitment initiated in January 2013 and completed as per the protocol in November 2013. The last subject completed the final visit in February 2014 and the 12-month safety follow-up phone contact after the last vaccination in December 2014.
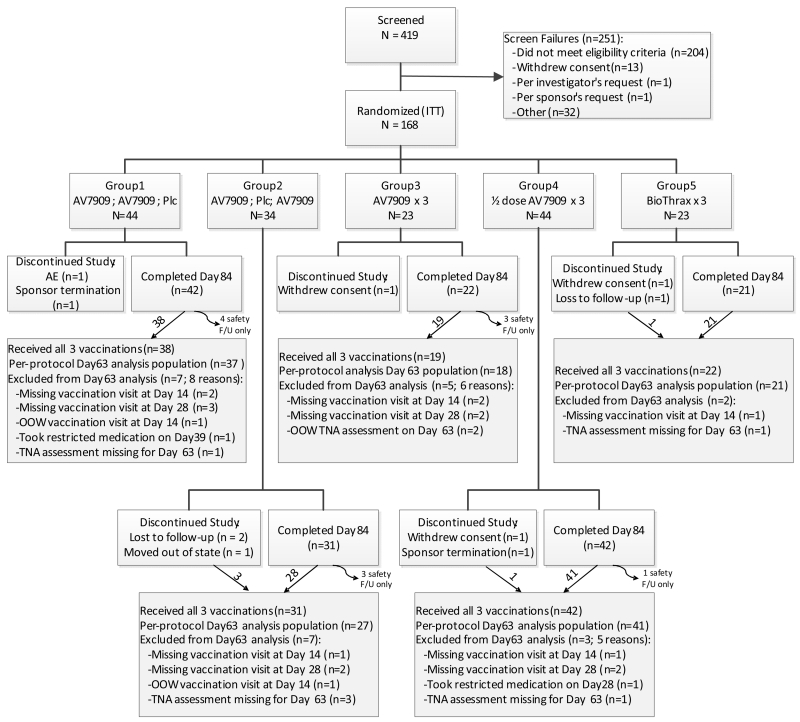
Of 419 screened subjects, 168 met eligibility requirements and were randomized to one of the 5 groups. All 168 subjects received at least one vaccination, hence were included in the safety population. A total 158 (94.0%) subjects completed through Day 84 (Figure 1). Because subjects could be followed for safety after discontinuing vaccinations for any reason, not all the completer subjects received all vaccinations: 147 (87.5%) subjects completed Day 84 having received all vaccinations, whereas 11 (6.5%) subjects discontinued vaccinations while still remaining in the study through Day 84.
Figure 1.
Subject Disposition Through Day 84.
F/U=follow up; ITT=intent to treat; OOO=out of window; Plc=placebo; TNA=toxin neutralizing antibody. Subjects were randomized to 1 of 5 study groups at a 4:3:2:4:2 ratio. One-hundred sixty-eight subjects were randomized and received at least one vaccination; 158 subjects completed the in-clinic visits through Day 84; and 147 subjects completed the in-clinic visits through Day 84 and received all 3 vaccinations.
Ten (6.0%) subjects discontinued prior to Day 84 (Figure 1). Five subjects withdrew having received all 3 vaccinations (3 were lost to follow up; 1 was withdrawn by the sponsor; and 1 moved out of state) and 5 withdrew without receiving all 3 vaccinations (3 withdrew consent, 1 experienced an SAE [cellulitis secondary to animal bite], and 1 was withdrawn by the sponsor).
Subjects were equally distributed by age and gender and other baseline characteristics across the treatment groups (Table 1). The population was predominantly white (91.7%) followed by Black or African American (7.1%) and of non-Hispanic/Latino descent (82.7%).
Table 1. Subject Demographics and Baseline Characteristics – ITT Population.
