Abstract
STUDY QUESTION
Among women who carry pathogenic mitochondrial DNA (mtDNA) point mutations and healthy oocyte donors, what are the levels of support for developing oocyte mitochondrial replacement therapy (OMRT) to prevent transmission of mtDNA mutations?
SUMMARY ANSWER
The majority of mtDNA carriers and oocyte donors support the development of OMRT techniques to prevent transmission of mtDNA diseases.
WHAT IS KNOWN ALREADY
Point mutations of mtDNA cause a variety of maternally inherited human diseases that are frequently disabling and often fatal. Recent developments in (OMRT) as well as pronuclear transfer between embryos offer new potential options to prevent transmission of mtDNA disease. However, it is unclear whether the non-scientific community will approve of embryos that contain DNA from three people.
STUDY DESIGN, SIZE, DURATION
Between 1 June 2012 through 12 February 2015, we administered surveys in cross-sectional studies of 92 female carriers of mtDNA point mutations and 112 healthy oocyte donors.
PARTICIPANTS/MATERIALS, SETTING, METHODS
The OMRT carrier survey was completed by 92 female carriers of an mtDNA point mutation. Carriers were recruited through the North American Mitochondrial Disease Consortium (NAMDC), the United Mitochondrial Disease Foundation (UMDF), patient support groups, research and private patients followed at the Columbia University Medical Center (CUMC) and patients' referrals of maternal relatives. The OMRT donor survey was completed by 112 women who had donated oocytes through a major in vitro fertilization clinic.
MAIN RESULTS AND THE ROLE OF CHANCE
All carriers surveyed were aware that they could transmit the mutation to their offspring, with 78% (35/45) of women, who were of childbearing age, indicating that the risk was sufficient to consider not having children, and 95% (87/92) of all carriers designating that the development of this technique was important and worthwhile. Of the 21 surveyed female carriers considering childbearing, 20 (95%) considered having their own biological offspring somewhat or very important and 16 of the 21 respondents (76%) were willing to donate oocytes for research and development. Of 112 healthy oocyte donors who completed the OMRT donor survey, 97 (87%) indicated that they would donate oocytes for generating a viable embryo through OMRT.
LIMITATIONS, REASONS FOR CAUTION
Many of the participants were either patients or relatives of patients who were already enrolled in a research-oriented database, or who sought care in a tertiary research university setting, indicating a potential sampling bias. The survey was administered to a select group of individuals, who carry, or are at risk for carrying, mtDNA point mutations. These individuals are more likely to have been affected by the mutation or have witnessed first-hand the devastating effects of these mutations. It has not been established whether the general public would be supportive of this work. This survey did not explicitly address alternatives to OMRT.
WIDER IMPLICATIONS OF THE FINDINGS
This is the first study indicating a high level of interest in the development of these methods among women affected by the diseases or who are at risk of carrying mtDNA mutations as well as willingness of most donors to provide oocytes for the development of OMRT.
STUDY FUNDING/COMPETING INTEREST(S)
This work was conducted under the auspices of the NAMDC (Study Protocol 7404). NAMDC (U54NS078059) is part of the NCATS Rare Diseases Clinical Research Network (RDCRN). RDCRN is an initiative of the Office of Rare Diseases Research (ORDR) and NCATS. NAMDC is funded through a collaboration between NCATS, NINDS, NICHD and NIH Office of Dietary Supplements. The work was also supported by the Bernard and Anne Spitzer Fund and the New York Stem Cell Foundation (NYSCF). Dr Hirano has received research support from Santhera Pharmaceuticals and Edison Pharmaceuticals for studies unrelated to this work. None of the other authors have conflicts of interest.
TRIAL REGISTRATION NUMBER
Not applicable.
Keywords: mitochondria, nuclear transfer, genetic disorders, mitochondrial DNA, mitochondrial disease, in vitro fertilization, oocyte, mitochondrial replacement therapy, mtDNA
Introduction
Mitochondrial disorders, due to defects of the respiratory chain and oxidative phosphorylation, are typically multisystem diseases that often cause severe disability and early death (DiMauro et al., 2006, 2013; Schon et al., 2012). These disorders can be caused by primary defects in nuclear DNA (nDNA) or mitochondrial DNA (mtDNA). Unlike nDNA mutations that are inherited in Mendelian or X-linked patterns, mtDNA defects are transmitted exclusively via maternal inheritance. Another characteristic of most mtDNA mutations is heteroplasmy, the mixture of mutant and normal mtDNA in tissues that have hundreds or thousands of copies of mtDNA in each cell. Heteroplasmy of mtDNA varies from patient to patient and, often, from tissue-to-tissue within an individual. As a consequence of mtDNA heteroplasmy, tissue distribution and maternal inheritance, matrilineal relatives within a pedigree harboring a certain mtDNA mutation frequently manifest clinical diversity ranging from asymptomatic carriers to severely affected infants (Shanske et al., 2004; Kaufmann et al., 2009). Another critical factor contributing to the maternal transmission of mtDNA diseases is the oocyte mutation burden, which can vary widely within an individual female mutation carrier (Blok et al., 1997; Brown et al., 2001; Hellebrekers et al., 2012). The variability in the oocyte mutation burden is dictated by a genetic bottleneck, whose mechanism remains uncertain and debated (Wai et al., 2008; Folmes et al., 2013). Because of skewed segregation of mtDNA mutations through the bottleneck, women with low mutation levels can have offspring with high mutation loads; thus, low heteroplasmy in an asymptomatic female mtDNA mutation carrier does not preclude transmission of an mtDNA disease (Nishigaki et al., 2003; Choi et al., 2010; Sallevelt et al., 2013). As a consequence, within a family with a pathogenic mtDNA mutation, there may be a large number of ‘at risk’ women, all of whom should be considered obligate carriers even with negative genetic testing in blood (Shanske et al., 2004).
