Abstract
Introduction:
We aimed to evaluate the predictive value of the Cancer of the Prostate Risk Assessment Postsurgical Score (CAPRA-S) for patients treated with radical prostatectomy followed by subsequent external beam radiotherapy (EBRT).
Methods:
A total of 373 patients treated with EBRT between January 2000 and June 2015 were identified in the institutional database. Followup and complete CAPRA-S score were available for 334 (89.5%) patients. CAPRA-S scores were sorted into previously defined categories of low- (score 0–2), intermediate- (3–5), and high-risk (6–12). Time to biochemical recurrence (BCR) was defined as prostate-specific antigen (PSA) >0.20 ng/mL after EBRT. Survival analyses were performed using the Kaplan-Meier method and comparisons were made using the log-rank test.
Results:
Overall median time from surgery to EBRT was 18 months (interquartile range [IQR] 8–36) and median followup since EBRT was 48 months (IQR 28–78). CAPRA-S predicted time to BCR (<0.001), time to palliative androgen-deprivation therapy (ADT) (p=0.017), and a trend for significantly predicting overall survival (OS, p=0.058). On multivariate analysis, the CAPRA-S was predictive of time to BCR only (low-risk vs. intermediate-risk; hazard ratio [HR] 0.14, 95% confidence interval [CI] 0.043–0.48, p=0.001). The last PSA measurement before EBRT as a continuous and grouped variable proved highly significant in predicting all outcomes tested, including OS (p≤0.002).
Conclusions:
CAPRA-S predicts time to BCR and freedom from palliative ADT, and is borderline significant for OS. Together with the PSA before EBRT, CAPRA-S is a useful, predictive tool. The main limitation of this study is its retrospective design.
Introduction
The Cancer of the Prostate Risk Assessment Postsurgical (CAPRA-S) score is a post-surgical prediction tool that uses preoperative prostate-specific antigen (PSA) and pathologic parameters from radical prostatectomy specimen histopatho-logical exam to predict cancer recurrence and mortality.1 Developed by Cooperberg et al, this tool generates a score of 0–12. In general, each two-point increase indicates at least a doubling of recurrence risk. The CAPRA-S offers good discriminatory accuracy, calibration, and ease of calculation for clinical and research settings, and has been validated externally following radical prostatectomy (RP).2–4
Assuming that biochemical recurrence (BCR) after RP will mostly be related to local disease recurrence, external beam radiotherapy (EBRT) after RP would be expected to improve outcome. Our objective is to evaluate if the CAPRA-S score further predicts outcome for patients treated both with RP and EBRT, and whether other factors might be identified in this setting. To the best of our knowledge, this has not yet been reported in the literature.
Methods
Following ethical review board approval, we retrospectively reviewed all men included in our institutional database who received EBRT either in the adjuvant or salvage setting, between January 2000 and June 2015. Among the 373 patients identified, followup was available for 347 patients. Moreover, incomplete data prevented us from calculating CAPRA-S in another 13 patients, thus leaving 334 men available for final analysis (89.5%). Of those, 136 (40.7%) were included in prospective trials (RTOG 9601, RTOG 0534, and NCIC-RADICALS). A total of 56 patients received androgen-deprivation therapy (ADT) for more than six months.
During chart review, we recorded the constituting variables of CAPRA-S (i.e., pre-EBRT PSA and PSA before surgery, pathological Gleason score, status of surgical margins, as well as presence or absence of extracapsular extension, seminal vesicle involvement [SVI] and lymph node involvement). Table 1 demonstrates point distribution of CAPRA-S among the cohort. Patients were categorized as low-risk (CAPRA-S score 0–2), intermediate-risk (score 3–5), and high-risk (score ≥6), as in the original publication.1 Further collected data included the last PSA at the initiation of salvage EBRT, age at time of EBRT, use of concomitant ADT, date of BCR defined as an increasing PSA of at least 0.2 ng/mL, initiation of palliative ADT, and presence of radiological metastatic disease.
Table 1.
