Abstract
STUDY QUESTION
Do women with BRCA1 or BRCA2 mutations have reduced ovarian reserve, as measured by circulating anti-Müllerian hormone (AMH) concentration?
SUMMARY ANSWER
Women with a germline mutation in BRCA1 have reduced ovarian reserve as measured by AMH.
WHAT IS KNOWN ALREADY
The DNA repair enzymes encoded by BRCA1 and BRCA2 are implicated in reproductive aging. Circulating AMH is a biomarker of ovarian reserve and hence reproductive lifespan.
STUDY DESIGN, SIZE, DURATION
This was a cross-sectional study of AMH concentrations of 693 women at the time of enrolment into the Kathleen Cuningham Foundation Consortium for research in the Familial Breast Cancer (kConFab) cohort study (recruitment from 19 August 1997 until 18 September 2012). AMH was measured on stored plasma samples between November 2014 and January 2015 using an electrochemiluminescence immunoassay platform.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Eligible women were from families segregating BRCA1 or BRCA2 mutations and had known mutation status. Participants were aged 25–45 years, had no personal history of cancer, retained both ovaries and were not pregnant or breastfeeding at the time of plasma storage. Circulating AMH was measured for 172 carriers and 216 non-carriers from families carrying BRCA1 mutations, and 147 carriers and 158 non-carriers from families carrying BRCA2 mutations. Associations between plasma AMH concentration and carrier status were tested by linear regression, adjusted for age at plasma storage, oral contraceptive use, body mass index and cigarette smoking.
MAIN RESULTS AND THE ROLE OF CHANCE
Mean AMH concentration was negatively associated with age (P < 0.001). Mutation carriers were younger at blood draw than non-carriers (P ≤ 0.031). BRCA1 mutation carriers had, on average, 25% (95% CI: 5%–41%, P = 0.02) lower AMH concentrations than non-carriers and were more likely to have AMH concentrations in the lowest quartile for age (OR 1.84, 95% CI: 1.11–303, P = 0.02). There was no evidence of an association between AMH concentration and BRCA2 mutation status (P = 0.94).
LIMITATIONS, REASONS FOR CAUTION
AMH does not directly measure the primordial follicle pool. The clinical implications of the lower AMH concentrations seen in BRCA1 mutation carriers cannot be assessed by this study design.
WIDER IMPLICATIONS OF THE FINDINGS
Women with a germline mutation in BRCA1 may have reduced ovarian reserve. This is consistent with other smaller studies in the literature and has potential implications for fertility and reproductive lifespan.
STUDY FUNDING/COMPETING INTEREST(S)
kConFab is supported by a grant from the Australian National Breast Cancer Foundation, and previously by the National Health and Medical Research Council (NHMRC), the Queensland Cancer Fund, the Cancer Councils of New South Wales, Victoria, Tasmania and South Australia, and the Cancer Foundation of Western Australia. K.A.P. is an Australian National Breast Cancer Foundation Practitioner Fellow. J.L.H. is a NHMRC Senior Principal Research Fellow. M.H. is a NHMRC Practitioner Fellow. R.A.A. reports personal fees from Roche Diagnostics & Beckman Coulter outside the submitted work and C.S. reports other earnings from Melbourne IVF outside the submitted work. The remaining authors have nothing to declare and no conflicts of interest.
Keywords: BRCA1, BRCA2, anti-Müllerian hormone, ovarian reserve, fertility, DNA repair, reproduction
Introduction
Germline mutations in the BRCA1 or BRCA2 genes substantially increase the risk of breast cancer, high grade serous ovarian cancer, fallopian tube cancer and primary peritoneal cancer (Antoniou et al., 2003). Less is known about the non-cancer-related implications, but preliminary data suggest that ovarian reserve, and hence fertility, may be reduced in BRCA1 mutation carriers (Oktay et al., 2010, 2014; Titus et al., 2013; Pavone et al., 2014; Wang et al., 2014). If confirmed, this could have clinical consequences for pregnancy planning, reproductive lifespan and perhaps ovarian function following chemotherapy.
BRCA1 and BRCA2 are integral in the repair of DNA double-strand breaks through homologous recombination, and thus are important members of the ATM-mediated DNA damage signalling pathway (Jackson and Bartek, 2009). The importance of inefficient DNA double-strand break repair in carcinogenesis is well understood (Jackson and Bartek, 2009) but recently, inefficient DNA repair has also been shown to contribute to oocyte aging. The protective function of double-strand DNA repair proteins, including BRCA1, declines with age, leading to accumulation of lethal DNA double-strand breaks and oocyte apoptosis (Titus et al., 2013). Data from mouse models suggest that inheriting a BRCA1 germline mutation may accelerate this process: BRCA1 heterozygous mutant mice have smaller litter sizes, produce fewer oocytes in response to ovarian stimulation and their oocytes accumulate DNA damage more quickly than wild-type mice or mice with germline BRCA2 mutations (Titus et al., 2013).