Although mtDNA diseases are considered rare, mtDNA mutations are not uncommon. To date, more than 250 point mutations have been identified (Schon et al., 2012). Epidemiological studies have estimated that at least 1 in 5000 working age individuals harbors an mtDNA mutation (Gorman et al., 2015) and have assessed carrier frequency at birth to be ∼1 in 200 for the 10 most common mtDNA point mutations (Elliott et al., 2008). Unfortunately, there is no cure for these disorders and treatment is limited to symptomatic and supportive measures. In this setting, efforts aimed at eliminating disease transmission is paramount.
Options to reduce the risk of transmitting mtDNA point mutations have been suboptimal for female carriers. Genetic counseling has been limited to discussions on the unpredictability of disease severity in offspring. Prenatal diagnosis (PND), through CVS and amniocentesis, has not been useful for diseases due to transfer RNA (tRNA) gene mutations (Shanske et al., 2013). Fetal muscle biopsy has even been used to assess mutation load (Shanske et al., 2013), however this is technically difficult, not available at many centers, and not yet firmly established as a means to assess mtDNA mutation burdens. Furthermore, PND is not suitable for couples who oppose selective abortion.
Preimplantation genetic diagnosis (PGD) has been proposed as a means to reduce mutation load by implanting only embryos having blastomeres with low levels of heteroplasmic mtDNA mutations in biopsied trophoectoderm cells or blastocyts (Poulton and Bredenoord, 2010; Hellebrekers et al., 2012; Sallevelt et al., 2013). Although there are reports of six apparently healthy infants born after PGD revealed ≤12% heteroplasmic mtDNA mutations in blastocysts (Thorburn et al., 2009; Monnot et al., 2011; Hellebrekers et al., 2012; Treff et al., 2012; Sallevelt et al., 2013), one of those children developed complex developmental and multisystemic problems with m.3243A > G heteroplasmy levels of 46–47% in blood and 42–52% in urine (Mitalipov et al., 2014). This case suggests that blastocyst biopsy for PGD may be less accurate than samples of cleavage stage embryos for the m.3243A > G mutation in tRNALeu(UUR) gene. In contrast, PGD with blastocyst biopsy may be more reliable for other mutations in polypeptide-encoding genes, such as the m.8993T > G and m.8993T > C in the ATPase 6 gene (MT-ATP6), that have shown relatively uniform distribution in all tissues, stable mutation loads and thresholds for phenotypic expression that are generally predictable (Steffann et al., 2006; Thorburn et al., 2009; Poulton and Bredenoord, 2010). Nevertheless, PGD is apparently not reliable for most mtDNA point mutations that reside in tRNA genes. PGD has four additional major limitations: (i) carriers may not produce oocytes with mtDNA mutation loads below threshold levels for implantation; (ii) PGD does not allow alterations of the mutant mtDNA load; (iii) PGD may produce an inadequate number of embryos and (iv) as illustrated in the aforementioned PGD case, blastocyst biopsies may be less accurate for predicting mtDNA heteroplasmy in offspring than cleavage stage embryo biopsies. Given the limited options, a conservative approach for mtDNA mutation carriers has been to avoid having biological offspring, although this is an approach that many families find undesirable.
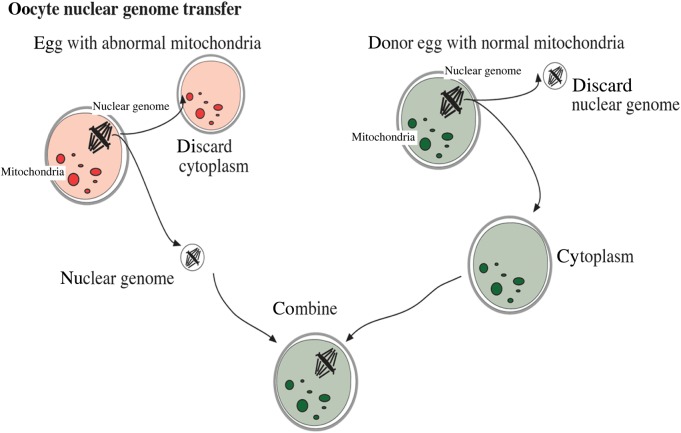
More recently, the development of nuclear transfer methods aimed at reducing mtDNA transmission load has gained momentum (Craven et al., 2010; Paull et al., 2013; Tachibana et al., 2013). In this scenario, chromosomal-spindle tethered nDNA of the carrier oocyte would be inserted into an enucleated healthy donor oocyte, then fertilized through in vitro fertilization (IVF), and implanted (Fig. 1). Alternatively, IVF generated pronuclei can be transferred into the cytoplasm of an enucleated donor embryo. In both cases, the resulting embryo contains DNA of three individuals, with the donor providing mtDNA, which would dramatically reduce the mtDNA mutation load. Because mtDNA encodes genes that contribute exclusively to the oxidative-phosphorylation production of ATP, it is unlikely that other physical characteristics of the child would be directly altered by either procedure.
