Abstract
Introduction:
In the TROPIC study, cabazitaxel improved overall survival in abiraterone-naïve metastatic castration-resistant prostate cancer (mCRPC) patients post-docetaxel. To evaluate cabazitaxel in routine clinical practice, an international, single-arm trial was conducted. Efficacy, safety, and quality of life (QoL) data were collected from Canadian patients enrolled. Overall survival and progression-free survival were not collected as part of this study. Importantly, prior abiraterone use was obtained and its impact on clinical parameters was examined.
Methods:
Sixty-one patients from nine Canadian centres were enrolled, with prior abiraterone use known for 60 patients. Prostate-specific antigen (PSA) response rate, safety, and impact on QoL life were analyzed as a function of prior abiraterone use.
Results:
Overall, 92% of patients were ECOG 0/1, 88% had bone metastases, and 25% visceral metastases. Patients treated without prior abiraterone (NoPriorAbi) (n=35, 58%) and with prior abiraterone (PriorAbi) (n=25, 42%) had similar baseline characteristics, except for age and prior cumulative docetaxel dose. Median number of cabazitaxel cycles received was similar between groups (NoPriorAbi=6, PriorAbi=7), as was PSA response rate (NoPriorAbi=36.4%, PriorAbi=45.0%, p=0.54). Almost one-third (31%) of patients received prophylactic granulocyte colony-stimulating factors. Most frequent Grade 3/4 toxicities were neutropenia (14.8%); anemia, febrile neutropenia, fatigue (each at 9.8%); and diarrhea (8.2%). No treatment-related adverse event leading to death was observed. QoL and pain were improved with no difference seen between groups. Treatment discontinuation was mainly due to disease progression (45.9%) and adverse events (32.8%).
Conclusions:
In routine clinical practice, cabazitaxel’s risk-benefit ratio in mCRPC patients previously treated with docetaxel seems to be maintained independent of prior abiraterone use.
Introduction
Prostate cancer is a heterogeneous disease presenting with inter- and intra-individual variations due to molecular heterogeneity, which impacts on patients’ responsiveness to therapy. Sensitivity to androgen blockade varies based on quantitative and qualitative characteristics of androgen receptor (AR) expression.1,2 In patients with recurrent or metastatic prostate cancer, androgen-deprivation therapy (ADT) remains the mainstay of treatment.2,3 However, it is not curative and inevitably androgen-independent progression occurs.3 Patients are then categorized as having castration-resistant prostate cancer (CRPC).
In patients with mCRPC, docetaxel was the first agent approved that demonstrated a survival benefit.4 It has been the first-line treatment of choice for over a decade. However, in the last few years, several novel agents have provided new hope for patients with mCRPC.5,6 Hormonal agents, such as abiraterone acetate, which induces remission of prostate cancer via CYP17 inhibition of extragonadal and intratumoural androgen synthesis, has been shown in randomized trials to improve overall survival when given either before or after docetaxel.7 Similarly, the hormonal agent enzalutamide, which targets the androgen receptor, has also shown a survival benefit both pre- and post-docetaxel. From a chemotherapy standpoint, cabazitaxel is a novel taxane with in vitro efficacy in docetaxel resistant cell lines.8 In a large, randomized trial (TROPIC), cabazitaxel improved overall survival compared to mitoxantrone in men with mCRPC progressing on or after first-line docetaxel.9
One of the key challenges currently facing clinicians treating mCRPC is not a lack of novel treatment options, but rather knowing how best to sequence the novel agents to maximize benefit. While we await large, prospective, randomized trials addressing sequencing questions, we can look at our smaller prospective trials to better understand the efficacy and safety of the novel treatments when used in sequence. In the current study, mCRPC patients received cabazitaxel post-docetaxel in routine clinical practice. Prostate-specific antigen (PSA) response rates, safety, and quality of life (QoL) data, as well as prior abiraterone use were collected on the Canadian cohort. Exploratory hypothesis-generating analyses have been conducted to evaluate the impact of prior abiraterone use on these key clinical outcomes, thus, providing preliminary information and adding to a growing body of evidence on how to best sequence these treatments.
Methods
Design and objectives
This study reports on post-hoc analyses conducted on Canadian patients enrolled in the Cabazitaxel International Expanded-Access Program (EAP) study (NCT01254279). This prospective, single-arm, open-label, multicentre clinical trial intended to provide early access to cabazitaxel for patients with mCRPC progressing on or after docetaxel (a similar patient population to the TROPIC study). In addition, safety data were collected and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, Version 4.0 (NCI CTCAE v. 4.0), of cabazitaxel. Only in Canada, efficacy data based on PSA response and QoL data were collected and evaluated based on prior abiraterone use. PSA response was measured and collected by the investigator, as per his/her clinical practice, at baseline and at each cycle. Overall survival and progression-free survival were not collected as part of this study. Patients were treated until disease progression, death, unacceptable toxicity, investigator’s decision, or up to 10 cycles. Patients were followed for up to 30 days after the last administration of study treatment.
