Abstract
Introduction:
Laparoscopic radical nephrectomy (LRN) and laparoscopic nephroureterectomy (LNU) are similar procedures and some surgeons may believe the perioperative risks are the same. The purpose of this study is to characterize and compare complications following LRN and LNU.
Methods:
A historical cohort of patients who received either LRN or LNU between 2006 and 2012 was reviewed from the National Surgical Quality Improvement Program (NSQIP) database. Patient characteristics, surgical characteristics, and perioperative outcomes up to 30 days postoperatively were abstracted. Unadjusted and adjusted associations between procedure (LRN or LNU) and any adverse event were determined.
Results:
During the study period, 4904 patients met study inclusion criteria; 4159 (84.8%) received a LRN while 745 (15.2%) received a LNU. Overall, 651 (13.3%) patients experienced at least one postoperative complication. LNU was associated with more complications than LRN (21% and 12%, respectively, p value <0.01). The most common complications were: bleeding requiring blood transfusion (9.0% LNU vs. 6.0% LRN), urinary tract infection (4.6% LNU vs. 1.5% LRN), wound infection (1.3% LNU vs. 1.8% LRN), and unplanned intubation (2.3% LNU vs. 0.9% LRN). After adjusting for potential confounders, LNU was associated with higher risk of any complication compared to LRN (relative risk [RR] 1.41, 95% confidence interval [CI] 1.16–1.72). Other variables independently associated with an increased risk of complications included: increasing patient age (RR 1.01, 95% CI 1.01–1.02), American Society of Anesthesiologists (ASA) classification ≥3 (RR 1.34, 95% CI 1.10–1.63), higher preoperative creatinine (RR 1.11, 95% CI 1.06–1.17), >4 units of blood transfused within 72 hours before surgery (RR 1.93, 95% CI 1.29–2.86), and operative time >6 hours (RR 2.17, 95% CI 1.71–2.75).
Conclusions:
Postoperative complications within 30 days of surgery are common after LNU and LRN. Despite having technical similarities, LNU carries a significantly higher risk of developing short-term complications compared to LRN. This information should be considered when counseling patients prior to surgery. Notable limitations of this study included the lack of information on tumour stage and management of the distal ureter.
Introduction
Cancers of the upper urinary tract are common, with an estimated 63 920 new cases involving the kidney/renal pelvis and at least 3000 new cases involving the ureter in the U.S. in 2014.1 Tumours originating in the renal parenchyma are usually treated with radical or partial nephrectomy, and tumours originating in the upper tract urothelium (ureter and renal pelvis) are usually treated with nephroureterectomy, where the ureter is removed in addition to the kidney.2–4
Laparoscopic renal surgery has become the standard of care for patients with low-stage malignancies because this approach is associated with easier convalescence and equivalent cancer outcomes.5 Both laparoscopic radical nephrectomy (LRN) and laparoscopic nephroureterectomy (LNU) have been associated with reduced blood loss, complications, postoperative pain, and length of hospital stay compared to open surgery.3,4,6,7
While LRN and LNU are performed for different malignancies, the operative steps are similar, with the exception of the distal ureter and bladder dissection required for LNU. Because LRN and LNU are technically similar, some surgeons and patients may believe the perioperative risks are the same. Furthermore, since LRN is performed more often than LNU, surgeons may be disproportionately influenced by the experience of LRN patients.
The objective of this study was to compare postoperative complications up to 30 days following surgery for patients undergoing LRN and LNU. We hypothesized that the frequency and type of postoperative complications experienced after LRN and LNU would be different. Directly comparing postoperative complications of LRN and LNU will allow clinicians to better prepare and counsel patients for surgery.
Methods
The American College of Surgeons National Surgical Quality Improvement Program (NSQIP) is a validated program that measures perioperative outcomes at over 500 academic and community hospitals located predominantly in North America. The NSQIP database includes patient demographics, patient comorbidities, surgical procedure type, and perioperative adverse events up to 30 days after surgery. A combination of automated data collection and abstraction by trained study nurses is used to populate the data from each hospital. At some centres, data from all procedures is collected, while a rotating eight-day cycle of cases is used at other centres.8 Previous data audits have revealed an inter-rater reliability of approximately 98% for surgical complications. NSQIP hospitals are the source of data used in this analysis; however, NSQIP administrators have not reviewed the methodology of this study.
