Abstract
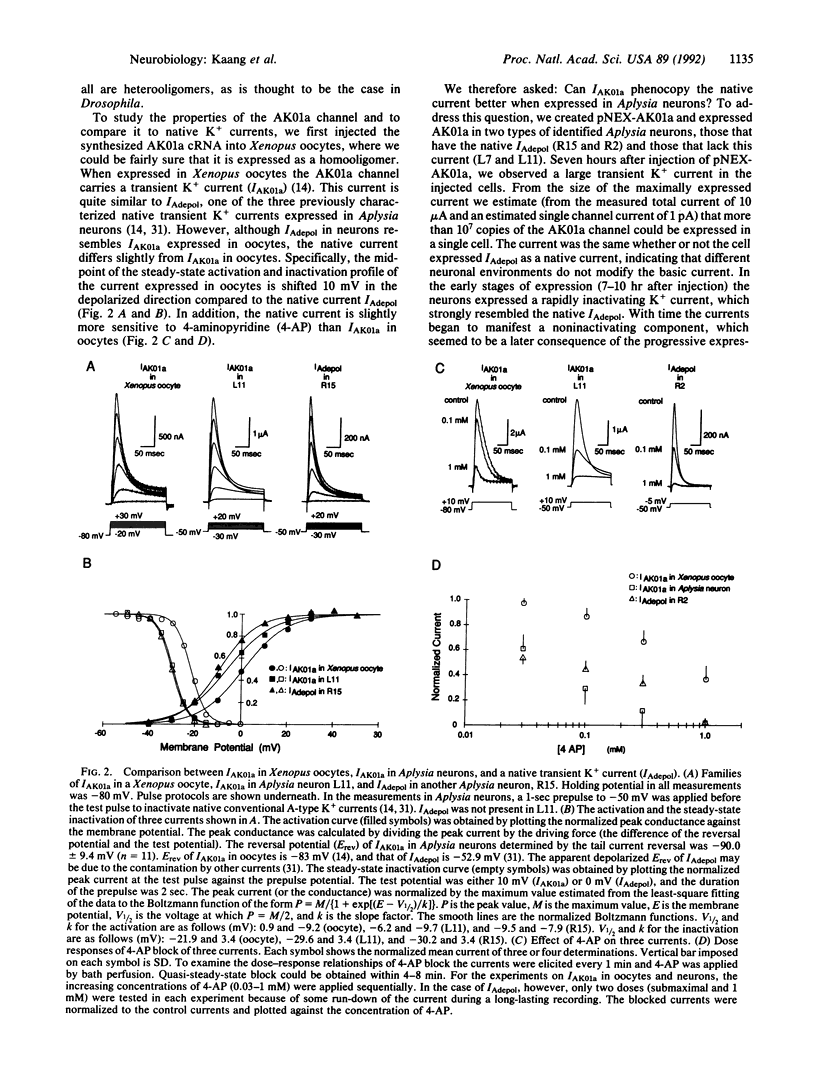
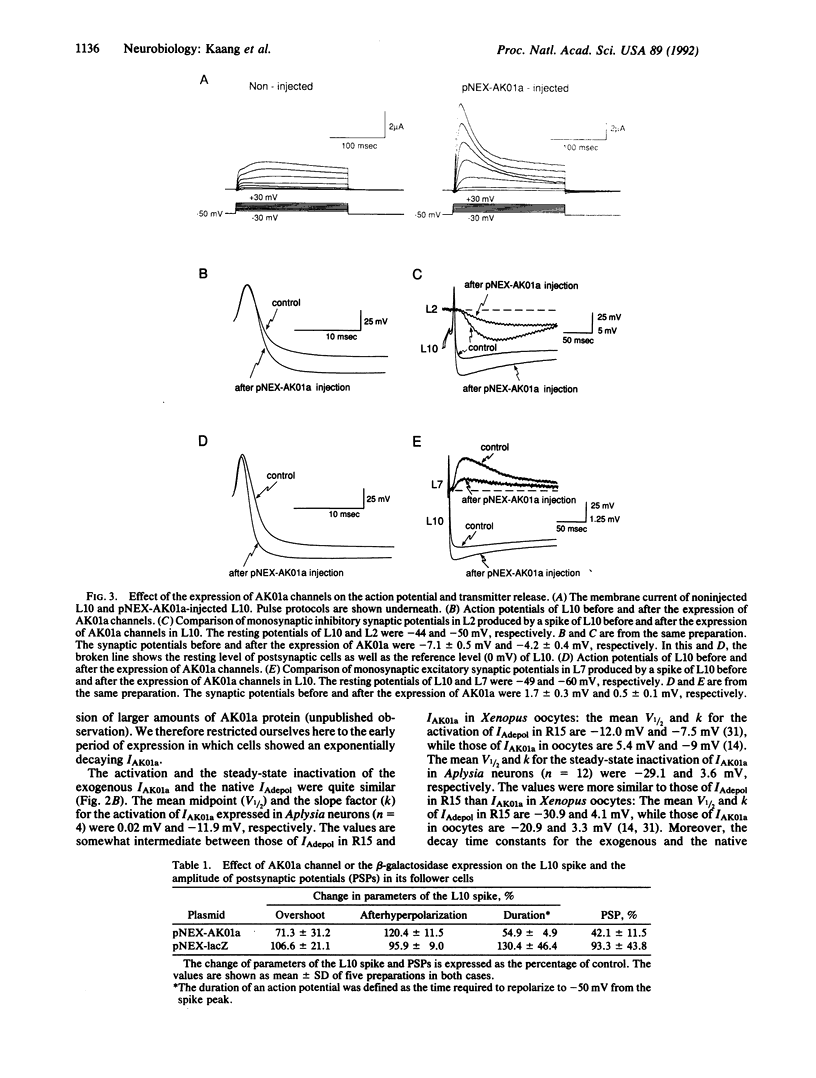
Although potassium channels play a variety of roles in shaping the electrical properties of neurons, little is known about how these channels are constituted in neurons. To examine the assembly and physiological function of A-type K+ channels in mature differentiated neurons, we have developed a highly efficient gene transfer method for Aplysia neurons that has allowed us to express about 10(7) copies of the cloned Aplysia Shaker (Sh) K+ channel (AK01a) in single identified cells. We find that expression of AK01a phenocopies one of the native transient K+ currents (IAdepol), suggesting that the native channel carrying IAdepol is assembled as a homooligomer of AK01a. Overexpression of AK01a has substantial effect on the action potential, shortening its duration, enhancing its hyperpolarizing afterpotential, and depressing by more than half the amount of transmitter release by the action potential from the terminals. Thus, the AK01a channel not only contributes to the firing properties within a given neuron but also can regulate the signaling between interconnected cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkon D. L. Learning in a marine snail. Sci Am. 1983 Jul;249(1):70-4, 76-8, 80-4. doi: 10.1038/scientificamerican0783-70. [DOI] [PubMed] [Google Scholar]
- Alkon D. L., Sakakibara M., Forman R., Harrigan J., Lederhendler I., Farley J. Reduction of two voltage-dependent K+ currents mediates retention of a learned association. Behav Neural Biol. 1985 Sep;44(2):278–300. doi: 10.1016/s0163-1047(85)90296-1. [DOI] [PubMed] [Google Scholar]
- Baumann A., Krah-Jentgens I., Müller R., Müller-Holtkamp F., Seidel R., Kecskemethy N., Casal J., Ferrus A., Pongs O. Molecular organization of the maternal effect region of the Shaker complex of Drosophila: characterization of an I(A) channel transcript with homology to vertebrate Na channel. EMBO J. 1987 Nov;6(11):3419–3429. doi: 10.1002/j.1460-2075.1987.tb02665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter D. A., Byrne J. H. Differential effects of cAMP and serotonin on membrane current, action-potential duration, and excitability in somata of pleural sensory neurons of Aplysia. J Neurophysiol. 1990 Sep;64(3):978–990. doi: 10.1152/jn.1990.64.3.978. [DOI] [PubMed] [Google Scholar]