Figure 1.
Oocyte mitochondrial replacement therapy technique.
In mice with a heteroplasmic mtDNA mutation, nuclear transfer from embryos with mutant mtDNA into enucleated normal embryos has proven effective in sparing progeny from mitochondrial respiration defects; however, mutant mtDNA was still detectable in blastocysts, and offspring carried an average of 11% mutated mtDNA in samples from their tails (Sato et al., 2005). In addition, transfer of pronuclei between fertilized human embryos proved successful in significantly reducing levels of the original mtDNA in resultant blastocysts (Craven et al., 2010); however, the embryos showed reduced developmental potential possibly because the transfer might have introduced an abnormal number of centrosomes leading to multipolar spindles (Egli et al., 2011) and aneuploidy (Ganem et al., 2009).
In rhesus monkeys, spindle chromosome complex transfers between unfertilized oocytes followed by IVF was successful in producing live offspring without detectable transfer of mtDNA (Tachibana et al., 2009). Using human oocytes, spindle chromosome transfer experiments initially demonstrated feasibility of mtDNA replacement, but frequently induced premature oocyte activation (Tachibana et al., 2013), possibly due to karyotype abnormalities. Paull et al. (2013) demonstrated that the premature oocyte activation could be reduced by partial depolarization of the spindle-chromosome complex via cooling or cryopreservation of oocytes prior to chromosomal-spindle transfer. Furthermore, the mt-genome-exchanged oocytes could be converted into pleuripotent stem cells, which, in turn, could be differentiated into neuron, cardiomyocytes or β-cells with <0.5% heteroplasmy in most of the resultant cell lines, and <1% in all.
Transfer of a first or second polar bodies from oocytes carrying mtDNA mutations to enucleated donor oocytes has been proposed as another technique to prevent mtDNA disease transmission; however, as this technique has only been attempted in mice and in no other mammals, its applicability to humans is premature (Wang et al., 2014; Wolf et al., 2015).
In parallel with the scientific advances, ethical questions have been raised regarding potential application of nuclear transfer in the prevention of mitochondrial diseases. One question is that of identity; does prevention of mitochondrial diseases affect the identity of children produced through mitochondrial replacement therapy (Bredenoord et al., 2011a,b)? In addition, nuclear transfer utilizes three, not two, sets of parental DNA, which has led to legal, biological and social questions about parentage. Additional questions have been raised about how to follow these children for potential mtDNA disease once they are born. Due to the many ethical quandaries arising from the development of oocyte mitochondrial replacement therapy (OMRT), it is important to gauge interest in this potential method of disease prevention in women who are known carriers or at risk of carrying mitochondrial mutations, as well as in oocyte donors.
Materials and Methods
The OMRT survey was developed and provided to women at risk for carrying mtDNA point mutations, to assess the following issues: (i) if carriers of childbearing age considered not having children because of the risk of transmitting a mitochondrial disease; (ii) whether carrier women, who had children before knowing their mutation status, would have considered not having them had they known of the risk; (iii) do carriers support the development of these techniques; (iv) would carriers donate oocytes to develop these techniques and (v) are carriers interested in using this technique to have a child. Carriers of mtDNA mutations were recruited through the North American Mitochondrial Disease Consortium (NAMDC), the United Mitochondrial Disease Foundation (UMDF), patient support groups, research and private patients followed at the Columbia University Medical Center (CUMC), and through patient contact with maternal relatives. All participants were at least 18 years of age and signed institutional review board (IRB) consent or participated under an IRB consent waiver. The surveys were administered in person in 2014 and 2015 at CUMC and at UMDF Annual Meetings, via e-mail and postal mailed copies and by telephone interviews. Descriptions of OMRT were provided to all study participants though written consent forms, in-person discussion or IRB-approved telephone scripts, and all participants were allowed to ask questions about OMRT and the survey questions. Because this survey focused on attitudes of female mtDNA mutation carriers toward OMRT, descriptions of alternatives to prevent transmission of mtDNA diseases (e.g. adoption, not having children, PGD and IVF with oocyte donors) were not presented in this study. Women carriers of childbearing age followed at CUMC were aware of reproductive options through routine clinical care and genetic counseling provided by study investigators (K.E. and M.H.). In addition, these reproductive options were presented to carriers and their families in an oral communication at the 2014 UMDF Meeting, where six carriers completed the survey. After reviewing and authorizing the consent form (by written signature or by telephone consent using an IRB-approved telephone script), each patient was asked to answer seven close-ended questions. Carriers who were of childbearing age and considering having children, were asked to complete four additional close-ended questions.