Data was queried from NSQIP Participant Data Use Files from 2006 to 2012. All LRN (CPT code 50545) and LNU (CPT code 50548) cases were included. Since tumour stage information is not available in the NSQIP database, patients were chosen based on whether they were treated with a laparoscopic approach, as this likely selected for patients with similarly localized disease. Open procedures were not included because significant heterogeneity in tumour stage would be expected for those procedures. Partial nephrectomy or isolated ureterectomy patients were also excluded, as these operations are associated with unique complication profiles and were outside of our primary study question.
Numerous complications are abstracted per-protocol in the NSQIP. NSQIP nurses use specific definitions to determine if a complication occurred or not. The definitions are modified as needed over time, but are consistently used across all participating hospitals. Complications evaluated in this study included superficial and deep surgical site infection, wound disruption, pneumonia, unplanned intubation, pulmonary embolism, requiring ventilator for >48 hours, progressive renal insufficiency, acute renal failure, urinary tract infection, stroke with neurological deficits, cardiac arrest requiring cardiopulmonary resuscitation (CPR), myocardial infarction, intraoperative or postoperative bleeding within 72 hours requiring transfusion, and deep vein thrombosis (DVT).8
The data were summarized using descriptive statistics. Patient and surgical factors were compared between the LRN and LNU groups using t-tests and chi-squared analyses. The associations between surgery type (LRN vs. LNU) and the occurrence of any complication by 30 days were calculated using log binomial regression and presented as relative risks (RR) with 95% confidence intervals (CI). To more accurately characterize the association between surgery type and complications, a multivariable model adjusting for statistically significant and clinically relevant potential confounders was created. Variables with >25% missing data were excluded from the multivariable model. A p value of ≤0.05 was considered to be statistically significant. All statistical calculations were performed using SAS 9.3 (SAS institute Inc., Cary, NC, U.S.).
Results
From 2006 to 2012, 4159 patients who received LRN and 745 who received LNU were abstracted into the NSQIP database and were included in this study. Table 1 lists baseline patient and surgical factors stratified by procedure type. Those who had LNU were older, more likely to be current smokers, and had higher American Society of Anesthesiologists (ASA) physical status classification system scores.
Table 1.
Baseline patient and surgical characteristics of LNU and LRN cases in NSQIP from 2006–2012
| Variable | Laparoscopic nephroureterectomy n (%) | Laparoscopic radical nephrectomy n (%) | p value |
|---|---|---|---|
| TOTAL | 745 (15.2) | 4159 (84.8) | |
|
| |||
| Patient characteristics | |||
|
| |||
| Age (years) | |||
| Mean age (SD) | 68.7 (SD 14.1) | 61.1 (SD 14.3) | |
| <65 | 233 (31.3) | 2343 (56.3) | <0.01 |
| 65–70 | 97 (13.0) | 579 (13.9) | |
| 70–75 | 132 (17.7) | 493 (11.9) | |
| >75 | 283 (38.0) | 744 (17.9) | |
| Race | |||
| White | 599 (90.9) | 3135 (86.6) | <0.01 |
| Other | 60 (9.1) | 485 (13.4) | |
| Missing: 625 (12.7) | |||
| Gender | |||
| Female | 291 (39.1) | 1747 (42.1) | 0.13 |
