Abstract
BACKGROUND
Annual updates on cancer occurrence and trends in the United States are provided through an ongoing collaboration among the American Cancer Society (ACS), the Centers for Disease Control and Prevention (CDC), the National Cancer Institute (NCI), and the North American Association of Central Cancer Registries (NAACCR). This annual report highlights the increasing burden of liver and intrahepatic bile duct (liver) cancers.
METHODS
Cancer incidence data were obtained from the CDC, NCI, and NAACCR; data about cancer deaths were obtained from the CDC’s National Center for Health Statistics (NCHS). Annual percent changes in incidence and death rates (age-adjusted to the 2000 US Standard Population) for all cancers combined and for the leading cancers among men and women were estimated by joinpoint analysis of long-term trends (incidence for 1992–2012 and mortality for 1975–2012) and short-term trends (2008–2012). In-depth analysis of liver cancer incidence included an age-period-cohort analysis and an incidence-based estimation of person-years of life lost because of the disease. By using NCHS multiple causes of death data, hepatitis C virus (HCV) and liver cancer-associated death rates were examined from 1999 through 2013.
RESULTS
Among men and women of all major racial and ethnic groups, death rates continued to decline for all cancers combined and for most cancer sites; the overall cancer death rate (for both sexes combined) decreased by 1.5% per year from 2003 to 2012. Overall, incidence rates decreased among men and remained stable among women from 2003 to 2012. Among both men and women, deaths from liver cancer increased at the highest rate of all cancer sites, and liver cancer incidence rates increased sharply, second only to thyroid cancer. Men had more than twice the incidence rate of liver cancer than women, and rates increased with age for both sexes. Among non-Hispanic (NH) white, NH black, and Hispanic men and women, liver cancer incidence rates were higher for persons born after the 1938 to 1947 birth cohort. In contrast, there was a minimal birth cohort effect for NH Asian and Pacific Islanders (APIs). NH black men and Hispanic men had the lowest median age at death (60 and 62 years, respectively) and the highest average person-years of life lost per death (21 and 20 years, respectively) from liver cancer. HCV and liver cancer-associated death rates were highest among decedents who were born during 1945 through 1965.
CONCLUSIONS
Overall, cancer incidence and mortality declined among men; and, although cancer incidence was stable among women, mortality declined. The burden of liver cancer is growing and is not equally distributed throughout the population. Efforts to vaccinate populations that are vulnerable to hepatitis B virus (HBV) infection and to identify and treat those living with HCV or HBV infection, metabolic conditions, alcoholic liver disease, or other causes of cirrhosis can be effective in reducing the incidence and mortality of liver cancer.
Keywords: cancer, incidence, liver cancer, mortality, National Program of Cancer Registries (NPCR), North American Association of Central Cancer Registries (NAACCR), Surveillance, Epidemiology, and End Results (SEER), survival, trends, viral hepatitis
INTRODUCTION
This marks the 18th year that the American Cancer Society (ACS), the Centers for Disease Control and Prevention (CDC), the National Cancer Institute (NCI), and the North American Association of Central Cancer Registries (NAACCR) have collaborated to develop the Annual Report to the Nation on the Status of Cancer.1–17 These reports provide annual updates on cancer incidence, deaths, and trends of the most common cancers in the United States. In addition, each publication features an in-depth analysis of a selected special topic. This year’s report provides a detailed analysis of the incidence of liver and intrahepatic bile duct (liver) cancers and rates of liver cancer and hepatitis C virus (HCV) infection-associated deaths.
Worldwide, liver cancer is the fifth most common cancer among men, the ninth most common cancer among women, and the second most common cause of cancer death for men and women combined.18 Although liver cancer occurs more frequently in less developed regions of the world,18 it is still a significant health outcome in the United States. In 2012, a total of 28,012 persons in the United States (excluding Nevada) were diagnosed with liver cancer (20,207 men and 7805 women), and 22,972 died of this disease (15,563 men and 7409 women).19 The relative 5-year survival rate for liver cancer is 16.6% (95% confidence interval [CI], 16.3%–16.9%).19 Forty-three percent of patients with liver cancer are diagnosed at a localized stage, for which the 5-year relative survival rate is 30.5%.20 Those diagnosed at a regional stage (27%) and a distant stage (18%) have 5-year relative survival rates of 10.7% and 3.1%, respectively.20
Viral hepatitis is an important cause of hepatocellular carcinoma (HCC), the most common histologic type of liver cancer. Chronic infections with hepatitis B virus (HBV) or HCV are well documented risk factors for HCC. Globally, it is reported that both infections contribute to greater than 60% of HCC cases.21 In the United States, it has also been reported that chronic HBV and HCV infections are major risk factors for HCC and are correlated with increasing trends in HCC incidence.22,23 Data from national US surveys indicate that from 850,000 to 2.2 million persons are living with chronic HBV infection,24,25 and from 2.7 to 3.5 million persons are living with chronic HCV infection.26,27 The age-specific prevalence of HCV infection also suggests that persons born during 1945 through 1965 are more likely than other birth cohorts to be diagnosed with HCV infection.28 In the absence of improved testing and appropriate treatment, HCV infections among this generation will continue to account for a substantial proportion of deaths from liver cancer.29 Consequently, the CDC and the United States Preventive Services Task Force (USPSTF) recently recommended a 1-time HCV test for persons born during 1945 through 1965.30,31 Other important risk factors for liver cancer include excessive alcohol consumption, obesity, nonalcoholic fatty liver disease, rare metabolic disorders, and type 2 diabetes mellitus.32 In this report, we closely examine incidence and mortality trends from liver cancer among different groups, discuss the potential risk factors contributing to changes in liver cancer rates, and present ongoing public health interventions aimed at reducing the burden of liver cancer in the United States.
MATERIALS AND METHODS
Data Sources, Codes, and Selection Criteria
Cancer incidence data
Population-based cancer incidence data were obtained from registries that participate in the CDC’s National Program of Cancer Registries (NPCR) and/or the NCI’s Surveillance, Epidemiology, and End Results (SEER) program and voluntarily submit their data to NAACCR. Participating registries met NAACCR’s data-quality criteria for the December 2014 submission cycle.33 Site and histology were coded according to the International Classification of Diseases for Oncology (ICD-O) edition in use at the time of diagnosis, converted to the third edition coding,34 and categorized according to SEER site groups.20 Only cases defined as malignant under ICD-O-2 and ICD-O-3 were included in this report.
Incidence rates were calculated for all sites combined, for childhood cancers (ages 0–14 and 0–19 years), and for the most common cancers for each of the 5 major racial and ethnic groups (white, black, Asian and Pacific Islander [API], American Indian/Alaska Native [AI/AN], and Hispanic). Rates for Hispanic ethnicity included individuals from all races identified as Hispanic, except in the special section, as noted. Rates for AI/ANs were based on cases and deaths occurring in counties covered by the Indian Health Service Contract Health Service Delivery Areas (CHSDA), because it has been demonstrated that these areas have more accurate classification of AI/AN race.10,35,36
Cancer incidence data were not available uniformly for every calendar year, geographic area, or racial and ethnic group in the United States. Long-term (1992–2012) incidence trends for all racial and ethnic groups combined were estimated by using data from the SEER-13 registries, which cover approximately 14% of the US population.37 Five-year (2008–2012) average annual incidence rates, 5-year (2008–2012) and 10-year (2003–2012) incidence trends for all racial and ethnic groups combined, and 10-year trends for each of the 5 major racial and ethnic populations were calculated by using combined data from the NPCR and SEER registries as submitted to NAACCR. Together, participating registries cover 97% (for 2008–2012) and 92% (for 2003–2012) of the US population.
Cancer mortality data
Cause of death was based on death certificate information reported to state vital statistics offices and compiled into a national file for the entire United States by the CDC National Center for Health Statistics’ (NCHS) National Vital Statistics System.38 The underlying causes of death were selected according to the International Classification of Disease (ICD) codes and rules in use at the time of death (ICD-8 through ICD-10) and categorized according to SEER site groups to maximize comparability between ICD and ICD-O versions.20 Death rates were calculated for all cancer sites combined, for childhood cancers, and the most common cancers among men and women identified by the incidence analysis. We examined long-term (1975–2012) mortality trends for all racial and ethnic groups combined and 5-year (2008–2012) average annual age-adjusted death rates and 10-year (2003–2012) mortality trends for each of the 5 major racial and ethnic groups.
Population data
The population estimates that were used in this report represent a modification of the intercensal and Vintage 2013 annual times series of July 1; and county population estimates by age, sex, race, and Hispanic origin were produced by the US Census Bureau’s Population Estimates Program in collaboration with the CDC’s NCHS and with support from the NCI.39 The estimates incorporate intercensal (July 1, 2000–2009) and Vintage 2013 (July 1, 2010–2013) bridged, single-race estimates that are derived from the original multiple race categories in the 2000 and 2010 US Censuses (as specified in the 1997 Office of Management and Budget standards for the collection of data about race and ethnicity).40 For most states, population estimates as of July 1 of each year were used to calculate incidence rates, which were presumed to reflect the average population of a defined geographic area for a calendar year; however, some adjustments were made to refine these estimates, as has been done in previous reports.16,17,39
Liver and Intrahepatic Bile Duct Cancer-Specific Incidence and Mortality Data
The special analysis for this report included all invasive liver and intrahepatic bile duct (liver) cancer cases (ICD-O-3 site codes C220 and C221, excluding histology codes 9050–9055; 9140; 9590–9989). Analyses in this section were restricted to 4 large, nonoverlapping racial or ethnic groups with a sufficient number of cases to produce informative results: non-Hispanic (NH) white, NH black, NH API, and Hispanic. Small counts produced unstable rate estimates for the NH AI/AN group and have been excluded from certain analyses.
To help understand differences in liver cancer incidence rates and trends by birth cohort, we conducted age-period-cohort analyses. We grouped liver cancer incidence data from the SEER-13 registries into 5-year age groups (ages 35–39 through 80–84 years) and 5-year calendar periods (1993–1997, 1998–2002, 2003–2007, and 2008–2012), spanning 13 partially overlapping, 10-year birth cohorts (from 1908–1917 [referred to as “1913,” the mid-year of birth] through 1968–1977). To analyze age-specific liver cancer incidence rates by period, data from the SEER-13 registries were grouped into 5-year age groups (35–39 through 80–84 and ≥85 years) and 5-year periods (1992–1996, 2000–2004, and 2008–2012), which were the beginning, middle, and end of the 20-year span.
To examine the distribution of liver cancer deaths by race and ethnicity, SEER-18 incidence-based mortality data for liver cancer deaths (ICD-10 codes C22) during 2008 through 2012 were analyzed. All patients in this analysis were diagnosed with and subsequently died of liver cancer, lessening the misclassification of death from liver cancer metastasis as death from primary liver cancer. Patients who had death certificate and autopsy-only diagnoses were excluded. SEER-18 registries cover 28% of the US population.
Because HCV infection is a major contributing factor for HCC in the United States, we also examined HCV and liver cancer-associated mortality for 1999 through 2013. The cancer mortality data and population files used in this analysis were consistent with the files used for other cancer mortality estimates in this report. The data were analyzed to describe mortality by birth year category and year of death and by birth year category and age at death for decedents aged ≥35 years who had both HCV and liver cancer listed among causes of death. Birth year and cohort were assigned by subtracting the age at death from the year of death. The underlying cause of death and multiple causes of death were selected according to ICD-10 codes41 and selection rules in use at the time of death.42 HCV and liver cancer-associated deaths were defined as having HCV (ICD-10 codes B17.1 or B18.2) and liver cancer (ICD-10 codes C22.0–C22.9) listed together anywhere on the death record as either the underlying cause or among the multiple causes of death.
Statistical Methods
Incidence and death rates and trends
Age-adjusted rates were expressed per 100,000 persons on the basis of the 2000 US Standard Population and were generated by using SEER*Stat software, version 8.2.1.43 Corresponding 95% CIs were calculated as modified γ intervals.44 For stability and reliability, incidence rates were suppressed if there were fewer than 16 cases for the time interval, and incidence trends were suppressed if there were fewer than 10 cases for at least a year within the time interval. Death rates were suppressed if there were fewer than 10 deaths.
Trends in age-adjusted cancer incidence and death rates were estimated using joinpoint regression.45,46 Up to 5 joinpoints were allowed in models for 1975 to 2012, up to 3 joinpoints were allowed in models for 1992 to 2012, and up to 2 joinpoints were allowed in models for 2003 to 2012. The resulting trends were described according to the annual percent change (APC). The average APC (AAPC) was estimated as a weighted geometric average of the APCs, with the weights equal to the length of each line segment during the prespecified, fixed interval.47 Long-term incidence trends were calculated by using both observed and delay-adjusted SEER-13 data.48 Descriptions of the long-term incidence trends were based on the delay-adjusted data unless otherwise noted. Delay-adjustment factors are not currently published for NPCR; thus, all 5-year and 10-year trends were based on observed NPCR and SEER combined data without delay adjustment. We used the t test and the Z test, respectively, to test whether the APC and AAPC were statistically different from zero. All statistical tests were 2-sided. In describing trends, the term increase or decrease was used when the slope of the trend (APC or AAPC) was statistically significant (2-sided P < .05). For nonstatistically significant trends, terms such as stable, nonsignificant increase, and nonsignificant decrease were used.
Liver Cancer Age-Period-Cohort and Estimated Years-of-Life-Lost Analysis
Liver cancer incidence rates were examined by sex, age, race or ethnicity, state, and year of diagnosis by using the same methods described above. The NCI’s online age-period-cohort analysis tool was used to calculate cohort rate ratios, comparing liver cancer incidence rates in each birth cohort relative to the 1943 birth cohort, and adjusting for age and period effects.49 The 1943 birth cohort was selected as the reference because it immediately preceded the known increase in the prevalence of HCV infection for individuals born during 1945 through 1965.28
Proportions of incidence-based deaths from liver cancer and median age at death were determined by sex and race or ethnicity.50 Person-years of life lost (PYLL) to liver cancer were calculated by linking the “expectation of life at age x” column of the US 2010 complete life tables to single-year age-at-death data.20,51,52 All-race life tables were used to calculate overall PYLL, whereas single-sex, all-race tables were used for gender-specific analyses. The average PYLL (APYLL) per death was calculated as the PYLL divided by the number of deaths.
HCV and liver cancer-associated death rates during 1999 through 2013 for those aged ≥35 years according to 3 birth-year categories (those born before 1945, during 1945–1965, and during 1966–1978) were examined by year at death. To remove the effect of age, HCV and liver cancer-associated death rates also were examined using slightly more refined birth-year categories (those born before 1945, during 1945–1949, during 1950–1959, during 1960–1965, and during 1966–1978) and by age at death. Crude HCV and liver cancer-associated death rates were calculated by dividing the number of deaths in each category by the US Census population estimate for each year. A similar methodology was applied in a previous study that examined the burden of mortality associated with viral hepatitis in the United States from 1999 to 2007.53
RESULTS
Long-Term Trends of Cancer Incidence Rates for the Most Common Cancers
Trends in delay-adjusted cancer incidence rates using data from SEER-13 registries that submitted data to the NCI from 1992 to 2012 are presented in Table 1. Cancer incidence rates decreased among men during each period, but at different rates. In contrast, cancer incidence rates among women increased slightly (0.8% per year) from 1992 to 1998, then remained stable. Among children in both age groups, (ages 0–14 and 0–19 years), incidence rates increased from 1992 to 2012 with an APC of 0.8%.
TABLE 1.