| Characteristic Parameter |
AV7909; AV7909;Plc (N=44) |
AV7909;Plc; AV7909 (N=34) |
AV7909 × 3 (N=23) |
½ dose AV7909 × 3 (N=44) |
BioThrax × 3 (N=23) |
|---|---|---|---|---|---|
| Gender, n (%) | |||||
| Male | 22 (50.0) | 17 (50.0) | 12 (52.2) | 22 (50.0) | 12 (52.2) |
| Female | 22 (50.0) | 17 (50.0) | 11 (47.8) | 22 (50.0) | 11 (47.8) |
| Age (years) | |||||
| Mean/Median | 33.4/30.5 | 32.7/30.0 | 29.7/29.0 | 32.8/30.5 | 32.5/30.0 |
| SD | 9.02 | 9.66 | 10.22 | 9.21 | 10.37 |
| Min-Max | 20-50 | 18-48 | 18-50 | 19-50 | 18-48 |
| Age Group, n (%) | |||||
| ≤ 30 years | 22 (50.0) | 18 (52.9) | 12 (52.2) | 22 (50.0) | 12 (52.2) |
| >30 years | 22 (50.0) | 16 (47.1) | 11 (47.8) | 22 (50.0) | 11 (47.8) |
| Weight (kg) | |||||
| Mean/Median | 85.4/82.5 | 79.8/75.2 | 86.7/80.0 | 82.9/78.0 | 87.1/81.0 |
| SD | 22.8 | 20.2 | 26.3 | 19.6 | 18.3 |
| Min- Max | 56.0-145.0 | 43.6-125.0 | 52.8-173.0 | 51.0-129.0 | 60.0-133.0 |
| Race, n (%) | |||||
| White | 40 (90.9) | 33 (97.1) | 20 (87.0) | 40 (90.9) | 21 (91.3) |
| Black or African American |
3 (6.8) | 1 (2.9) | 3 (13.0) | 3 (6.8) | 2 (8.7) |
| Othera | 1 (2.3) | 0 | 0 | 1 (2.3) | 0 |
| Ethnicity, n (%) | |||||
| Not Hispanic or Latino |
35 (79.5) | 27 (79.4) | 19 (82.6) | 39 (88.6) | 19 (82.6) |
| Hispanic or Latino |
9 (20.5) | 7 (20.6) | 4 (17.4) | 5 (11.4) | 4 (17.4) |
Plc=placebo; ITT=intent-to-treat; max=maximum; min=minimum.
AV7909 full dose: 0.5 mL (0.5 mL AVA + 0.25 mg CPG 7909); AV7909 half dose: 0.25 mL (0.25 mL AVA + 0.125 mg CPG 7909). BioThrax dose: 0.5 mL. All doses were injected intramuscularly in the deltoid on Days 0, 14, 28.
Other includes Asian (n=1) and Native Hawaiian or Other Pacific Islander (n=1).
3.2. Safety
3.2.1. Adverse events
During the in-clinic portion of the study, two subjects reported SAEs requiring hospitalization. Following vaccination 2, a subject in Group 1 was withdrawn after developing cellulitis secondary to an animal bite. After receiving all vaccinations, a subject in Group 5 experienced pyelonephritis. Both events were assessed by the investigator as being unrelated to the vaccine.
After Day 84, an additional 2 SAEs were reported. One SAE was reported for the infant of a subject (Group 1) who became pregnant after birth control restrictions were lifted. The subject delivered at 36 weeks via precautionary cesarean section because of a bicornuate uterus and previous cesarean section history. The infant’s complication of neonatal atelectasis requiring continuous positive airway pressure was considered unrelated to the vaccine since this condition is not uncommon in premature births. A separate SAE of rectal neoplasm was reported at the final safety follow-up phone contact for the subject who also reported cellulitis. The event was assessed by the investigator as being unrelated to the vaccine.
No AESIs of potential autoimmune etiology were reported up through the last safety follow-up phone contact.
The incidences of AEs and, as assessed by the investigator, potentially treatment-related AEs were lower in the BioThrax group in comparison with the AV7909 treatment groups. The highest incidence of AEs was in Group 1 (36/44; 81.8%), followed by Group 4 (35/44; 79.5%), Group 2 (26/34; 76.5%), Group 3 (17/23; 73.9%), and Group 5 (15/23; 65.2%). The common AEs occurring in 5% or more of subjects in any group are provided in Table 2.
Table 2. Common Adverse Events Occurring in ≥ 5% of Subjects in any Study Group - Safety Population.