Circulating anti-Müllerian hormone (AMH) is the best currently available biomarker to forecast age at menopause and thus the reproductive lifespan (Sowers et al., 2008; Broer et al., 2011; Tehrani et al., 2013). It is widely used to predict ovarian response in assisted reproductive technologies (Iliodromiti et al., 2014), although its relationship to natural fertility is less clear (Steiner et al., 2011; Hagen et al., 2012). AMH production begins prenatally, peaks in the mid-20s and then declines to the menopause (Kelsey et al., 2011). AMH is produced by the granulosa cells of growing pre-antral and early antral follicles, but not by the primordial follicles which are the true arbiter of female reproductive lifespan. Thus AMH directly assesses ovulatory potential within about a 6-month timeframe (Findlay et al., 2015). Nevertheless AMH approximates primordial follicle number and can be used to assess ovarian reserve in women aged 25 years and over (Broer et al., 2011; Hansen et al., 2011). Circulating levels remain relatively constant across the menstrual cycle (Tsepelidis et al., 2007) and also between cycles in the same woman (Fanchin et al., 2005). Current oral contraceptive pill (OCP) use and cigarette smoking are associated with lower AMH concentrations (Dolleman et al., 2013), whereas markedly elevated levels are found in polycystic ovary syndrome (PCOS) (Iliodromiti et al., 2013).
This study was conducted to determine whether women with a mutation in BRCA1 or BRCA2 have reduced ovarian reserve, as measured by circulating AMH concentrations, compared with women who do not carry a BRCA1 or BRCA2 mutation. It was hypothesized that AMH concentrations would be lower in mutation carriers compared with non-carriers, that AMH would be lower in BRCA1 mutation carriers than in BRCA2 mutation carriers, and that the difference between AMH concentrations of mutation carriers and non-carriers would be greater at older ages.
Methods
Subjects
Eligible women were from Australian and New Zealand families, with multiple cases of breast cancer, who were enrolled in the Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer (kConFab) Cohort Study (Mann et al., 2006). Recruitment to that cohort, which currently includes 1636 families, commenced on 19 August 1997 and is ongoing. Individuals had been recruited to kConFab after at least one family member attended a clinical consultation in any of 24 Family Cancer Centres. At the time of cohort entry, participants provided blood for both genetic testing and storage of plasma. Participants in the cohort have been followed up every three years (Phillips et al., 2005). All participants provided written informed consent, and the kConFab Cohort Study has Institutional Review Board approval at recruiting sites.
Women eligible for the study reported here either had a pathogenic mutation, splice site mutation or large deletion in BRCA1 or BRCA2 (‘mutation carriers’), or were a blood relative of a mutation carrier and had themselves been tested and found not to carry the identified family-specific mutation (‘non-carriers’). At the time of blood draw, they had to be aged 25–45 years, have two intact ovaries, no personal history of any cancer (apart from non-melanoma skin cancer), no history of primary amenorrhoea and not pregnant or breastfeeding. The Institutional Review Board of the Peter MacCallum Cancer Centre approved this study.
Data collection
At cohort entry, kConFab Cohort Study participants completed an epidemiologic questionnaire (John et al., 2004). Data collected include demographics, height and weight, personal and family cancer history, oophorectomy status, menstrual history, parity, breastfeeding history, OCP use and fertility treatment and cigarette smoking. Additional data were collected on the day of blood draw, including current pregnancy status, date of commencement of last menstrual cycle, self-reported menopausal status and current OCP use.
AMH analysis
A blood sample was collected at enrolment into the kConFab Cohort Study. Plasma aliquots were stored at −80°C within 48 h. AMH measurements, blinded to participant mutation status, were undertaken between November 2014 and January 2015, at the Melbourne IVF Endocrine Laboratory utilizing a fully automated Elecsys® AMH assay on the Cobas e electrochemiluminescence immunoassay platform (Gassner and Jung, 2014; Anderson et al., 2015). The lowest level of detection was 0.07 pmol/l and the intra-assay and inter-assay imprecision coefficients of variation at 7.0 pmol/l were 4.6 and 5.6% respectively. Plasma samples had been stored for a mean of 11.4 years (standard deviation [SD]: 3.6) prior to AMH analysis and none had been previously thawed. Quality assurance testing was performed on 30 non-study samples for which both plasma and serum were available; AMH concentrations using serum samples were approximately 5% higher than those using plasma. It was considered that this would not affect the study conclusions given that plasma was used for both the comparison groups.