- Collin C., Ikeno H., Harrigan J. F., Lederhendler I., Alkon D. L. Sequential modification of membrane currents with classical conditioning. Biophys J. 1988 Nov;54(5):955–960. doi: 10.1016/S0006-3495(88)83031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesGroseillers L., Cowan D., Miles M., Sweet A., Scheller R. H. Aplysia californica neurons express microinjected neuropeptide genes. Mol Cell Biol. 1987 Aug;7(8):2762–2771. doi: 10.1128/mcb.7.8.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisch T. M., Prywes R., Roeder R. G. c-fos sequence necessary for basal expression and induction by epidermal growth factor, 12-O-tetradecanoyl phorbol-13-acetate and the calcium ionophore. Mol Cell Biol. 1987 Oct;7(10):3490–3502. doi: 10.1128/mcb.7.10.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner D. Bilateral symmetry and interneuronal organization in the buccal ganglia of Aplysia. Science. 1971 Aug 6;173(3996):550–553. doi: 10.1126/science.173.3996.550. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochner B., Klein M., Schacher S., Kandel E. R. Action-potential duration and the modulation of transmitter release from the sensory neurons of Aplysia in presynaptic facilitation and behavioral sensitization. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8410–8414. doi: 10.1073/pnas.83.21.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. How might the diversity of potassium channels be generated? Trends Neurosci. 1990 Oct;13(10):415–419. doi: 10.1016/0166-2236(90)90123-r. [DOI] [PubMed] [Google Scholar]
- Kamb A., Iverson L. E., Tanouye M. A. Molecular characterization of Shaker, a Drosophila gene that encodes a potassium channel. Cell. 1987 Jul 31;50(3):405–413. doi: 10.1016/0092-8674(87)90494-6. [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Frazier W. T., Waziri R., Coggeshall R. E. Direct and common connections among identified neurons in Aplysia. J Neurophysiol. 1967 Nov;30(6):1352–1376. doi: 10.1152/jn.1967.30.6.1352. [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Schwartz J. H. Molecular biology of learning: modulation of transmitter release. Science. 1982 Oct 29;218(4571):433–443. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- Klein M., Camardo J., Kandel E. R. Serotonin modulates a specific potassium current in the sensory neurons that show presynaptic facilitation in Aplysia. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5713–5717. doi: 10.1073/pnas.79.18.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koester J., Kandel E. R. Further identification of neurons in the abdominal ganglion of Aplysia using behavioral criteria. Brain Res. 1977 Jan 31;121(1):1–20. doi: 10.1016/0006-8993(77)90435-8. [DOI] [PubMed] [Google Scholar]
- Leavitt J., Gunning P., Porreca P., Ng S. Y., Lin C. S., Kedes L. Molecular cloning and characterization of mutant and wild-type human beta-actin genes. Mol Cell Biol. 1984 Oct;4(10):1961–1969. doi: 10.1128/mcb.4.10.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Simon S. M. Transmission by presynaptic spike-like depolarization in the squid giant synapse. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2415–2419. doi: 10.1073/pnas.79.7.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