Patient treatment schedule
Patients received cabazitaxel at a dose of 25 mg/m2 during a one-hour intravenous (IV) infusion on Day 1 of every three-week cycle. This was given with prednisone or prednisolone at a dose of 10 mg orally daily. One dose reduction (to 20 mg/m2) per patient was permitted throughout the study. Dose delays not exceeding two weeks were permitted.
Primary prophylaxis with granulocyte colony-stimulating factor (G-CSF) was considered (but not obligatory) in patients with high-risk clinical features (age ≥65 years, poor performance status, previous episodes of febrile neutropenia, extensive prior radiation ports, poor nutritional status, or other serious comorbidities) that could predispose patients to increased complications from neutropenia.
Inclusion and exclusion criteria
Patients having mCRPC were included in the study if they were 18 years of age or older, had an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2, had progressed during or after docetaxel, had ongoing surgical or medical castration, had adequate bone marrow, liver and renal function, had signed informed consent, and had a life expectancy of three months or longer.
Patients were excluded as per the TROPIC study exclusion criteria. The study was approved by an Independent Ethics Committee at each site in accordance with the Declaration of Helsinki, Good Clinical Practices. All patients signed informed consent before study entry.
Statistical analysis
A formal sample size calculation was not done for the Canadian portion of the study, as it represented a subgroup of a larger international trial. The safety population consisted of patients who had received any cabazitaxel.
PSA-evaluable patients were patients who had a baseline and on-treatment assessments for PSA. PSA response was defined as per the Prostate Cancer Working Group 2 guidelines (PCWG2),10 which is a ≥50% decline in PSA from baseline maintained for at least three weeks (two consecutive cycles three weeks apart) and measured by the same laboratory, and without evidence of other disease progression documented at time of confirmatory values. PSA progression was defined as a ≥25% increase in PSA and an absolute increase of ≥2 ng/mL in PSA from the nadir in patients with a decline following baseline. In patients without a decline following baseline, PSA progression was defined as a ≥25% increase in PSA and an absolute increase of ≥2 ng/mL in PSA after 12 weeks of treatment.
Prostate cancer-specific QoL data was collected using the Functional Assessment of Cancer Therapy – Prostate (FACT-P) self-administered questionnaire. This validated questionnaire11 is frequently used to assess QoL in men with prostate cancer. A higher score on the FACT-P and its subscales indicates better QoL. Baseline assessment with the questionnaire was performed and patients were asked to complete the questionnaire at each clinic visit, before visiting the physician and before treatment administration, and at the followup visit after the last treatment cycle. The QoL population consisted of patients having responded to at least 80% of the items in the FACT-P questionnaire at baseline and on-treatment. For the QoL analyses, a t-test compared the percent of patients that showed an improvement by a minimally clinically important difference (Table 1).
Table 1.
Quality of life values
| Parameters n (%) | Patients (%) (n=53)¶ | Prior use of abiraterone | p value | |
|---|---|---|---|---|
|
| ||||
| No (n=33) | Yes (n=20) | |||
| FACT-P ≥16 points* | 7 (13.0) | 3 (9.1) | 4 (20.0) | 0.2807 |
| FACT-P ≥10 points* | 14 (25.9) | 6 (18.2) | 8 (40.0) | 0.1454 |
| FACT-P ≥6 points* | 20 (37.0) | 10 (30.3) | 10 (50.0) | 0.2151 |
| PCS subscale ≥2 points* | 26 (50.0) | 15 (46.9) | 11 (57.9) | 0.7011 |
| PCS pain subscale ≥2 points* | 12 (23.1) | 7 (21.9) | 5 (26.3) | 0.8505 |
| Pain response rate‡ | 4 (21.1) | 3 (20.0) | 1 (25.0) | 0.7425 |
Minimally clinically important difference observed on two consecutive cycles where an increase in score on the FACT-P instrument and its subscales indicates improvement in quality of life.
For the FACT-P questionnaire, an evaluable patient is defined as a patient who responds to at least 80 % of the items. FACT–P is a 39-item questionnaire that consists of FACT–G (general), a 27-item self-report questionnaire that measures general health-related quality of life in cancer patients, and the Prostate Cancer Subscale (PCS), a 12-item subscale specifically designed to measure prostate cancer-specific quality of life. The FACT–P total score includes the FACT–G and the PCS. The FACT–P PCS pain-related score includes four questions from the FACT–P interrogating pain specifically.
Pain response was established only for patients with median present pain intensity (PPI) score of 2 or more or mean analgesic score (AS) of 10 points or more at baseline, or both, and was defined as a two-point or greater reduction from baseline median PPI score without an increased AS or a decrease of 50% or more in the AS without an increase in the PPI score, maintained for at least three weeks. FACT-P: Functional Assessment of Cancer Therapy – Prostate.
Pain response rate was evaluated using the McGill-Melzack Present Pain Intensity Index (PPI) and analgesic use was derived from consumption normalized to morphine equivalents, as in TROPIC. The percentage of patients meeting the pain response rate criteria are specified in Table 1. Results are presented for the safety, QoL, and PSA-evaluable populations and variations in the number of patients between study parameters was due to the fact that data were not always available for each individual patient.