| Male | 453 (60.9) | 2404 (57.9) | |
| Body mass index (BMI) | |||
| Mean BMI (SD) | 28.4 (SD 6.2) | 30.0 (SD 6.8) | |
| <25 | 219 (29.6) | 939 (22.8) | <0.01 |
| 25–<30 | 282 (38.1) | 1434 (34.8) | |
| 30–<35 | 154 (20.8) | 932 (22.6) | |
| ≥35 | 86 (11.5) | 822 (19.9) | |
| ASA score | |||
| 1–2 | 243 (32.7) | 1590 (38.3) | <0.01 |
| 3–5 | 501 (67.3) | 2566 (61.7) | |
| Bleeding disorder | 24 (3.2) | 119 (2.9) | 0.59 |
| Preoperative weight loss (>10% over six months) | 12 (1.6) | 99 (2.4) | 0.19 |
| Preoperative sepsis | 5 (0.7) | 46 (1.1) | 0.28 |
| Preoperative pneumonia | 0 (0.0) | 1 (0.1) | 1.00 |
| Missing | 353 (47.4) | 1942 (46.7) | |
| Preoperative ascites | 1 (0.1) | 4 (0.1) | 0.56 |
| Chronic steroid use | 16 (2.1) | 225 (5.4) | <0.01 |
| Diabetes | 152 (20.4) | 819 (19.7) | 0.65 |
| Dialysis preoperatively | 27 (3.6) | 274 (6.6) | <0.01 |
| Acute renal failure | 1 (0.1) | 18 (0.4) | 0.34 |
| Disseminated cancer | 18 (2.4) | 169 (4.1) | 0.03 |
| Chemotherapy (≤30 days before surgery) | |||
| Yes | 7 (1.8) | 18 (0.8) | 0.09 |
| Missing | 353 (47.4) | 1942 (46.7) | |
| Radiotherapy (≤90 days preoperatively) | |||
| Yes | 0 (0.0) | 9 (0.4) | 0.37 |
| Missing | 357 (47.9) | 1959 (47.1) | |
| Dyspnea | 83 (11.1) | 393 (9.4) | 0.15 |
| Alcohol use (>2 drinks/day) | |||
| Yes | 12 (3.1) | 73 (3.3) | 0.81 |
| Missing | 353 (47.4) | 1942 (46.7) | |
| Current smoker | 180 (24.2) | 807 (19.4) | <0.01 |
| Smoking history | |||
| ≤50 pack years | 292 (89.0) | 1712 (93.5) | <0.01 |
| >50 pack years | 36 (11.0) | 119 (6.5) | |
| Missing | 417 (56.0) | 2328 (56.0) | |
| Functional status | |||
| Independent | 727 (98.0) | 4053 (97.7) | 0.60 |
| Dependent | 15 (2.0) | 97 (2.3) | |
| Hemiplegia | 4 (1.0) | 18 (0.8) | 0.56 |
| Missing | 353 (47.4) | 1942 (46.7) | |
| Cerebrovascular accident/stroke with neurological deficit | 7 (1.8) | 51 (2.3) | 0.52 |
| Missing | 353 (47.4) | 1942 (46.7) | |
| Cerebrovascular accident/stroke with no neurological deficit | 14 (3.6) | 55 (2.5) | 0.21 |
| Missing | 353 (47.4) | 1942 (46.7) | |
| Chronic obstructive pulmonary disease (severe) | 54 (7.2) | 231 (5.6) | 0.07 |
| Transient ischemic attacks | 12 (3.1) | 71 (3.2) | 0.88 |
| Missing | 353 (47.4) | 1942 (46.7) | |
| Congestive heart failure (≤30 days preoperatively) | 3 (0.4) | 34 (0.8) | 0.23 |
| Preoperative albumin concentration (g/dl) | |||
| >4.1 | 156 (37.3) | 952 (39.8) | |
| 3.8–4.1 | 90 (21.5) | 565 (23.6) | 0.09 |
| 3.5–3.7 | 93 (22.3) | 411 (17.2) | |
| <3.5 | 79 (18.9) | 467 (19.5) | |
| Missing | 327 (43.9) | 1764 (42.4) | |
| Preoperative creatinine level (mg/dl) | |||
| ≤1.3 | 522 (73.3) | 3120 (78.43) | |
| >1.3–<2 | 143 (20.1) | 503 (12.6) | <0.01 |
| ≥2 | 47 (6.6) | 355 (8.9) | |
| Missing | 33 (4.4) | 181 (4.3) | |
| Emergency case | 5 (0.7) | 20 (0.5) | 0.57 |
| >4 units PRBC transfusion (≤72 hours preoperatively) | 13 (1.7) | 33 (0.8) | 0.01 |
|
| |||
| Surgical characteristics | |||
|
| |||
| Operative time | |||
| ≤6 hours | 623 (89.9) | 3476 (96.8) | <0.01 |
| >6 hours | 70 (10.1) | 116 (3.2) | |
| Missing | 52 (7) | 567 (13.6) | |
Missing data not shown for variables with <1% missing data. ASA: American Society of Anesthesiologists; LRN: laparoscopic radical nephrectomy; LNU: laparoscopic nephroureterectomy; NSQIP: National Surgical Quality Improvement Program; PRBC: packed red blood cells; SD: standard deviation.