| MedDRA System Organ Class Preferred Term |
AV7909; AV7909;Plc (N=44) n (%) |
AV7909;Plc; AV7909 (N=34) n (%) |
AV7909 × 3 (N=23) n (%) |
½ dose AV7909 × 3 (N=44) n (%) |
BioThrax × 3 (N=23) n (%) |
|---|---|---|---|---|---|
|
Subjects with any
adverse event |
36 (81.8) | 26 (76.5) | 17 (73.9) | 35 (79.5) | 15 (65.2) |
| Cardiac Disorders | 3 (6.8) | 1 (2.9) | 2 (8.7) | 5 (11.4) | 0 |
| Bradycardia | 2 (4.5) | 0 | 1 (4.3) | 5 (11.4) | 0 |
|
Infections and
Infestations |
19 (43.2) | 13 (38.2) | 5 (21.7) | 15 (34.1) | 4 (17.4) |
| Nasopharyngitis | 7 (15.9) | 3 (8.8) | 0 | 2 (4.5) | 1 (4.3) |
| Upper respiratory tract infection |
10 (22.7) | 5 (14.7) | 2 (8.7) | 7 (15.9) | 2 (8.7) |
| Urinary tract infection | 0 0 | 1 (2.9) | 2 (8.7) | 1 (2.3) | 0 |
| Viral infection | 1 (2.3) | 2 (5.9) | 0 | 2 (4.5) | 0 |
| Vulvovaginal mycotic infection |
0 | 0 | 0 | 0 | 2 (18.2)a |
| Investigations | 19 ( 43.2) | 13 (38.2) | 9 (39.1) | 19 (43.2) | 7 (30.4) |
| Aspartate aminotransferase increased |
2 (4.5) | 2 (5.9) | 1 (4.3) | 1 (2.3) | 0 |
| Blood pressure diastolic decreased |
5 ( 11.4) | 3 (8.8) | 2 (8.7) | 6 (13.6) | 0 |
| Blood pressure increased |
3 (6.8) | 0 | 0 | 0 | 0 |
| Heart rate decreased | 1 (2.3) | 4 (11.8) | 0 | 1 (2.3) | 2 (8.7) |
| Protein urine present | 0 | 0 | 0 | 0 | 2 (8.7) |
| Red blood cells urine positive |
2 (4.5) | 0* | 0 | 0* | 4 (17.4) |
| Respiratory rate increased |
6 (13.6) | 2 (5.9) | 4 (17.4) | 10 (22.7) | 2 (8.7) |
|
Musculoskeletal and
Connective Tissue Disorders |
3 (6.8) | 5 (14.7) | 2 (8.7) | 5 ( 11.4) | 0 |
| Back pain | 2 (4.5) | 2 (5.9) | 0 | 2 (4.5) | 0 |
|
Respiratory, Thoracic
and Mediastinal Disorders |
3 (6.8) | 5 (14.7) | 3 (13.0) | 2 (4.5) | 3 (13.0) |
| Oropharyngeal pain | 1 (2.3) | 2 (5.9) | 1 (4.3) | 2 (4.5) | 0 |
| Pharyngeal erythema | 0 | 0 | 2 (8.7) | 0 | 0 |
MedDRA=Medical Dictionary for Regulatory Activities; Plc=placebo.
Adverse events were assessed at every study visit after signing informed consent from Screening through Day 84.
All adverse events occurring prior to receipt of investigational product are excluded from this table.
Adverse events were coded using MedDRA version 15.1.
The denominator for this gender-specific AE term is the number of women.
P < 0.05 for comparison with the BioThrax group (Fisher’s Exact test; analysis performed post hoc).
Vaccinations in all groups were well-tolerated; 92.1% (105/114) of AV7909 subjects and 86.7% of BioThrax subjects (13/15) having AEs reported Grade 1 or 2 AEs. Nine AEs led to vaccine discontinuation in seven (7/168; 4.2%) subjects across Groups 1, 2, and 3, a proportionally higher number in Group 1 (5/44; 11.4%) compared with Group 2 (1/34; 2.9%) or Group 3 (1/23; 4.3%). Six of the nine AEs that led to discontinuation of vaccination were assessed as related to the vaccine, involving 4 subjects with Grade 1 generalized pruritus and rash (Group 2), Grade 2 rash (Group 1), Grade 2 elevated transaminases (Group 1), and Grade 3 injection site erythema (Group 3).
No clinically relevant differences among groups in the type or incidence of vital signs or clinical laboratory or physical exam abnormalities were observed.
3.2.2. Injection site and systemic reactogenicity (e-diary data)
Reactogenicity data represent both the common and expected reactions with vaccination. Self-reported e-diary data were considered more relevant than the in-clinic reactogenicity physician assessments because self-reports occurred soon after each injection and were more frequent. Eighteen e-diary temperature measurements out of over 3500 planned assessments were taken or recorded improperly by subjects and excluded from analysis as they fell outside the range of clinically plausible values.