Abiraterone use was obtained during the trial as part of the patients’ medical history. A post-hoc analysis was conducted in Canadian patients after the database was locked to evaluate the impact of the prior use of abiraterone on cabazitaxel’s efficacy assessed based on PSA response, QoL, and safety. Unpaired t-tests were used to compare parameters between cabazitaxel patients in the PriorAbi and NoPriorAbi groups.
Results
Patient and disease characteristics
A total of 61 Canadian patients were enrolled in the international EAP from nine Canadian sites between May 2011 and February 2012. Of these 61 patients, prior abiraterone exposure status was known in 60 patients who were divided into two groups: NoPriorAbi (n=35, 58.3%) and PriorAbi (n=25, 41.7%). Patients’ baseline characteristics, disease and treatment history are presented in Table 2. The median age was 65 years with 18% of patients being 75 or older. All patients were previously treated with docetaxel (Table 2). Most patients (91.8%) had an ECOG performance status of 0 or 1. Median time since mCPRC diagnosis was 2.2 years (range 0.4–18.0). The median time from initial diagnosis of prostate cancer was 6.3 years (range 0.8–21.5). The median cumulative dose of docetaxel was 750 mg/m2 (range 136–3406 mg/m2). The median number of docetaxel cycles was eight (range 3–24). The median time from last docetaxel dose to first cabazitaxel cycle was 7.82 months (range 0.9–56.8) with 41.0% of patients receiving the first cabazitaxel cycle within six months of their last docetaxel dose. The time between the last docetaxel dose and progression was ≥6 months in 45.9% (28/61) of patients, 3–6 months in 18.0% (11/61) of patients, <3 months in 24.6% of patients (15/61), and 11.5% (7/61) of patients received their last docetaxel dose after progression. More than half of the patients presented with two or more metastatic sites and nearly 25% presented with visceral metastases. Significantly, more patients received a higher cumulative dose of docetaxel in the group with no prior use of abiraterone. Patient characteristics were similar between the NoPriorAbi and PriorAbi groups except for age and cumulative docetaxel dose. NoPriorAbi patients tended to be younger and received a higher cumulative docetaxel dose (Table 2).
Table 2.
Patient characteristics (safety population)
| Characteristics | All patients (n=61) | Prior use of abiraterone | p value | |
|---|---|---|---|---|
|
| ||||
| No (n=35) | Yes (n=25†) | |||
| Age, yr, median (range) | 65 (42–81) | 64 (42–78) | 69 (47–81) | 0.0253* |
| Age, n (%) | ||||
| < 65 years | 27 (44.3) | 18 (51.4) | 9 (36.0) | |
| 65 years to <75 years | 23 (37.7) | 15 (42.9) | 8 (32.0) | 0.0310** |
| ≥75 years | 11 (18.0) | 2 (5.7) | 8 (32.0) | |
| ECOG performance status, n (%) | ||||
| 0 | 18 (29.5) | 11 (31.4) | 6 (24.0) | |
| 1 | 38 (62.3) | 20 (57.1) | 18 (72.0) | 1.0000** |
| 2 | 5 (8.2) | 4 (11.4) | 1 (4.0) | |
| Time since diagnosis, years, median (range) | ||||
| Initial | 6.3 (0.8–21.5) | 5.7 (0.8–18.8) | 6.6 (1.6–21.3) | 0.1850* |
| mCRPC | 2.2 (0.4–18.0) | 2.1 (0.4–6.6) | 2.6 (1.0–18.0) | 0.2769* |
| Number of metastatic sites, n (%) | ||||
| 1 | 26 (42.6) | 16 (45.7) | 10 (40.0) | 0.6623** |
| ≥2 | 35 (57.4) | 19 (54.3) | 15 (60.0) | |
| Location of metastatic sites≠, n (%) | ||||
| Bones | 54 (88.5) | 29 (82.9) | 24 (96.0) | 0.1211** |
| Visceral | 15 (24.6) | 8 (22.9) | 7 (28.0) | 0.6529** |
| Lymph nodes | 23 (37.7) | 15 (42.9) | 7 (28.0) | 0.2430** |
| Other | 6 (9.8) | 4 (11.4) | 2 (8.0) | 0.6652 |
| Time between last docetaxel dose and progression, n (%) | ||||
| <0 months | 7 (11.5) | 6 (17.1) | 1 (4.0) | |
| 0 to <3 months | 15 (24.6) | 10 (28.6) | 4 (16.0) | 0.054** |
| 3 to <6 months | 11 (18.0) | 5 (14.3) | 6 (24.0) | |
| At least 6 months | 28 (45.9) | 14 (40.0) | 14 (56.0) | |
| Type of progression, n (%) | ||||
| Bone scan and measurable lesions | 29 (47.5) | 19 (54.3) | 10 (40.0) | 0.2790** |
| Clinical progression | 15 (24.6) | 6 (17.1) | 9 (36.0) | 0.0991** |
| Increased PSA | 52 (85.2) | 29 (82.9) | 22 (88.0) | 0.5855** |
| Type of castration, n (%) | ||||
| Medical | 56 (91.8) | 33 (94.3) | 22 (88.0) | |
| Surgical | 5 (8.2) | 2 (5.7) | 3 (12.0) | 0.3891** |
| Cumulative docetaxel dose (mg/m2) by category, n (%)+ | ||||
| <225 | 1 (1.7) | 0 (0.0) | 1 (4.3) | |
| ≥225–450 | 10 (16.9) | 5 (14.3) | 5 (21.7) | |
| ≥450–675 | 11 (18.6) | 4 (11.4) | 7 (30.4) | 0.0486** |
| ≥675–900 | 19 (32.2) | 14 (40.0) | 5 (21.7) | |
| ≥900 | 18 (30.5) | 12 (34.3) | 5 (21.7) | |
| Median (range) | 750 (136–3406) | 750 (254–2250) | 602 (136–3406) | 0.2415** |
One patient was withdrawn from the study analysis due to lack of information on his prior use of abiraterone.