A second survey was developed and provided to assess whether healthy oocyte donors supported oocyte donation to develop these techniques and for the production of an implantable embryo. The surveys were given to oocyte donors by staff at a single IVF Clinic. The survey contained a written lay summary of OMRT and donors were encouraged to ask questions about the technique. Descriptions of alternatives to prevent transmission of mtDNA diseases (e.g. adoption, not having children, PGD and IVF with oocyte donors) were not presented to most of the donors.
The results of both surveys were independently tabulated and analyzed by biostatisticians (J.K., A.S. and J.L.P.T.) for statistical significance. The results were interpreted by a multidisciplinary team including mitochondrial disease clinician-investigators (M.H. and S.D.), an IVF clinician and member of the Ethics Committee of the American Society of Reproductive Medicine (M.V.S.), a stem cell biologist (D.E.), a clinical neurologist (M.S.), two clinical coordinators (J.G. and D.A.) and the biostatistical team.
Results
Carrier women
The ‘OMRT carrier survey’ was completed by 92 women (termed ‘female carriers’) who carry or are at risk of carrying a pathogenic mtDNA point mutation.
Thirteen mtDNA point mutations were represented among carriers: m.3243A > G (n = 52), m.11778G > A (n = 20), m.8344A > G (n = 5), m.8993T > G (n = 2), m.3460G > A (n = 2), m.14484T > C (n = 2), m.8993T > C (n = 2), m.9176T > C (n = 2), m.3288A > G (n = 1), m.12276G > A (n = 1), m.8363G > A (n = 1), m.10191T > C (n = 1) and m.11484T > C (n = 1) (Table I). The most common mutation was the m.3243A > G which can cause a severe neurological disorder known as mitochondrial encephalomyopathy lactic acidosis and stroke-like episodes, characterized clinically by atypical strokes usually in childhood or early adulthood. All carriers were familiar with the concept of maternal inheritance and the risk of transmitting the mutation to their offspring. Carriers were asked whether their knowledge of mutation status affected or would have affected their decision to have children, had they known of the risk. There were 45 women of childbearing age, of whom 78% (35/45) had thought about not having children because of the transmission risk (Table II). Additionally, of the 51 women who had already had children before knowing about the risk, 73% (37/51) said that, had they known the risk of transmission, they would have thought about not having children. There were seven carriers who did not plan on having children regardless of their mutation status.
Table I.
mtDNA point mutations represented in female carriers.
| Mutation | n |
|---|---|
| m.3243A > G | 52 |
| m.11778G > A | 20 |
| m.8344 A > G | 5 |
| m.8993T > G | 2 |
| m.3460G > A | 2 |
| m.14484T > C | 2 |
| m.8993T > C | 2 |
| m.9176T > C | 2 |
| m.12276G > A | 1 |
| m.8363G > A | 1 |
| m.10191T > C | 1 |
| m.11484T > C | 1 |
| m.3288A > G | 1 |
| Total | 92 |
Table II.
Responses of 92 mtDNA mutation carriers.
| Question | Respondents (n) | Response |
|
|---|---|---|---|
| Yes | No | ||
| Childbearing category (n = 45) | |||
| A. For women who are of childbearing age: Have you ever thought about NOT having a child because you were concerned about passing an mtDNA mutation to them? | 45 | 35 (78%) | 10 (22%) |
| All carriers (n = 92) | |||
| For women who had children before knowing they carried or were at risk of carrying an mtDNA mutation: Would you have thought about NOT having children because you were concerned about passing an mtDNA mutation to them | 51 | 37 (73%) | 14 (22%) |
| Are you someone who did not/do not plan on having children regardless of your mtDNA carrier status? | 92 | 7 (8%) | 85 (92%) |
| Do you think that the project described above is important and worthwhile? | 92 | 87 (95%) | 5 (5%) |
| Are you interested in becoming an egg donor? You would receive hormone injections then have an outpatient procedure to obtain the eggs | 92 | 22 (24%) | 70 (76%) |
| Carriers considering having children (n = 21) | |||
| How important is it that your children are your biological offspring? | Very important N = 11 (52%) Somewhat important N = 9 (43%) Not important N = 1 (5%) |
||
| Would you be interested in using this technology to try to have a child? | Yes- 19 (90%) No- 2 (10%) |
||
| Would you be interested in allowing your eggs to be used for basic laboratory research in the process of developing an implantable zygote (fertilized egg)? | Yes- 16 (76%) No- 5 (24%) |
||
Support for OMRT was evident as 95% (87/92) of carriers indicated that development of the technique was important and worthwhile. Of the respondents, 24% (22/92) indicated that they would donate oocytes to the development of the OMRT, knowing that this procedure would require hormone injections and an outpatient procedure. Of 21 women who were considering having children, all were supportive of these efforts. The desire to have biological offspring was evident among the carriers, with 52% (11/21) indicating that it was very important and 43% (9/21) saying that it was somewhat important. Furthermore, 16 of these 21(76%) carrier respondents were willing to donate oocytes to support the development of this technique and 90% (19/21) were interested in using this technology to have a child (P = 0.003). Seven women with mtDNA variants that are not considered disease causing completed surveys which are not included here.