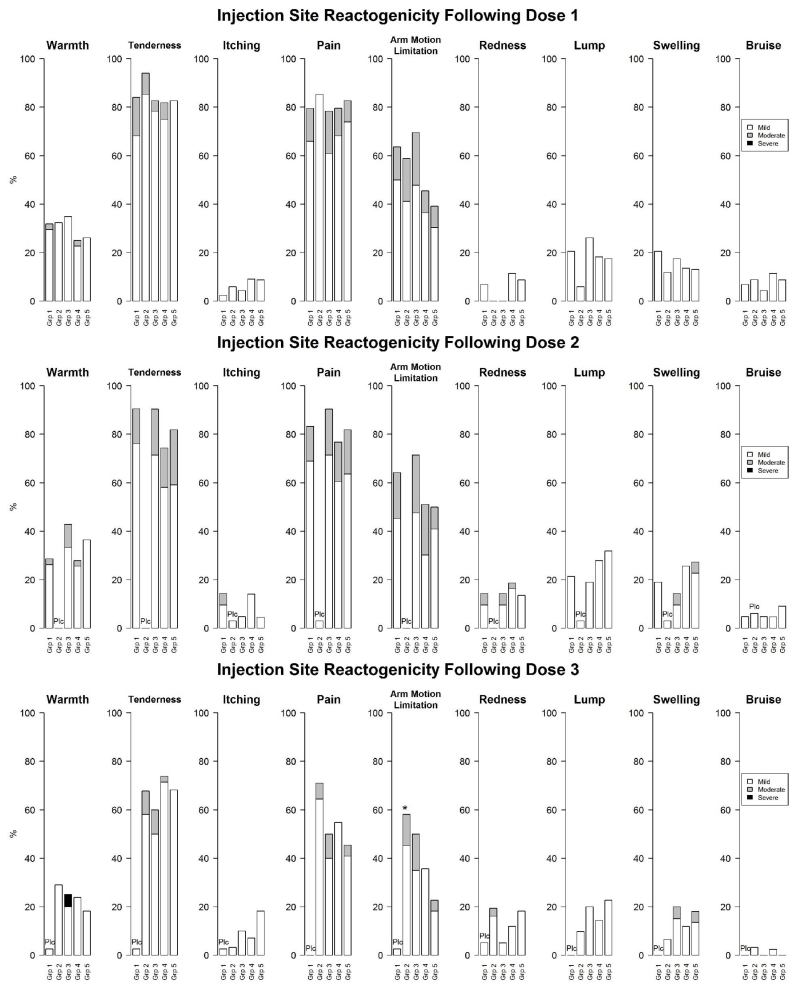
One subject (Group 3) discontinued treatment as a result of reactogenicity: Grade 3 injection site erythema of diameter > 13 cm. The systemic reactions and injection site reactions are summarized by vaccination and severity in Figure 2. Over half of subjects overall reported tenderness, injection site pain, arm motion limitation, and muscle ache after the first vaccination. After the third vaccination, only tenderness was reported in more than half of subjects overall.
Figure 2.
a. Reactogenicity After Each Vaccination (e-Diary Data) - Solicited Systemic Reactions.
Grp=group. “Plc” appearing over the treatment group denotes a placebo dose (saline for injection) was administered to mask the vaccine schedule.
Group 1=full dose AV7909 0/14 days; Group 2=full dose AV7909 0/28 days; Group 3=full dose AV7909 0/14/28 days; Group 4=half dose AV7909 0/14/28 days; Group 5=full dose BioThrax 0/14/28 days. All doses were administered intramuscularly.
Three subjects reported severe events later downgraded to moderate after discussion with the investigator: fatigue/tiredness and headache after vaccination 2 in Group 1; headache after vaccination 2 in Group 1; fatigue/tiredness and muscle ache after vaccination 2 in Group 1 (corrected data shown in the figure).
Eighteen temperature values were excluded from analysis because of clinical infeasibility (2 improbably high, 16 improbably low).
*P < 0.05 for comparison with Group 5 (Fisher’s Exact test; analysis performed post hoc). Inferential statistics were not performed in comparison to placebo.
b. Reactogenicity After Each Vaccination (e-Diary Data) - Solicited Injection Site Reactions.
Grp=group. “Plc” appearing over the treatment group denotes a placebo dose (saline for injection) was administered to mask the vaccine schedule.
Group 1=full dose AV7909 0/14 days; Group 2=full dose AV7909 0/28 days; Group 3=full dose AV7909 0/14/28 days; Group 4=half dose AV7909 0/14/28 days; Group 5=full dose BioThrax 0/14/28 days. All doses were administered intramuscularly.
One subject in Group 1 reported events after vaccination 2 of severe pain and severe arm motion limitation later downgraded to moderate after discussion with the investigator (corrected data shown in the figure).
*P < 0.05 for comparison with Group 5 (Fisher’s Exact test; analysis performed post hoc). Inferential statistics were not performed in comparison to placebo.