Prevalence of >5%.
The dose of docetaxel for two patients was missing.
p value based on Student t-est.
p value based on Cochran Mantel Haenszel statistic. ECOG: Eastern Cooperative Oncology Group; mCRPC: metastatic castration-resistant prostate cancer; PSA: prostate-specific antigen.
Exposure to cabazitaxel
Patients received a median number of cabazitaxel cycles of six (range 1–27). The median cumulative dose of cabazitaxel was 259.0 mg/m2 (range 43.0–1269.0 mg/m2). At baseline all patients received 25 mg/m2 cabazitaxel. Main reasons for treatment discontinuation were disease progression in 45.9% (NoPriorAbi=48.6%, PriorAbi=44.0%) and adverse events in 32.8% (NoPriorAbi=25.7%, PriorAbi=40.0%).
Efficacy
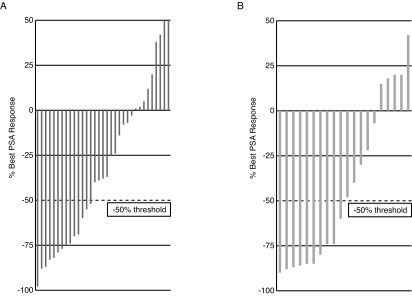
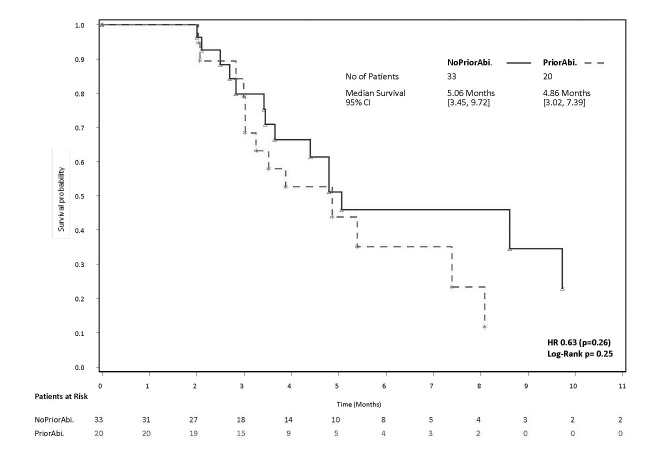
PSA response, defined as a ≥50% decline in PSA from baseline maintained for at least three weeks and evaluable for 53 patients, was achieved in 39.6% of overall patients, 36.4% of patients without the prior use of abiraterone, and 45.0% with prior abiraterone use (p=0.54). Waterfall plots of best PSA response are presented in Figs. 1A and 1B. The median time to PSA progression, evaluable for 53 patients, was 5.1 months for no prior abiraterone use compared to 4.9 months for prior abiraterone use (hazard ratio [HR] 0.63; p=0.26) (Fig. 2).
Figs. 1A,B.
Waterfall plot of best prostate-specific antigen response to cabazitaxel therapy in each patient (A) with and (B) without prior exposure to abiraterone.
Fig. 2.
Kaplan-Meier curves for time to prostate-specific antigen progression (months) with cabazitaxel by prior exposure to abiraterone.
Quality of life
The proportions of the patients reaching a minimal important improvement on the FACT-P total score, Prostate Cancer Subscale (PCS) and PCS pain subscale scores were similar in both groups regardless of prior abiraterone use, as were the pain response rates (Table 1).
Safety
Overall, treatment discontinuation due to treatment-emergent adverse events (TEAEs) was 32.8% (NoPriorAbi=25.7%, PriorAbi=40.0%; p=0.27) (Table 3). TEAEs of any grade were observed in all of the 61 patients (100%). TEAEs of Grade 3 and more were observed in 68.9% (42/61) of patients overall. This rate was 60% (21/35) in patients with no prior use of abiraterone and 80% (20/25) among prior abiraterone users (p=0.16). Most frequent Grade 3/4 toxicities were neutropenia (14.8%); anemia, febrile neutropenia, and fatigue (each at 9.8%); and diarrhea (8.2%). G-CSF was administered prophylactically at Cycle 1 in 19 patients (31.1%). No treatment-related adverse event leading to death was observed.
Table 3.