Statistical methods
Comparisons of participant characteristics by carrier status were made by applying Fisher's exact test for categorical variables and Wilcoxon rank-sum test for numerical variables. For each gene (BRCA1 and BRCA2), a difference in mean AMH concentration between mutation carriers and non-carriers was tested by linear regression, modelling the natural logarithm of AMH as the outcome variable and carrier status as the explanatory variable. This log transformation was applied to correct for the asymmetry in the distribution of AMH values, as consistently reported in other studies (Su et al., 2013; Whitworth et al., 2015). The exponential of the regression coefficient for carrier status (and its 95% confidence interval (CI) limits) was taken as an estimate of mean AMH concentration on the natural scale for carriers relative to non-carriers. For three samples, the AMH concentration was below the lower limit of detection of 0.07 pmol/l and these were set to a value of 0.07 pmol/l for the purposes of the analysis. Multivariable models incorporated factors known to affect ovarian reserve, including age at blood draw (by including a linear and quadratic term for the age range considered, as suggested by Kelsey et al (Kelsey et al., 2011)), body mass index (BMI) at cohort entry, cigarette smoking at cohort entry (never/past/current regular cigarette use), and OCP use at time of blood draw (no/yes). Robust standard errors were estimated to account for the inclusion of multiple women from the same families. Sensitivity analyses were conducted, excluding women who were using the OCP at the time of blood draw and, separately, excluding women who reported being post-menopausal or had unknown menopausal status at blood draw. To assess whether any difference in mean AMH concentration between carriers and non-carriers was more pronounced at older ages, we fit an additional parameter for the interaction between mutation carrier status and the linear term for age. We also estimated the odds ratio (OR) for having an AMH level in the lowest quartile for age in years, by logistic regression, with the same covariates as in the primary analysis. All P-values were two-sided and those less than 0.05 were considered statistically significant. Statistical analyses were performed by R.L.M.
Results
Participants
At the time of AMH analysis, there were 1021 women aged 25–45 years enrolled in the kConFab Cohort Study who were blood relatives in a family with a mutation in either BRCA1 or BRCA2. Of these, 328 were excluded due to: unilateral or bilateral oophorectomy prior to blood draw (134), a personal history of cancer prior to blood draw (166), pregnancy or breastfeeding at the time of blood draw (14), pathogenic mutations in both the BRCA1 and BRCA2 genes (4), or inadequate sample for AMH testing (10). Thus 693 participants were included in the final study sample, including 172 carriers of a pathogenic mutation in BRCA1, 216 women who tested negative for the known BRCA1 mutation in their family, 147 carriers of a pathogenic mutation in BRCA2 and 158 women who tested negative for the known BRCA2 mutation in their family.
Participant characteristics are shown in Table I. The mean age at blood draw was 35.1 years and mutation carriers were younger than non-carriers (P ≤ 0.03). Of the subjects, 24% reported current cigarette use at cohort entry and this did not differ between mutation carriers and non-carriers (P ≥ 0.31). BRCA1 mutation carriers had lower BMI than non-carriers (P = 0.02), but there was no significant difference in BMI between BRCA2 mutation carriers and non-carriers (P = 0.99). There were no significant differences between carriers of BRCA1 and BRCA2 mutations and non-carriers for OCP use at the time of blood draw (P ≥ 0.08) or for surrogate measures of ovarian function, including parity (P ≥ 0.33), age at first birth (P ≥ 0.76) and history of infertility treatments (P ≥ 0.64).
Table I.
Sample characteristics.
|
BRCA1 |
BRCA2 |
|||||
|---|---|---|---|---|---|---|
| Carriers | Non-Carriers | P-value | Carriers | Non-Carriers | P-value | |
| n = 172 | n = 216 | n = 147 | n = 158 | |||
| Year of birth* | 1968 (7.2) | 1966 (6.9) | 0.003 | 1969 (7.0) | 1967 (7.0) | 0.01 |
| Age at blood draw (years)* | 34.2 (5.7) | 35.8 (5.8) | 0.006 | 34.4 (5.6) | 35.8 (5.6) | 0.03 |
| Years sample was stored* | 11.4 (3.9) | 11.9 (3.3) | 0.15 | 10.8 (3.7) | 11.4 (3.6) | 0.17 |
| Cigarette use**, n (%) | ||||||
| Never | 84 (49) | 97 (45) | 75 (51) | 76 (48) | ||
| Past | 39 (23) | 64 (30) | 45 (31) | 46 (29) | ||
| Current | 49 (28) | 55 (25) | 0.31 | 27 (18) | 36 (23) | 0.65 |
| Body mass index* (kg/m2) | 24.8 (5.2) | 26.2 (6.4) | 0.02 | 25.4 (6.0) | 25.4 (5.3) | 0.99 |
| OCP use at blood draw, n (%) | ||||||
| No | 150 (87) | 183 (85) | 118 (80) | 139 (88) | ||
| Yes | 22 (13) | 33 (15) | 0.56 | 29 (20) | 19 (12) | 0.08 |
| Infertility treatment, n (%) | ||||||
| Ever | 10 (6) | 10 (5) | 8 (5) | 11 (7) | ||
| Never | 161 (94) | 205 (95) | 0.65 | 135 (92) | 142 (90) | 0.64 |
| Don't know | 1 (1) | 1 (0.5) | 4 (3) | 5 (3) | ||
| Parity, n (%) | ||||||
| Nulliparous | 46 (27) | 65 (30) | 53 (36) | 48 (30) | ||
| Parous | 126 (73) | 151 (70) | 0.50 | 94 (64) | 110 (69) | 0.33 |
| Age at first birth* (years) | 24.9 (4.7) | 25.0 (5.1) | 0.76 | 25.7 (4.4) | 25.6 (4.7) | 0.87 |
All values <0.05 are statistically significant.
n, number; OCP, oral contraceptive pill.
*Mean (standard deviation).