The vast majority of subjects reported reactogenicity events that were mild or moderate in severity (157/168 [93.5%] for injection site reactions and 140/168 [83.3%] for systemic reactions). One subject (Group 3) reported a Grade 3 injection site reaction of warmth after the third vaccination. Five subjects reported Grade 3 systemic reactions: 4 subjects in Group 1 (fever after vaccination 1; fatigue/tiredness, muscle ache, and headache after vaccination 1; headache after vaccination 1; and fever after vaccination 3) and 1 subject in Group 3 (fatigue/tiredness after vaccination 2). Three subjects downgraded their event reports from Grade 3 to Grade 2 after discussing their symptoms with investigators. There was no discernible pattern regarding incidence or severity of reactogenicity events that indicated a safety concern for any of the groups.
3.3. Immunogenicity
Across all groups, reasons for exclusion from analysis in the PP population (N=144 out of 168 randomized subjects) predominantly involved missing vaccination visits and missing TNA assessments (refer to Figure 1 for exclusion reasons).
Peak immune responses reflected immunization schedules (refer to Figure 3). Highest GMT peak based upon TNA NF50 occurred after two administrations of AV7909 on Days 0/28 (Group 2; 5.30; 95% CI: 4.09, 6.86), which was slightly greater than after three administrations on Days 0/14/28 (Group 3; 4.06; 95% CI: 2.52, 6.53). Other schedules had lower peak responses: Group 4 (2.40; 95% CI: 1.91, 3.02), Group 1 (1.83; 95% CI: 1.32, 2.53), and Group 5 (1.17; 95% CI: 0.74, 1.84). Notably, AV7909 administered on Days 0/14 (Group 1) resulted in a higher immune response on Day 28 compared with BioThrax IM vaccine administered on the same schedule (Group 5).
Figure 3.
TNA ED50 and NF50 Geometric Mean Titers and 95% Confidence Intervals, Per Protocol Day 63 Population.
CI=confidence interval; ED50=50% effective dose; GMT=geometric mean titer; IM=intramuscular; NF50=50% neutralization factor; TNA=toxin neutralizing antibody.
The Per Protocol Population at Day 63 is all randomized subjects who did not have any deviation of 1) history of anthrax vaccination; 2) missing or out of window vaccination at Day 14 or 28; 3) incorrect vaccine dose at one or more visits; 4) vaccine dose associated with a temperature excursion; 5) prohibited medications; or 6) missing Day 63 immunogenicity data.
The horizontal line represents a TNA NF50 threshold of 0.56, established for BioThrax at Day 63.
The numbers of subjects analyzed are as follows:
Day 63: Group 1, 37 subjects; Group 2, 27 subjects; Group 3, 18 subjects; Group 4, 41 subjects; and Group 5, 21 subjects.
Day 28: Group 1, 37 subjects; Group 2, 27 subjects; Group 3, 18 subjects; Group 4, 41 subjects; and Group 5, 21 subjects.
Day 42: Group 1, 37 subjects; Group 2, 26 subjects; Group 3, 18 subjects; Group 4, 41 subjects; and Group 5, 20 subjects.
In addition, the time point at which peak immunity was reached reflected immunization schedules. Group 2 (vaccinated Days 0/28) reached peak NF50 values on Day 42 compared with Day 35 for Group 3 (vaccinated Days 0/14/28). Although the peak was lower, Group 1 reached peak NF50 values on Day 28, which were equivalent to non-peak NF50 values of Group 3 at the same time point.
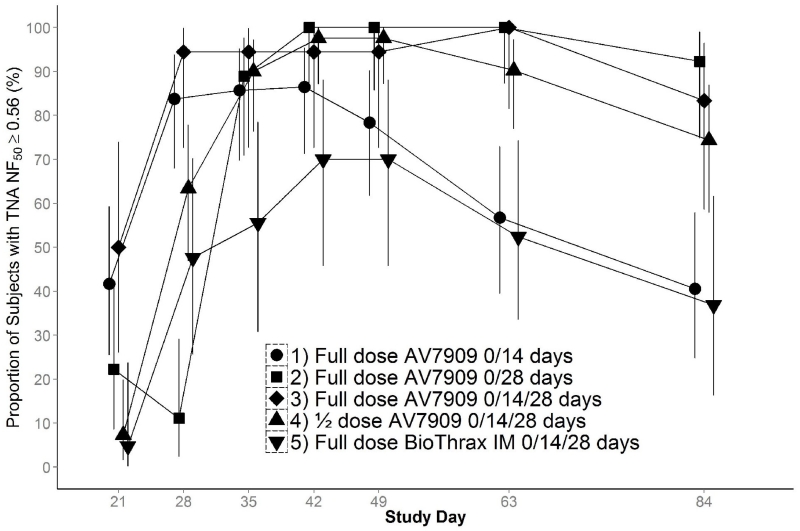
For the primary immunogenicity analysis, success was defined as the demonstration of the lower 95% confidence limit of the percentage of subjects with TNA NF50 values ≥ 0.56 to be ≥ 40% at Day 63. Accordingly, the study-defined success criterion was met at Day 63 in Groups 2 (point estimate 100%; lower limit 87.2%), 3 (point estimate 100%; lower limit 81.5%), and 4 (point estimate 90.2%; lower limit 76.9%). Group 1 marginally missed the criterion, with a point estimate of 56.8% and lower 95% confidence limit 39.5%. The same success criterion was achieved for Groups 1, 3 and 4 at Day 28 and all groups at Day 42 (see Figure 4).