Clinical and pathological characteristics (n=334)
| Characteristic | Number of patients (%) | Points on CAPRA-S |
|---|---|---|
| Age at EBRT median (IQR) | 64 (range 59–68) | |
| <70 years | 277 (82.9) | |
| ≥70 years | 57 (17.1) | |
| Preoperative PSA (ng/mL) median (IQR) | 7 (5–12) | |
| 0–6 | 118 (35.3) | 0 |
| 6.01–10 | 106 (31.7) | 1 |
| 10.01–20 | 79 (23.7) | 2 |
| >20 | 31 (9.3) | 3 |
| Gleason score in surgical specimen | ||
| 5–6 | 72 (21.6) | 0 |
| 3+4 | 93 (27.8) | 1 |
| 4+3 | 89 (26.6) | 2 |
| 8–10 | 80 (24.0) | 3 |
| Extracapsular extension | ||
| Yes | 205 (61.4) | 1 |
| Seminal vesicle extension | ||
| Yes | 69 (20.7) | 2 |
| Surgical margin status | ||
| Positive | 213 (63.8) | 2 |
| Lymph node status | ||
| Positive | 13 (3.9) | 1 |
| CAPRA-S | ||
| 0–2 (low risk) | 52 (15.6) | |
| 3–5 (intermediate risk) | 149 (44.6) | |
| ≥6 (high risk) | 132 (39.5) | |
| Concomitant ADT | ||
| Yes | 121 | |
| 4 months | 8 (6.6) | |
| 6 months | 57 (47.1) | |
| 24 months | 56 (46.3) |
ADT: androgen-deprivation therapy; EBRT: external beam radiotherapy; IQR: interquartile range; PSA: prostate-specific antigen.
Most patients received EBRT to the prostate bed up to a total dose of 66 Gy in 2 Gy/fraction, five sessions a week; alternatively, 64.8–66.6 Gy in 1.8 Gy/fraction were administered for patients enrolled in two out of three aforementioned studies, as per that particular protocol. Standard conformal three-dimensional or intensity-modulated radiotherapy (IMRT) technique was used with a 4–6 MV linear accelerator. If a macroscopic recurrence within the prostate bed was evidenced by computed tomography (CT) scan or rectal examination, EBRT was increased to a total dose of 70–72 Gy in 2 Gy/fraction (in three patients). Whether pelvic lymph nodes were included (and treated up to 44–46 Gy) was left to the discretion of the treating radiation oncologist, as was the prescription of concomitant ADT, according to the randomized arm of the treatment protocol. Survival analysis was performed using the Kaplan-Meier method and comparisons were made using the log rank test. Statistical significance was defined for p values ≤0.05. Statistical analysis was done using SPSS 17.0 for Windows (IBM SPSS, Chicago, IL).
Results
Median age at time of EBRT was 64 years (interquartile range [IQR] 59–68). Median time from surgery to EBRT was 18 months (IQR 8–36). Median followup after EBRT was 48 months (IQR 28–78) (Table 1). Concomitant ADT was administered in 36.2% of cases, for a median duration of nine months (IQR 6–24).
Forty patients (12.0%) received EBRT within four months of surgery; among them, 10 patients had a PSA of 0.0 ng/ml, four patients between 0.01 and 0.03 ng/ml, and six patients between 0.04 and 0.10 ng/ml. The CAPRA-S in these patients was >5 in 80% (16/20). Table 2 lists the PSA values before EBRT. Median PSA value was 0.35 ng/mL (IQR 0.2–0.7).
Table 2.
PSA before EBRT (ng/ml)
| Group | Last PSA before EBRT (ng/mL) | (%) |
|---|---|---|
| 1 | 0.00–0.20 | 26.2 |
| 2 | 0.21–0.50 | 38.9 |
| 3 | 0.51–0.99 | 18.9 |
| 4 | 1.00–2.00 | 11.5 |
| 5 | >2.00 | 4.5 |
ERBT: external beam radiotherapy; PSA: prostate-specific antigen.