**Regular cigarette smoking—at least one per day for 3 months or longer.
AMH concentrations were negatively associated with age overall and for both carriers and non-carriers of BRCA1 and BRCA2 mutations (P < 0.001). AMH concentrations were, on average, 28% lower (exp(β) = 0.72, 95% CI = 0.58–0.89; P = 0.003) for current OCP users compared with non-users. AMH concentrations were not associated with the length of time between blood draw and AMH analysis (P = 0.08), smoking status (P = 0.55) or BMI (P = 0.92).
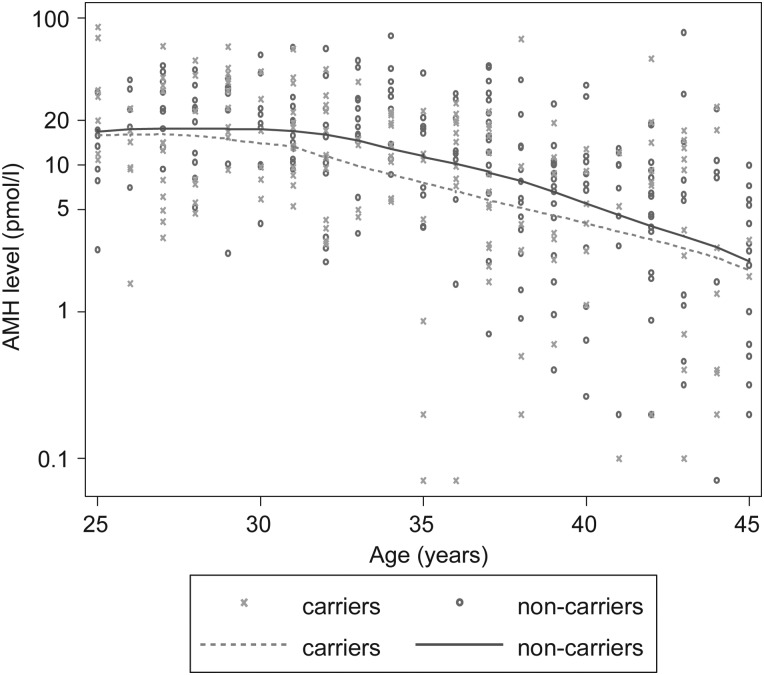
After adjusting for age at blood draw, BRCA1 carrier status was associated with AMH concentration (Fig. 1); on average BRCA1 mutation carriers had 25% lower AMH concentrations than non-carriers (exp(β) = 0.75, 95% CI = 0.59–0.95; P = 0.02). There was no evidence that this association varied with age (P-interaction = 0.61). As shown in Table II, further adjustment for OCP use at time of blood draw, BMI at cohort entry and cigarette smoking ever, had little effect on these estimates (exp(β) = 0.75, 95% CI = 0.58–0.97), nor did adjustment for length of time from blood draw to analysis (exp(β) = 0.76, 95% CI = 0.59–0.97) or exclusion of current OCP users and women who reported they were post-menopausal (exp(β) = 0.74, 95% CI = 0.58–0.94). Based on the quadratic model fit for age using our data for non-carriers, the difference in average AMH concentration was approximately equivalent to that between a 37 year-old compared with a 35 year-old woman.
Figure 1.
AMH levels in BRCA1 mutation carrier families, by mutation carrier status. Solid and dashed lines are drawn by locally weighted regression of log-transformed AMH levels. Carriers: crosses and dashed line; non-carriers: circles and solid line.
Table II.
Estimated coefficient (β) for mutation carriers versus non-carriers from linear regression modelling the natural logarithm of AMH as the outcome variable.
| Model | BRCA1 β (95% CI), P-value |
BRCA2 β (95% CI), P-value |
|---|---|---|
| All women, adjusted for: | ||
|
−0.29 (−0.53, −0.05), 0.02 | −0.05 (−0.31, 0.21), 0.71 |
|
−0.28 (−0.54, −0.03), 0.03 | −0.01 (−0.26, 0.24), 0.94 |
|
−0.28 (−0.53, −0.03), 0.03 | 0.00 (−0.25, 0.25), 0.99 |
| Excluding current OCP users & post-menopausal and unknown menopausal status at time of blood draw# | −0.30 (−0.55, −0.06), 0.02 | −0.03 (−0.31, 0.26), 0.85 |
CI, confidence interval.
*Adjustment for age included a linear and quadratic term.
#Adjusted for age, OCP use, BMI, smoking.