Oocyte donors
The ‘OMRT donor Survey’ was completed by 112 women who have donated oocytes through a major IVF clinic (Table III). All participants were at least 18 years of age. Of the 112 oocyte donors, 103 (92%) indicated that they would donate for basic laboratory research in which viable embryos are not produced while 97 (87%) responded that they would donate for the production of implantable embryos. All donors who were willing to allow the production of a viable embryo also indicated that they would donate to research. Of the 103 oocyte donors willing to donate for research in which an embryo is not produced, 97 (94%) were also willing to donate their oocytes for the production of an implantable embryo that has the father and carrier mother's nDNA and the participant's mtDNA. In contrast, none (0 of 9) of the donors who would not donate their oocytes for research (Fisher's exact test P < 0.0001) would donate their oocytes to generate an implantable embryo. Only 15 oocyte donors (13%) would not allow an implantable embryo and, of these, 9 (8%) noted that they would also not donate for research purposes.
Table III.
Responses of 112 healthy oocyte donors.
| Question | Yes | No | No response |
|---|---|---|---|
| 1. Would you be willing to donate your eggs for basic laboratory research in which a viable zygote would NOT be produced? | 103 (92%) | 9 (8%) | 0 (0%) |
| 2. Would you be willing to donate your eggs for the production of an implantable zygote that has the father and the carrier mother's nDNA and your mtDNA? | 97 (87%) | 15 (13%) | 0 (0%) |
Discussion
Mitochondrial replacement through nuclear transfer between oocytes or embryos is an emerging novel technique that can potentially reduce or eliminate the transmission of mutant mtDNA from female carriers to offspring. It is a disease-preventing option that, in theory, is more effective than PND and PGD.
Nuclear transfer techniques have recently been evaluated in animal models, and human cells in vitro. If clinically implemented, nuclear transfer would represent the first use of the implantation of genetically modified embryos. Considering that the resulting child will have DNA from three individuals, legal, ethical and social concerns would be significant. To assess the level of support for oocyte mtDNA replacement therapies given these concerns, we surveyed women carrying, or at risk for carrying, mtDNA point mutations as well as healthy oocyte donors. In both groups, there was overwhelming support for developing these techniques; 95% of female mutation carriers responded that development of OMRT was important and worthwhile. Among carriers considering having children, the vast majority (95%) considered having biological offspring sharing their nDNA to be important (52% very important and 43% somewhat important). Despite the strong desire of having biological offspring, the risk of transmitting the mtDNA mutation to their children caused 78% of carrier women to consider not having children. Furthermore, of female carriers who had children prior to knowing the risk of maternal transmission, 73% responded that, had they known the risk of transmission of the mtDNA mutation, they would have thought about not having children. The desire for biological offspring may have been overestimated because alternatives, such as oocyte donation and adoption which provide offspring that do not share DNA with the mother, were not presented in the study questionnaire as alternatives to most of the carrier women.
Although only 24% of all female carriers indicated willingness to donate oocytes for the development of this OMRT, the majority (76%) of female carriers who were considering having children were willing to donate oocytes for the development of this field, and for the creation of an implantable embryo. The potential negative implications of OMRT did not deter the vast majority of the oocyte donors, as 92% would donate for basic laboratory research in which a viable embryo is not produced while 87% would donate for production of implantable embryos.
Internationally, investigators have wrestled with the unique genetic and ethical challenges of OMRT (Shoubridge, 2009; Bredenoord et al., 2011a,b; Lancet, 2012). In late 2012, the UK Human Fertilisation and Embryology authority (HFEA) initiated a public debate regarding the ethical and social implications of using mitochondrial replacement techniques in families at risk of a mitochondrial disease (Pitts-Tucker, 2012; Tavare, 2012). There was broad public support and HFEA has advised the government to legalize mitochondrial replacement for use in humans; and, in fact, in February 2015, the UK Parliament approved legislation that permits this technique under tight regulations. Although the plethora of emerging ethical issues surrounding OMRT is beyond of the scope of this paper, it is noteworthy that two groups have affirmed the ethical nature of this technique in potentially preventing transmission of mtDNA diseases. Bredenoord et al. (2011a, b) contributed to the ethical debate, by arguing that, although we consider the nuclear genome as the sole ‘germ-line’, we cannot exclude the fact that mitochondrial transfer alters the mitochondrial germ-line and laws to protect the ‘germ-line’ exist in many countries. Further, Bredenoord stated that it is not known whether mitochondrial transfer alters the identity of an individual; for instance does mtDNA interact with nDNA in a way that alters the action of nDNA? Despite these issues, Bredenoord et al. (2011a,b) conclude that the modification of the mitochondrial germ-line is ethical as it promotes the possibility of an ‘open future’ for the individual that might not have been otherwise possible. In addition, the Nuffield Council on Bioethics issued an extensive report that stated ‘The Working Group is similarly skeptical of locating any distinctions about the ethical acceptability of interventions on different genomes in notions of identity, because developing a possibly life-limiting disorder (or not) can make such a significant difference to the life of the future person’ and concluded ‘Provided that the techniques are proved to be safe and effective, and an appropriate level of information and support is offered, it would be ethical for families to use these techniques as treatment.’ (Watts et al., 2012). At the same time, the report emphasized the need for further research to establish efficacy and safety of these techniques.