Figure 4.
Proportion of Subjects with TNA NF50 ≥ 0.56, Per Protocol Day 63 Population.
The Per Protocol Population at Day 63 is all randomized subjects who did not have any deviation of 1) history of anthrax vaccination; 2) missing or out of window vaccination at Day 14 or 28; 3) incorrect vaccine dose at one or more visits; 4) vaccine dose associated with a temperature excursion; 5) prohibited medications; or 6) missing Day 63 immunogenicity data.
The numbers of subjects analyzed are Group 1, 37 subjects; Group 2, 27 subjects; Group 3, 18 subjects; Group 4, 41 subjects; and Group 5, 21 subjects.
Success was pre-defined as the lower bound of the 95% confidence limit being ≥ 40% at Day 63.
4. DISCUSSION
AV7909 vaccine is expected to achieve, compared with BioThrax vaccine, higher serum TNA levels, an accelerated immune response, and fewer injections to confer protection. This would potentially result in shorter courses of antimicrobials, hence reduced side effects and non-compliance. An additional goal is to reduce the amount of each vaccine component needed to establish protective immunity in a post-exposure setting. The results of this study are broadly applicable to a relatively young (≤ 50 years), mostly white, healthy adult population. The primary outcome was based upon achieving serum immune responses at Day 63 that meet or exceed the threshold of protection derived from previously conducted animal studies for BioThrax vaccine [6].
No autoimmune-related AEs were reported and otherwise no new safety signals were identified with use of the CpG-adjuvanted vaccine. Subjects reported more AEs in the AV7909 groups vs. the BioThrax group. However, no pattern by type or severity of AE emerged across groups. While a previous study [8, 10] has shown significantly enhanced injection site reactogenicity for BioThrax vaccine when administered using the SC vs. IM route, phase 3 testing will evaluate AV7909 IM versus the recently licensed BioThrax vaccine schedule for PEP administered SC. Overall, all AV7909 doses and schedules in the current study were well tolerated.
All AV7909 doses and schedules evaluated met the success criterion at Day 63 by a large margin (Groups 2-4) or narrowly missed the criterion (Group 1). The BioThrax group (Group 5) failed to meet this success criterion on Day 63, which differs from previous BioThrax vaccine studies [5, 6] and is likely explained by low sample size and/or difference in route of administration (IM vs. SC) [8].
To achieve the goal of PEP, immune responses must be rapid and durable. Protection at early time points is important for individuals who are not adherent with or cannot tolerate PEP antimicrobials. At time points beyond the currently recommended 60-day antimicrobial course, latent B. anthracis spores may germinate [9]. The TNA NF50 GMTs for subjects receiving AV7909 administered on Days 0/14 (Group 1 and Group 3) and administered only on Day 0 (Group 2) showed greater immune responses at the early Day 21 assessment time point compared to subjects administered BioThrax vaccine. Starting on Day 42, the TNA NF50 GMT curves for Groups 1 and 5 essentially overlapped (Figure 3), illustrating identical rates of immune decay.
Highest serum peak TNA NF50 titers were achieved similarly with two vaccinations (Days 0/28) or three vaccinations (Days 0/14/28) but were achieved 7 days earlier (Day 35) with 3 vaccinations. TNA NF50 responses for these two groups were equivalent by Day 42 and were equivalently durable. The Day 0/14 regimen was associated with higher circulating antibody levels between Days 21 and 35 than the Day 0/28 regimen. This early protection is critical in individuals who are not able to take the full course of the currently-recommended 60-day antimicrobial regimen for PEP. Since non-compliance is common and cannot be predicted a priori, the Day 0/14 regimen provides reassurance that early protection will be present even in poorly compliant patients.