Surveillance, Epidemiology, and End Results (SEER) Cancer Incidence Rate Trends With Joinpoint Analyses From 1992 to 2012 for the Most Common Cancers, by Sex, for All Racial and Ethnic Groups Combineda
| Sex/Cancer Site or Type | Joinpoint Analyses (1992–2012)b
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Trend 1
|
Trend 2
|
Trend 3
|
Trend 4
|
AAPCc
|
||||||
| Years | APCd | Years | APCd | Years | APCd | Years | APCd | 2003–2012 | 2008–2012 | |
| All sitese | ||||||||||
| Both sexes | 1992–1994 | −3.1f | 1994–1998 | 0.4 | 1998–2009 | −0.4f | 2009–2012 | −2.3f | −1.0g | −1.8g |
| (Delay-adjusted) | 1992–1994 | −3.1f | 1994–1998 | 0.4 | 1998–2009 | −0.3f | 2009–2012 | −1.5f | −0.7g | −1.2g |
| Men | 1992–1994 | −5.7f | 1994–2009 | −0.5f | 2009–2012 | −4.1f | −1.7g | −3.2g | ||
| (Delay-adjusted) | 1992–1994 | −5.8f | 1994–2009 | −0.5f | 2009–2012 | −3.1f | −1.4g | −2.5g | ||
| Women | 1992–1998 | 0.7f | 1998–2012 | −0.3f | −0.3g | −0.3g | ||||
| (Delay-adjusted) | 1992–1998 | 0.8f | 1998–2003 | −0.6 | 2003–2012 | 0.0 | 0.0 | 0.0 | ||
| Children (ages 0–14 years) | 1992–2012 | 0.7f | 0.7g | 0.7g | ||||||
| (Delay-adjusted) | 1992–2012 | 0.8f | 0.8g | 0.8g | ||||||
| Children (ages 0–19 years) | 1992–2012 | 0.7f | 0.7g | 0.7g | ||||||
| (Delay-adjusted) | 1992–2012 | 0.8f | 0.8g | 0.8g | ||||||
| Top 17 cancers among menh | ||||||||||
| Prostate | 1992–1995 | −11.1f | 1995–2000 | 2.1 | 2000–2010 | −2.0f | 2010–2012 | −12.4f | −4.4g | −7.3g |
| (Delay-adjusted) | 1992–1995 | −11.1f | 1995–2000 | 2.1 | 2000–2010 | −1.8f | 2010–2012 | −11.2f | −4.0g | −6.6g |
| Lung and bronchus | 1992–2009 | −1.9f | 2009–2012 | −4.2f | −2.7g | −3.6g | ||||
| (Delay-adjusted) | 1992–2009 | −1.9f | 2009–2012 | −3.3f | −2.4g | −3.0g | ||||
| Colon and rectum | 1992–1995 | −2.6f | 1995–1998 | 1.4 | 1998–2008 | −2.5f | 2008–2012 | −4.0f | −3.2g | −4.0g |
| (Delay-adjusted) | 1992–1995 | −2.6f | 1995–1998 | 1.4 | 1998–2008 | −2.5f | 2008–2012 | −3.6f | −3.0g | −3.6g |
| Urinary bladder | 1992–2007 | 0.1 | 2007–2012 | −1.7f | −0.9g | −1.7g | ||||
| (Delay-adjusted) | 1992–2007 | 0.1 | 2007–2012 | −1.2f | −0.6g | −1.2g | ||||
| Melanoma of the skin | 1992–2008 | 2.6f | 2008–2012 | −0.4 | 1.2g | −0.4 | ||||
| (Delay-adjusted) | 1992–1996 | 5.0f | 1996–2012 | 2.0f | 2.0g | 2.0g | ||||
| Non-Hodgkin lymphoma | 1992–1995 | 2.8 | 1995–1998 | −2.1 | 1998–2010 | 0.6f | 2010–2012 | −4.1 | −0.5 | −1.8 |
| (Delay-adjusted) | 1992–1995 | 2.8 | 1995–1998 | −2.2 | 1998–2010 | 0.7f | 2010–2012 | −2.8 | −0.1 | −1.1 |
| Kidney and renal pelvis | 1992–2004 | 1.9f | 2004–2008 | 4.4f | 2008–2012 | −1.4 | 1.5g | −1.4 | ||
| (Delay-adjusted) | 1992–1999 | 1.1f | 1999–2008 | 3.2f | 2008–2012 | −0.1 | 1.7g | −0.1 | ||
| Oral cavity and pharynx | 1992–2001 | −1.8f | 2001–2012 | 0.2 | 0.2 | 0.2 | ||||
| (Delay-adjusted) | 1992–2003 | −1.5f | 2003–2012 | 0.8f | 0.8g | 0.8g | ||||
| Leukemia | 1992–2012 | 0.1 | 0.1 | 0.1 | ||||||
| (Delay-adjusted) | 1992–2006 | 0.2 | 2006–2012 | 1.8f | 1.3g | 1.8g | ||||
| Pancreas | 1992–2003 | 0.0 | 2003–2006 | 2.9 | 2006–2012 | 0.1 | 1.0 | 0.1 | ||
| (Delay-adjusted) | 1992–2001 | 0.0 | 2001–2012 | 1.2f | 1.2g | 1.2g | ||||
| Liver and intrahepatic bile duct | 1992–1999 | 4.6f | 1999–2002 | 0.5 | 2002–2007 | 5.4f | 2007–2012 | 1.8f | 3.4g | 1.8g |
| (Delay-adjusted) | 1992–2012 | 3.7f | 3.7g | 3.7g | ||||||
| Stomach | 1992–2012 | −1.7f | −1.7g | −1.7g | ||||||
| (Delay-adjusted) | 1992–2012 | −1.7f | −1.7g | −1.7g | ||||||
| Esophagus | 1992–2012 | −0.2 | −0.2 | −0.2 | ||||||
| (Delay-adjusted) | 1992–2012 | −0.2 | −0.2 | −0.2 | ||||||
| Brain and other nervous system | 1992–2012 | −0.3f | −0.3g | −0.3g | ||||||
| (Delay-adjusted) | 1992–2012 | −0.2f | −0.2g | −0.2g | ||||||
| Myeloma | 1992–2012 | 0.7f | 0.7g | 0.7g | ||||||
| (Delay-adjusted) | 1992–2006 | 0.4 | 2006–2012 | 3.0f | 2.1g | 3.0g | ||||
| Thyroid | 1992–1995 | −3.0 | 1995–2012 | 5.2f | 5.2g | 5.2g | ||||
| (Delay-adjusted) | 1992–1995 | −3.2 | 1995–2012 | 5.3f | 5.3g | 5.3g | ||||
| Larynx | 1992–2003 | −3.1f | 2003–2012 | −1.9f | −1.9g | −1.9g | ||||
| (Delay-adjusted) | 1992–2003 | −3.2f | 2003–2012 | −1.7f | −1.7g | −1.7g | ||||
| Top 18 cancers among womenh | ||||||||||
| Breast | 1992–1999 | 1.3f | 1999–2004 | −2.2f | 2004–2012 | 0.2 | −0.1 | 0.2 | ||
| (Delay-adjusted) | 1992–1999 | 1.3f | 1999–2004 | −2.2f | 2004–2012 | 0.3 | 0.0 | 0.3 | ||
| Lung and bronchus | 1992–2007 | 0.0 | 2007–2012 | −2.4f | −1.3g | −2.4g | ||||
| (Delay-adjusted) | 1992–2007 | 0.0 | 2007–2012 | −1.9f | −1.1g | −1.9g | ||||
| Colon and rectum | 1992–1995 | −1.8f | 1995–1998 | 1.8 | 1998–2008 | −2.0f | 2008–2012 | −4.1f | −2.9g | −4.1g |
| (Delay-adjusted) | 1992–1995 | −1.8f | 1995–1998 | 1.8 | 1998–2008 | −2.0f | 2008–2012 | −3.8f | −2.8g | −3.8g |
| Corpus and uterus, NOS | 1992–1997 | 0.8 | 1997–2004 | −0.8 | 2004–2012 | 1.8f | 1.5g | 1.8g | ||
| (Delay-adjusted) | 1992–2006 | −0.1 | 2006–2012 | 2.3f | 1.5g | 2.3g | ||||
| Thyroid | 1992–1999 | 4.1f | 1999–2009 | 6.9f | 2009–2012 | 1.4 | 5.0g | 2.7g | ||
| (Delay-adjusted) | 1992–1999 | 4.1f | 1999–2009 | 6.9f | 2009–2012 | 1.8 | 5.2g | 3.0g | ||
| Non-Hodgkin lymphoma | 1992–2004 | 1.3f | 2004–2012 | −0.8f | −0.5 | −0.8g | ||||
| (Delay-adjusted) | 1992–2004 | 1.3f | 2004–2012 | −0.4 | −0.2 | −0.4 | ||||
| Melanoma of the skin | 1992–1997 | 4.0f | 1997–2010 | 1.6f | 2010–2012 | −3.1 | 0.5 | −0.8 | ||
| (Delay-adjusted) | 1992–2005 | 2.4f | 2005–2012 | 0.5 | 0.9g | 0.5 | ||||
| Ovarye | 1992–2012 | −1.0f | −1.0g | −1.0g | ||||||
| (Delay-adjusted) | 1992–2012 | −0.9f | −0.9g | −0.9g | ||||||
| Kidney and renal pelvis | 1992–1998 | 1.2 | 1998–2008 | 3.1f | 2008–2012 | −1.3 | 1.2g | −1.3 | ||
| (Delay-adjusted) | 1992–2000 | 1.6f | 2000–2007 | 3.6f | 2007–2012 | −0.1 | 1.5g | −0.1 | ||
| Pancreas | 1992–2012 | 0.7f | 0.7g | 0.7g | ||||||
| (Delay-adjusted) | 1992–1999 | −0.1 | 1999–2012 | 1.1f | 1.1g | 1.1g | ||||
| Leukemia | 1992–2012 | 0.3f | 0.3g | 0.3g | ||||||
| (Delay-adjusted) | 1992–2012 | 0.7f | 0.7g | 0.7g | ||||||
| Urinary bladder | 1992–2004 | −0.2 | 2004–2012 | −1.2f | −1.1g | −1.2g | ||||
| (Delay-adjusted) | 1992–2004 | −0.2 | 2004–2012 | −1.1f | −1.0g | −1.1g | ||||
| Cervix uteri | 1992–2012 | −2.4f | −2.4g | −2.4g | ||||||
| (Delay-adjusted) | 1992–2012 | −2.4f | −2.4g | −2.4g | ||||||
| Oral cavity and pharynx | 1992–2012 | −0.7f | −0.7g | −0.7g | ||||||
| (Delay-adjusted) | 1992–2005 | −1.1f | 2005–2012 | 0.5 | 0.1 | 0.5 | ||||
| Brain and other nervous system | 1992–2012 | −0.2 | −0.2 | −0.2 | ||||||
| (Delay-adjusted) | 1992–2012 | 0.0 | 0.0 | 0.0 | ||||||
| Myeloma | 1992–2012 | 0.4f | 0.4g | 0.4g | ||||||
| (Delay-adjusted) | 1992–2012 | 0.7f | 0.7g | 0.7g | ||||||
| Stomach | 1992–2012 | −0.7f | −0.7g | −0.7g | ||||||
| (Delay-adjusted) | 1992–2012 | −0.6f | −0.6g | −0.6g | ||||||
| Liver and intrahepatic bile duct | 1992–1996 | 6.9f | 1996–2012 | 2.4f | 2.4g | 2.4g | ||||
| (Delay-adjusted) | 1992–2012 | 3.0f | 3.0g | 3.0g | ||||||
Abbreviations: AAPC, average annual percent change; APC, annual percent change; NOS, not otherwise specified.
Source: Surveillance, Epidemiology, and End Results (SEER)-13 areas covering about 14% of the US population (Connecticut, Hawaii, Iowa, Utah, and New Mexico; the Alaska Native Tumor Registry; rural Georgia; and the metropolitan areas of San Francisco, Los Angeles, San Jose-Monterey, Detroit, Atlanta, and Seattle-Puget Sound).
Joinpoint analyses with up to 3 joinpoints yielding up to 4 trend segments (Trends 1–4) were based on rates per 100,000 persons and were age-adjusted to the 2000 US standard population (19 age groups: ages <1 year, 1–4 years, 5–9 years, …, 80–84 years, and ≥85 years; Census publication p25-1130 [US Bureau of the Census, Current Population Reports, p25-1130. Washington, DC: US Government Printing Office; 2000]). For joinpoint analysis, the Joinpoint Regression Program was used (version 4.2.0.0, April 2015; Statistical Research and Applications Branch, National Cancer Institute, Bethesda, MD).
The AAPC is a weighted average of the APCs calculated by joinpoint regression.
The APC is based on rates that were age-adjusted to the 2000 US standard population (19 age groups; Census publication p25-1130).
All sites exclude myelodysplastic syndromes and borderline tumors; ovary excludes borderline tumors.
The APC is statistically significantly different from zero (2-sided t test; P < .05).
The AAPC is statistically significantly different from zero (2-sided Z test; P < .05).
Cancers are listed in descending rank order of sex-specific, age-adjusted incidence rates for 2008 through 2012 for all racial and ethnic groups combined (using data from the National Program of Cancer Registries [NPCR] and SEER Program areas reported by the North American Association of Central Cancer Registries [NAACCR] as meeting high-quality incidence data standards for 2008–2012). More than 15 cancers may appear under men and women to include the top 15 cancers in each racial and ethnic group.
During 2003 through 2012, the AAPC indicated that overall cancer incidence rates for all persons combined decreased on average 0.7% per year (Table 1). Among men, overall cancer incidence decreased on average by 1.4% per year during 2003 through 2012; however, among women, rates were stable during this 10-year period. Among men, incidence rates for 7 of the 17 most common cancers decreased from 2003 to 2012 (prostate, colon and rectum [colorectal], lung and bronchus [lung], stomach, larynx, urinary bladder [bladder], and brain and other nervous system [brain]). The most striking decline was for prostate cancer, which had an average 6.6% decline per year for the most recent 5-year period (2008–2012). In contrast, incidence rates increased among men during 2003 through 2012 for 8 cancers (thyroid, liver, myeloma, melanoma of the skin [melanoma], kidney and renal pelvis [kidney], leukemia, pancreas, and oral cavity and pharynx). Among women, incidence rates for 6 of the 18 most common cancers decreased during 2003 through 2012 (colorectal, cervix uteri [cervix], lung, bladder, ovary, and stomach), whereas incidence rates among women increased during 2003 through 2012 for 8 cancers (thyroid; liver; corpus and uterus, not otherwise specified [uterus]; kidney; pancreas; melanoma; leukemia; and myeloma). Incidence rates were stable for all other sites.
Long-Term Trends of Cancer Death Rates for the Most Common Cancers
The long-term (1975–2012) mortality trend analysis revealed that the overall cancer death rate in the United States has generally declined since the early 1990s for adults and since the 1970s for children (Table 2). More recently, from 2003 to 2012, death rates continued to decline by an average of 1.5% per year overall, by 1.3% per year for children ages 0 to 14 years, and by 2.0% per year for children ages 0 to 19 years. Among men, death rates declined overall from 2003 to 2012 (1.8% per year). Relatively large declines among men were also observed for cancers of the prostate (3.5% per year), stomach (3.3% per year), colorectum (2.9% per year), lung (2.7% per year), and larynx (2.5% per year) and for non-Hodgkin lymphoma (2.2% per year). Declines in death rates during this time among men were ≤1% per year for leukemia, myeloma, and cancers of the esophagus and kidney. Death rates among men remained stable during this time for melanoma and for cancers of the bladder, brain, and oral cavity and pharynx. Death rates increased among men during 2003 through 2012 for cancers of the liver (2.8% per year); soft tissue, including the heart (1.1% per year); and pancreas (0.3% per year).
TABLE 2.