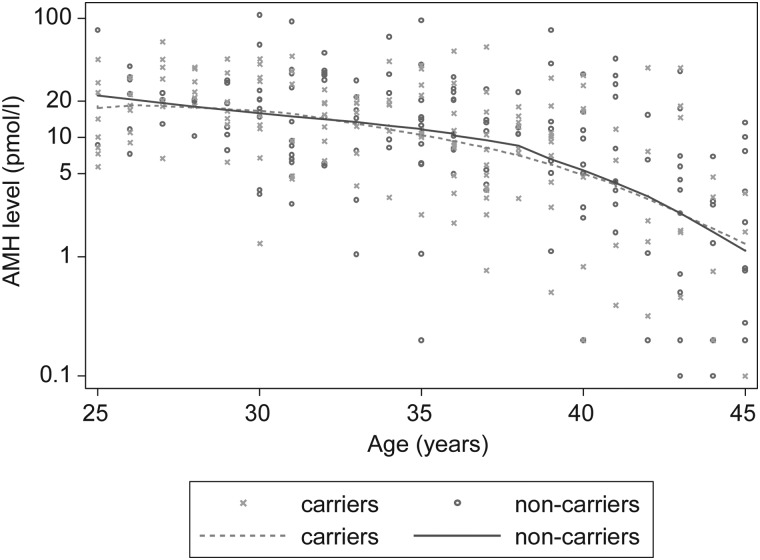
There was no difference in average AMH concentrations between BRCA2 mutation carriers and non-carriers (exp(β) = 0.99, 95% CI = 0.77–1.27; P = 0.94), after adjusting for age (Fig. 2), and for OCP use, BMI and cigarette smoking. Results were consistent after further adjustment for time from blood draw to analysis and the exclusion of current OCP users and post-menopausal women (P ≥ 0.85).
Figure 2.
AMH levels in BRCA2 mutation carrier families, by mutation carrier status. Solid and dashed lines are drawn by locally weighted regression of log-transformed AMH levels. Carriers: crosses and dashed line; non-carriers: circles and solid line.
The estimated OR for having an AMH concentration in the lowest quartile for age was 1.84 (95% CI 1.11–3.03, P = 0.02) for BRCA1 mutation carriers and 0.87 (95% CI 0.51–1.47, P = 0.59) for BRCA2 mutation carriers.
Information was not available regarding whether women had PCOS, which is known to result in high AMH concentrations, however excluding women with AMH concentrations in the highest quartile from the analysis did not substantially change the estimates obtained.
Discussion
BRCA1 and BRCA2 mutation carriers are rare in the general population (about 0.1 and 0.2% respectively) (Antoniou et al., 2008), although they are much more prevalent in some subgroups due to founder effects, for example 1.2 and 1.5% respectively in Ashkenazi Jews (Roa et al., 1996). Whilst the increased cancer risk implications of having these germline mutations are well-described, much less is known about the non-cancer implications. This is the first large study to find that BRCA1 germline mutations are associated with lower than expected AMH, an established biomarker of ovarian reserve. Low AMH concentrations have not been shown to affect natural fecundability in young women (Hagen et al., 2012) but are associated with reduced fecundability in older women in their 30s (Steiner et al., 2011). The reduced concentrations of AMH observed in this study were equivalent, for example, to a two year age increase for a woman in her mid 30s. Thus it is possible that the findings of our study might not translate to clinically relevant fertility implications for younger women, but may be important for the subgroup of BRCA1 mutation carriers who wish to conceive in their late 30s or 40s when fertility is reduced even in the general population.
Our findings are consistent with pioneering observations in 2010 that breast cancer patients with germline BRCA1 mutations undergoing ovarian stimulation for fertility preservation prior to chemotherapy had lower oocyte yields compared with women not known to be BRCA1 mutation carriers or who carried a BRCA2 mutation (Oktay et al., 2010). Low oocyte yields from ovarian stimulation predict lower likelihood of pregnancy and earlier age at menopause (de Boer et al., 2002). Some studies have suggested that women with BRCA1 mutations experience earlier menopause than non-carriers (Rzepka-Gorska et al., 2006; Finch et al., 2013; Lin et al., 2013). In a previous study of the kConFab cohort, we did not find a difference in age at natural menopause between BRCA1 or BRCA2 mutation carriers and their non-carrier relatives (Collins et al., 2013). However, that observation could have been confounded; for example, mutation carriers who seemed destined for an earlier menopause (e.g. because their menses were becoming irregular) may have been more likely to choose cancer risk-reducing bilateral salpingo-oophorectomy at an early age (and therefore be censored from the analysis), biasing the study findings toward the null. Another limitation of our previous study was that only 19% of the cohort had undergone natural menopause.
Our new findings are consistent with some smaller studies. Wang et al found lower AMH concentrations in a group of 62 BRCA1 mutation carriers compared with 54 unrelated non-carriers, but no difference in AMH levels between 27 BRCA2 mutation carriers and non-carriers (Wang et al., 2014). Titus et al found similar results in a study of 15 BRCA1 mutation carriers, 9 BRCA2 mutation carriers and 60 non-carriers (Titus et al., 2013). Pavone et al found that AMH concentrations were similar between 66 BRCA1 mutation carriers and 59 non-carriers, but lower for BRCA1 mutation carriers aged 35–39 years (Pavone et al., 2014). Another study found no difference in the AMH concentrations between 41 BRCA1 and BRCA2 mutation carriers (pooled) and 324 controls (Michaelson-Cohen et al., 2014). Our study overcomes several methodologic limitations of these previous reports; specifically we had a larger sample size, detailed information about potential confounders such as age, OCP use, BMI, and cigarette smoking, and we used non-carriers (controls) from the same families as mutation carriers (cases) which, by design, adjusts in part for unmeasured genetic factors that might influence ovarian reserve.