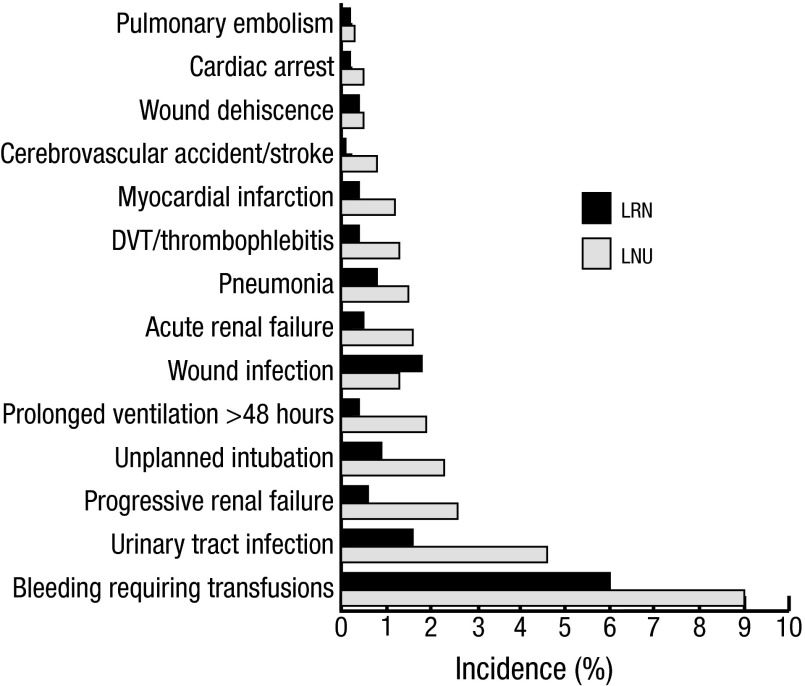
One hundred fifty-four (20.7%) of the LNU patients experienced at least one complication compared to 497 (12.0%) of LRN patients (RR 1.73; 95% CI 1.47–2.04). For both groups, the most common complication was bleeding requiring at least one transfusion within 72 hours of surgery (9% for LNU vs. 6% for LRN, p value <0.01). For the LNU group, other common complications included urinary tract infections (4.6%), progressive renal insufficiency (2.6%), and unplanned intubations (2.3%). In the LRN group, wound infections (1.8%) and urinary tract infections (1.5%) were most common (Table 2, Fig. 1).
Table 2.
Complications after LNU compared to LRN cases in NSQIP from 2006–2012
| Variable | LNU n (%) | LRN n (%) | Relative risk* (95% CI) | p value |
|---|---|---|---|---|
| Hematologic complications | ||||
| Blood transfusion | 67 (9.0) | 251 (6.0) | 1.49 (1.15–1.92) | <0.01 |
| DVT/thrombophlebitis | 10 (1.3) | 16 (0.4) | 3.49 (1.59–7.66) | <0.01 |
| Pulmonary embolism | 2 (0.3) | 10 (0.2) | 1.12 (0.25–5.09) | 0.89 |
| Cerebrovascular accident/stroke with neurological deficit | 6 (0.8) | 5 (0.1) | 6.70 (2.05–21.90) | <0.01 |
| Infectious complications | ||||
| Urinary tract infection | 34 (4.6) | 63 (1.5) | 3.01 (2.00–4.54) | <0.01 |
| Wound infection | 10 (1.3) | 75 (1.8) | 0.74 (0.39–1.43) | 0.38 |
| Pneumonia | 11 (1.5) | 34 (0.8) | 1.81 (0.92–3.55) | 0.09 |
| Respiratory complications | ||||
| Unplanned intubation | 17 (2.3) | 39 (0.9) | 2.43 (1.38–4.28) | <0.01 |
| Prolonged ventilation >48 hours | 14 (1.9) | 17 (0.4) | 4.60 (2.28–9.29) | <0.01 |
| Renal complications | ||||
| Progressive renal failure | 19 (2.6) | 26 (0.6) | 4.08 (2.27–7.33) | <0.01 |
| Acute renal failure | 12 (1.6) | 21 (0.5) | 3.19 (1.58–6.46) | <0.01 |
| Cardiac and other complications | ||||
| Myocardial infarction | 9 (1.2) | 18 (0.4) | 2.79 (1.26–6.19) | 0.01 |
| Wound dehiscence | 4 (0.5) | 15 (0.4) | 1.49 (0.50–4.47) | 0.48 |
| Cardiac arrest requiring CPR | 4 (0.5) | 10 (0.2) | 2.23 (0.70–7.10) | 0.17 |
| Any complication | 154 (20.7) | 497 (12.0) | 1.73 (1.47–2.04) | <0.01 |
| Length of total hospital stay | Median: 4 IQR 3–6 | Median: 3 IQR 2–4 | <0.01 |
Denotes the relative risk of LNU when compared to LRN. CI: confidence interval; CPR: cardiopulmonary resuscitation; DVT: deep vein thrombosis; IQR: interquartile range; LRN: laparoscopic radical nephrectomy; LNU: laparoscopic nephroureterectomy; NSQIP: National Surgical Quality Improvement Program.