Treatment-emergent adverse events (safety population)
| Treatment-emergent adverse events* | Grade ≥3 Patients (%) (n=61) | Prior use of abiraterone | |
|---|---|---|---|
|
| |||
| No (n=35) | Yes (n=25) | ||
| Patients with TEAEs | 42 (68.9) | 21 (60.0) | 20 (80.0)† |
| Hematological TEAEs | |||
| Neutropenia | 9 (14.8) | 4 (11.4) | 5 (20.0) |
| Anemia | 6 (9.8) | 3 (8.6) | 3 (12.0) |
| Febrile neutropenia | 6 (9.8) | 3 8.6) | 3 (12.0) |
| White blood cell count decreased | 3 (4.9) | 2 (5.7) | 1 (4.0) |
| Non-hematological TEAEs | |||
| Fatigue | 6 (9.8) | 3 (8.6) | 3 (12.0) |
| Diarrhea | 5 (8.2) | 3 (8.6) | 2 (8.0) |
| Back pain | 3 (4.9) | 0 (0.0) | 3 (12.0) |
| Dehydration | 4 (6.6) | 1 (2.9) | 3 (12.0) |
| Spinal cord compression | 4 (6.6) | 2 (5.7) | 1 (4.0) |
| Nausea | 3 (4.9) | 2 (5.7) | 1 (4.0) |
| Vomiting | 3 (4.9) | 2 (5.7) | 1 (4.0) |
| Cellulitis | 3 (4.9) | 2 (5.7) | 1 (4.0) |
| Sepsis | 3 (4.9) | 2 (5.7) | 1 (4.0) |
| Pain in extremity | 1(1.6) | 1 (2.9) | 0 (0.0) |
| Asthenia | 2 (3.3) | 2 (5.7) | 0 (0.0) |
| Pulmonary embolism | 2 (3.3) | 1 (2.9) | 1 (4.0) |
| Renal failure acute | 2 (3.3) | 0 (0.0) | 2 (8.0) |
TEAEs with a frequency ≥2% for Grade ≥3 adverse events. TEAEs are classified based on the frequency of grade ≥3 TEAEs.
p=0.16. Significance of specific hematological and nonhematological TEAEs was not assessed as analyses would be underpowered due to the small number of events in each group. TEAE: treatment-emergent adverse events.
Discussion
In this study, we evaluated the efficacy, safety, and impact on QoL of cabazitaxel administered to mCRPC patients enrolled in the Canadian arm of an international, expanded-access program. Importantly, we conducted exploratory analyses to provide further insight into whether or not prior abiraterone use impacted key outcome measures, namely efficacy, safety, QoL. Canada was the only country to collect PSA and FACT-P QoL data.
From an efficacy perspective, we observed that the activity of cabazitaxel seems to be maintained in the post-abiraterone setting, despite patients having undergone more treatment regimens, as demonstrated by the PSA response, number of cycles, and QoL data. A ≥50% PSA decline was achieved in nearly 40% of patients, both in those who received prior abiraterone (45.0% of patients) or not (36.4%) (p=0.5371). This is in line with European reports, where 35% of 79 patients from France12 and 39% of the 59 patients from U.K.13 achieved a ≥50% PSA decline with cabazitaxel following AR targeted therapies. Additionally, our overall 40.7% of PSA response is similar to the TROPIC study (39.2%) and to the German EAP report, where 37.6% (35/93) of patients showed a ≥50% PSA reduction that was maintained for eight weeks after chemotherapy discontinuation.9,11 There was no significant difference in median number of treatment cycles among patients who had prior use of abiraterone (six cycles, range 1–27) or not (seven cycles, range 1–13) (Table 4). These results are similar to the number of treatment cycles in the TROPIC study (six cycles, range 3–10).9 Overall, these results suggest that the use of cabazitaxel after prior abiraterone appears efficacious. These results should, however, be appraised in the context of important limitations, which include a limited sample size, non-randomized design, and post-hoc analyses.
Table 4.
Cabazitaxel exposure
| Characteristics | All patients (N=61) | Prior use of abiraterone | p value | |
|---|---|---|---|---|
|
| ||||
| No (n=35) | Yes (n=25) | |||
| Cycles of cabazitaxel, n, median (range) | 6.0 (1–27) | 6 (1–27) | 7 (1–13) | 0.2517 |
| Cumulative dose of cabazitaxel, median (mg/m2) | 259.0 (43–1269) | 268.8 (43–1269) | 259.0 (45–550) | 0.1762 |
| Patients receiving more than 10 cycles of cabazitaxel, n (%) | 15 (24.6) | 10 (28.6) | 5 (20.0) | 0.4535 |
| Relative dose intensity, median* | 100 (80–100) | 100 (82–100) | 100 (80–100) | 0.6991 |
| Dose delay, n (%) | 28 (50.0)** | 16 (45.7) | 12 (57.1) | 0.4118 |
| Dose delays due to: | ||||
| Hematotoxicity | 6 (21.4) | 1 (6.3) | 5 (41.7) | 0.3157 |
| Non-hematotoxicity | 4 (14.3) | 2 (12.5) | 2 (16.7) | |
| Other | 18 (64.3) | 13 (81.2) | 5 (41.7) | |
| Dose reduction, n (%) | 15 (26.8)** | 8 (22.9) | 7 (33.3) | 0.3957 |
| Dose reduction due to: | ||||
| Hematotoxicity | 6 (40.0) | 3 (37.5) | 3 (42.9) | 0.9699 |
| Non-hematotoxicity | 6 (40.0) | 4 (50.0) | 2 (28.6) | |
| Other | 3 (20.0) | 1 (12.5) | 2 (28.6) | |
At least 50% of the patients did not have the dose reduction.