During followup, palliative ADT was initiated for BCR in 20.9% of cases; median time between EBRT and initiation of palliative ADT was 38 months (IQR 20–66). Metastatic disease was found on imaging in 8.0% of cases; median time from EBRT to distant metastatic spreading was 44 months (IQR 24–75).
Twenty-two patients (6.6%) died during follow-up, nine from metastatic prostate cancer, seven from other cancers, and one patient from cardio-vascular disease; in 5 of them, the primary cause of death could not be determined. Median time from EBRT to death was 48 months (IQR 28–78).
Freedom from BCR, palliative ADT (Fig. 1), metastatic spreading, and overall survival (OS) are reported in Table 3, according to CAPRA-S score and other clinical factors. CAPRA-S as a categorical variable (low-, intermediate-, high-risk group) was predictive of freedom from BCR and palliative ADT use, and borderline significant (p=0.058) of OS. When considering only the CAPRA-S high-risk group, OS at five and 10 years were 90% and 83%, respectively. In this high-risk group, 62% had not received ADT for recurrence at five years and 47% at 10 years.
Fig. 1.
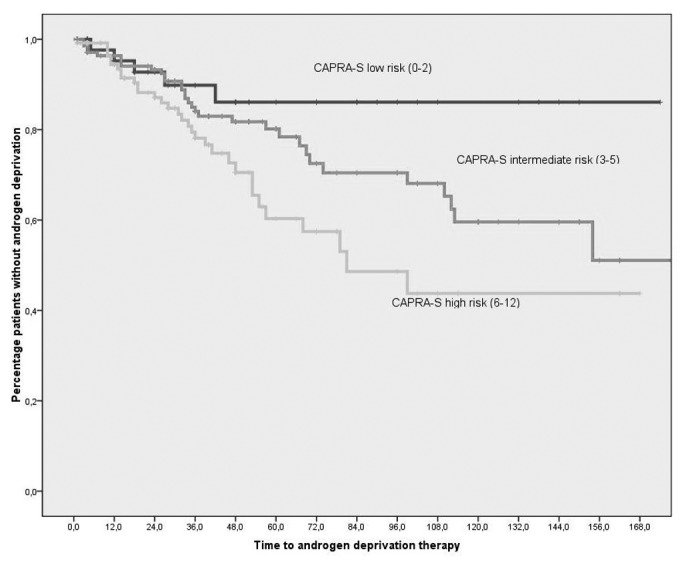
Freedom from androgen-deprivation therapy after external beam radiotherapy in months according to CAPRA-S groups (p=0.017, log-rank test).
Table 3.
Influence of CAPRA-S and other clinical factors on OS, time to metastasis, and time to palliative ADT.
| OS (p value) | Time to metastasis (p value) | Time to ADT (p value) | Time to BCR (p value) | |
|---|---|---|---|---|
| CAPRA-S* | 0.33 | 0.36 | 0.127 | 0.006 |
| CAPRA-S† | 0.058 | 0.15 | 0.017 | <0.001 |
| PSA (CAPRA-S categories) | 0.48 | 0.56 | 0.59 | 0.53 |
| Gleason (CAPRA-S categories) | 0.10 | 0.001 | <0.001 | 0.011 |
| ECE | 0.37 | 0.58 | 0.29 | 0.019 |
| SVI | 0.16 | 0.003 | 0.003 | <0.001 |
| SM | 0.47 | 0.003 | 0.049 | 0.46 |
| LNI | 0.16 | <0.001 | 0.28 | 0.010 |
| PSA pre-EBRT cont. | <0.001 | <0.001 | <0.001 | 0.002 |
| PSA pre-EBRT, cat. | <0.001 | 0.026 | 0.030 | 0.08 |
| Concurrent ADT to EBRT | 0.23 | 0.028 | <0.001 | 0.82 |
| Age (<70 vs. ≥70 years) | 0.21 | 0.77 | 0.72 | 0.16 |
Continuous variable;
Categorical variable. Results reaching statistical significance (p<0.05) are in bold. ADT: androgen-deprivation therapy; BCR: biochemical recurrence; EBRT: external beam radiotherapy; ECE: extracapsular extension; LNI: lymph node invasion; OS: overall survival; PSA: prostate-specific antigen; SM: positive surgical margins; SVI: seminal vesicle invasion.