US Cancer Death Rate Trends With Joinpoint Analyses From 1975 to 2012 for the Most Common Cancers, by Sex, for All Racial and Ethnic Groups Combineda
| Sex/Cancer Site or Type | Joinpoint Analyses (1975–2012)b
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trend 1
|
Trend 2
|
Trend 3
|
Trend 4
|
Trend 5
|
Trend 6
|
AAPCc
|
||||||||
| Years | APCd | Years | APCd | Years | APCd | Years | APCd | Years | APCd | Years | APCd | 2003–2012 | 2008–2012 | |
| All sites | ||||||||||||||
| Both sexes | 1975–1984 | 0.5e | 1984–1991 | 0.3e | 1991–1994 | −0.5 | 1994–1998 | −1.3e | 1998–2001 | −0.8 | 2001–2012 | −1.5e | −1.5f | −1.5f |
| Men | 1975–1979 | 1.0e | 1979–1990 | 0.3e | 1990–1993 | −0.5 | 1993–2001 | −1.5e | 2001–2012 | −1.8e | −1.8f | −1.8f | ||
| Women | 1975–1990 | 0.6e | 1990–1994 | −0.2 | 1994–2002 | −0.8e | 2002–2012 | −1.4e | −1.4f | −1.4f | ||||
| Children (ages 0–14 years) | 1975–1996 | −2.9e | 1996–2012 | −1.3e | −1.3f | −1.3f | ||||||||
| Children (ages 0–19 years) | 1975–1998 | −2.7e | 1998–2002 | 0.0 | 2002–2012 | −2.0e | −2.0f | −2.0f | ||||||
| Top 17 cancers among meng | ||||||||||||||
| Lung and bronchus | 1975–1978 | 2.5e | 1978–1984 | 1.2e | 1984–1990 | 0.4e | 1990–1993 | −1.1 | 1993–2005 | −1.9e | 2005–2012 | −2.9e | −2.7f | −2.9f |
| Prostate | 1975–1987 | 0.9e | 1987–1991 | 3.0e | 1991–1994 | −0.5 | 1994–1998 | −4.2e | 1998–2012 | −3.5e | −3.5f | −3.5f | ||
| Colon and rectum | 1975–1978 | 0.8 | 1978–1984 | −0.3 | 1984–1990 | −1.3e | 1990–2002 | −2.0e | 2002–2005 | −3.9e | 2005–2012 | −2.6e | −2.9f | −2.6f |
| Pancreas | 1975–1986 | −0.8e | 1986–2000 | −0.3e | 2000–2012 | 0.3e | 0.3f | 0.3f | ||||||
| Leukemia | 1975–1980 | 0.5 | 1980–1987 | −0.7e | 1987–1995 | 0.1 | 1995–2012 | −0.9e | −0.9f | −0.9f | ||||
| Liver and intrahepatic bile duct | 1975–1979 | 0.3 | 1979–1987 | 2.3e | 1987–1996 | 3.9e | 1996–1999 | 0.6 | 1999–2007 | 2.4e | 2007–2012 | 3.1e | 2.8f | 3.1f |
| Non-Hodgkin lymphoma | 1975–1991 | 2.7e | 1991–1997 | 1.6e | 1997–2006 | −2.9e | 2006–2012 | −1.9e | −2.2f | −1.9f | ||||
| Urinary bladder | 1975–1983 | −1.4e | 1983–1987 | −2.8e | 1987–1993 | 0.2 | 1993–1997 | −1.1 | 1997–2012 | 0.0 | 0.0 | 0.0 | ||
| Esophagus | 1975–1985 | 0.7e | 1985–1994 | 1.2e | 1994–2005 | 0.4e | 2005–2012 | −1.0e | −0.7f | −1.0f | ||||
| Kidney and renal pelvis | 1975–1991 | 1.1e | 1991–2001 | −0.1 | 2001–2012 | −0.8e | −0.8f | −0.8f | ||||||
| Brain and other nervous system | 1975–1977 | 4.4 | 1977–1982 | −0.4 | 1982–1991 | 1.3e | 1991–2007 | −1.0e | 2007–2012 | 0.7 | 0.0 | 0.7 | ||
| Stomach | 1975–1987 | −2.4e | 1987–1990 | −0.4 | 1990–2012 | −3.3e | −3.3f | −3.3f | ||||||
| Myeloma | 1975–1994 | 1.5e | 1994–2012 | −1.0e | −1.0f | −1.0f | ||||||||
| Melanoma of the skin | 1975–1990 | 2.2e | 1990–2002 | 0.0 | 2002–2009 | 1.0e | 2009–2012 | −1.6 | 0.1 | −0.9 | ||||
| Oral cavity and pharynx | 1975–1977 | 0.7 | 1977–1993 | −2.0e | 1993–2000 | −2.9e | 2000–2009 | −1.3e | 2009–2012 | 1.7 | −0.3 | 1.0 | ||
| Larynx | 1975–1994 | −0.8e | 1994–2012 | −2.5e | −2.5f | −2.5f | ||||||||
| Soft tissue including heart | 1975–1980 | 7.6e | 1980–1997 | 1.2e | 1997–2002 | −3.4e | 2002–2012 | 1.1e | 1.1f | 1.1f | ||||
| Top 17 cancers among womeng | ||||||||||||||
| Lung and bronchus | 1975–1982 | 6.0e | 1982–1990 | 4.2e | 1990–1995 | 1.7e | 1995–2003 | 0.3e | 2003–2007 | −0.8 | 2007–2012 | −1.9e | −1.4f | −1.9f |
| Breast | 1975–1990 | 0.4e | 1990–1995 | −1.8e | 1995–1998 | −3.3e | 1998–2012 | −1.9e | −1.9f | −1.9f | ||||
| Colon and rectum | 1975–1984 | −1.0e | 1984–2001 | −1.8e | 2001–2012 | −2.9e | −2.9f | −2.9f | ||||||
| Pancreas | 1975–1984 | 0.8e | 1984–2000 | 0.1 | 2000–2012 | 0.4e | 0.4f | 0.4f | ||||||
| Ovary | 1975–1982 | −1.2e | 1982–1992 | 0.4e | 1992–1998 | −1.2e | 1998–2002 | 1.1 | 2002–2012 | −2.0e | −2.0f | −2.0f | ||
| Leukemia | 1975–1980 | 0.7 | 1980–1999 | −0.4e | 1999–2012 | −1.2e | −1.2f | −1.2f | ||||||
| Non-Hodgkin lymphoma | 1975–1994 | 2.2e | 1994–1997 | 0.8 | 1997–2012 | −3.1e | −3.1f | −3.1f | ||||||
| Corpus and uterus, NOS | 1975–1989 | −1.6e | 1989–1997 | −0.7e | 1997–2009 | 0.3e | 2009–2012 | 2.5e | 1.1f | 2.0f | ||||
| Liver and intrahepatic bile duct | 1975–1978 | −1.5 | 1978–1988 | 1.4e | 1988–1995 | 3.9e | 1995–2000 | 0.4 | 2000–2008 | 1.4e | 2008–2012 | 3.1e | 2.2f | 3.1f |
| Brain and other nervous system | 1975–1992 | 1.0e | 1992–2006 | −1.1e | 2006–2012 | 0.1 | −0.3 | 0.1 | ||||||
| Myeloma | 1975–1993 | 1.5e | 1993–2002 | −0.5 | 2002–2009 | −2.7e | 2009–2012 | 2.0 | −1.2f | 0.8 | ||||
| Kidney and renal pelvis | 1975–1995 | 1.1e | 1995–2012 | −1.0e | −1.0f | −1.0f | ||||||||
| Stomach | 1975–1987 | −2.8e | 1987–1990 | −0.4 | 1990–2012 | −2.6e | −2.6f | −2.6f | ||||||
| Cervix uteri | 1975–1982 | −4.3e | 1982–1996 | −1.6e | 1996–2003 | −3.8e | 2003–2012 | −0.9e | −0.9f | −0.9f | ||||
| Urinary bladder | 1975–1986 | −1.7e | 1986–2012 | −0.4e | −0.4f | −0.4f | ||||||||
| Oral cavity and pharynx | 1975–1990 | −0.9e | 1990–2005 | −2.4e | 2005–2012 | −1.0e | −1.3f | −1.0f | ||||||
| Gallbladder | 1975–2002 | −2.7e | 2002–2012 | −1.2e | −1.2f | −1.2f | ||||||||
Abbreviations: AAPC, average annual percent change; APC, annual percent change; NOS, not otherwise specified.
Source: National Center for Health Statistics public-use data file for the total United States, 1975–2012.
Joinpoint analyses with up to 5 joinpoints yielding up to 6 trend segments (Trends 1–6) were based on rates per 100,000 persons and were age-adjusted to the 2000 US standard population (19 age groups: ages <1 year, 1–4 years, 5–9 years, …, 80–84 years, ≥85 years; Census publication p25-1130 [US Bureau of the Census, Current Population Reports, p25-1130. Washington, DC: US Government Printing Office; 2000]). For joinpoint analysis, the Joinpoint Regression Program was used (version 4.2.0.0, April 2015; Statistical Research and Applications Branch, National Cancer Institute, Bethesda, MD).
The AAPC is a weighted average of the APCs calculated by joinpoint regression.
The APC is based on rates that were age-adjusted to the 2000 US standard population (19 age groups; Census publication p25-1130).
The APC is statistically significantly different from zero (2-sided t test; P < .05).
The AAPC is statistically significantly different from zero (2-sided Z test; P < .05).
Cancers are listed in descending rank order of sex-specific, age-adjusted death rates for 2008 through 2012 for all racial and ethnic groups combined. More than 15 cancers may appear under men and women to include the top 15 cancers in each racial and ethnic group.
Among women, during 2003 through 2012, death rates declined overall (1.4% per year) and for non-Hodgkin lymphoma (3.1% per year), colorectal cancer (2.9% per year), leukemia (1.2% per year), and myeloma (1.2% per year), and for cancers of the stomach (2.6% per year), ovary (2.0% per year), breast (1.9% per year), lung (1.4% per year), oral cavity and pharynx (1.3% per year), gallbladder (1.2% per year), kidney (1.0% per year), cervix (0.9% per year), and bladder (0.4% per year), and remained stable for brain cancer, but increased for cancers of the liver (2.2% per year), uterus (1.1% per year), and pancreas (0.4% per year).
Current Cancer Incidence Rates and Trends by Race and Ethnicity
By using data from both SEER and NPCR registries, 5-year (2008–2012) average annual incidence rates and 5-year (2008–2012) and 10-year (2003–2012) incidence trends were analyzed by site, sex, and race and ethnicity (Table 3). During 2008 through 2012, observed rates of all cancers combined in all racial groups were lower among women than among men (412.6 vs 512.7 per 100,000). Black men had the highest overall cancer incidence rate (573.6 per 100,000) of any racial or ethnic group, and non-Hispanics had higher rates than Hispanics (464.7 vs 353.7 per 100,000). Among women, white women had the highest overall cancer incidence rate during this time of any racial or ethnic group (418.4 per 100,000). Prostate cancer remained the most common cancer among men in each racial and ethnic group, and the rates were substantially higher than for any other type of cancer. Lung and colorectal cancer were the second and third most common cancers, respectively, among men of all racial and ethnic groups, except among Hispanic men, in whom these ranks were reversed. Breast cancer was the most common cancer among women of all racial and ethnic groups. Like in men, lung cancer was the second most common cancer among women, followed by colorectal cancer, except among API and Hispanic women, in whom the ranks were reversed. Rankings of other cancers for both men and women varied by race and ethnicity. White children had higher cancer incidence rates than children of other racial and ethnic groups.
TABLE 3.
Cancer Incidence Rates for 2008 to 2012 and Fixed-Interval Trends From 2003 to 2012 for the Top Cancers by Sex, Race, and Ethnicity for Areas in the United States With High-Quality Incidence Dataa
| Sex/Cancer Site or Typec |
All Races and Ethnicities
|
Whiteb
|
Blackb
|
APIb
|
AI/AN (CHSDA)b
|
Hispanicb
|
Non-Hispanicb
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rank | Rated | 2003– 2012 AAPCe |
2008– 2012 AAPCe |
Rank | Rated | 2003– 2012 AAPCe |
Rank | Rated | 2003– 2012 AAPCe |
Rank | Rated | 2003– 2012 AAPCe |
Rank | Rated | 2003– 2012 AAPCe |
Rank | Rated | 2003– 2012 AAPCe |
Rank | Rated | 2003– 2012 AAPCe |
|
| All sitesf | ||||||||||||||||||||||
| Both sexes | 454.0 | −0.9g | −2.1g | 454.5 | −0.9g | 466.6 | −1.2g | 292.1 | −0.9g | 385.6 | −1.6g | 353.7 | −1.3g | 464.7 | −0.8g | |||||||
| Men | 512.7 | −1.7g | −3.4g | 506.5 | −1.6g | 573.6 | −2.2g | 309.7 | −1.9g | 416.2 | −2.8g | 400.8 | −2.4g | 524.1 | −1.6g | |||||||
| Women | 412.6 | −0.3g | −0.8g | 418.4 | −0.2 | 394.9 | −0.2 | 283.1 | 0.2 | 367.2 | −0.4 | 324.7 | −0.5g | 422.3 | −0.2g | |||||||
| Children (ages 0–14 years) | 16.0 | 0.5 | 0.5 | 16.6 | 0.4 | 12.6 | 0.5 | 12.8 | 0.8 | 11.1 | −1.3 | 15.6 | −0.1 | 16.2 | 0.6g | |||||||
| Children (ages 0–19 years) | 17.4 | 0.4g | 0.4g | 18.2 | 0.3 | 13.1 | 0.3 | 13.8 | 1.1 | 12.6 | −0.5 | 16.8 | 0.3 | 17.6 | 0.5g | |||||||
| Top 17 cancers among men | ||||||||||||||||||||||
| Prostate | 1 | 131.5 | −3.2g | −7.0g | 1 | 121.4 | −3.6g | 1 | 205.1 | −3.5g | 1 | 67.8 | −4.5g | 1 | 90.5 | −5.8g | 1 | 112.1 | −4.7g | 1 | 133.5 | −3.1g |
| Lung and bronchus | 2 | 76.7 | −2.5g | −3.4g | 2 | 76.2 | −2.5g | 2 | 91.2 | −2.8g | 2 | 47.4 | −1.8g | 2 | 66.2 | −2.6g | 3 | 43.3 | −3.1g | 2 | 79.6 | −2.4g |
| Colon and rectum | 3 | 48.3 | −3.6g | −3.6g | 3 | 47.1 | −3.8g | 3 | 59.1 | −3.5g | 3 | 39.0 | −2.6g | 3 | 50.4 | −1.9g | 2 | 44.6 | −3.0g | 3 | 48.8 | −3.6g |
| Urinary bladder | 4 | 36.4 | −1.1g | −1.1g | 4 | 38.6 | −1.1g | 5 | 19.5 | 0.0 | 6 | 15.4 | −1.0g | 6 | 18.3 | −1.9g | 5 | 20.1 | −2.3g | 4 | 37.8 | −1.0g |
| Melanoma of the skin | 5 | 25.4 | 1.7g | 0.6 | 5 | 28.4 | 1.7g | 25 | 1.1 | −1.0 | 20 | 1.5 | −1.7 | 13 | 6.8 | −1.0 | 17 | 4.7 | −1.3 | 5 | 27.6 | 2.0g |
| Non-Hodgkin lymphoma | 6 | 23.1 | −0.7g | −1.5g | 6 | 23.7 | −0.7g | 6 | 17.0 | −0.4 | 5 | 15.7 | 0.2 | 7 | 17.0 | −2.1 | 6 | 19.9 | −0.8g | 6 | 23.4 | −0.6g |
| Kidney and renal pelvis | 7 | 21.5 | 1.0g | −0.5g | 7 | 21.6 | 1.0g | 4 | 23.6 | 1.6g | 9 | 10.8 | 1.3 | 4 | 29.7 | −1.3 | 4 | 20.6 | 0.6g | 7 | 21.7 | 1.1g |
| Oral cavity and pharynx | 8 | 16.9 | 0.6g | 0.6g | 8 | 17.3 | 0.9g | 9 | 15.0 | −2.5g | 8 | 10.9 | 0.2 | 8 | 14.7 | 1.1 | 11 | 10.9 | −0.5 | 8 | 17.6 | 0.8g |
| Leukemia | 9 | 16.8 | −0.3g | −0.3g | 9 | 17.3 | −0.4g | 12 | 12.9 | −0.1 | 11 | 9.6 | 0.6 | 11 | 11.2 | −2.5 | 9 | 12.7 | −0.7 | 9 | 17.0 | −0.3g |
| Pancreas | 10 | 14.0 | 0.8g | 0.5g | 10 | 13.8 | 0.9g | 7 | 16.8 | 0.0 | 10 | 9.8 | 0.1 | 10 | 11.3 | −1.1 | 10 | 12.0 | −0.2 | 10 | 14.2 | 0.9g |
| Liver and intrahepatic bile duct | 11 | 11.5 | 3.5g | 2.3g | 11 | 10.3 | 3.7g | 8 | 16.2 | 3.9g | 4 | 20.6 | −1.3g | 5 | 18.7 | 3.2 | 7 | 19.3 | 1.8g | 11 | 10.8 | 3.5g |
| Stomach | 12 | 9.3 | −1.3g | −1.3g | 13 | 8.4 | −1.1g | 10 | 14.8 | −2.3g | 7 | 14.5 | −3.8g | 9 | 12.0 | −4.2g | 8 | 13.5 | −2.9g | 12 | 8.9 | −1.4g |
| Esophagus | 13 | 8.3 | −1.4g | −2.9g | 12 | 8.5 | −0.9 | 14 | 7.8 | −5.3g | 15 | 3.8 | −2.2 | 12 | 7.2 | −0.2 | 14 | 5.3 | −2.3g | 13 | 8.6 | −1.3g |
| Brain and other nervous system | 14 | 7.8 | −0.7g | −1.4g | 14 | 8.3 | −0.7g | 15 | 4.8 | 0.0 | 14 | 4.4 | −0.5 | 16 | 5.3 | −0.4 | 13 | 5.9 | −1.3g | 14 | 8.1 | −0.5g |
| Myeloma | 15 | 7.7 | 0.8g | 0.8g | 16 | 7.1 | 0.7g | 11 | 14.6 | 0.5 | 13 | 4.5 | 0.5 | 14 | 6.3 | −1.5 | 12 | 7.5 | 0.5 | 15 | 7.8 | 0.8g |
| Thyroid | 16 | 6.8 | 5.2g | 2.8g | 15 | 7.3 | 5.3g | 17 | 3.6 | 4.6g | 12 | 6.3 | 6.4g | 18 | 4.0 | 2.5 | 16 | 5.1 | 5.2g | 16 | 7.1 | 5.4g |
| Larynx | 17 | 6.3 | −2.8g | −3.6g | 18 | 6.2 | −2.5g | 13 | 9.1 | −3.9g | 18 | 2.3 | −2.0 | 15 | 5.8 | −2.2 | 15 | 5.2 | −3.7g | 17 | 6.4 | −2.4g |
| Top 18 cancers among women | ||||||||||||||||||||||
| Breast | 1 | 123.1 | 0.1 | 0.1 | 1 | 124.2 | 0.0 | 1 | 121.8 | 0.8g | 1 | 88.3 | 1.1g | 1 | 91.9 | −0.3 | 1 | 91.9 | −0.1 | 1 | 126.6 | 0.2 |
| Lung and bronchus | 2 | 54.1 | −0.9g | −2.2g | 2 | 55.7 | −0.9g | 2 | 50.3 | −1.0g | 3 | 28.3 | −0.1 | 2 | 52.7 | −0.5 | 3 | 26.0 | −1.3g | 2 | 56.7 | −0.8g |
| Colon and rectum | 3 | 36.6 | −3.2g | −3.8g | 3 | 35.7 | −3.2g | 3 | 43.3 | −3.6g | 2 | 29.2 | −2.6g | 3 | 40.1 | −2.3g | 2 | 30.6 | −2.8g | 3 | 37.2 | −3.1g |
| Corpus and uterus, NOS | 4 | 25.3 | 1.1g | 1.1g | 4 | 25.8 | 1.0g | 4 | 24.3 | 2.4g | 5 | 17.7 | 2.3g | 4 | 22.9 | 1.5 | 4 | 21.1 | 1.6g | 4 | 25.7 | 1.1g |
| Thyroid | 5 | 20.3 | 5.6g | 2.9g | 5 | 21.3 | 5.6g | 6 | 12.7 | 5.9g | 4 | 20.4 | 5.7g | 7 | 12.9 | 5.2g | 5 | 19.3 | 5.3g | 5 | 20.6 | 5.7g |
| Non-Hodgkin lymphoma | 6 | 16.0 | −1.0g | −1.7g | 7 | 16.5 | −1.1g | 8 | 11.8 | −0.3 | 6 | 10.8 | −0.1 | 6 | 13.5 | −3.0g | 6 | 15.2 | −0.7 | 7 | 16.1 | −1.0g |
| Melanoma of the skin | 7 | 15.9 | 1.4g | −0.1 | 6 | 18.3 | 1.5 | 27 | 1.0 | −1.3 | 21 | 1.2 | −1.4 | 16 | 5.2 | 0.5 | 18 | 4.0 | −1.7g | 6 | 17.5 | 1.8g |
| Ovaryf | 8 | 11.9 | −2.0g | −2.0g | 8 | 12.3 | −2.1g | 11 | 9.4 | −1.3g | 7 | 9.0 | −0.9g | 8 | 11.8 | −0.3 | 8 | 10.6 | −2.1g | 8 | 12.0 | −2.0g |
| Kidney and renal pelvis | 9 | 11.3 | 0.9g | −1.1 | 9 | 11.4 | 1.0g | 7 | 12.7 | 2.2g | 13 | 4.9 | 0.1 | 5 | 18.3 | 0.6 | 7 | 11.8 | 1.3g | 9 | 11.3 | 0.9g |
| Pancreas | 10 | 10.9 | 0.7g | 0.7g | 11 | 10.6 | 0.7g | 5 | 14.2 | 0.6 | 8 | 8.7 | 1.3g | 9 | 9.6 | −1.6 | 9 | 10.3 | 0.3 | 10 | 11.0 | 0.8g |
| Leukemia | 11 | 10.3 | 0.0 | 0.0 | 10 | 10.6 | −0.1 | 12 | 8.3 | 0.6 | 12 | 6.2 | 0.5 | 11 | 8.9 | 0.6 | 11 | 8.9 | −0.1 | 11 | 10.3 | 0.0 |
| Urinary bladder | 12 | 9.0 | −1.4g | −1.4g | 12 | 9.5 | −1.4g | 14 | 6.6 | −0.8g | 15 | 3.8 | −1.5 | 17 | 4.9 | 1.6 | 14 | 5.1 | −2.3g | 12 | 9.4 | −1.2g |
| Cervix uteri | 13 | 7.7 | −1.3g | −1.3g | 13 | 7.5 | −1.1g | 10 | 9.8 | −2.3g | 11 | 6.3 | −3.0g | 10 | 9.4 | −0.5 | 10 | 10.2 | −3.9g | 13 | 7.4 | 1.1g |
| Oral cavity and pharynx | 14 | 6.3 | 0.4g | 0.4g | 14 | 6.4 | 0.7g | 15 | 5.1 | −1.2g | 14 | 4.9 | −0.6 | 15 | 5.2 | −1.1 | 17 | 4.2 | 0.2 | 14 | 6.6 | 0.5g |
| Brain and other nervous system | 15 | 5.6 | −1.0g | −1.8g | 15 | 6.1 | −0.8g | 17 | 3.5 | −0.7 | 16 | 3.1 | −0.9g | 18 | 3.8 | 1.6 | 16 | 4.5 | −1.6g | 15 | 5.8 | −0.7g |
| Myeloma | 16 | 5.1 | 0.3 | 0.5 | 16 | 4.4 | 0.0 | 9 | 10.9 | 0.9g | 17 | 2.9 | 0.1 | 14 | 5.3 | −1.8 | 15 | 5.1 | −0.6 | 16 | 5.1 | 0.4 |
| Stomach | 17 | 4.6 | −0.9g | 0.2 | 17 | 4.0 | −1.0g | 13 | 7.9 | −1.2g | 9 | 8.5 | −2.8g | 13 | 6.6 | −1.4 | 12 | 7.8 | −2.2g | 17 | 4.3 | −1.0g |
| Liver and intrahepatic bile duct | 18 | 3.9 | 3.0g | 2.3g | 18 | 3.5 | 3.6g | 16 | 4.8 | 3.4g | 10 | 7.9 | −1.2 | 12 | 8.9 | 2.2 | 13 | 7.2 | 2.4g | 18 | 3.6 | 2.9g |
Abbreviations: AAPC, average annual percent change; AI/AN, American Indian/Alaska Native; API, Asian/Pacific Islander; CHSDA, Indian Health Service Contract Health Services Delivery Area; NOS, not otherwise specified.