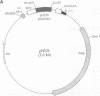
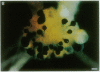
- MacGregor G. R., Caskey C. T. Construction of plasmids that express E. coli beta-galactosidase in mammalian cells. Nucleic Acids Res. 1989 Mar 25;17(6):2365–2365. doi: 10.1093/nar/17.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papazian D. M., Schwarz T. L., Tempel B. L., Jan Y. N., Jan L. Y. Cloning of genomic and complementary DNA from Shaker, a putative potassium channel gene from Drosophila. Science. 1987 Aug 14;237(4816):749–753. doi: 10.1126/science.2441470. [DOI] [PubMed] [Google Scholar]
- Pfaffinger P. J., Furukawa Y., Zhao B., Dugan D., Kandel E. R. Cloning and expression of an Aplysia K+ channel and comparison with native Aplysia K+ currents. J Neurosci. 1991 Apr;11(4):918–927. doi: 10.1523/JNEUROSCI.11-04-00918.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacher S., Proshansky E. Neurite regeneration by Aplysia neurons in dissociated cell culture: modulation by Aplysia hemolymph and the presence of the initial axonal segment. J Neurosci. 1983 Dec;3(12):2403–2413. doi: 10.1523/JNEUROSCI.03-12-02403.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro E., Castellucci V. F., Kandel E. R. Presynaptic membrane potential affects transmitter release in an identified neuron in Aplysia by modulating the Ca2+ and K+ currents. Proc Natl Acad Sci U S A. 1980 Jan;77(1):629–633. doi: 10.1073/pnas.77.1.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegelbaum S. A., Camardo J. S., Kandel E. R. Serotonin and cyclic AMP close single K+ channels in Aplysia sensory neurones. Nature. 1982 Sep 30;299(5882):413–417. doi: 10.1038/299413a0. [DOI] [PubMed] [Google Scholar]
- Tsukada T., Horovitch S. J., Montminy M. R., Mandel G., Goodman R. H. Structure of the human vasoactive intestinal polypeptide gene. DNA. 1985 Aug;4(4):293–300. doi: 10.1089/dna.1985.4.293. [DOI] [PubMed] [Google Scholar]
- Van Nguyen T., Kobierski L., Comb M., Hyman S. E. The effect of depolarization on expression of the human proenkephalin gene is synergistic with cAMP and dependent upon a cAMP-inducible enhancer. J Neurosci. 1990 Aug;10(8):2825–2833. doi: 10.1523/JNEUROSCI.10-08-02825.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachtel H., Kandel E. R. A direct synaptic connection mediating both excitation and inhibition. Science. 1967 Dec 1;158(3805):1206–1208. doi: 10.1126/science.158.3805.1206. [DOI] [PubMed] [Google Scholar]
- Zhong Y., Wu C. F. Alteration of four identified K+ currents in Drosophila muscle by mutations in eag. Science. 1991 Jun 14;252(5012):1562–1564. doi: 10.1126/science.2047864. [DOI] [PubMed] [Google Scholar]