Fig. 1.
Complications after laparoscopic nephroureterectomy (LNU) and laparoscopic radical nephrectomy (LRN) cases in NSQIP from 2006–2012.
A multivariable model was created to adjust for patient and surgical factors that may confound the association between procedure type and risk of complication. After adjusting for potential confounders, LNU was independently associated with a higher risk of experiencing any complication vs. LRN (RR 1.41, 95% CI 1.16–1.72). The following patient and surgical factors were also independently associated with complications: older age, ASA class 3–5, higher preoperative creatinine, transfusion of >4 units of red blood cells within 72 hours prior of surgery, and >6 hours of operating time (Table 3).
Table 3.
Multivariable analysis of patient and surgical factors associations with complications following LNU and LRN in NSQIP from 2006–2012
| Variable | Relative risk | 95% CI | p value |
|---|---|---|---|
| LNU vs. LRN | 1.41 | 1.16–1.72 | <0.01 |
| Age (increase of one year) | 1.01 | 1.01–1.02 | <0.01 |
| Non-White vs. White | 0.82 | 0.6–1.08 | 0.16 |
| BMI >35 vs. <25 | 0.91 | 0.70–1.18 | 0.49 |
| ASA class 3–5 vs. 1–2 | 1.34 | 1.10–1.63 | <0.01 |
| Chronic steroid use | 1.13 | 0.79–1.61 | 0.50 |
| Dialysis preoperatively | 0.68 | 0.43–1.09 | 0.11 |
| Disseminated cancer | 1.36 | 0.96–1.93 | 0.08 |
| Current smoker | 0.91 | 0.73–1.14 | 0.43 |
| Preoperative creatinine (increase by one) | 1.11 | 1.06–1.17 | <0.01 |
| Transfusion of >4 units PRBC within 72 hours prior to surgery | 1.93 | 1.29–2.86 | <0.01 |
| >6 hours of operating time | 2.17 | 1.71–2.75 | <0.01 |
ASA: American Society of Anesthesiologists; BMI: body mass index; LNU: laparoscopic nephroureterectomy; LRN: laparoscopic radical nephrectomy; NSQIP: National Surgical Quality Improvement Program; PRBC: packed red blood cells.
Discussion
Accurate knowledge of perioperative risk is important for valid patient consent and to identify patients most in need of prophylactic interventions. Since similar anatomic dissection is performed during LRN and LNU, surgeons may believe the associated risks to patients are similar. Furthermore, since LRN is performed more frequently than LNU, the experience of LRN patients may be predominately used by surgeons when counseling patients prior to a LNU. Using a large contemporary cohort of patients, LNU was associated with a 40% higher risk of perioperative complications compared to LRN.
The observed complications in this study are consistent with those reported in previous studies of laparoscopic renal surgery. To our knowledge, this is the first study that has compared complications of LRN and LNU. Previous studies examining each procedure alone report complication rates of 11–31% and 21–37% for LRN and LNU, respectively.6, 9–12 Higher morbidity with LNU may be due to the distal ureter and bladder dissection7 or may be related to differences in patient populations. Indeed, LNU patients in NSQIP were older, had worse functional status, and were more likely to smoke. Additionally, upper tract urothelial carcinoma is generally more challenging to clinically stage than renal parenchymal tumours.13 LNU patients might, therefore, have more advanced disease than was expected based on preoperative images and this advanced stage could contribute to the higher postoperative morbidity. Lymph node dissection may also be more commonly performed during LNU and this aspect of the procedure may add to morbidity.
The results of this study are more generalizable than previous studies because they were derived from a diverse group of hospitals and surgeons. In addition, NSQIP data are collected to prevent bias by recording all cases or an eight-day cycle at some centres. Finally, trained research nurses collect the data from each site, and previous studies have shown the data to be highly accurate with very low inter-rater variability.8
There are some notable limitations in this study. Certain preoperative variables of interest are not recorded in NSQIP, such as tumour subtype, stage, and grade. Also, some complications of interest were not available, such as gastrointestinal ileus.6,9,10 In addition, for the LNU procedures, NSQIP did not clearly delineate how the distal ureter was managed. Many approaches for the LNU have been described in the literature, including traditional open excision, pure laparoscopic excision, extravesical stapling, transvesical ligation and detachment, transurethral resection of the ureteric orifice (pluck technique), or ureteric intussusception and stripping.7,14 As these techniques may be associated with different risks of complication, it is possible that these findings are not applicable to all approaches. For some outcomes, there was a small absolute difference in risk that was statistically significant due to the large sample size. Care should be taken to interpret these findings in the context of absolute risk. Finally, no data was available to determine the severity of each adverse event.