Source: National Program of Cancer Registries (NPCR) and Surveillance, Epidemiology, and End Results (SEER) areas reported by the North American Association of Central Cancer Registries (NAACCR) as meeting high-quality incidence data standards for the specified time periods (2008–2012 rates for all races/ethnicities, white, black, AI/AN, API, Hispanic, and non-Hispanic [48 states: Alabama, Alaska, Arizona, California, Colorado, Connecticut, Delaware, District of Columbia, Florida, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Mississippi, Missouri, Montana, Nebraska, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, South Dakota, Tennessee, Texas, Utah, Vermont, Virginia, Washington, West Virginia, Wisconsin, and Wyoming]; 2003–2012 AAPCs for all races/ethnicities, white, black, AI/AN, API, Hispanic, and non-Hispanic [45 states: Alabama, Alaska, Arizona, California, Colorado, Connecticut, Delaware, District of Columbia, Florida, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Missouri, Montana, Nebraska, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, South Dakota, Texas, Utah, Vermont, Virginia, Washington, West Virginia, Wisconsin, Wyoming]).
White, black, API, and AI/AN (CHSDA 2012 counties) include Hispanic and non-Hispanic; the race and ethnicity categories are not mutually exclusive. AI/AN (CHSDA 2012) statistics exclude data from Kansas.
Cancers are listed in descending rank of sex-specific, age-adjusted rates for 2008 through 2012 for all racial and ethnic groups combined. More than 15 cancers may appear under men and women to include the top 15 cancers in each racial and ethnic group.
Rates are per 100,000 persons and were age-adjusted to the 2000 US standard population (19 age groups: ages <1 year, 1–4 years, 5–9 years, …, 80–84 years, ≥85 years; Census publication p25-1130 [US Bureau of the Census, Current Population Reports, p25-1130. Washington, DC: US Government Printing Office; 2000]).
The AAPC is a weighted average of the annual percent change (APC) calculated by joinpoint analyses with up to 2 joinpoints yielding up to 3 trend segments based on rates per 100,000 persons and age adjusted to the 2000 US standard population (19 age groups; Census publication p25-1130). For the joinpoint analysis, the Joinpoint Regression Program was used (version 4.2.0.0, April 2015; Statistical Research and Applications Branch, National Cancer Institute, Bethesda, MD).
For all sites, myelodysplastic syndromes are included for the rate calculations but not for the APC calculations; they are excluded from cancer-specific analyses. Ovary excludes borderline tumors.
The AAPC is statistically significantly different from zero (2-sided Z test; P < .05).
Overall cancer incidence rates among men declined in each racial and ethnic group during 2003 through 2012. During 2003 through 2012, the incidence rates for the 4 most common cancers among men decreased (prostate, lung, colorectal, and bladder) for all races except among black men, for whom bladder cancer incidence rates remained stable. Thyroid cancer incidence rates among men increased >5% annually during 2003 through 2012 for all racial and ethnic groups except for AI/AN, for whom the increase was not statistically significant. Trends in incidence rates for most cancers among men in each racial and ethnic group were similar in direction to those of all men combined, but some trends were not statistically significant. Oral cancer incidence rates decreased among black men (−2.5% per year), and liver cancer incidence rates decreased among API (−1.3% per year), but they increased or remained stable among other racial and ethnic groups.
Among women, the overall cancer incidence rates declined slightly from 2003 to 2012 for women in all racial and ethnic groups combined, but this decline was not statistically significant for most racial and ethnic groups. Whereas breast cancer incidence rates remained stable during this period for women overall, rates increased among black women (0.8% per year) and API women (1.1% per year). During 2003 through 2012, lung cancer incidence rates decreased among women of all racial and ethnic groups, except API and AI/AN. Colorectal cancer incidence rates decreased among women of all racial and ethnic groups; thyroid cancer incidence rates increased among women of all groups; and uterus cancer incidence rates increased among women of all groups, except AI/AN. Like in men, trends in incidence rates during the past decade for most cancers in each racial and ethnic group were similar in direction to those for all women combined, but some trends were not statistically significant. Oral cancer incidence rates decreased among black women but increased or remained stable among most racial and ethnic groups, and melanoma incidence rates increased among white women but decreased among Hispanic women.
Current Cancer Death Rates and Trends by Race and Ethnicity
Five-year (2008–2012) death rates and fixed-interval mortality trends from 2003 to 2012 were analyzed by sex and by race and ethnicity (Table 4). For all cancer sites combined, cancer death rates (per 100,000) for 2008 to 2012 were higher among men than among women (207.9 vs 145.4). During 2008 through 2012, black men and women had the highest cancer death rates compared with all other racial and ethnic groups. Lung cancer was the leading cause of cancer death among both men and women of all racial and ethnic groups, except among Hispanic women, for whom mortality from breast cancer was higher. Among men, lung cancer was followed by prostate cancer and then colorectal cancer as the leading causes of cancer death, except among API men, for whom liver cancer replaced prostate cancer as the second leading cause of cancer death. Among women of all racial and ethnic groups, except Hispanic women, lung cancer was followed by breast and colorectal cancers as the leading causes of cancer death; among Hispanic women, breast cancer mortality was followed by lung cancer and colorectal cancer mortality.
TABLE 4.
US Cancer Death Rates for 2008 to 2012 and Fixed-Interval Trends From 2003 to 2012 for the Top Cancers by Sex, Race, and Ethnicitya
| Sex/Cancer Site or Typec |
All Races and Ethnicities
|
Whiteb
|
Blackb
|
APIb
|
AI/AN (CHSDA)b
|
Hispanicb
|
Non-Hispanicb
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rank | Rated | 2003– 2012 AAPCe |
2008– 2012 AAPCe |
Rank | Rated | 2003– 2012 AAPCe |
Rank | Rated | 2003– 2012 AAPCe |
Rank | Rated | 2003– 2012 AAPCe |
Rank | Rated | 2003– 2012 AAPCe |
Rank | Rated | 2003– 2012 AAPCe |
Rank | Rated | 2003– 2012 AAPCe |
|
| All sites | ||||||||||||||||||||||
| Both sexes | 171.2 | −1.5f | −1.5f | 170.9 | −1.4f | 202.0 | −2.1f | 106.6 | −1.1f | 156.1 | −1.1f | 119.3 | −1.2f | 175.4 | −1.4f | |||||||
| Men | 207.9 | −1.7f | −1.7f | 206.4 | −1.6f | 261.5 | −2.6f | 128.4 | −1.5f | 186.7 | −1.1 | 148.0 | −1.5f | 212.4 | −1.7f | |||||||
| Women | 145.4 | −1.4f | −1.4f | 145.6 | −1.3f | 166.3 | −1.6f | 91.2 | −0.8f | 133.9 | −1.3f | 99.4 | −1.0f | 149.3 | −1.4f | |||||||
| Children (ages 0–14 years) | 2.2 | −1.8f | −1.8f | 2.2 | −1.8f | 2.1 | −2.0f | 1.9 | −0.6 | 1.7 | —g | 2.2 | −2.2f | 2.2 | −1.7f | |||||||
| Children (ages 0–19 years) | 2.4 | −1.5f | −0.2 | 2.4 | −1.6f | 2.3 | −2.0f | 2.1 | 0.6 | 1.8 | −0.6 | 2.5 | −1.8f | 2.3 | −2.0f | |||||||
| Top 17 cancers among menc | ||||||||||||||||||||||
| Lung and bronchus | 1 | 59.8 | −2.7f | −2.9f | 1 | 59.7 | −2.6f | 1 | 73.1 | −3.4f | 1 | 34.0 | −2.0f | 1 | 49.1 | −0.9 | 1 | 29.5 | −3.1f | 1 | 62.2 | −2.6f |
| Prostate | 2 | 21.4 | −3.4f | −3.4f | 2 | 19.8 | −3.3f | 2 | 46.3 | −3.9f | 4 | 9.4 | −3.5f | 2 | 20.2 | −2.8f | 2 | 17.8 | −3.0f | 2 | 21.6 | −3.4f |
| Colon and rectum | 3 | 18.6 | −2.8f | −2.8f | 3 | 18.0 | −3.0f | 3 | 26.9 | −2.6f | 3 | 13.0 | −1.1f | 3 | 18.8 | −2.5 | 3 | 15.6 | −1.5f | 3 | 18.8 | −2.8f |
| Pancreas | 4 | 12.6 | 0.3f | 0.3f | 4 | 12.5 | 0.5f | 4 | 15.0 | −0.5 | 5 | 8.4 | 0.0 | 5 | 9.3 | −1.6 | 5 | 9.8 | 0.2 | 4 | 12.8 | 0.4f |
| Leukemia | 5 | 9.4 | −0.9f | −0.9f | 5 | 9.7 | −0.8f | 7 | 7.9 | −1.6f | 7 | 5.1 | 0.7 | 8 | 6.7 | 1.6 | 8 | 6.1 | −0.7 | 5 | 9.5 | −0.8f |
| Liver and intrahepatic bile duct | 6 | 8.8 | 2.8f | 2.8f | 7 | 8.1 | 3.0f | 5 | 12.5 | 2.7f | 2 | 14.5 | −0.9f | 4 | 13.9 | 4.2f | 4 | 12.9 | 1.7f | 6 | 8.5 | 2.8f |
| Non-Hodgkin lymphoma | 7 | 7.9 | −2.1f | −2.1f | 6 | 8.2 | −2.1f | 10 | 5.7 | −1.0 | 8 | 5.0 | −2.2f | 9 | 5.7 | 0.5 | 7 | 6.3 | −1.1f | 7 | 8.0 | −2.2f |
| Urinary bladder | 8 | 7.7 | 0.0 | 0.0 | 8 | 8.1 | 0.1 | 12 | 5.3 | −0.7 | 9 | 3.0 | 1.1 | 11 | 4.2 | 3.4 | 11 | 3.9 | −1.2 | 8 | 7.9 | 0.1 |
| Esophagus | 9 | 7.5 | −0.8f | −0.8f | 9 | 7.7 | 0.0 | 9 | 7.0 | −4.5f | 12 | 2.8 | −2.4 | 10 | 5.6 | −4.9f | 10 | 4.3 | 0.1 | 9 | 7.7 | −0.7f |
| Kidney and renal pelvis | 10 | 5.7 | −0.7f | −0.7f | 10 | 5.9 | −0.7f | 11 | 5.6 | −1.3f | 11 | 2.9 | 1.5 | 6 | 8.7 | −0.9 | 9 | 5.0 | −1.4 | 10 | 5.8 | −0.7f |
| Brain and other nervous system | 11 | 5.3 | 0.1 | 0.1 | 11 | 5.7 | 0.2 | 15 | 3.1 | 0.0 | 13 | 2.4 | 0.2 | 14 | 3.2 | 1.6 | 13 | 3.4 | 0.3 | 11 | 5.5 | 0.2 |
| Stomach | 12 | 4.6 | −3.1f | −3.1f | 14 | 4.0 | −3.2f | 6 | 9.2 | −3.3f | 6 | 7.9 | −4.3f | 7 | 7.4 | −3.2 | 6 | 7.2 | −3.1f | 13 | 4.3 | −3.3f |
| Myeloma | 13 | 4.2 | −0.9f | −0.9f | 13 | 4.0 | −0.9f | 8 | 7.6 | −1.4f | 14 | 2.2 | 1.6 | 13 | 3.2 | −6.8f | 12 | 3.5 | 0.2 | 14 | 4.3 | −0.9f |
| Melanoma of the skin | 14 | 4.1 | 0.1 | −0.9 | 12 | 4.6 | 0.3 | 22 | 0.5 | −1.6 | 20 | 0.4 | —g | 16 | 1.5 | —g | 17 | 1.0 | 0.5 | 12 | 4.3 | 0.3 |
| Oral cavity and pharynx | 15 | 3.8 | −0.4 | 1.1 | 15 | 3.7 | 0.0 | 13 | 5.0 | −3.4f | 10 | 2.9 | −1.5 | 12 | 3.6 | 0.5 | 14 | 2.4 | −1.4f | 15 | 3.9 | −0.2 |
| Larynx | 16 | 1.9 | −2.8f | −2.8f | 16 | 1.8 | −2.5f | 14 | 3.6 | −4.2f | 16 | 0.8 | 0.6 | 15 | 1.7 | —g | 15 | 1.7 | −2.9f | 16 | 2.0 | −2.7f |
| Soft tissue including heart | 18 | 1.5 | 1.0f | 1.0f | 18 | 1.6 | 1.1f | 16 | 1.5 | 0.1 | 15 | 1.0 | 2.2 | 17 | 1.5 | —g | 16 | 1.2 | 3.2f | 18 | 1.6 | 1.0f |
| Top 17 cancers among womenc | ||||||||||||||||||||||
| Lung and bronchus | 1 | 37.8 | −1.4f | −1.9f | 1 | 39.1 | −1.3f | 1 | 35.8 | −1.8f | 1 | 18.2 | −0.5f | 1 | 32.1 | −1.3f | 2 | 13.7 | −1.4f | 1 | 39.8 | −1.2f |
| Breast | 2 | 21.9 | −1.9f | −1.5 | 2 | 21.3 | −1.9f | 2 | 30.2 | −1.4f | 2 | 11.4 | −1.4f | 3 | 15.0 | −3.4f | 1 | 14.5 | −1.3f | 2 | 22.5 | −1.8f |
| Colon and rectum | 3 | 13.1 | −2.8f | −2.8f | 3 | 12.7 | −2.8f | 3 | 17.8 | −3.4f | 3 | 9.4 | −1.3f | 2 | 15.6 | 1.4 | 3 | 9.6 | −2.2f | 3 | 13.3 | −2.8f |
| Pancreas | 4 | 9.6 | 0.3f | 0.3f | 4 | 9.4 | 0.4f | 4 | 12.3 | −0.2 | 4 | 7.3 | 1.0f | 4 | 7.8 | 0.1 | 4 | 7.7 | 0.0 | 4 | 9.8 | 0.4f |
| Ovary | 5 | 7.7 | −2.1f | −2.1f | 5 | 8.0 | −2.1f | 6 | 6.7 | −1.6f | 7 | 4.6 | −1.3f | 5 | 6.7 | −0.9 | 5 | 5.6 | −1.4f | 5 | 7.9 | −2.1f |
| Leukemia | 6 | 5.2 | −1.0f | −1.0f | 6 | 5.4 | −0.9f | 8 | 4.7 | −1.3f | 9 | 3.2 | 1.5f | 12 | 3.2 | −5.1 | 9 | 4.0 | −0.1 | 6 | 5.3 | −1.0f |