Only 56 patients were treated with two or more cycles.
Overall, we found the frequency of adverse events Grade 3 or higher in patients that received cabazitaxel was 68.9%. This frequency was 80% in patients with prior abiraterone use and 60% in patients with no prior abiraterone use (p=0.16). The limited sample size in both groups prevents conclusions on the statistical significance of this difference. Patient characteristics, however, may have influenced this numerical difference. Patients with prior abiraterone were significantly older and presented with numerically worse ECOG performance status than patients without prior abiraterone use. These observations are not surprising considering that cabazitaxel would be administered as a later line of therapy in patients that received prior abiraterone.
Most importantly, the adverse events were manageable with a reported frequency of febrile neutropenia of 9.8%, and Grade 3 or higher neutropenia of 14.8%. G-CSF was used as primary or secondary prophylaxis in 31% of patients. In the TROPIC study, more post-docetaxel cabazitaxel patients compared to mitoxantrone patients presented with hematological Grade 3 or higher adverse events, with Grade ≥3 neutropenia affecting 82% of patients.9 It is important to note that in the TROPIC trial,9 prophylactic G-CSF use was not permitted during the first cycle, contrary to the EAP. The safety profile of cabazitaxel in the Canadian EAP patients is consistent with the ones reported by our European counterparts.11,14–18 Heidenreich et al showed that in Germany, cabazitaxel in routine clinical practice in mCRPC patients post-docetaxel had a manageable toxicity profile.11 Grade ≥3 neutropenia occurred in 7.2% of patients (G-CSF was administered prophylactically in 13.5% of patients at Cycle 1).11 Other published EAP reports have shown the rates of Grade 3 or higher neutropenia range from 33.9–4.1%14,15,18 with prophylactic use of G-CSF in 16.3–62.4%14,15,18 of patients. Non-hematological adverse events of Grade ≥3 occurred at a similar manageable frequency in our study compared to previous EAP reports11,14–18 and the TROPIC trial.9 Overall, this EAP study has demonstrated that cabazitaxel in routine clinical practice has a manageable safety profile that does not appear to be impacted by the prior use of abiraterone acetate. However, this conclusion is limited by the small sample size in each of these subgroups and further research is warranted.
With the recent results in pre-19,20 and post-chemotherapy21,22 settings of the novel hormonal therapies, and the possible emergence of new hormonal agents to treat mCRPC,5 the optimal sequencing of these agents remains a major issue. Some studies have suggested that giving two agents sequentially that both target the androgen signaling pathway (i.e., abiraterone followed by enzalutamide or the opposite) may not be the best option for mCRPC patients because of cross-resistance.23–26 In the study by Loriot et al,23 only three of the 38 patients (8%) who received abiraterone following progression on both enzalutamide and docetaxel attained a PSA response (≥50% decline in PSA confirmed after ≥4 weeks). Similarly, in the study by Bianchini et al,24 only 12.8% of the 39 patients achieved a PSA response when they received enzalutamide following progression on both abiraterone and docetaxel. For treatment sequences involving enzalutamide or abiraterone as a second-line agent following the opposite agent, several other studies have reported that 17–28% of patients achieved a PSA response.26–34 A report from Schnadig et al showed that more patients reached a third-line treatment if they received docetaxel followed immediately by cabazitaxel, compared to docetaxel followed by abiraterone.35 This retrospective analysis in 667 post-docetaxel mCRPC patients showed that 31% of patients who received cabazitaxel second-line received abiraterone in third-line, compared to only 12% of patients who received cabazitaxel third-line if they received abiraterone second-line.35
Despite the suggested effect of cabazitaxel on the androgen receptor pathway,36 it appears that cabazitaxel may be less likely to be affected by prior hormonal therapy treatment. In vitro studies had shown that cabazitaxel was able to decrease cell viability in both enzalutamide-resistant and enzalutamide-sensitive cells, but abiraterone did not show the same decrease in cell viability following enzalutamide.12 It appears that cabazitaxel has its effects on prostate cancer cells mainly via pathways independent of the androgen receptor, which could reduce the cross-resistance phenomenon observed with hormonal therapies.12 This would be a viable explanation as to why prior abiraterone did not impact cabazitaxel efficacy; and similar PSA responses were reported in other studies when cabazitaxel was used post-hormonal therapies in mCRPC patients.12,13
In conclusion, this EAP study, although limited by its small sample size, provides additional data on the efficacy and safety of cabazitaxel in mCRPC patients previously treated with docetaxel, with or without prior abiraterone use. This is also the first time FACT-P data is reported relative to cabazitaxel and in relation to previous exposure to abiraterone. As CRPC remains a major health concern worldwide36–40 and numerous new therapeutic agents are now available,5,6 research to understand what treatment should be given at what time remains important to optimize patient management.