Conclusion
Postoperative complications are common in laparoscopic renal surgery, with LNU having a higher incidence than LRN. Surgeons should be aware of these differences and incorporate this information in preoperative counseling and perioperative management of their patients.
Footnotes
Competing interests: Dr. Morash has been an Advisory Board member for Abbvie, Astellas, Ferring, Janssen, and Sanofi; and has participated in clinical trials for Abbvie. The remaining authors declare no competing financial or personal interests.
This paper has been peer-reviewed.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Krogh J, Kvist E, Rye B. Transitional cell carcinoma of the upper urinary tract: Prognostic variables and postoperative recurrences. Br J Urol. 1991;67:32–6. doi: 10.1111/j.1464-410X.1991.tb15064.x. [DOI] [PubMed] [Google Scholar]
- 3.Tsujihata M, Nonomura N, Tsujimura A, et al. Laparoscopic nephroureterectomy for upper tract transitional cell carcinoma: Comparison of laparoscopic and open surgery. Eur Urol. 2006;49:332–6. doi: 10.1016/j.eururo.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 4.McDougall E, Clayman R, Elashry O. Laparoscopic nephroureterectomy for upper tract transitional cell cancer: The Washington University experience. J Urol. 1995;154:975–80. doi: 10.1016/S0022-5347(01)66949-0. [DOI] [PubMed] [Google Scholar]
- 5.Parsons JK, Varkarakis I, Rha KH, et al. Complications of abdominal urologic laparoscopy: Longitudinal five-year analysis. Urology. 2004;63:27–32. doi: 10.1016/j.urology.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Hung-Jui T, Wolf J, Zaojun Y, et al. Complications and failure to rescue after laparoscopic versus open radical nephrectomy. J Urol. 2011;186:1254–60. doi: 10.1016/j.juro.2011.05.074. [DOI] [PubMed] [Google Scholar]
- 7.Phe V, Cussenot O, Bitker MO, et al. Does the surgical technique for management of the distal ureter influence the outcome after nephroureterectomy? BJU Int. 2010;108:130–8. doi: 10.1111/j.1464-410X.2010.09835.x. [DOI] [PubMed] [Google Scholar]
- 8.National Surgical Quality Improvement Program. Chicago IL: American College of Surgeons; 2005. Available from: www.acsnsqip.org Accessed September 27, 2014. [Google Scholar]
- 9.Permpongkosol S, Link R, Su L, et al. Complications of 2775 urological laparoscopic procedures: 1993 to 2005. J Urol. 2007;177:580–5. doi: 10.1016/j.juro.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 10.Pareek G, Hedican S, Gee J, et al. Meta-analysis of the complications of laparoscopic renal surgery: Comparison of procedures and techniques. J Urol. 2006;175:1208–13. doi: 10.1016/S0022-5347(05)00639-7. [DOI] [PubMed] [Google Scholar]
- 11.Kim F, Rha KH, Hernandez F, et al. Laparoscopic radical versus partial nephrectomy: Assessment of complications. J Urol. 2003;170:408–11. doi: 10.1097/01.ju.0000076017.26789.6a. [DOI] [PubMed] [Google Scholar]
- 12.Lin K, Lehman E, Krabbe LM, et al. Preoperative factors associated with complications following radical nephroureterectomy. J Urol. 2014;191:e895. doi: 10.1016/j.juro.2014.02.2425. [DOI] [Google Scholar]
- 13.Lughezzani G, Burger M, Margulis V, et al. Prognostic factors in upper urinary tract urothelial carcinomas: A comprehensive review of the current literature. Eur Urol. 2012;62:100–14. doi: 10.1016/j.eururo.2012.02.030. [DOI] [PubMed] [Google Scholar]
- 14.Ghavamian R. Complications of Laparoscopic and Robotic Urologic Surgery. New York, NY: Springer; 2010. Complications of laparoscopic radical nephrectomy and nephroureterectomy; pp. 113–126. [Google Scholar]