| Non-Hodgkin lymphoma | 7 | 4.8 | −2.8f | −2.8f | 7 | 5.0 | −2.8f | 12 | 3.5 | −2.3f | 8 | 3.4 | −1.9f | 8 | 3.6 | −5.5f | 7 | 4.3 | −1.2f | 7 | 4.9 | −2.9f |
| Corpus and uterus, NOS | 8 | 4.4 | 1.1f | 1.1f | 8 | 4.1 | 0.9f | 5 | 7.7 | 1.6f | 10 | 2.8 | 3.1f | 10 | 3.5 | —g | 10 | 3.5 | 2.3f | 8 | 4.4 | 1.0f |
| Liver and intrahepatic bile duct | 9 | 3.5 | 2.0f | 3.4f | 10 | 3.3 | 2.2f | 10 | 4.3 | 1.6f | 5 | 6.1 | −1.4 | 6 | 6.3 | −1.2 | 6 | 5.6 | 1.1f | 10 | 3.4 | 2.0f |
| Brain and other nervous system | 10 | 3.5 | −0.2 | −0.2 | 9 | 3.8 | −0.2 | 15 | 2.1 | 0.4 | 12 | 1.6 | 0.2 | 14 | 1.9 | —g | 12 | 2.4 | 0.1 | 9 | 3.6 | −0.2 |
| Myeloma | 11 | 2.7 | −1.2f | 0.8 | 12 | 2.4 | −1.2f | 7 | 5.3 | −1.5f | 13 | 1.4 | 0.2 | 13 | 2.3 | −6.6 | 14 | 2.3 | −1.3 | 11 | 2.7 | −1.1f |
| Kidney and renal pelvis | 12 | 2.5 | −1.4f | −1.4f | 11 | 2.6 | −1.4f | 13 | 2.5 | −1.1 | 14 | 1.2 | −0.1 | 7 | 4.7 | 1.9 | 13 | 2.4 | −0.8 | 12 | 2.5 | −1.4f |
| Stomach | 13 | 2.4 | −2.6f | −2.6f | 15 | 2.1 | −2.7f | 9 | 4.4 | −2.8f | 6 | 4.7 | −3.3f | 9 | 3.6 | −3.5f | 8 | 4.2 | −2.7f | 15 | 2.3 | −2.8f |
| Cervix uteri | 14 | 2.3 | −0.9f | −0.9f | 14 | 2.1 | −0.6f | 11 | 4.0 | −2.2f | 11 | 1.8 | −3.1f | 11 | 3.5 | −1.4 | 11 | 2.7 | −2.3f | 13 | 2.3 | −0.8f |
| Urinary bladder | 15 | 2.2 | −0.5f | −0.5f | 13 | 2.2 | −0.3 | 14 | 2.5 | −1.5f | 16 | 0.9 | −1.5 | 18 | 1.1 | —g | 15 | 1.3 | −1.3 | 14 | 2.3 | −0.3 |
| Oral cavity and pharynx | 18 | 1.4 | −1.2f | −1.2f | 18 | 1.3 | −1.1f | 18 | 1.3 | −2.3f | 15 | 1.2 | −1.2 | 17 | 1.2 | —g | 19 | 0.8 | 0.2 | 18 | 1.4 | −1.2f |
| Gallbladder | 20 | 0.7 | −1.2f | −1.2f | 20 | 0.7 | −1.5f | 19 | 1.0 | 0.8 | 20 | 0.8 | −2.2 | 15 | 1.8 | −4.4 | 16 | 1.3 | 0.2 | 20 | 0.7 | −1.6f |
Abbreviations: AAPC, average annual percent change; AI/AN, American Indian/Alaska Native; API, Asian/Pacific Islander; CHSDA, Indian Health Service Contract Health Services Delivery Area; NOS, not otherwise specified.
Source: National Center for Health Statistics public-use data file for the total United States, 1975–2012.
White, black, API, and AI/AN (CHSDA 2012 counties) populations include Hispanic and non-Hispanic; the race and ethnicity categories are not mutually exclusive.
Cancers are listed in descending rank order of sex-specific, age-adjusted incidence rates for 2008 through 2012 for all racial and ethnic groups combined. More than 15 cancers may appear under men and women to include the top 15 cancers in each racial and ethnic group.
Rates are per 100,000 persons and were age-adjusted to the 2000 US standard population (19 age groups: ages <1 year, 1–4 years, 5–9 years, …, 80–84 years, ≥85 years; Census publication p25-1130 [US Bureau of the Census, Current Population Reports, p25-1130. Washington, DC: US Government Printing Office; 2000]).
The AAPC is a weighted average of the annual percent change and is calculated by joinpoint analyses with up to 2 joinpoints yielding up to 3 trend segments based on rates per 100,000 persons and age adjusted to the 2000 US standard population (19 age groups; Census publication p25-1130). For joinpoint analysis, the Joinpoint Regression Program was used (version 4.2.0.0, April 2015; Statistical Research and Applications Branch, National Cancer Institute, Bethesda, MD).
The AAPC is statistically significantly different from zero (2-sided Z test; P < .05).
The statistic could not be calculated. The AAPC is based on <10 cases for at least 1 year within the time interval.
From 2003 to 2012, death rates declined for the most common cancers (lung, prostate, colorectal, breast) among men and women of all racial and ethnic groups except for statistically insignificant decreases in lung cancer and colorectal cancer among AI/AN men and in colorectal cancer among AI/AN women. Death rates for most cancer sites declined or were stable from 2003 to 2012 among men and women of each racial and ethnic group, except for liver cancer, which increased for most racial and ethnic groups except for API men and women and AI/AN women; pancreatic cancer, which increased among white men and women and API women; soft tissue cancer, which increased among white and Hispanic men; uterine cancer, which increased among white, black, API, and Hispanic women; and leukemia, which increased among API women.
Incidence of Liver Cancer
Rates of incident liver and intrahepatic bile duct (liver) cancer were examined by mutually exclusive racial or ethnic groups, gender, and age during 2008 through 2012 to better describe demographic patterns (Table 5). Among men and women, liver cancer incidence rates were highest among NH AI/ANs, followed by NH APIs, and Hispanics. Among all racial or ethnic groups, liver cancer incidence rates among men were more than twice those among women. Among men, liver cancer incidence rates increased during 2008 through 2012 among NH white, NH black, and NH AI/AN men, but decreased among NH API men, and were stable among Hispanic men. A different pattern emerged for liver cancer incidence rates among women, which increased among Hispanic women and among NH white and NH black women. Among both men and women, liver cancer incidence rates increased significantly from 2008 to 2012, beginning at age 55 years, and the largest AAPC was observed among the group ages 60 to 64 years among men and the group ages 55 to 59 years among women. Liver cancer incidence rates among both men and women increased with age for almost all groups until age ≥85 years. State-specific liver cancer incidence rates ranged 3-fold, from 3.3 to 12.5 per 100,000 persons (Fig. 1). Liver cancer incidence rates were highest in Pacific states, in states on the southern US border, in the District of Columbia, and in a few states in the Northeast, including Delaware, Connecticut, Massachusetts, and New York.
TABLE 5.
Average Annual Number of Liver and Intrahepatic Bile Duct Cancer Cases (N = 24,777), Incidence Rates and Average Annual Percentage Change in Incidence Rate From 2008 to 2012 by Sex, Race or Ethnicity, and Age Group for Areas in the United States With High-Quality Incidence Dataa
| Characteristic | Both Sexes
|
Men
|
Women
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Average Annual No. of Cases | Rateb | 2008–2012 AAPCc | Average Annual No. of Cases | Rateb | 2008–2012 AAPCc | Average Annual No. of Cases | Rateb | 2008–2012 AAPCc | |
| Overalld | 24,777 | 7.4 | 2.3e | 17,835 | 11.4 | 2.2e | 6,941 | 3.9 | 2.2e |
| Race or ethnicity | |||||||||
| NH white | 15,417 | 6.0 | 2.8e | 11,102 | 9.3 | 2.8e | 4,315 | 3.2 | 3.5e |
| NH black | 3,604 | 9.9 | 3.9e | 2,671 | 16.5 | 3.9e | 932 | 4.8 | 3.4e |
| NH AI/AN (CHSDA) | 184 | 14.9 | 3.4e | 119 | 21.0 | 4.0e | 64 | 9.9 | 2.7 |
| NH API | 1,891 | 13.8 | −1.2e | 1,315 | 20.9 | −1.2e | 576 | 8.0 | −1.1 |
| Hispanicd | 3,682 | 12.7 | 0.7 | 2,627 | 19.3 | −0.1 | 1,054 | 7.2 | 2.4e |
| Age group, y | |||||||||
| <40 | 551 | 0.4 | 0.2 | 336 | 0.4 | −0.5 | 215 | 0.3 | 1.3 |
| 40–44 | 358 | 1.8 | −1.5e | 253 | 2.5 | −2.6e | 105 | 1.0 | 1.5 |
| 45–49 | 1,109 | 5.1 | −2.5e | 862 | 8.1 | −3.3e | 247 | 2.3 | 0.7 |
| 50–54 | 2,897 | 13.6 | −2.5 | 2,345 | 22.4 | −3.5 | 552 | 5.1 | 1.3 |
| 55–59 | 4,588 | 24.1 | 3.9e | 3,726 | 40.5 | 3.2e | 862 | 8.8 | 6.5e |
| 60–64 | 3,997 | 24.7 | 8.9e | 3,129 | 40.4 | 9.7e | 867 | 10.3 | 5.9e |
| 65–69 | 3,034 | 25.0 | 3.8e | 2,176 | 38.1 | 3.8e | 858 | 13.4 | 3.5e |
| 70–74 | 2,597 | 28.7 | 2.5e | 1,742 | 42.2 | 2.5e | 855 | 17.4 | 2.1e |
| 75–79 | 2,311 | 32.6 | 2.1e | 1,449 | 47.0 | −0.4 | 861 | 21.5 | 2.4e |
| 80–84 | 1,845 | 33.2 | 2.7e | 1,084 | 48.8 | 2.4e | 761 | 22.8 | 2.5e |
| ≥85 | 1,491 | 27.9 | 1.8e | 733 | 41.9 | 1.8e | 758 | 21.1 | 1.4e |
Abbreviations: AI/AN, American Indian/Alaska Native; AAPC, average annual percent change; API, Asian/Pacific Islander; CHSDA, Contract Health Services Delivery Area; NH, non-Hispanic.
Source: National Program of Cancer Registries and Surveillance, Epidemiology, and End Results areas reported by North American Association of Central Cancer Registries as meeting high-quality incidence data standards for the specified time. Rates are listed from 2008 to 2012 for NH whites, NH blacks, NH AI/AN (CHSDA 2012 counties), NH API, and Hispanics (48 states: Alabama, Alaska, Arizona, California, Colorado, Connecticut, Delaware, District of Columbia, Florida, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Mississippi, Missouri, Montana, Nebraska, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, South Dakota, Tennessee, Texas, Utah, Vermont, Virginia, Washington, West Virginia, Wisconsin, and Wyoming).
Rates are per 100,000 persons and were age-adjusted to the 2000 US standard population (19 age groups: ages <1 year, 1–4 years, 5–9 years, …, 80–84 years, ≥85 years; Census publication p25-1130 [US Bureau of the Census, Current Population Reports, p25-1130. Washington, DC: US Government Printing Office; 2000]).
The AAPC is a weighted average of the APCs calculated by joinpoint analysis with up to 2 joinpoints, yielding up to 3 trend segments based on rates per 100,000 persons and age-adjusted to the 2000 US standard population (19 age groups; Census publication p25-1130). For joinpoint analysis, the Joinpoint Regression Program was used (version 4.2.0.0, April 2015; Statistical Research and Applications Branch, National Cancer Institute, Bethesda, MD).
This table excludes unknown Hispanic and Hispanic other/unknown race.
The AAPC is statistically significantly different from zero (2-sided Z test; P < .05).
Figure 1.
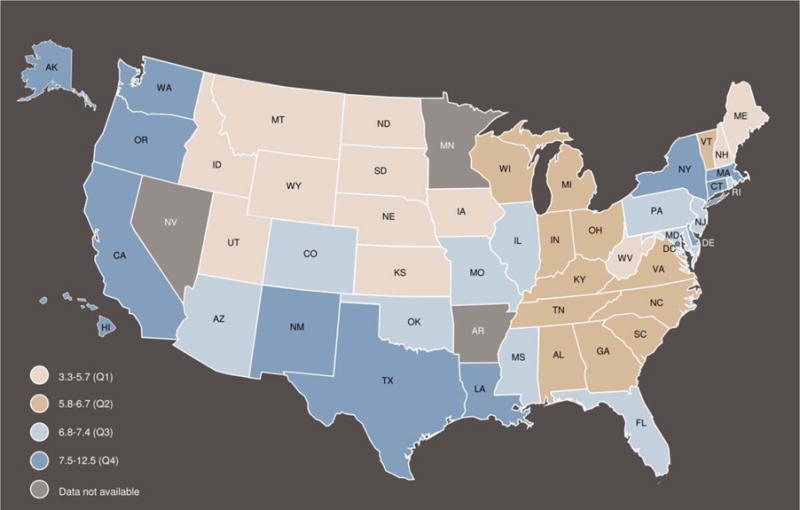
Age-adjusted incidence rates for 2008 to 2012 of liver and intrahepatic bile duct cancer are illustrated by state for areas in the United States with high-quality incidence data. Rates are per 100,000 persons and were age-adjusted to the 2000 US standard population (19 age groups: ages <1 year, 1–4 years, 5–9 years, …, 80–84 years, ≥85 years [Census publication p25-1130; US Bureau of the Census, Current Population Reports, p25-1130. Washington, DC: US Government Printing Office; 2000]). Source: National Program of Cancer Registries and Surveillance, Epidemiology, and End Results areas reported by the North American Association of Central Cancer Registries as meeting high-quality incidence data standards for the specified time. Rates from 2008 to 2012 for non-Hispanic (NH) whites, NH blacks, NH American Indians/Alaska Natives (Contract Health Services Delivery Area 2012 counties), NH Asians/Pacific Islanders, and Hispanics (48 states: Alabama, Alaska, Arizona, California, Colorado, Connecticut, Delaware, District of Columbia, Florida, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Mississippi, Missouri, Montana, Nebraska, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, South Dakota, Tennessee, Texas, Utah, Vermont, Virginia, Washington, West Virginia, Wisconsin, and Wyoming).