Acknowledgments
François Leblond and Pasha Javadi provided assistance with medical writing.
Footnotes
Competing interests: This study was sponsored by Sanofi. This study is registered on clinicaltrial.gov as NCT01254279.
Dr. Saad has been an Advisory Board member for Janssen and Sanofi; and has received research funding, as well as honoraria from Sanofi. Dr. Berry has been an Advisory Board member for and received honoraria from Sanofi. Dr. Levesque has been an Advisory Board member for Astellas, Janssen, and Sanofi; and has received honoraria from Sanofi, as well as research funding from Janssen. Dr. Aucoin has been an Advisory Board member for Sanofi. Dr. Czaykowski has received honoraria from Sanofi. Dr. Sridhar has been an Advisory Board member for Astellas, Janssen, and Sanofi; and has received research funding from Sanofi. Mrs. Alloul and Mr. Stewart are full-time employees of Sanofi-aventis Canada Inc. The remaining authors declare no competing financial or personal interests.
Previous publication: These results were presented in part as a poster at the 2014 ASCO Annual Meeting (Saad et al. J Clin Oncol 2014;32:5:abstr 5062).
This paper has been peer-reviewed.
References
- 1.Sun Y, Niu J, Huang J. Neuroendocrine differentiation in prostate cancer. Am J Transl Res. 2009;1:148–62. [PMC free article] [PubMed] [Google Scholar]
- 2.Tombal B. What is the pathophysiology of a hormone-resistant prostate tumour? Eur J Cancer. 2011;47:S179–88. doi: 10.1016/S0959-8049(11)70163-0. [DOI] [PubMed] [Google Scholar]
- 3.Zong Y, Goldstein AS. Adaptation or selection—mechanisms of castration-resistant prostate cancer. Nat Rev Urol. 2013;10:90–8. doi: 10.1038/nrurol.2012.237. [DOI] [PubMed] [Google Scholar]
- 4.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 5.Bishr M, Saad F. Overview of the latest treatments for castration-resistant prostate cancer. Nat Rev Urol. 2013;10:522–8. doi: 10.1038/nrurol.2013.137. [DOI] [PubMed] [Google Scholar]
- 6.Malik Z, Payne H, Ansari J, et al. Evolution of the treatment paradigm for patients with metastatic castration-resistant prostate cancer. Adv Ther. 2013;30:1041–66. doi: 10.1007/s12325-013-0070-z. [DOI] [PubMed] [Google Scholar]
- 7.Rathkopf D, Scheer HI. Androgen receptor antagonists in castration-resistant prostate cancer. Cancer J. 2013;19:43–9. doi: 10.1097/PPO.0b013e318282635a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vrignaud P, Sémiond D, Lejeune P, et al. Preclinical antitumor activity of cabazitaxel, a semisynthetic taxane active in taxane-resistant tumours. Clin Cancer Res. 2013;19:2973–83. doi: 10.1158/1078-0432.CCR-12-3146. [DOI] [PubMed] [Google Scholar]
- 9.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomized open-label trial. Lancet. 2010;376:1147–54. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 10.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–59. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heidenreich A, Scholz H-J, Rogenhofer S, et al. Cabazitaxel plus prednisone for metastatic castration-resistant prostate cancer progressing after docetaxel: Results from the German compassionate-use program. Eur Urol. 2013;63:977–82. doi: 10.1016/j.eururo.2012.08.058. [DOI] [PubMed] [Google Scholar]
- 12.Al Nakouzi N, Le Moulec S, Albigès L, et al. Cabazitaxel remains active in patients progressing after docetaxel followed by novel androgen receptor pathway-targeted therapies. Eur Urol. 2015;68(2):228–35. doi: 10.1016/j.eururo.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 13.Pezaro CJ, Omlin AG, Altavilla A, et al. Activity of cabazitaxel in castration-resistant prostate cancer progressing after docetaxel and next-generation endocrine agents. Eur Urol. 2014;66:459–65. doi: 10.1016/j.eururo.2013.11.044. [DOI] [PubMed] [Google Scholar]
- 14.Bracarda S, Gernone A, Gasparro D, et al. Real-world cabazitaxel safety: The Italian early-access program in metastatic castration-resistant prostate cancer. Future Oncol. 2014;10:975–83. doi: 10.2217/fon.13.256. [DOI] [PubMed] [Google Scholar]
- 15.Heidenreich A, Bracarda S, Mason M, et al. Safety of cabazitaxel in senior adults with metastatic castration-resistant prostate cancer: Results of the European compassionate-use programme. Eur J Cancer. 2014;50:1090–9. doi: 10.1016/j.ejca.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Houede N, Eymard J, Zoubier T. Safety data of cabazitaxel (Jevtana) in patients treated for metastatic castration resistant prostate cancer after docetaxel treatment: Result of a cohort of patients during the temporary authorization for use in France (ATU) Ann Oncol. 2012;23:ix314–5. [Google Scholar]