Serum antibody decay rates for the AV7909 groups and BioThrax group were similar suggesting that the addition of the CPG 7909 adjuvant does not have a negative effect on the rate of antibody clearance within humans after the acquisition of a peak serum concentration. Of note, BioThrax vaccine in this study was administered IM, which is not the licensed route of administration for PEP. Comparison to the IM route of BioThrax vaccine allowed for a direct assessment of the relative contribution of CPG7909 to BioThrax vaccine after 2 doses (Days 21 and 28). A previous study [8, 10] demonstrated that the immune response to BioThrax vaccine administered SC trended higher than when administered IM. The anti-PA enzyme-linked immunosorbent assay geometric mean concentrations were 49.7 vs. 30.8 μg/mL for BioThrax SC vaccine vs. BioThrax IM vaccine at Week 4 (after 2 doses on Weeks 0/2) and 94.3 vs. 84.5 μg/mL, respectively, at Week 8 (after 3 doses on Weeks 0/2/4). Phase 3 testing will compare AV7909 IM to BioThrax SC vaccine using the licensed schedule for PEP.
The threshold of protection used in this study (NF50 0.56) was derived from previous BioThrax studies that utilized a pre-exposure prophylaxis model in which BioThrax vaccine was administered to rabbits on Day 0 and Day 28 and challenged with B. anthracis spores on Day 70 [6]. At the time of planning for the current study, a threshold of protection had not been derived for AV7909 and the applicability of the BioThrax vaccine threshold for AV7909 was uncertain.
Nonclinical studies to determine a TNA NF50 threshold specific for AV7909 using appropriate animal models are ongoing. Current (unpublished) data suggest that a TNA NF50 value that correlates to an equivalent probability of survival (70%) is lower for AV7909 compared with BioThrax vaccine. This is not unexpected based upon CPG 7909 effects which expanded the high affinity B cell memory population in mice [11] and enhanced antibody affinity maturation in humans [12]. Although these effects have not been established for AV7909, they may partly explain why survival is higher at similar TNA NF50 values for AV7909 vs. BioThrax vaccine. AV7909 delivered IM in humans [4] on Days 0 and 14 resulted in a 1.77-fold and 2.22-fold higher average avidity for PA than BioThrax vaccine on Day 28 (p=0.068) and Day 56 (p=0.13), respectively, based upon a small population of 7-16 subjects (unpublished data). This preliminary observation is consistent with the possibility that AV7909 is equally protective at lower TNA values. Regardless of the mechanism responsible for a lower threshold of protection as measured by the TNA NF50 value for AV7909 vs. BioThrax vaccine, a lower threshold will likely provide both rapid and prolonged protective immunity for AV7909 using the Day 0/14 schedule. While the Day 0/28 AV7909 schedule is associated with an even more durable immune response, it likely does not afford the level of early protection seen with the Day 0/14 schedule of AV7909.
Of interest, the TNA NF50 GMT curve for Group 4 was essentially identical to that for the BioThrax group except was offset with higher titers at each time point. This schedule of three half doses of AV7909 administered 2 weeks apart may be a viable vaccine-sparing strategy in the event of limited supply during a large-scale anthrax attack.
5. CONCLUSIONS
AV7909 vaccine was safe and well tolerated. The primary and secondary immunogenicity outcomes of this study provide the necessary rationale for further clinical studies of an AV7909 2-dose regimen given 2 weeks apart using larger sample sizes. The 0/14 day schedule for full dose AV7909 showed a comparable immune response to a 0/14/28 day BioThrax vaccine schedule but had a higher and earlier peak.
HIGHLIGHTS.
AV7909 is a combination of BioThrax® (Anthrax Vaccine Absorbed) and CPG 7909 adjuvant.
AV7909 vaccine is being developed for anthrax post-exposure prophylaxis.
The safety and immunogenicity of 2 doses and 3 vaccine schedules were evaluated.
Immunogenicity results indicate further study of a two dose schedule is warranted.
Both doses and all vaccine schedules were well tolerated.
ACKNOWLEDGEMENTS
The authors wish to acknowledge Sukjoon Park for program management, Peter Lill and Nancy Daczkowski for clinical project management, Brenda Wolling for regulatory support, Mario Skiadopoulos and Mike Lacy for critical review of the manuscript, and Marianne Baker, Mohamed Elsafy, and Mirjana Nesin for contributions to study design, safety oversight, and data review. The authors also acknowledge safety review contributions of the ISMs (Stephen Richardson, Robert Feldman, Yvonne Davis, Mitchell Feller, and Timothy Owens) and the members of the SMC (Sharon Frey, Samer El-Kamary, Jonathan Zenilman). This work was supported by NIAID contract number HHSN272201000035C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors are current or former employees of the study sponsor (Robert Hopkins, Gurdyal Kalsi, Victor Montalvo-Lugo, Yukun Wu, Mona Sharma, Laurence Lemiale) or study investigators compensated for their participation in the research by the study sponsor (Derek Muse, Eric Sheldon, Frank Hampel) and declare no other conflicts of interest.