The age distribution of liver cancer diagnosed during 2008 through 2012 varied across racial or ethnic groups (Fig. 2). Although liver cancer incidence rates generally increased with age, the rate of increase varied by racial or ethnic group. Furthermore, liver cancer incidence rates during 2008 through 2012 among NH blacks peaked at an earlier age (60–64 years), and then decreased at older ages. The NH AI/AN population also had an increase at younger ages (ages 55–59 years); however, liver cancer incidence rates continued to increase among this group up to ages 80 to 84 years. The statistical uncertainty in the liver cancer incidence rates by age group among the NH AI/AN population was mostly caused by the smaller number of cases at each age, as indicated in the Figure 2 inset. Because of this statistical instability, the incidence rates for NH AI/AN populations are not provided separately in later analyses. NH API populations experienced the highest liver cancer incidence rates for those aged <50 years and ≥65 years (after NH AI/AN populations), but Hispanic and NH blacks had higher rates in the group ages 50 to 64 years. The lowest liver cancer incidence rates for each age group were observed among NH white populations.
Figure 2.
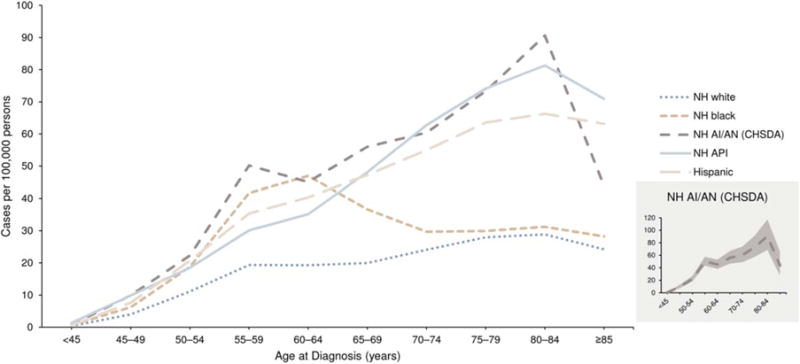
Age-specific incidence rates from 2008 to 2012 of liver and intrahepatic bile duct cancer are illustrated by race or ethnicity for areas in the United States with high-quality incidence data. Rates are per 100,000 persons and were age-adjusted to the 2000 US standard population (19 age groups: ages <1 year, 14 years, 5–9 years, …, 80–84 years, ≥85 years [Census publication p25-1130; US Bureau of the Census, Current Population Reports, p25-1130. Washington, DC: US Government Printing Office; 2000]). Source: National Program of Cancer Registries and Surveillance, Epidemiology, and End Results areas reported by the North American Association of Central Cancer Registries as meeting high-quality incidence data standards for the specified time. Rates from 2008 to 2012 for non-Hispanic (NH) whites, NH blacks, NH American Indians/Alaska Natives (AI/AN) (Contract Services Delivery Area [CHSDA] 2012 counties), NH Asians/Pacific Islanders (API), and Hispanics (48 states: Alabama, Alaska, Arizona, California, Colorado, Connecticut, Delaware, District of Columbia, Florida, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Mississippi, Missouri, Montana, Nebraska, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, South Dakota, Tennessee, Texas, Utah, Vermont, Virginia, Washington, West Virginia, Wisconsin, and Wyoming).
Figure 3 illustrates the cohort rate ratios and 95% CIs by race or ethnicity for liver cancer incidence relative to the experience of the 1943 reference birth cohort. Among NH white, NH black, and Hispanic men and women, the cohort rate ratio was <1.0 for the 1913 to 1938 birth cohorts. The rate ratio then sharply increased for birth cohorts 1948 through 1953. The rate ratio for birth cohorts 1953 through 1968 slightly decreased among NH whites and Hispanics but decreased sharply among NH blacks. In contrast, the cohort relative risk remained unchanged for NH APIs, signifying a minimal birth-cohort effect.
Figure 3.
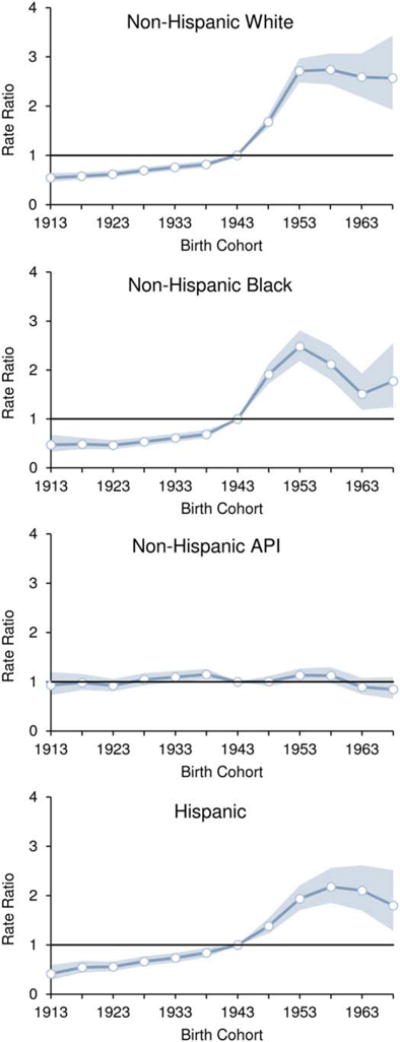
Cohort rate ratios (horizontal blue lines) and 95% confidence intervals (blue shading) are illustrated for the incidence of liver and intrahepatic bile duct cancer by race or ethnicity relative to the experience of the 1943 reference birth cohort (Surveillance, Epidemiology, and End Results [SEER]-13 areas, 1992–2012). The vertical lines indicate a rate ratio of 1 (no difference between a select birth cohort and the reference cohort). API indicates Asian/Pacific Islander. Source: SEER-13 areas covering about 14% of the US population (Connecticut, Hawaii, Iowa, Utah, and New Mexico; the Alaska Native Tumor Registry; rural Georgia; and the metropolitan areas of San Francisco, Los Angeles, San Jose-Monterey, Detroit, Atlanta, and Seattle-Puget Sound).
Figure 4 illustrates liver cancer incidence rates by age for cases diagnosed during 3 time intervals: 1992 to 1996, 2000 to 2004, and 2008 to 2012. Liver cancer incidence rates in general, as noted above, continued to increase with advancing age for each diagnosis period. However, during 2008 through 2012, liver cancer incidence rates among NH blacks were highest among persons ages 55 to 59 years, who are in the 1953 birth cohort (born during 1948–1957). A similar pattern is observed among NH whites and Hispanics, for whom there was a sharp increase among those ages 55 to 59 years during 2008 through 2012, although liver cancer incidence rates continued to increase with age. Among NH whites, NH blacks, and Hispanics, liver cancer incidence rates were generally highest for the most recent (2008–2012) diagnosis years and lowest for the 1992 to 1996 diagnosis years. Among Hispanics, there was a large difference between diagnosis years for every age; whereas, among NH whites and NH blacks, the largest difference was for those ages 50 to 69 years. The differences by diagnosis year were less pronounced among NH APIs than among other subgroups.
Figure 4.
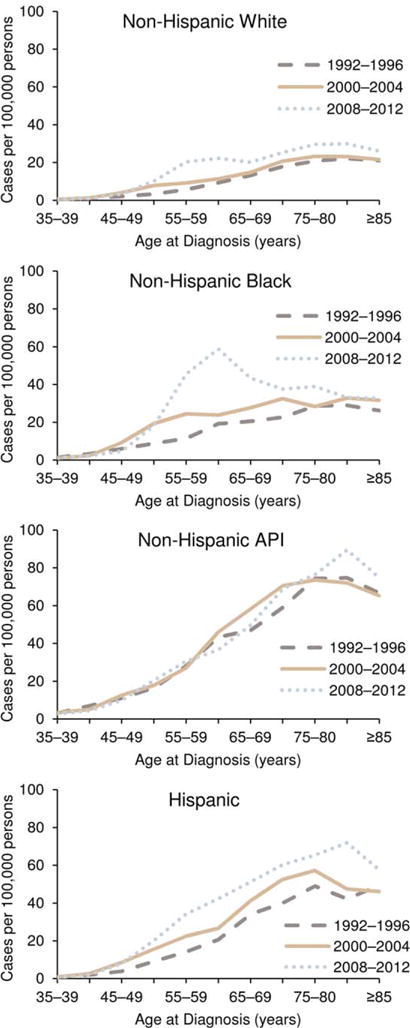
Age-specific liver and intrahepatic bile duct cancer incidence rates are illustrated by race or ethnicity and by age and year of diagnosis in the Surveillance, Epidemiology, and End Results (SEER)-13 areas from 1992 to 2012. Rates are per 100,000 persons. API indicates Asian/Pacific Islander. Source: SEER-13 areas covering about 14% of the US population (Connecticut, Hawaii, Iowa, Utah, and New Mexico; the Alaska Native Tumor Registry; rural Georgia; and the metropolitan areas of San Francisco, Los Angeles, San Jose-Monterey, Detroit, Atlanta, and Seattle-Puget Sound).
Table 6 presents incidence-based mortality data for liver cancer in the SEER-18 areas during 2008 through 2012. More than half of these deaths (53%) occurred among NH whites. The median age at death from liver cancer was younger among NH blacks (median age, 61 years) and Hispanics (median age, 64 years) than among NH whites (median age, 66 years) and NH APIs (median age, 68 years). The median age at death was 9 years older among women (median age, 72 years) than among men (median age, 63 years), but this difference ranged from 4 years among NH blacks to 10 years among NH APIs. More than 379,000 person-years of life were lost to liver cancer during 2008 through 2012. The average person-years of life that were lost to liver cancer death was higher among NH blacks (average, 22 PYLL) and Hispanics (average, 20 PYLL) than among NH APIs and NH whites (average, 18 PYLL for both). Although 74% of total PYLL were among men, the APYLL was similar among men (19 APYLL) and women (17 APYLL).
TABLE 6.
Distribution of Deaths, Median Age at Death, and Person-Years of Life Lost From Liver and Intrahepatic Bile Duct Cancer, by Sex, and Race or Ethnicity, Surveillance, Epidemiology, and End Results-18 Areas, 2008 to 2012a
| Sex, Race, and Ethnicityb | Deaths by Race, %c | Deaths by Sex, %c | Median Age at Death, y | Total No. of PYLLd | PYLL by Race, %d | PYLL by Sex, %d | Average PYLL Deathd |
|---|---|---|---|---|---|---|---|
| Total | 100 | 65 | 379,464 | 100 | 19 | ||
| Men | 73 | 63 | 270,741 | 74 | 19 | ||
| Women | 27 | 72 | 93,294 | 26 | 17 | ||
| Non-Hispanic, white | 53 | 66 | 192,278 | 51 | 18 | ||
| Men | 73 | 64 | 137,046 | 74 | 18 | ||
| Women | 27 | 73 | 47,180 | 26 | 17 | ||
| Non-Hispanic, black | 13 | 61 | 55,030 | 15 | 22 | ||
| Men | 76 | 60 | 40,038 | 76 | 21 | ||
| Women | 24 | 64 | 12,733 | 24 | 21 | ||
| Non-Hispanic, API | 15 | 68 | 56,248 | 15 | 18 | ||
| Men | 69 | 65 | 39,543 | 73 | 19 | ||
| Women | 31 | 75 | 14,570 | 27 | 16 | ||
| Hispanic | 18 | 64 | 70,588 | 19 | 20 | ||
| Men | 73 | 62 | 50,486 | 74 | 20 | ||
| Women | 27 | 71 | 17,308 | 26 | 18 |
Abbreviations: API, Asian/Pacific Islander; PYLL, person-years of life lost.
Source: Surveillance, Epidemiology, and End Results (SEER)-18 areas covering about 28% of the US population (10 state registries [Connecticut, Georgia, Greater California, Hawaii, Iowa, Kentucky, Louisiana, New Jersey, New Mexico, and Utah], 3 Native American registries [the Alaska Native Tumor Registry, Arizona Indians, and the Cherokee Nation Cancer Registry], and 5 metropolitan area registries [metropolitan Atlanta and rural Georgia, San Francisco-Oakland and San Jose-Monterey, Los Angeles, Detroit, and Seattle-Puget Sound]).
The table excludes individuals of unknown Hispanic and Hispanic other/unknown race.
Values indicate incidence-based mortality.
PYLL and average PYLL estimates are 5-year totals estimated by using overall, male, and female all-races life tables.
HCV and liver cancer-associated mortality from 1999 to 2013 is illustrated in Figure 5. Among persons for whom both HCV and liver cancer were listed as causes of death, those born during 1945 through 1965 had the largest increase in mortality from 1999 to 2013 relative to the other birth-year categories (Fig. 5A). Considering the differences in age between birth cohorts, those born during 1945 through 1965 had substantially higher rates of HCV and liver cancer-associated deaths than the preceding or subsequent birth cohorts, particularly for the mid-generational group (Fig. 5B).
Figure 5.
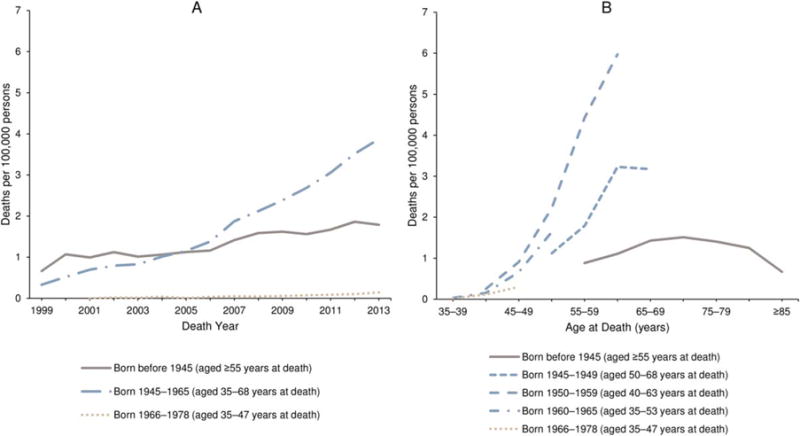
Crude death rates associated with hepatitis C virus and liver or intrahepatic bile duct cancer are illustrated among decedents aged ≥35 years according to (A) birth cohort and year and (B) birth cohort and age for the United States from 1999 to 2013. Rates are per 100,000 persons. Hepatitis C virus was defined according to the International Classification of Diseases 10th Revision (ICD-10) codes B17.1 and B18.2. Liver and intrahepatic bile duct cancers were defined by any ICD-10 code in the C22 ICD-10 category. Patients who died from liver or intrahepatic bile duct cancers had hepatitis C and liver or intrahepatic bile duct cancer listed together as any cause of death on their death certificate. Source: National Center for Health Statistics public-use data file for the total United States, 1999 to 2013.
DISCUSSION
Ongoing reductions in both the incidence and mortality of cancer overall, and specifically deaths from the most common cancers (female breast, prostate, lung, and colorectal cancers) in most racial and ethnic groups, represent continued progress in specific areas of public health and oncology care.2–17 For example, successful and comprehensive public health tobacco-control strategies remain an important contributing factor to the observed declines in lung cancer incidence and mortality among both men and women.11,54–56 To aid in the earlier diagnosis of lung cancer among those who remain at an increased risk from tobacco use, in 2013, the USPSTF recommended routine screening with low-dose computed tomography of persons at high-risk because of their age and cigarette smoking history.57 Continuing public health surveillance is needed to monitor the success of these efforts to increase screening in appropriate populations; and resulting reductions in mortality may reflect a decrease in lung cancer mortality rates.
Colorectal cancer incidence and death rates also continue to decline. There is strong evidence to support the finding that screening for colorectal cancer reduces both the incidence and the mortality of the disease,58 and increases in colorectal cancer survival rates during the past 2 decades have been attributed largely to screening.59–61 Still, a recent report about the use of colorectal cancer screening indicated that 35% of US adults were not up to date with one of the colorectal cancer screening tests recommended by the USPSTF.62 To help increase colorectal cancer screening compliance, the CDC funds the Colorectal Cancer Control Program to implement evidence-based interventions and provide screening and follow-up services for a limited number of eligible individuals.63 In addition, in 2014, the National Colorectal Cancer Roundtable, a coalition aimed at reducing colorectal cancer incidence and mortality, initiated a nationwide goal of increasing colorectal cancer screening prevalence to 80% by 2018.64
Breast cancer, as the most commonly diagnosed and second leading cause of cancer death among women, remains an important public health issue. Advances in treatment and early detection contribute to the ongoing decrease in breast cancer mortality among women overall.65 However, not all racial and ethnic groups benefit equally in this favorable trend: black women have a higher breast cancer death rate than white women.17 Efforts are underway to better understand the factors contributing to these observed differences, including surveillance of breast cancer subtypes by different racial and ethnic groups,17 and to promote the use of evidence-based interventions aimed at groups struggling with access to care, such as CDC’s National Breast and Cervical Cancer Early Detection Program.66
The continued decrease in prostate cancer incidence likely reflects the continued reduction in the use of prostate-specific antigen testing.67–70 In 2008, the USPSTF concluded that there was insufficient evidence to recommend prostate cancer screening among men aged ≥75 years; and, in 2012, it further recommended against screening for all men,71 likely contributing to the observed reduction in incidence rates since that time.15–17,72 Close public health surveillance of both prostate stage-specific incidence and death rates will be particularly important during future years to ensure a balance is achieved between reducing unnecessary screening and ensuring appropriate and early diagnosis and treatment of more aggressive cases of this important cancer among men.