- 17.Bahl A, Masson S, Malik Z, et al. Cabazitaxel for metastatic castration-resistant prostate cancer (mCRPC): Final quality-of-life (QoL) results with safety data from the United Kingdom (UK) Early Access Programme (EAP) ( NCT01254279) BJU Int. 2015;116:880–7. doi: 10.1111/bju.13069*. [DOI] [PubMed] [Google Scholar]
- 18.Wissing MD, van Oort IM, Gerritsen WR, et al. Cabazitaxel in patients with metastatic castration-resistant prostate cancer: Results of a compassionate use program in the Netherlands. Clin Genitourin Cancer. 2013;11:238–50. doi: 10.1016/j.clgc.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–48. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–33. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: Final overall survival analysis of the COU-AA-301 randomized, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13:983–92. doi: 10.1016/S1470-2045(12)70379-0. [DOI] [PubMed] [Google Scholar]
- 22.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 23.Loriot Y, Bianchini D, Ileana E, et al. Antitumour activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide (MDV3100) Ann Oncol. 2013;24:1807–12. doi: 10.1093/annonc/mdt136. [DOI] [PubMed] [Google Scholar]
- 24.Bianchini D, Lorente D, Rodriguez-Vida A, et al. Antitumour activity of enzalutamide (MDV3100) in patients with metastatic castration-resistant prostate cancer (CRPC) pre-treated with docetaxel and abiraterone. Eur J Cancer. 2014;50:78–84. doi: 10.1016/j.ejca.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 25.Noonan KL, North S, Bitting RL, et al. Clinical activity of abiraterone acetate in patients with metastatic castration-resistant prostate cancer progressing after enzalutamide. Ann Oncol. 2013;24:1802–7. doi: 10.1093/annonc/mdt138. [DOI] [PubMed] [Google Scholar]
- 26.Schrader AJ, Boegemann M, Ohlmann C, et al. Enzalutamide in castration-resistant prostate cancer patients progressing after docetaxel and abiraterone. Eur Urol. 2014;65:30–6. doi: 10.1016/j.eururo.2013.06.042. [DOI] [PubMed] [Google Scholar]
- 27.Cheng HH, Gulati R, Azad A, et al. Activity of enzalutamide in men with metastatic castration-resistant prostate cancer is affected by prior treatment with abiraterone and/or docetaxel. Prostate Cancer Prostatic Dis. 2015;18:122–7. doi: 10.1038/pcan.2014.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stevenson R, Fackrell DG, Ford D, et al. The sequential use of abiraterone and enzalutamide in metastatic castrate resistant prostate cancer patients: Experience from seven U.K. centres. J Clin Oncol. 2014;32:125. [Google Scholar]
- 29.Scholz MC, Lam RY, Turner JS, et al. Enzalutamide in men with prostate cancer resistant to docetaxel and abiraterone. J Clin Oncol. 2014;32:247. [Google Scholar]
- 30.Badrising S, van der Noort V, van Oort IM, et al. Clinical activity and tolerability of enzalutamide (MDV3100) in patients with metastatic, castration-resistant prostate cancer who progress after docetaxel and abiraterone treatment. Cancer. 2014;120:968–75. doi: 10.1002/cncr.28518. [DOI] [PubMed] [Google Scholar]
- 31.Thomsen FB, Roder MA, Rathenborg P, et al. Enzalutamide treatment in patients with metastatic castration-resistant prostate cancer progressing after chemotherapy and abiraterone acetate. Scand J Urol. 2014;48:268–75. doi: 10.3109/21681805.2013.860189. [DOI] [PubMed] [Google Scholar]
- 32.Roeder MA, Thomsen FB, Brasso K, et al. Biochemical response to enzalutamide therapy in patients with mCRPC following docetaxel and abiraterone treatment. J Clin Oncol. 2014;32:202. [Google Scholar]
- 33.Thomson D, Charnly N, Parikh O. Enzalutamide after failure of docetaxel and abiraterone in metastatic castrate resistant prostate cancer (mCRPC): Results from an expanded access program. J Clin Oncol. 2014;32:188. doi: 10.1016/j.ejca.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 34.Sandhu GS, Parikh RA, Appleman LJ, et al. Enzalutamide after abiraterone in patients with metastatic castrate-resistant prostate cancer (mCRPC) J Clin Oncol. 2014;32:240. [Google Scholar]
- 35.Schnadig ID, Bhor M, Vogelzang NJ, et al. Sequencing of cabazitaxel and abiraterone acetate following docetaxel in metastatic castration-resistant prostate cancer (mCRPC) J Clin Oncol. 2013;31:79. [Google Scholar]
- 36.Zhu ML, Horbinski CM, Garzotto M, et al. Tubulin-targeting chemotherapy impairs androgen receptor activity in prostate cancer. Cancer Res. 2010;70:7992–8002. doi: 10.1158/0008-5472.CAN-10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 38.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 39.Canadian Cancer Society’s Advisory Committee on Cancer Statistics . Canadian Cancer Statistics 2013. Toronto, ON: Canadian Cancer Society; 2013. http://www.cancer.ca/en/cancer-information/cancer-101/canadian-cancer-statistics-publication/?region=on. Accessed February 25, 2016. [Google Scholar]
- 40.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]