Prior to clinical use, additional research needs to be done to establish both the safety and efficacy parameters for OMRT. In vivo data are currently limited to a few monkeys born from spindle transfer to an enucleated oocyte (Tachibana et al., 2009). The UK HFEA has established draft guidelines and safeguards regarding the development and use of pronuclear, but not oocyte, MRT techniques (HFEA, 2013, 2015).
Genetic evaluation is a rapidly evolving field encroaching upon mainstream clinical use. As genetic panels, including whole exome sequencing, address the needs of individual patient populations and become less costly, these tests may become first tier evaluation for many patients. In this setting, it is possible that the detection of mtDNA carriers will rise. In this context, MRT may be viewed as an option for a wider audience than that surveyed in this study, including women who have no clinical symptoms related to mitochondrial disorders and have no affected family members.
A limitation of this work is that the survey was administered to a select group of individuals; those known to be or at risk for harboring mtDNA point mutations. These individuals are more likely to have been affected by the mutation or have witnessed first-hand the devastating effects of these mutations. It has not been established in the USA whether the general public would be supportive of this work. Many of the participants were either patients or relatives of patients who were already enrolled in a research-oriented database or who sought care in a tertiary research university setting, indicating a potential sampling bias. Finally, the views of the participants regarding risks of MRT and ethical aspects of the procedures were not explored in this study.
Despite the many ethical issues surrounding the use of oocyte nuclear transfer for the prevention of mitochondrial diseases, the UK HFEA and Parliament and the Nuffield Council of Bioethics and individual ethicists have deemed study of these techniques to be ethical with the application of certain safety precautions (Bredenoord et al., 2011a,b; Watts et al., 2012; HFEA, 2013, 2015). This study demonstrates the high level of interest in the development of these methods among women affected by the diseases or who are at risk of carrying mtDNA mutations, and the willingness of most donors to provide oocytes for the development of OMRT. Given the exceedingly high level of interest among women at risk of having children with mtDNA mutations, the severe disability and high level of mortality among patients affected by mitochondrial disorders, and the lack of other successful treatment or prevention methods available, future studies should be conducted to assess efficacy and safety of this promising approach to prevent mtDNA diseases.
Authors' roles
The study was conceived by M.H., D.E. and M.V.S. Survey questions were designed and revised by M.H., D.A., J.G., M.S., K.E., D.E. and M.V.S. The survey was administered by K.E., J.G. and M.V.S. The results of both surveys were independently tabulated and analyzed for statistical significance by biostatisticians, J.K., A.S. and J.L.P.T. The results were interpreted by M.H., S.D., J.G., D.A., M.S., K.E., J.K., A.S., J.L.P.T. and D.E., and M.V.S., K.E. and M.H. drafted the manuscript, which was reviewed and edited by all authors.
Funding
This work was conducted under the auspices of the North American Mitochondrial Disease Consortium (NAMDC Study Protocol 7404). NAMDC (U54NS078059) is a part of the NCATS Rare Diseases Clinical Research Network (RDCRN). RDCRN is an initiative of the Office of Rare Diseases Research (ORDR) and NCATS. NAMDC is funded through a collaboration between NCATS, NINDS, NICHD and NIH Office of Dietary Supplements. The work was also supported by the Bernard and Anne Spitzer Fund and New York Stem Cell Foundation (NYSCF). In addition, M.H. acknowledges support from NIH grants P01 HD32062, R01 HD057543 and R01 HD056103 (NICHD), the Office of Dietary Supplements, the Marriott Mitochondrial Disease Clinic Research Fund (MMDCRF), the Nunno Foundation, the Mileti Fund and the Muscular Dystrophy Association.
Conflict of interest
M.H. has received research support from Santhera Pharmaceuticals and Edison Pharmaceuticals for studies unrelated to the work presented in this manuscript. None of the other authors have conflicts of interest related to this manuscript. The sponsors did not contribute to the design and conduct of the study, the collection, management, analysis or interpretation of the data; or the preparation, review or approval of the manuscript.