All of the CAPRA-S subcategories were significant predictors of several endpoints, whereas extracapsular extension (ECE) was only predictive of BCR.
Using predefined PSA categories before EBRT (according to Table 2), we found that each rise to a higher group increases the hazard of BCR by 28.6% (hazard ratio [HR] 1.286; 95% confidence interval [CI] 1.009–1.641). When evaluating the PSA before EBRT as a continuous variable, each increase of 1 ng/mL translated into a 20% increase in the risk of BCR (HR 1.199; 95% CI 1.070–1.343).
In a multivariate analysis including age (<70 vs. ≥70 years) and both the pre-EBRT PSA level and the CAPRA-S score as categorical variables, the CAPRA-S was predictive of time to BCR (low-risk vs. intermediate-risk; HR 0.14, 95% CI 0.043–0.48, p=0.001), but not of time to palliative ADT (p=0.28), metastasis-free survival (p=0.18), or OS (p=0.94). The pre-EBRT PSA was predictive of time to palliative ADT (p=0.015), metastasis-free survival (p=0.014), and OS (p=0.037). Age was not predictive of any endpoints.
Discussion
We found that the CAPRA-S score predicted the freedom from BCR and palliative ADT, but not the time to metastases on univariate analysis. There was a trend towards significance for OS (p=0.058). On multivariate analysis, the CAPRA-S was only predictive of time to BCR. The pre-EBRT PSA was the most significant prognostic factor predicting for time to palliative ADT, metastasis-free survival, and OS.
The management of a detectable PSA after RP remains a difficult challenge, as not all patients may benefit from salvage EBRT. Furthermore, up to 40% of patients will experience BCR after RP5,6 and a rising PSA will precede clinical progression in most cases. Still, a small subset of patients with detectable PS3365A recurrence after RP will not exhibit biochemical or clinical progression, even after 10 years of followup.7
The CAPRA-S score has been developed to stratify patients and help predict the risk of prostate cancer recurrence and mortality following RP, based on pathological data from the surgical specimen and preoperative PSA. Our aim was to evaluate the CAPRA-S score in a population of patients with mostly high-risk of BCR and to assess whether the CAPRA-S score retains its predictive value after both RP and salvage EBRT. To the best of our knowledge, we are unaware of any similar study published before. Our study shows that even in patients with CAPRA-S >5, and thus at high-risk for recurrence and mortality, only 47% received ADT at 10-year followup; even in high-risk patients, EBRT is efficient in only about half of cases. In patients with a CAPRA-S of ≥8 (n=58), even though only 32% showed no BCR at five years, 44% of patients were still without ADT.
The fact that most factors, with the exception of the pre-EBRT PSA, were not predictive of OS but of other endpoints is not surprising. Palliative ADT occurs early in the course of disease, delaying the occurrence of metastases and death by years. The median followup of 48 months after EBRT might have been too short to detect an influence of the CAPRA-S on time to metastasis or OS. These patients continue to be followed and we will later be able to update these endpoints. In one of the series with the longest followup (median 126 months), only 56% experienced BCR after salvage EBRT; OS averaged 13.6 years from the time of PSA failure.8 Conversely, we might also interpret the present results as showing the benefit of EBRT in a majority of patients presenting with persistent or recurrent prostate cancer.
Patients who received concomitant ADT and EBRT experienced a longer time to distant metastases (p=0.02), without translating into improved OS (p=0.22). Regarding the absence of benefit for OS, we might hypothesize that the expected positive effect from the concomitant ADT on OS was countered by an excess of mortality from either the cardiovascular causes or from a followup that was too short.