Despite the successful reductions in the occurrence of and deaths from the most common cancers, several cancer sites show unfavorable trends, particularly liver cancer. Liver cancer death rates are increasing at the highest rate of all cancer sites among both men and women. Indeed, the incidence of liver cancer is also increasing at rates second only to those of thyroid cancer. The differences in liver cancer incidence observed by sex, race or ethnicity, and birth cohort highlighted in this annual report suggest differences in the distribution of a variety of risk factors among different groups and over time.
HCC is the most common histologic type of primary liver cancer, and HCC trends are largely affected by the changing prevalence of risk factors. In the United States, a critical risk factor is HCV infection.73 Risks for HCV transmission are primarily associated with parenteral exposures, including receipt of contaminated blood and injection drug use. The incidence of HCV infection was highest during the 1960s to 1980s, before the virus was discovered and preventive measures, including HCV screening of the blood supply, became possible. Consequently, the prevalence of HCV infection is particularly high among individuals born during 1945 through 1965.28,74,75 Thus, in 2012, the CDC recommended 1-time HCV testing for individuals born during 1945 through 1965 (ages 47–67 years in 2012).31 The following year, the USPSTF issued similar recommendations.76 This annual report provides evidence of an increased burden of HCV and liver cancer-associated mortality among this birth cohort; although, according to the 2013 National Health Interview Survey, only 12.3% of persons born during 1945 through 1965 reported HCV testing.77 Increased liver cancer incidence among this birth cohort is particularly elevated for NH whites, NH blacks, and Hispanics. Findings of a high burden of HCC among certain racial or ethnic groups is consistent with race-specific HCV infection prevalence estimates from US surveys.78
Among APIs, HBV is the dominant HCC-related virus, particularly among those not born in the United States.79 Because hepatitis B vaccination of infants is now widespread, the prevalence of HBV infection is declining in most Asian countries among vaccinated cohorts of children and adolescents.80,81 Accordingly, hepatitis B vaccination is considered a best buy for liver cancer prevention.82 Consequently, HBV-related HCC among APIs may decline in coming generations. However, in the immediate term, HBV infection prevalence remains high among unvaccinated adults from these countries, representing an ongoing risk of liver cancer.83 HBV testing followed by HBV therapy is associated with a reduction from 50% to 80% in the risk of liver cancer.84,85 In 2008, the CDC recommended HBV testing for persons born in sub-Saharan Africa, Asia, and countries in other world regions where HBV infection prevalence is ≥2%. In 2014, the USPSTF also recommended HBV testing for these target populations.86
Cirrhosis is a precursor for most liver cancers. Screening of individuals at risk of cirrhosis increases the chance of detecting liver cancer at an early stage when it is more amenable to therapy. Population groups at risk of cirrhosis include persons with metabolic disease, history of liver disease, history of heavy alcohol use, exposure to HCV or HBV, and some rare genetic disorders.87 Increasing evidence suggests that other risk factors, such as type 2 diabetes mellitus and metabolic syndrome (ie, clustering of at least 3 of the following: obesity, elevated blood pressure, elevated fasting plasma glucose, high serum triglycerides, and low high-density lipoprotein levels), are also important contributing factors, particularly when considering the prevalence of these types of medical conditions among the population by using the calculation of a population-attributable fraction (PAF).32 A recent SEER-Medicare analysis of HCC cases diagnosed during 1994 through 2007 among persons aged ≥68 years examined odds ratios (ORs) as well as PAFs for the most common risk factors for HCC.32 Among this study population, the strongest predictors of HCC were HCV infection (OR, 39.9), HBV infection (OR, 11.2), alcohol-related disease (OR, 4.1), metabolic disease (OR, 3.5), and diabetes and/or obesity (OR, 2.3). However, when the prevalence of each risk factor was considered, diabetes and/or obesity had the highest PAF (36.6%), followed by alcohol-related disease (PAF, 23.5%), HCV infection (PAF, 22.4%), HBV infection (PAF, 6.3%), and metabolic disease (PAF, 3.2%).
Although there are a variety of risk factors for liver cancer, approximately 22% of HCC among those aged ≥65 years in the United States is attributed to HCV,32 and an estimated 1.6 million persons will be eligible for HCV treatment by 2020.88 Previous interferon-based therapies are associated with considerable toxicity,89 yet current standards of HCV treatment include from 8 to 12 weeks of all-oral medications with fewer side effects, which can produce a sustained virologic response in >85% of HCV-infected individuals who complete treatment.90–92 It is estimated that, compared with interferon-based therapy, newer antiviral therapies, such as sofosbuvir-ledipasvir, could prevent an additional 310 cases of HCC per 10,000 treated HCV cases. However, fewer than half of persons living with HCV are aware of their infection; and, among those who know their status, many do not receive appropriate clinical management of HCV infection and associated liver disease.93 Although drug expenditures for treatment of HCV infection have declined as a result of mandated rebates for Medicaid and privately negotiated prices by health plans, the cost of HCV medications may limit the number of patients receiving recommended antiviral therapy.94 Barriers to HCV testing and access to adequate care with affordable medications must be overcome to prevent HCV-related liver cancer.95
The burden of liver cancer is not equally distributed across the US population, reflecting the heterogeneous prevalence of liver cancer risk factors among groups. Men have nearly a 3-fold higher rate of liver cancer incidence than women. Liver cancer incidence rates generally increase with age. Historically, APIs have been the racial group most affected by liver cancer; however, that may change if the decreasing trend in this group continues as trends increase for other racial groups. Indeed, our analysis indicates that, among individuals ages 55 to 64 years, NH blacks and Hispanics had higher rates than APIs. NH black and Hispanic men have the youngest median age at liver cancer death and the highest APYLL to liver cancer. Although NH whites have lower liver cancer rates than other racial or ethnic groups, they make up a large segment of the US population and account for the majority of deaths and PYLL to liver cancer in the United States.
Limitations
The completeness, quality, and geographic coverage of cancer incidence data exceed those available for other chronic diseases. The NPCR and SEER data set used for general trends is the most complete and current data set available, covering the vast majority of the US population. However, as with any surveillance data set, local level variations in data quality, incomplete geographic or population reporting, and the complexity of estimating the underlying populations at risk may have influenced the results reported here. For instance, reporting from smaller hospitals or providers unaffiliated with a cancer center may be less complete or may have a longer lag in reporting time. Corrections for late reporting were incorporated into the rates and trends that included delay adjustment; however, this adjustment was not possible for data used to estimate race-specific or ethnicity-specific incidence trends, and differing results may occur, particularly for sites typically diagnosed in a physician’s office, like melanoma.
Another issue is the compatibility of the cancer data and the population data by race. Since 2000, the US Census has provided the opportunity for respondents to self-select multiple race categories, which has created incompatibility between the classification of race in the population denominators from the Census (self-reported) with the incidence numerators from registry data (from medical record) and the mortality numerators from the National Vital Statistics System (recorded by medical examiner). The methods for developing single-race estimates from these data are complex and can create additional uncertainties in racial estimates and resultant rates.96,97 The broad Hispanic and API categories may mask important epidemiologic variation in risk by country of origin.98,99
We present rates by race separately from Hispanic ethnicity for the cancer incidence and mortality section (Tables 1–4), so the race categories include Hispanics. Thus, as the proportion of the Hispanic population in the United States increases over time, this may influence the reported trends. Shifts in demographics can influence surveillance trends, and our results must be interpreted with this in mind. Furthermore, death rates for the AI/AN, API, and Hispanic populations may be underestimated because of reporting problems on the death certificate and should be interpreted with caution.100
Long-term trends were reported on the basis of SEER-13 registries, which represent only 14% of the US population, and the liver cancer incidence-based mortality analysis was based on SEER-18 registries, which represent approximately 28% of the US population. Thus, these analyses may be influenced by the population composition of the SEER areas. More geographic population coverage was available for 5-year and 10-year trends using the combined NPCR and SEER data; however, because of data quality concerns, data from 5 states were excluded from 10-year trends (resulting in 92% coverage), and data from 2 states were excluded for 5-year trends (resulting in 97% coverage), which may influence reported trends. However, >90% of the United States was represented; therefore, these exclusions likely have only a minor effect on national trends.
The limitations of mortality data include the incomplete ascertainment of cases, misclassification, and missing causes of death.101,102 However, these biases may be mitigated by analyzing trends in which the problems have been assumed to be relatively constant over time.53,103 Finally, despite using the largest national data set available for cancer rates, small numbers for less common sites were an issue for the smaller racial categories, especially AI/AN. Small numbers raise statistical issues, particularly random variation. When evaluating trends, it is vital to assess the magnitude and direction along with the notation of statistical significance, particularly for smaller subgroups.
Future Directions
This annual report brings attention to the growing burden of liver cancer in the United States. Most cases of liver cancer are preventable.104 One of the most important and preventable risk factors for liver cancer is chronic HCV infection; yet, despite this knowledge, new HCV infections continue to occur.28,105 Prevention strategies aimed at reducing the frequency of behaviors that increase the risk of HCV transmission can be effective in reducing HCV infection. Early diagnosis of HCV infection with referral to treatment services can decrease a patient’s risk for subsequent health outcomes, such as liver cancer, and can decrease transmission of the virus to others. Cures of HCV infection are associated with a 75% reduction in the risk of liver cancer.106,107 In addition to appropriate medical care and management for HCV infection, other strategies to reduce the burden of liver cancer in the United States include promoting hepatitis B vaccination strategies; reducing unhealthy behaviors, such as tobacco use and excessive alcohol use; and reducing obesity by promoting healthy eating and physical activity. Furthermore, improvements in the public health surveillance of both acute and chronic viral hepatitis infections can help to better monitor the incidence and prevalence of infections and to evaluate interventions.
Although increases in liver cancer incidence and mortality are concerning, the continuing decline in cancer death rates for all sites combined and for the leading cancer sites overall demonstrate progress in cancer prevention and control in general. However, even if incidence rates remain stable or decline, the number of individuals diagnosed with cancer and living after a diagnosis will continue to rise because of improvements in treatment and because of population growth and aging.108 Thus, primary, secondary, and tertiary cancer prevention efforts at the patient and population-based levels are important. Continued focus on primary prevention efforts to reduce or eradicate risk factors before cancer occurs is critical. Cancer is a heterogeneous disease, and each cancer site has a unique set of risk factors. However, many modifiable cancer risk factors contribute to the burden of many cancer sites, including tobacco use, excess alcohol consumption, poor diet, excess body weight, and physical inactivity.109,110 Reductions in these unhealthy behaviors and improvements in healthy lifestyles over time can reduce the burden of cancer.
Although many cancer risk factors are modifiable or avoidable, many are not. Much of the morbidity and mortality associated with cancer can be prevented by detecting the disease at an early stage, when treatment is more effective.30,57,111,112 On the basis of systematic reviews of the net benefits and harms of screening, the USPSTF currently recommends population-based screening for colorectal, female breast, and cervical cancers among persons at average risk of developing these cancers and for lung cancer among persons at high-risk on the basis of their cigarette smoking history and age.113 Regardless of how the cancer is diagnosed, reducing morbidity and mortality associated with the disease depends on timely and appropriate treatment. Removing barriers to receipt of appropriate cancer screening and treatment, including financial barriers, is crucial.114 Programs like the CDC’s National Breast and Cervical Cancer Early Detection Program66 and the CDC’s Colorectal Cancer Control Program63 can provide needed services, such as free or low-cost early cancer detection examinations, patient navigation services, and treatment.
For individuals diagnosed with cancer (cancer survivors), methods to mitigate the negative effects of a diagnosis and its treatment and to prevent the recurrence of cancer are also critical. Cancer survivors often face long-term adverse physical, psychosocial, and financial effects from their cancer diagnosis and treatment.115–121 In addition, cancer survivors are at an increased risk of subsequent primary cancers.122,123 Cancer can have a long-term impact on the health and well being of the cancer survivor. This has important implications for ongoing preventive care for all cancers and comorbid conditions.
Finally, disease surveillance is essential to the practice of public health for guiding prevention and control activities, monitoring trends, and evaluating outcomes.124,125 Information obtained from cancer incidence registries and vital statistics systems provide decision makers with vital information necessary to lead and manage effective cancer prevention and control programs. Cancer registry systems are among the most sophisticated and standardized surveillance systems in the United States. Routine monitoring of cancer incidence and mortality helps to evaluate outcomes of public health-oriented cancer prevention efforts and to identify areas and populations with high cancer incidence or mortality rates that can benefit from targeted interventions to promote healthy environments and behaviors.
Supplementary Material
Acknowledgments
FUNDING SUPPORT
This work was supported by the Centers for Disease Control and Prevention, the National Cancer Institute, the American Cancer Society, and the North American Association of Central Cancer Registries.
We gratefully acknowledge the contributions of the state and regional cancer registry staff for their work in collecting the data used in this study. In addition, we thank Martin Krapcho, Rick Firth, and Steve Scoppa of Information Management Services, Inc, for assistance in compiling the data used in this report.
Footnotes
This article has been contributed to by US Government employees and their work is in the public domain in the USA.
The findings and conclusions in this article are those of the authors and do not necessarily represent the official positions of the author’s agencies (the Centers for Disease Control and Prevention, the National Cancer Institute, the American Cancer Society, or the North American Association of Central Cancer Registries).
Additional supporting information may be found in the online version of this article.
AUTHOR CONTRIBUTIONS
A. Blythe Ryerson: Conceptualization, methodology, writing–original draft, visualization, supervision, and project administration.
Christie R. Eheman: Conceptualization, investigation, resources, writing–original draft, writing–review and editing, supervision, and project administration.
Sean F. Altekruse: Formal analysis and writing–original draft.
John W. Ward: Conceptualization, validation, formal analysis, writing–original draft, writing–review and editing, and supervision.
Ahmedin Jemal: Conceptualization, methodology, validation, investigation, writing–original draft, writing–review and editing, and visualization.
Recinda L. Sherman: Conceptualization, methodology, writing–original draft, and writing–review and editing.
S. Jane Henley: Methodology, formal analysis, writing–original draft, writing–review and editing, and visualization.
Deborah Holtzman: Conceptualization, methodology, validation, writing–original draft, writing–review and editing, and visualization.
Andrew Lake: Software, validation, formal analysis, data curation, writing–review and editing, visualization.
Anne-Michelle Noone: Writing–original draft and writing–review and editing.
Robert N. Anderson: Conceptualization and writing–review and editing.
Jiemin Ma: Conceptualization and writing–review and editing.
Kathleen N. Ly: Methodology, software, validation, formal analysis, resources, writing–original draft, and writing–review and editing.
Kathleen A. Cronin: Conceptualization, methodology, writing–review and editing, and visualization.
Lynne Penberthy: Conceptualization, resources, writing–review and editing, and supervision.
Betsy A. Kohler: Conceptualization, methodology, validation, resources, writing–review and editing, visualization, supervision, project administration, and funding acquisition.