Acknowledgements
The authors thank the mtDNA mutation carriers and anonymous oocyte donors for participating in the surveys and the United Mitochondrial Disease Foundation for facilitating recruitment of mutation carriers.
References
- Blok RB, Gook DA, Thorburn DR, Dahl HH. Skewed segregation of the mtDNA nt 8993 (T → G) mutation in human oocytes. Am J Hum Genet 1997;60:1495–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredenoord AL, Dondorp W, Pennings G, De Wert G. Ethics of modifying the mitochondrial genome. J Med Ethics 2011a;37:97–100. [DOI] [PubMed] [Google Scholar]
- Bredenoord AL, Dondorp W, Pennings G, De Wert G. Nuclear transfer to prevent mitochondrial DNA disorders: revisiting the debate on reproductive cloning. Reprod Biomed Online 2011b;22:200–207. [DOI] [PubMed] [Google Scholar]
- Brown DT, Samuels DC, Michael EM, Turnbull DM, Chinnery PF. Random genetic drift determines the level of mutant mtDNA in human primary oocytes. Am J Hum Genet 2001;68:533–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi BO, Hwang JH, Cho EM, Jeong EH, Hyun YS, Jeon HJ, Seong KM, Cho NS, Chung KW. Mutational analysis of whole mitochondrial DNA in patients with MELAS and MERRF diseases. Exp Mol Med 2010;42:446–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven L, Tuppen HA, Greggains GD, Harbottle SJ, Murphy JL, Cree LM, Murdoch AP, Chinnery PF, Taylor RW, Lightowlers RN et al. Pronuclear transfer in human embryos to prevent transmission of mitochondrial DNA disease. Nature 2010;465:82–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMauro S, Hirano M, Schon EA. Mitochondrial Medicine. Oxon: Informa Healthcare, 2006. [Google Scholar]
- DiMauro S, Schon EA, Carelli V, Hirano M. The clinical maze of mitochondrial neurology. Nat Rev Neurol 2013;9:429–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli D, Chen AE, Saphier G, Ichida J, Fitzgerald C, Go KJ, Acevedo N, Patel J, Baetscher M, Kearns WG et al. Reprogramming within hours following nuclear transfer into mouse but not human zygotes. Nat Commun 2011;2:488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott HR, Samuels DC, Eden JA, Relton CL, Chinnery PF. Pathogenic mitochondrial DNA mutations are common in the general population. Am J Hum Genet 2008;83:254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folmes CD, Martinez-Fernandez A, Perales-Clemente E, Li X, McDonald A, Oglesbee D, Hrstka SC, Perez-Terzic C, Terzic A, Nelson TJ. Disease-causing mitochondrial heteroplasmy segregated within induced pluripotent stem cell clones derived from a patient with MELAS. Stem Cells 2013;31:1298–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem NJ, Godinho SA, Pellman D. A mechanism linking extra centrosomes to chromosomal instability. Nature 2009;460:278–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman GS, Schaefer AM, Ng Y, Gomez N, Blakely EL, Alston CL, Feeney C, Horvath R, Yu-Wai-Man P, Chinnery PF et al. Prevalence of nuclear and mtDNA mutations related to adult mitochondrial disease. Ann Neurol 2015;77:753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellebrekers DM, Wolfe R, Hendrickx AT, de Coo IF, de Die CE, Geraedts JP, Chinnery PF, Smeets HJ. PGD and heteroplasmic mitochondrial DNA point mutations: a systematic review estimating the chance of healthy offspring. Hum Reprod Update 2012;18:341–349. [DOI] [PubMed] [Google Scholar]
- HFEA (Human Fertilisation and Embryology Authority). Mitochondria Replacement Consultation: Advice to Government. London: HFEA, 2013. [Google Scholar]
- HFEA (Human Fertilisation and Embryology Authority). The Human Fertilisation and Embryology (Mitochondrial Donation) Regulations 2015. London: HFEA, 2015.