High-fidelity double-strand DNA break repair is critical to mitosis and meiosis (Bolcun-Filas et al., 2014), and other genetic diseases characterized by deficient homologous recombination and DNA repair are known to be associated with subfertility due to accelerated oocyte apoptosis (Titus et al., 2013). A recent large scale genomic analysis by Day et al (Day et al., 2015) extended the findings of a prior genome-wide association study (Stolk et al., 2012) and showed that genetic variants in several DNA repair enzymes, including BRCA1, are associated with age at menopause in large populations, providing further evidence for the importance of DNA repair processes in determining reproductive lifespan. Oocyte meiosis is characterized by very prolonged arrest at meiosis I, from fetal life until ovulation which might be decades later. This prolonged arrest highlights the importance of maintaining chromosome/genetic integrity, which underpins oocyte health and survival over a very protracted period. Thus our finding of reduced AMH concentrations for carriers of mutations in BRCA1, a gene that is critically important in the repair of double-strand DNA breaks, has biologic plausibility. BRCA2 has a more limited role in double-strand DNA break repair compared with BRCA1 and BRCA2 mutation carriers tend to develop fewer cancers and at a later age, compared with BRCA1 mutation carriers (Antoniou et al., 2003). Thus it is credible that any effect of mutation status on ovarian reserve would be more pronounced in BRCA1 mutation carriers and this observation has been made in mouse models (Titus et al., 2013).
BRCA1 mutation carriers are at increased risk of ovarian and fallopian tube cancers and so are advised to consider bilateral salpingo-oophorectomy after completion of childbearing and preferably while premenopausal (National Comprehensive Cancer Network, 2008) because such timing also reduces breast cancer risk (Domchek et al., 2010). Some BRCA1 mutation carriers may therefore choose early childbearing in order to facilitate early bilateral salpingo-oophorectomy. Our study found that BRCA1 mutation carriers had, on average, 25% (95% CI: 5–41%, P = 0.02) lower AMH concentrations than non-carriers and were more likely to have AMH concentrations in the lowest quartile for age (OR 1.84, 95% CI: 1.11–303, P = 0.02). There was no evidence of an association between AMH concentration and BRCA2 mutation status (P = 0.94). Further research is required to fully understand the direct clinical implications of these findings, in terms of fertility, nevertheless they suggest that BRCA1 mutation carriers should try to avoid delaying pregnancy until later reproductive ages. Our findings also raise the hypothesis that BRCA1 mutation carriers may have a higher than average risk of chemotherapy-induced menopause (Anderson and Cameron, 2011); but this requires further study. Importantly our findings may shed new light on mechanisms of age-related fertility decline, the most common indication for assisted reproduction treatment.
Authors' roles
K.-A.P., I.M.C. and R.F. conceived and designed the study. R.L.M. analysed the data. K.-A.P. wrote the first draft of the manuscript. All authors contributed to the writing of the manuscript and agreed with manuscript results and conclusions.
Funding
kConFab is supported by a grant from the Australian National Breast Cancer Foundation, and previously by the National Health and Medical Research Council (NHMRC), the Queensland Cancer Fund, the Cancer Councils of New South Wales, Victoria, Tasmania and South Australia, and the Cancer Foundation of Western Australia. K.-A.P. is an Australian National Breast Cancer Foundation Practitioner Fellow. J.L.H. is a NHMRC Senior Principal Research Fellow. M.H. is a NHMRC Practitioner Fellow. Funding to pay the Open Access publication charges for this article was provided by the corresponding author.
Conflict of interest
R.A.A. reports personal fees from Roche Diagnostics & Beckman Coulter outside the submitted work and C.S. reports other earnings from Melbourne IVF outside the submitted work. The remaining authors have nothing to declare and no conflicts of interest.
Acknowledgements
We thank the kConFab study participants as well as Heather Thorne, Eveline Niedermayr, the kConFab research nurses and staff and the heads and staff of the Australian and New Zealand Family Cancer Clinics for their contributions to the kConFab resource. We also thank Professor Henry Burger for helpful discussions in the design phase of this study.