Clinicaltrials.gov identifier: NCT01770743.
REFERENCES
- 1.CDC, Centers for Disease Control [Internet] Use of anthrax vaccine in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. RR06. Vol. 59. MMWR; 2010. pp. 1–30. Available from: http://www.cdc.gov?mmwr/preview/mmwrhtml/rr5906a1.htm. [PubMed] [Google Scholar]
- 2.Shepard CW, Soriano-Gabarro M, Zell ER, Hayslett J, Lukacs S, Goldstein S, et al. Antimicrobial postesposure prophylaxis for anthrax: adverse events and adherence. Emerg Infect Dis. 2002;8:1124–1132. doi: 10.3201/eid0810.020349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rynkiewicz D, Rathkopf M, Sim I, Waytes AT, Hopkins RJ, Giri L, et al. Marked enhancement of the immune response to BioThrax® (Anthrax Vaccine Adsorbed) by the TLR9 agonist CPG 7909 in healthy volunteers. Vaccine. 2011;29(37):6313–6320. doi: 10.1016/j.vaccine.2011.05.047. [DOI] [PubMed] [Google Scholar]
- 4.Hopkins RJ, Daczkowski NF, Kaptur PE, Muse D, Sheldon E, LaForce C, Sari S, Rudge TL, Bernton E. Randomized, double-blind, placebo-controlled, safety and immunogenicity study of 4 formulations of anthrax vaccine adsorbed plus CPG 7909 (AV7909) in healthy adult volunteers. Vaccine. 2013;31(30):3051–58. doi: 10.1016/j.vaccine.2013.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hopkins RJ, Howard C, Hunter-Stitt E, Kaptur PE, Pleune B, Muse D, et al. Phase 3 trial evaluating the immunogenicity and safety of a three-dose BioThrax® regimen for post-exposure prophylaxis in healthy adults. Vaccine. 2014;32(19):2217–2224. doi: 10.1016/j.vaccine.2014.01.073. [DOI] [PubMed] [Google Scholar]
- 6.Ionin B, Hopkins RJ, Pleune B, Sivko GS, Reid FM, Clement KH, et al. Evaluation of immunogenicity and efficacy of anthrax vaccine adsorbed for postexposure prophylaxis. Clin Vac Immun. 2013 Jul;20(7):1016–1026. doi: 10.1128/CVI.00099-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.U.S. Department of Health and Human Services. Food and Drug Administration. Center for Biologics Evaluation and Research [Accessed on March15, 2012];Guidance for Industry. Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials. 2007 Sep; Available at: http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm091977.pdf.
- 8.Wright JG, Plikaytis BD, Rose CE, Parker SD, Babcock J, Keitel W, El Sahly H, Poland GA, Jacobson RM, Keyserling HL, Semenova VA, Li H, Schiffer J, Dababneh H, Martin SK, Martin SW, Marano N, Messonnier NE, Quinn CP. Effect of reduced dose schedules and intramuscular injection of anthrax vaccine adsorbed on immunological response and safety profile: a randomized trial. Vaccine. 2014 Feb 12;32(8):1019–28. doi: 10.1016/j.vaccine.2013.10.039. doi:10.1016/j.vaccine.2013.10.039. Epub 2013 Dec 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henderson DW, Peacock S, Belton FC. Observations on the prophylaxis of experimental pulmonary anthrax in the monkey. J Hyg. 1956;54(1):28–36. doi: 10.1017/s0022172400044272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marano N, Plikaytis BD, Martin SW, Rose C, Semenova VA, Martin SK, et al. for the Anthrax Vaccine Research Program Working Group Effects of a reduced dose schedule and intramuscular administration of anthrax vaccine adsorbed on immunogenicity and safety at 7 months: a randomized trial. JAMA. 2008 Oct 1;300(13):1532–43. doi: 10.1001/jama.300.13.1532. doi:10.1001/jama.300.13.1532. [DOI] [PubMed] [Google Scholar]
- 11.Tross D, Klinman DM. Effect of CpG oligonucleotides on vaccine-induced B cell memory. J Immunol. 2008;181(8):5785–90. doi: 10.4049/jimmunol.181.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegrist CA, Pihlgren M, Tougne C, Efler SM, Morris ML, AlAdhami MJ, Cameron DW, Cooper CL, Heathcote J, Davis HL, Lambert PH. Co-administration of CpG oligonucleotides enhances the late affinity maturation process of human anti-hepatitis B vaccine response. Vaccine. 2004;23(5):615–22. doi: 10.1016/j.vaccine.2004.07.014. [DOI] [PubMed] [Google Scholar]