- Kaufmann P, Engelstad K, Wei Y, Kulikova R, Oskoui M, Battista V, Koenigsberger DY, Pascual JM, Sano M, Hirano M et al. Protean phenotypic features of the A3243G mitochondrial DNA mutation. Arch Neurol 2009;66:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancet. Ethics of mitochondrial donation. Lancet 2012;379:2314. [DOI] [PubMed] [Google Scholar]
- Mitalipov S, Amato P, Parry S, Falk MJ. Limitations of preimplantation genetic diagnosis for mitochondrial DNA diseases. Cell Rep 2014;7:935–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnot S, Gigarel N, Samuels DC, Burlet P, Hesters L, Frydman N, Frydman R, Kerbrat V, Funalot B, Martinovic J et al. Segregation of mtDNA throughout human embryofetal development: m.3243A > G as a model system. Hum Mutat 2011;32:116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishigaki Y, Tadesse S, Bonilla E, Shungu D, Hersh S, Keats BJ, Berlin CI, Goldberg MF, Vockley J, DiMauro S et al. A novel mitochondrial tRNALeu(UUR) mutation in a patient with features of MERRF and Kearns–Sayre syndrome. Neuromuscul Disord 2003;13:334–340. [DOI] [PubMed] [Google Scholar]
- Paull D, Emmanuele V, Weiss KA, Treff N, Stewart L, Hua H, Zimmer M, Kahler DJ, Goland RS, Noggle SA et al. Nuclear genome transfer in human oocytes eliminates mitochondrial DNA variants. Nature 2013;493:632–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts-Tucker T. UK fertilisation authority launches consultation on mitochondrial replacement techniques. BMJ 2012;345:e6259. [DOI] [PubMed] [Google Scholar]
- Poulton J, Bredenoord AL. 174th ENMC international workshop: applying pre-implantation genetic diagnosis to mtDNA diseases: implications of scientific advances 19–21 March 2010, Naarden, The Netherlands. Neuromuscul Disord 2010;20:559–563. [DOI] [PubMed] [Google Scholar]
- Sallevelt SC, Dreesen JC, Drusedau M, Spierts S, Coonen E, van Tienen FH, van Golde RJ, de Coo IF, Geraedts JP, de Die-Smulders CE et al. Preimplantation genetic diagnosis in mitochondrial DNA disorders: challenge and success. J Med Genet 2013;50:125–132. [DOI] [PubMed] [Google Scholar]
- Sato A, Kono T, Nakada K, Ishikawa K, Inoue S, Yonekawa H, Hayashi J. Gene therapy for progeny of mito-mice carrying pathogenic mtDNA by nuclear transplantation. Proc Natl Acad Sci USA 2005;102:16765–16770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon EA, DiMauro S, Hirano M. Human mitochondrial DNA: roles of inherited and somatic mutations. Nat Rev Genet 2012;13:878–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanske S, Pancrudo J, Kaufmann P, Engelstad K, Jhung S, Lu J, Naini A, DiMauro S, De Vivo DC. Varying loads of the mitochondrial DNA A3243G mutation in different tissues: implications for diagnosis. Am J Med Genet 2004;130A:134–137. [DOI] [PubMed] [Google Scholar]
- Shanske S, Naini A, Chmait RH, Akman HO, Mansukhani M, Lu J, Hirano M, DiMauro S. Mutation in an mtDNA protein-coding gene: prenatal diagnosis aided by fetal muscle biopsy. J Child Neurol 2013;28:264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoubridge EA. Developmental biology: asexual healing. Nature 2009;461:354–355. [DOI] [PubMed] [Google Scholar]
- Steffann J, Frydman N, Gigarel N, Burlet P, Ray PF, Fanchin R, Feyereisen E, Kerbrat V, Tachdjian G, Bonnefont JP et al. Analysis of mtDNA variant segregation during early human embryonic development: a tool for successful NARP preimplantation diagnosis. J Med Genet 2006;43:244–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M, Sparman M, Sritanaudomchai H, Ma H, Clepper L, Woodward J, Li Y, Ramsey C, Kolotushkina O, Mitalipov S. Mitochondrial gene replacement in primate offspring and embryonic stem cells. Nature 2009;461:367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M, Amato P, Sparman M, Woodward J, Sanchis DM, Ma H, Gutierrez NM, Tippner-Hedges R, Kang E, Lee HS et al. Towards germline gene therapy of inherited mitochondrial diseases. Nature 2013;493:627–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavare A. Scientists are to investigate ‘three parent IVF’ for preventing mitochondrial diseases. BMJ 2012;344:e540. [DOI] [PubMed] [Google Scholar]
- Thorburn DR, Wilton L, Stock-Myer S. Healthy baby girl born following pre-implantation genetic diagnosis for mitochondrial DNA m.8993T > G mutation. Mol Genet Metab 2009;98:5–6. [Google Scholar]
- Treff NR, Campos J, Tao X, Levy B, Ferry KM, Scott RT Jr. Blastocyst preimplantation genetic diagnosis (PGD) of a mitochondrial DNA disorder. Fertil Steril 2012;98:1236–1240. [DOI] [PubMed] [Google Scholar]
- Wai T, Teoli D, Shoubridge EA. The mitochondrial DNA genetic bottleneck results from replication of a subpopulation of genomes. Nat Genet 2008;40:1484–1488. [DOI] [PubMed] [Google Scholar]
- Wang T, Sha H, Ji D, Zhang HL, Chen D, Cao Y, Zhu J. Polar body genome transfer for preventing the transmission of inherited mitochondrial diseases. Cell 2014;157:1591–1604. [DOI] [PubMed] [Google Scholar]
- Watts G, Braude P, Flinter F, Harding S, Lewens T, Parker M. Novel Techniques for the Prevention of Mitochondrial DNA Disorders: an Ethical Review. London: Nuffield Council on Bioethics, 2012. [Google Scholar]
- Wolf DP, Mitalipov N, Mitalipov S. Mitochondrial replacement therapy in reproductive medicine. Trends Mol Med 2015;21:68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]