References
- Anderson RA, Cameron DA. Pretreatment serum anti-mullerian hormone predicts long-term ovarian function and bone mass after chemotherapy for early breast cancer. J Clin Endocrinol Metab 2011;96:1336–1343. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Anckaert E, Bosch E, Dewailly D, Dunlop CE, Fehr D, Nardo L, Smitz J, Tremellen K, Denk B et al. . Prospective study into the value of the automated Elecsys antimullerian hormone assay for the assessment of the ovarian growing follicle pool. Fertil Steril 2015;103:1074–1080 e1074. [DOI] [PubMed] [Google Scholar]
- Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, Loman N, Olsson H, Johannsson O, Borg A et al. . Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet 2003;72:1117–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou AC, Cunningham AP, Peto J, Evans DG, Lalloo F, Narod SA, Risch HA, Eyfjord JE, Hopper JL, Southey MC et al. . The BOADICEA model of genetic susceptibility to breast and ovarian cancers: updates and extensions. Br J Cancer 2008;98:1457–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolcun-Filas E, Rinaldi VD, White ME, Schimenti JC. Reversal of female infertility by Chk2 ablation reveals the oocyte DNA damage checkpoint pathway. Science 2014;343:533–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broer SL, Eijkemans MJ, Scheffer GJ, van Rooij IA, de Vet A, Themmen AP, Laven JS, de Jong FH, Te Velde ER, Fauser BC et al. . Anti-mullerian hormone predicts menopause: a long-term follow-up study in normoovulatory women. J Clin Endocrinol Metab 2011;96:2532–2539. [DOI] [PubMed] [Google Scholar]
- Collins IM, Milne RL, McLachlan SA, Friedlander M, Hickey M, Weideman PC, Birch KE, Hopper JL, Phillips KA. Do BRCA1 and BRCA2 mutation carriers have earlier natural menopause than their noncarrier relatives? Results from the Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer. J Clin Oncol 2013;31:3920–3925. [DOI] [PubMed] [Google Scholar]
- Day FR, Ruth KS, Thompson DJ, Lunetta KL, Pervjakova N, Chasman DI, Stolk L, Finucane HK, Sulem P, Bulik-Sullivan B et al. . Large-scale genomic analyses link reproductive aging to hypothalamic signaling, breast cancer susceptibility and BRCA1-mediated DNA repair. Nat Genet 2015;47:1294–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer EJ, den Tonkelaar I, te Velde ER, Burger CW, Klip H, van Leeuwen FE, group OM-p. A low number of retrieved oocytes at in vitro fertilization treatment is predictive of early menopause. Fertil Steril 2002;77:978–985. [DOI] [PubMed] [Google Scholar]
- Dolleman M, Verschuren WM, Eijkemans MJ, Dolle ME, Jansen EH, Broekmans FJ, van der Schouw YT. Reproductive and lifestyle determinants of anti-Mullerian hormone in a large population-based study. J Clin Endocrinol Metab 2013;98:2106–2115. [DOI] [PubMed] [Google Scholar]
- Domchek SM, Friebel TM, Singer CF, Evans DG, Lynch HT, Isaacs C, Garber JE, Neuhausen SL, Matloff E, Eeles R et al. . Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA 2010;304:967–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanchin R, Taieb J, Lozano DH, Ducot B, Frydman R, Bouyer J. High reproducibility of serum anti-Mullerian hormone measurements suggests a multi-staged follicular secretion and strengthens its role in the assessment of ovarian follicular status. Hum Reprod 2005;20:923–927. [DOI] [PubMed] [Google Scholar]
- Finch A, Valentini A, Greenblatt E, Lynch HT, Ghadirian P, Armel S, Neuhausen SL, Kim-Sing C, Tung N, Karlan B et al. . Frequency of premature menopause in women who carry a BRCA1 or BRCA2 mutation. Fertil Steril 2013;99:1724–1728. [DOI] [PubMed] [Google Scholar]
- Findlay JK, Hutt KJ, Hickey M, Anderson RA. What is the ‘ovarian reserve’? Fertil Steril 2015;103:628–630. [DOI] [PubMed] [Google Scholar]
- Gassner D, Jung R. First fully automated immunoassay for anti-Mullerian hormone. Clin Chem Lab Med 2014;52:1143–1152. [DOI] [PubMed] [Google Scholar]
- Hagen CP, Vestergaard S, Juul A, Skakkebaek NE, Andersson AM, Main KM, Hjollund NH, Ernst E, Bonde JP, Anderson RA et al. . Low concentration of circulating antimullerian hormone is not predictive of reduced fecundability in young healthy women: a prospective cohort study. Fertil Steril 2012;98:1602–1608 e1602. [DOI] [PubMed] [Google Scholar]
- Hansen KR, Hodnett GM, Knowlton N, Craig LB. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril 2011;95:170–175. [DOI] [PubMed] [Google Scholar]
- Iliodromiti S, Kelsey TW, Anderson RA, Nelson SM. Can anti-Mullerian hormone predict the diagnosis of polycystic ovary syndrome? A systematic review and meta-analysis of extracted data. J Clin Endocrinol Metab 2013;98:3332–3340. [DOI] [PubMed] [Google Scholar]
- Iliodromiti S, Anderson RA, Nelson SM. Technical and performance characteristics of anti-Mullerian hormone and antral follicle count as biomarkers of ovarian response. Hum Reprod Update 2014;21:698–710. [DOI] [PubMed] [Google Scholar]
- Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature 2009;461:1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John EM, Hopper JL, Beck JC, Knight JA, Neuhausen SL, Senie RT, Ziogas A, Andrulis IL, Anton-Culver H, Boyd N et al. . The Breast Cancer Family Registry: an infrastructure for cooperative multinational, interdisciplinary and translational studies of the genetic epidemiology of breast cancer. Breast Cancer Res 2004;6:R375–R389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsey TW, Wright P, Nelson SM, Anderson RA, Wallace WH. A validated model of serum anti-mullerian hormone from conception to menopause. PLoS One 2011;6:e22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WT, Beattie M, Chen LM, Oktay K, Crawford SL, Gold EB, Cedars M, Rosen M. Comparison of age at natural menopause in BRCA1/2 mutation carriers with a non-clinic-based sample of women in northern California. Cancer 2013;119:1652–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann GJ, Thorne H, Balleine RL, Butow PN, Clarke CL, Edkins E, Evans GM, Fereday S, Haan E, Gattas M et al. . Analysis of cancer risk and BRCA1 and BRCA2 mutation prevalence in the kConFab familial breast cancer resource. Breast Cancer Res 2006;8:R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelson-Cohen R, Mor P, Srebnik N, Beller U, Levy-Lahad E, Eldar-Geva T. BRCA mutation carriers do not have compromised ovarian reserve. Int J Gynecol Cancer 2014;24:233–237. [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network. Genetic/familial high-risk assessment: breast and ovarian. NCCN Clinical Practice Guidelines in Oncology. V. 1.2008. Fort Washington: (PA: ): NCCN, 2008. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site (20 May 2015, date last accessed). [Google Scholar]
- Oktay K, Kim JY, Barad D, Babayev SN. Association of BRCA1 mutations with occult primary ovarian insufficiency: a possible explanation for the link between infertility and breast/ovarian cancer risks. J Clin Oncol 2010;28:240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oktay K, Moy F, Titus S, Stobezki R, Turan V, Dickler M, Goswami S. Age-related decline in DNA repair function explains diminished ovarian reserve, earlier menopause, and possible oocyte vulnerability to chemotherapy in women with BRCA mutations. J Clin Oncol 2014;32:1093–1094. [DOI] [PubMed] [Google Scholar]
- Pavone ME, Mittal N, Smith K, Giordano SB. Amh values in reproductive aged women with and without the brca1 mutation. Fertil Steril 2014;102:e156. [Google Scholar]
- Phillips KA, Butow PN, Stewart AE, Chang JH, Weideman PC, Price MA, McLachlan SA, Lindeman GJ, McKay MJ, Friedlander ML et al. . Predictors of participation in clinical and psychosocial follow-up of the kConFab breast cancer family cohort. Fam Cancer 2005;4:105–113. [DOI] [PubMed] [Google Scholar]
- Roa BB, Boyd AA, Volcik K, Richards CS. Ashkenazi Jewish population frequencies for common mutations in BRCA1 and BRCA2. Nat Genet 1996;14:185–187. [DOI] [PubMed] [Google Scholar]
- Rzepka-Gorska I, Tarnowski B, Chudecka-Glaz A, Gorski B, Zielinska D, Toloczko-Grabarek A. Premature menopause in patients with BRCA1 gene mutation. Breast Cancer Res Treat 2006;100:59–63. [DOI] [PubMed] [Google Scholar]
- Sowers MR, Eyvazzadeh AD, McConnell D, Yosef M, Jannausch ML, Zhang D, Harlow S, Randolph JF Jr. Anti-mullerian hormone and inhibin B in the definition of ovarian aging and the menopause transition. J Clin Endocrinol Metab 2008;93:3478–3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner AZ, Herring AH, Kesner JS, Meadows JW, Stanczyk FZ, Hoberman S, Baird DD. Antimullerian hormone as a predictor of natural fecundability in women aged 30–42 years. Obstet Gynecol 2011;117:798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolk L, Perry JR, Chasman DI, He C, Mangino M, Sulem P, Barbalic M, Broer L, Byrne EM, Ernst F et al. . Meta-analyses identify 13 loci associated with age at menopause and highlight DNA repair and immune pathways. Nat Genet 2012;44:260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su HI, Flatt SW, Natarajan L, DeMichele A, Steiner AZ. Impact of breast cancer on anti-mullerian hormone levels in young women. Breast Cancer Res Treat 2013;137:571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehrani FR, Solaymani-Dodaran M, Tohidi M, Gohari MR, Azizi F. Modeling age at menopause using serum concentration of anti-mullerian hormone. J Clin Endocrinol Metab 2013;98:729–735. [DOI] [PubMed] [Google Scholar]
- Titus S, Li F, Stobezki R, Akula K, Unsal E, Jeong K, Dickler M, Robson M, Moy F, Goswami S et al. . Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci Transl Med 2013;5:172ra121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsepelidis S, Devreker F, Demeestere I, Flahaut A, Gervy C, Englert Y. Stable serum levels of anti-Mullerian hormone during the menstrual cycle: a prospective study in normo-ovulatory women. Hum Reprod 2007;22:1837–1840. [DOI] [PubMed] [Google Scholar]
- Wang ET, Pisarska MD, Bresee C, Chen YD, Lester J, Afshar Y, Alexander C, Karlan BY. BRCA1 germline mutations may be associated with reduced ovarian reserve. Fertil Steril 2014;102:1723–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth KW, Baird DD, Steiner AZ, Bornman RM, Travlos GS, Wilson RE, Longnecker MP. Anti-mullerian hormone and lifestyle, reproductive, and environmental factors among women in rural South Africa. Epidemiology 2015;26:429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]