The CAPRA-S score showed a trend towards an influence on OS (p=0.058); however, none of the single factors contributing to the CAPRA-S total score were predictive of OS, nor were age or concomitant ADT. The only factor predicting for OS was the PSA before EBRT (p<0.001). A higher PSA at the start of EBRT might reflect micro-metastatic disease. The percentage of patients not on ADT for recurrence at five years according to the groups described in Table 2 was 72%, 78%, 51%, 55%, and 36%, respectively.
In a retrospective survey of 151 patients (median followup of 82 months), Lohm et al demonstrated a benefit on BCR from starting EBRT at lower PSA values; the best results were actually achieved with a PSA in the lowest quartile (<0.147 ng/ml) before EBRT9 and this was highly predictive of BCR (p< 0.0005), however, the pre-EBRT PSA had no impact on prostate cancer-specific survival (p=0.16) or OS (p=0.81).
We found that each 1 ng/ml increase of the last PSA before EBRT translated into a shorter time of freedom from BCR by 19.9% on average (HR 1.199; 95% CI 1.070–1.343). These results are in line with a systematic review and meta-analysis suggesting, on a multivariate analysis, that biochemical progression-free survival decreases with pre-EBRT PSA by 18.3% per 1 ng/mL (p < 0.001).10 Similar results were also reported in another systematic review performed by King, who found an average 2.6% loss of recurrence-free survival for each incremental 0.1 ng/ml PSA at the time of RT.11 Our paper contributes to the growing evidence that patients with PSA recurrence after surgery should be referred and treated at lower PSA levels to achieve better long-term outcomes.
This study is not without its limitations. This is a retrospective series, which may have introduced a selection bias. PSA assays differed during the studied years; 46% of our patients were treated with EBRT before 2010. Ultrasensitive PSA assays now allow for earlier detection of biochemical relapse after RP.12 Furthermore, the first postoperative ultra-sensitive PSA ≥0.03 ng/ml has been demonstrated recently to be an independent factor that identifies biochemical relapse more accurately, yielding a median lead time advantage of 18 months over the conventional definition of PSA ≥0.20 ng/ml.13
Followup for patients enrolled in prospective studies was more frequent and prolonged than for off-study-treated patients. Furthermore, different PSA cutoff values were used before starting palliative ADT. CT scan and bone scans were not performed on a fixed schedule, but left to the discretion of the treating physician. Finally, we did not have any control group (RP without EBRT), preventing us to evaluate whether low-risk patients, according to CAPRA-S score, benefit from EBRT after RP. In a larger, retrospective analysis of a cohort of 635 men who experienced biochemical and/or local recurrence after RP, where 397 received no salvage treatment, 160 were treated with EBRT alone, and 78 combined with ADT, Trock et al demonstrated that salvage EBRT alone was associated with a significant three-fold increase in prostate cancer-specific survival (HR 0.34, p=0.003), relative to those who received no salvage treatment.14 The beneficial effect of salvage EBRT appeared primarily confined to patients with a PSA doubling time <6 months, and treated within two years following BCR.14
Despite its merits, and being the first study reporting CAPRA-S outcomes in this unique population of multimodal therapy, this study has its limitations: single-institution study, small cohort, and patients predominantly treated for PSA recurrence or persistence and not in an adjuvant setting.
Prospective studies should investigate whether low-risk patients, according to CAPRA-S, would actually benefit from EBRT after RP.
Conclusion
For prostate cancer patients treated either with adjuvant or salvage EBRT, CAPRA-S groups predicted freedom from BCR and palliative ADT, and showed a trend towards significance for OS. The data also support a strong correlation between the PSA before EBRT and all outcomes, including OS. This seems to favour early salvage EBRT.
Acknowledgments
The authors would like to thank Dr. Fred Saad for his continuous support in maintaining the database.
Footnotes
Competing interests: Dr. Zimmermann has received grants/honoraria from Paladin; Dr. Zorn has been an Advisory Board member for American Medical Systems. The remaining authors declare no competing financial or personal interests.
This paper has been peer-reviewed.
References
- 1.Cooperberg MR, Hilton JF, Carroll PR. The CAPRA-S score: A straightforward tool for improved prediction of outcomes after radical prostatectomy. Cancer. 2011;117:5039–46. doi: 10.1002/cncr.26169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Punnen S, Freedland SJ, Presti JC, Jr, et al. Multi-institutional validation of the CAPRA-S score to predict disease recurrence and mortality after radical prostatectomy. Eur Urol. 2014;65:1171–7. doi: 10.1016/j.eururo.2013.03.058. [DOI] [PubMed] [Google Scholar]
- 3.Seong KT, Lim JH, Park CM, et al. External validation of the CAPRA-S score in Koreans undergoing radical prostatectomy. Korean J Urol. 2013;54:433–6. doi: 10.4111/kju.2013.54.7.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tilki D, Mandel P, Schlomm T, et al. External validation of the CAPRA-S score to predict biochemical recurrence, metastasis, and mortality after radical prostatectomy in a European cohort. J Urol. 2015;193:1970–5. doi: 10.1016/j.juro.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 5.Cooperberg MR, Freedland SJ, Pasta DJ, et al. Multi-institutional validation of the UCSF cancer of the prostate risk assessment for prediction of recurrence after radical prostatectomy. Cancer. 2006;107:2384–91. doi: 10.1002/cncr.22262. [DOI] [PubMed] [Google Scholar]
- 6.Novara G, Ficarra V, Mocellin S, et al. Systematic review and meta-analysis of studies reporting oncologic outcome after robot-assisted radical prostatectomy. Eur Urol. 2012;62:382–404. doi: 10.1016/j.eururo.2012.05.047. [DOI] [PubMed] [Google Scholar]
- 7.Shinghal R, Yemoto C, McNeal JE, et al. Biochemical recurrence without PSA progression characterizes a subset of patients after radical prostatectomy. Prostate-specific antigen. Urology. 2003;61:380–5. doi: 10.1016/S0090-4295(02)02254-9. [DOI] [PubMed] [Google Scholar]
- 8.Ying J, Wang CJ, Yan J, et al. Long-term outcome of prostate cancer patients who exhibit biochemical failure despite salvage radiation therapy after radical prostatectomy. Am J Clin Oncol. 2015 Jul 9; doi: 10.1097/COC.0000000000000207. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Lohm G, Lütcke J, Jamil B, et al. Salvage radiotherapy in patients with prostate cancer and biochemical relapse after radical prostatectomy: Long-term followup of a single-centre survey. Strahlenther Onkol. 2014;190:727–31. doi: 10.1007/s00066-014-0612-6. [DOI] [PubMed] [Google Scholar]
- 10.Ohri N, Dicker AP, Trabulski EJ, et al. Can early implementation of salvage radiotherapy for prostate cancer improve the therapeutic ratio? A systematic review and regression meta-analysis with radiobiological modelling. Eur J Cancer. 2012;48:837–44. doi: 10.1016/j.ejca.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.King CR. The timing of salvage radiotherapy after radical prostatectomy: A systematic review. Int J Radiat Oncol Biol Phys. 2012;84:104–11. doi: 10.1016/j.ijrobp.2011.10.069. [DOI] [PubMed] [Google Scholar]
- 12.Haese A, Huland E, Graefen M, et al. Ultrasensitive detection of prostate-specific antigen in the followup of 422 patients after radical prostatectomy. J Urol. 1999;161:1206–11. doi: 10.1016/S0022-5347(01)61635-5. [DOI] [PubMed] [Google Scholar]
- 13.Kang JJ, Reiter RE, Steinberg ML, et al. Ultrasensitive prostate-specific antigen after prostatectomy reliably identifies patients requiring postoperative radiotherapy. J Urol. 2015;193:1532–8. doi: 10.1016/j.juro.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trock BJ, Han M, Freedland SJ, et al. Prostate cancer-specific survival following salvage radiotherapy vs. observation in men with biochemical recurrence after radical prostatectomy. JAMA. 2008;18(299):2760–9. doi: 10.1001/jama.299.23.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]