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
References
- 1.Wingo PA, Ries LA, Rosenberg HM, Miller DS, Edwards BK. Cancer incidence and mortality, 1973–1995: a report card for the United States. Cancer. 1998;82:1197–1207. doi: 10.1002/(sici)1097-0142(19980315)82:6<1197::aid-cncr26>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 2.Wingo PA, Ries LA, Giovino GA, et al. Annual report to the nation on the status of cancer, 1973–1996, with a special section on lung cancer and tobacco smoking. J Natl Cancer Inst. 1999;91:675–690. doi: 10.1093/jnci/91.8.675. [DOI] [PubMed] [Google Scholar]
- 3.Ries LA, Wingo PA, Miller DS, et al. The annual report to the nation on the status of cancer, 1973–1997, with a special section on colorectal cancer. Cancer. 2000;88:2398–23424. doi: 10.1002/(sici)1097-0142(20000515)88:10<2398::aid-cncr26>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 4.Howe HL, Wingo PA, Thun MJ, et al. Annual report to the nation on the status of cancer (1973 through 1998), featuring cancers with recent increasing trends. J Natl Cancer Inst. 2001;93:824–842. doi: 10.1093/jnci/93.11.824. [DOI] [PubMed] [Google Scholar]
- 5.Edwards BK, Howe HL, Ries LA, et al. Annual report to the nation on the status of cancer, 1973–1999, featuring implications of age and aging on US cancer burden. Cancer. 2002;94:2766–2792. doi: 10.1002/cncr.10593. [DOI] [PubMed] [Google Scholar]
- 6.Weir HK, Thun MJ, Hankey BF, et al. Annual report to the nation on the status of cancer, 1975–2000, featuring the uses of surveillance data for cancer prevention and control. J Natl Cancer Inst. 2003;95:1276–1299. doi: 10.1093/jnci/djg040. [DOI] [PubMed] [Google Scholar]
- 7.Jemal A, Clegg LX, Ward E, et al. Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer. 2004;101:3–27. doi: 10.1002/cncr.20288. [DOI] [PubMed] [Google Scholar]
- 8.Edwards BK, Brown ML, Wingo PA, et al. Annual report to the nation on the status of cancer, 1975–2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst. 2005;97:1407–1427. doi: 10.1093/jnci/dji289. [DOI] [PubMed] [Google Scholar]
- 9.Howe HL, Wu X, Ries LA, et al. Annual report to the nation on the status of cancer, 1975–2003, featuring cancer among US Hispanic/Latino populations. Cancer. 2006;107:1711–1742. doi: 10.1002/cncr.22193. [DOI] [PubMed] [Google Scholar]
- 10.Espey DK, Wu XC, Swan J, et al. Annual report to the nation on the status of cancer, 1975–2004, featuring cancer in American Indians and Alaska Natives. Cancer. 2007;110:2119–2152. doi: 10.1002/cncr.23044. [DOI] [PubMed] [Google Scholar]
- 11.Jemal A, Thun MJ, Ries LA, et al. Annual report to the nation on the status of cancer, 1975–2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst. 2008;100:1672–1694. doi: 10.1093/jnci/djn389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohler BA, Ward E, McCarthy BJ, et al. Annual report to the nation on the status of cancer, 1975–2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst. 2011;103:714–736. doi: 10.1093/jnci/djr077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eheman C, Henley SJ, Ballard-Barbash R, et al. Annual report to the nation on the status of cancer, 1975–2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer. 2012;118:2338–2366. doi: 10.1002/cncr.27514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jemal A, Simard EP, Dorell C, et al. Annual report to the nation on the status of cancer, 1975–2009, featuring the burden and trends in human papillomavirus (HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst. 2013;105:175–201. doi: 10.1093/jnci/djs491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards BK, Noone AM, Mariotto AB, et al. Annual report to the nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120:1290–1314. doi: 10.1002/cncr.28509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohler BA, Sherman RL, Howlader N, et al. Annual report to the nation on the status of cancer, 1975–2011 featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state [serial online] J Natl Cancer Inst. 2015;107:djv048. doi: 10.1093/jnci/djv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012 [serial online] Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 19.US Cancer Statistics Working Group. United States Cancer Statistics: 1999–2012 Incidence and Mortality Web-based Report. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2015. Available at: www.cdc.gov/uscs Accessed September 2, 1015. [Google Scholar]
- 20.Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review 1975–2012 [Based on the November 2014 SEER data submission, posted to the SEER web site, April 2015] Bethesda, MD: National Cancer Institute; 2014. [Google Scholar]
- 21.de Martel C, Maucort-Boulch D, Plummer M, Franceschi S. World-wide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatology. 2015;62:1190–1200. doi: 10.1002/hep.27969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Bisceglie AM, Lyra AC, Schwartz M, et al. Hepatitis C-related hepatocellular carcinoma in the United States: influence of ethnic status. Am J Gastroenterol. 2003;98:2060–2063. doi: 10.1111/j.1572-0241.2003.t01-1-07641.x. [DOI] [PubMed] [Google Scholar]
- 23.El-Serag HB. Epidemiology of hepatocellular carcinoma in USA. Hepatol Res. 2007;37(suppl 2):S88–S94. doi: 10.1111/j.1872-034X.2007.00168.x. [DOI] [PubMed] [Google Scholar]
- 24.Kowdley KV, Wang CC, Welch S, Roberts H, Brosgart CL. Prevalence of chronic hepatitis B among foreign-born persons living in the United States by country of origin. Hepatology. 2012;56:422–433. doi: 10.1002/hep.24804. [DOI] [PubMed] [Google Scholar]
- 25.Roberts H, Kruszon-Moran D, Ly KN, et al. Prevalence of chronic hepatitis B virus (HBV) infection in US households—National Health and Nutrition Examination Survey (NHANES), 1988–2012. Hepatology. 2016;63:388–397. doi: 10.1002/hep.28109. [DOI] [PubMed] [Google Scholar]
- 26.Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160:293–300. doi: 10.7326/M13-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edlin BR, Eckhardt BJ, Shu MA, Holmberg SD, Swan T. Towards a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology. 2015;62:1353–1363. doi: 10.1002/hep.27978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 29.Rein DB, Wittenborn JS, Weinbaum CM, Sabin M, Smith BD, Lesesne SB. Forecasting the morbidity and mortality associated with prevalent cases of pre-cirrhotic chronic hepatitis C in the United States. Dig Liver Dis. 2011;43:66–72. doi: 10.1016/j.dld.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Moyer VA. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156:880–891. doi: 10.7326/0003-4819-156-12-201206190-00424. [DOI] [PubMed] [Google Scholar]
- 31.Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recomm Rep. 2012;61(RR-4):1–32. [PubMed] [Google Scholar]
- 32.Welzel TM, Graubard BI, Quraishi S, et al. Population-attributable fractions of risk factors for hepatocellular carcinoma in the United States. Am J Gastroenterol. 2013;108:1314–1321. doi: 10.1038/ajg.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.North American Association of Central Cancer Registries (NAACCR) NAACCR Data Quality Criteria. Available at: http://www.naaccr.org/Certification/Criteria.aspx. Accessed July 8, 2015.
- 34.World Health Organization. International Classification of Diseases for Oncology. 3rd. Geneva, Switzerland: World Health Organization Press; 2000. [Google Scholar]
- 35.Espey DK, Jim MA, Richards TB, Begay C, Haverkamp D, Roberts D. Methods for improving the quality and completeness of mortality data for American Indians and Alaska Natives. Am J Public Health. 2014;104(suppl 3):S286–S294. doi: 10.2105/AJPH.2013.301716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Espey DK, Wiggins CL, Jim MA, Miller BA, Johnson CJ, Becker TM. Methods for improving cancer surveillance data in American Indian and Alaska Native populations. Cancer. 2008;113(5 suppl):1120–1130. doi: 10.1002/cncr.23724. [DOI] [PubMed] [Google Scholar]
- 37.National Cancer Institute. SEER Registry Groupings for Analyses. Available at: http://seer.cancer.gov/registries/terms.html. Accessed July 8, 2015.
- 38.Murphy SL, Kochanek KD, Xu J, Heron M. Deaths: final data for 2012. Nat Vital Stat Rep. 2015;63:1–117. [PubMed] [Google Scholar]
- 39.Surveillance, Epidemiology, and End Results (SEER) Program; National Cancer Institute. Population estimates used in NCI’s SEER*Stat software. Available at: http://seer.cancer.gov/popdata/methods.html. Accessed July 13, 2015.
- 40.Ingram DD, Parker JD, Schenker N, et al. United States Census 2000 population with bridged race categories. Vital Health Stat 2. 2003;135:1–55. [PubMed] [Google Scholar]
- 41.World Health Organization. International Classification of Diseases. Geneva, Switzerland: World Health Organization; 1998. 10th Revision. [Google Scholar]
- 42.National Center for Health Statistics. Instructions for Classifying the Underlying Cause of Death, ICD-10, 2015. Available at: http://www.cdc.gov/nchs/data/dvs/2a_2015.pdf. Accessed October 20, 2015.
- 43.Surveillance Research Program. National Cancer Institute SEER*Stat software. Bethesda, MD: National Cancer Institute; 2015. ( www.seer.cancer.gov/seerstat) version 8.2.1. [Google Scholar]
- 44.Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006;15:547–569. doi: 10.1177/0962280206070621. [DOI] [PubMed] [Google Scholar]
- 45.National Cancer Institute. Joinpoint Regression Program, Version 4.2.0.2 (June 2015) Bethesda, MD: National Cancer Institute; 2015. Available at: http://surveillance.cancer.gov/joinpoint/. Accessed July 14, 2015. [Google Scholar]
- 46.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 47.Clegg LX, Hankey BF, Tiwari R, Feuer EJ, Edwards BK. Estimating average annual per cent change in trend analysis. Stat Med. 2009;28:3670–3682. doi: 10.1002/sim.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clegg LX, Feuer EJ, Midthune DN, Fay MP, Hankey BF. Impact of reporting delay and reporting error on cancer incidence rates and trends. J Natl Cancer Inst. 2002;94:1537–1545. doi: 10.1093/jnci/94.20.1537. [DOI] [PubMed] [Google Scholar]
- 49.Rosenberg PS, Check DP, Anderson WF. A web tool for age-period-cohort analysis of cancer incidence and mortality rates. Cancer Epidemiol Biomarkers Prev. 2014;23:2296–2302. doi: 10.1158/1055-9965.EPI-14-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chu KC, Miller BA, Feuer EJ, Hankey BF. A method for partitioning cancer mortality trends by factors associated with diagnosis: an application to female breast cancer. J Clin Epidemiol. 1994;47:1451–1461. doi: 10.1016/0895-4356(94)90089-2. [DOI] [PubMed] [Google Scholar]
- 51.National Cancer Institute. Cancer Trends Progress Report. Person-Years of Life Lost. Bethesda, MD: National Cancer Institute; 2015. Available at: http://www.progressreport.cancer.gov/end/life_lost. Accessed October 22, 2015. [Google Scholar]
- 52.Arias E. United States life tables, 2010. Natl Vital Stat Rep. 2014;63:1–63. [PubMed] [Google Scholar]
- 53.Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156:271–278. doi: 10.7326/0003-4819-156-4-201202210-00004. [DOI] [PubMed] [Google Scholar]
- 54.Centers for Disease Control and Prevention (CDC) State-specific trends in lung cancer incidence and smoking—United States, 1999–2008. MMWR Morb Mortal Wkly Rep. 2011;60:1243–1247. [PubMed] [Google Scholar]
- 55.Centers for Disease Control and Prevention (CDC) Vital signs: current cigarette smoking among adults aged ≥18 years with mental illness—United States, 2009–2011. MMWR Morb Mortal Wkly Rep. 2013;62:81–87. [PMC free article] [PubMed] [Google Scholar]
- 56.Henley SJ, Richards TB, Underwood JM, Eheman CR, Plescia M, McAfee TA. Lung cancer incidence trends among men and women—United States, 2005–2009. MMWR Morb Mortal Wkly Rep. 2014;63:1–5. [PMC free article] [PubMed] [Google Scholar]
- 57.Moyer VA. Screening for lung cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:330–338. doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 58.Zauber AG, Lansdorp-Vogelaar I, Knudsen AB, Wilschut J, van Ballegooijen M, Kuntz KM. Evaluating test strategies for colorectal cancer screening: a decision analysis for the US Preventive Services Task Force. Ann Intern Med. 2008;149:659–669. doi: 10.7326/0003-4819-149-9-200811040-00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 60.Littlejohn C, Hilton S, Macfarlane GJ, Phull P. Systematic review and meta-analysis of the evidence for flexible sigmoidoscopy as a screening method for the prevention of colorectal cancer. Br J Surg. 2012;99:1488–1500. doi: 10.1002/bjs.8882. [DOI] [PubMed] [Google Scholar]
- 61.Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369:1095–1105. doi: 10.1056/NEJMoa1301969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Centers for Disease Control and Prevention (CDC) Vital signs: colorectal cancer screening test use—United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:881–888. [PMC free article] [PubMed] [Google Scholar]
- 63.Centers for Disease Control and Prevention (CDC) CDC’s Colorectal Cancer Control Program. About the Program. Available at: http://www.cdc.gov/cancer/crccp/about.htm. Accessed September 23, 2015.
- 64.National Colorectal Cancer Roundtable. About the Roundtable. Available at: http://nccrt.org/about/. Accessed September 23, 2015.
- 65.Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 66.Centers for Disease Control and Prevention (CDC) CDC’s National Breast and Cervical Cancer Early Detection Program. About the Program. Available at: http://www.cdc.gov/cancer/nbccedp/about.htm. Accessed September 23, 2015.
- 67.Howard DH, Tangka FK, Guy GP, Ekwueme DU, Lipscomb J. Prostate cancer screening in men ages 75 and older fell by 8 percentage points after task force recommendation. Health Aff (Millwood) 2013;32:596–602. doi: 10.1377/hlthaff.2012.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barocas DA, Mallin K, Graves AJ, et al. Effect of the USPSTF grade D recommendation against screening for prostate cancer on incident prostate cancer diagnoses in the United States. J Urol. 2015;194:1587–1593. doi: 10.1016/j.juro.2015.06.075. [DOI] [PubMed] [Google Scholar]
- 69.Drazer MW, Huo D, Eggener SE. National prostate cancer screening rates after the 2012 US Preventive Services Task Force recommendation discouraging prostate-specific antigen-based screening. J Clin Oncol. 2015;33:2416–2423. doi: 10.1200/JCO.2015.61.6532. [DOI] [PubMed] [Google Scholar]
- 70.Li J, Berkowitz Z, Hall IJ. Decrease in prostate cancer testing following the US Preventive Services Task Force (USPSTF) recommendations. J Am Board Fam Med. 2015;28:491–493. doi: 10.3122/jabfm.2015.04.150062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.US Preventive Services Task Force. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:185–191. doi: 10.7326/0003-4819-149-3-200808050-00008. [DOI] [PubMed] [Google Scholar]
- 72.Jemal A, Fedewa SA, Ma J, et al. Prostate cancer incidence and PSA testing patterns in relation to USPSTF screening recommendations. JAMA. 2015;314:2054–2061. doi: 10.1001/jama.2015.14905. [DOI] [PubMed] [Google Scholar]
- 73.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma [serial online] Gastroenterology. 2012;142:1264–1273.e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ly KN, Xing J, Klevens RM, Jiles RB, Holmberg SD. Causes of death and characteristics of decedents with viral hepatitis, United States, 2010. Clin Infect Dis. 2014;58:40–49. doi: 10.1093/cid/cit642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wise M, Bialek S, Finelli L, Bell BP, Sorvillo F. Changing trends in hepatitis C-related mortality in the United States, 1995–2004. Hepatology. 2008;47:1128–1135. doi: 10.1002/hep.22165. [DOI] [PubMed] [Google Scholar]
- 76.Moyer VA. Screening for hepatitis C virus infection in adults: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:349–357. doi: 10.7326/0003-4819-159-5-201309030-00672. [DOI] [PubMed] [Google Scholar]
- 77.Jemal A, Fedewa SA. Prevalence of hepatitis C virus testing in cohorts born between 1945 and 1965 in the US [letter] Am J Prev Med. 2015;48:e7–e9. doi: 10.1016/j.amepre.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 78.McQuillan GM, Kruszon-Moran D, Kottiri BJ, Curtin LR, Lucas JW, Kington RS. Racial and ethnic differences in the seroprevalence of 6 infectious diseases in the United States: data from NHANES III, 1988–1994. Am J Public Health. 2004;94:1952–1958. doi: 10.2105/ajph.94.11.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Centers for Disease Control and Prevention (CDC) Screening for chronic hepatitis B among Asian/Pacific Islander populations—New York City, 2005. MMWR Morb Mortal Wkly Rep. 2006;55:505–509. [PubMed] [Google Scholar]
- 80.World Health Organization. Immunization Coverage Fact Sheet. Geneva, Switzerland: World Health Organization; 2015. Available at: http://www.who.int/mediacentre/factsheets/fs378/en/. Accessed September 3, 2015. [Google Scholar]
- 81.World Health Organization. 2015 Global Summary. Geneva, Switzerland: World Health Organization; 2015. WHO Vaccine-Preventable Diseases: Monitoring System. Available at: http://apps.who.int/immunization_monitoring/globalsummary/. Accessed September 3, 2015. [Google Scholar]
- 82.World Health Organization. Global Status Report on Noncommunicable Diseases 2010. Geneva, Switzerland: World Health Organization; 2011. Available at: http://www.who.int/nmh/publications/ncd_report_full_en.pdf. Accessed October 20, 2015. [Google Scholar]
- 83.Liang X, Bi S, Yang W, et al. Reprint of: Epidemiological serosurvey of hepatitis B in China—declining HBV prevalence due to hepatitis B vaccination. Vaccine. 2013;31(suppl 9):J21–J28. doi: 10.1016/j.vaccine.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 84.Gordon SC, Lamerato LE, Rupp LB, et al. Antiviral therapy for chronic hepatitis B virus infection and development of hepatocellular carcinoma in a US population. Clin Gastroenterol Hepatol. 2014;12:885–893. doi: 10.1016/j.cgh.2013.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lai CL, Yuen MF. Prevention of hepatitis B virus-related hepatocellular carcinoma with antiviral therapy. Hepatology. 2013;57:399–408. doi: 10.1002/hep.25937. [DOI] [PubMed] [Google Scholar]
- 86.LeFevre ML. Screening for hepatitis B virus infection in nonpregnant adolescents and adults: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:58–66. doi: 10.7326/M14-1018. [DOI] [PubMed] [Google Scholar]
- 87.McGlynn KA, London WT. The global epidemiology of hepatocellular carcinoma: present and future. Clin Liver Dis. 2011;15:223–243. doi: 10.1016/j.cld.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chhatwal J, Kanwal F, Roberts MS, Dunn MA. Cost-effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States. Ann Intern Med. 2015;162:397–406. doi: 10.7326/M14-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Drenth JP. HCV treatment—no more room for interferonologists? N Engl J Med. 2013;368:1931–1932. doi: 10.1056/NEJMe1303818. [DOI] [PubMed] [Google Scholar]
- 90.American Association for the Study of Liver Diseases and the Infectious Diseases Society of America. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. 2015 doi: 10.1002/hep.31060. Available at: http://www.hcvguidelines.org. Accessed November 2, 2015. [DOI] [PMC free article] [PubMed]
- 91.Afdhal N, Reddy KR, Nelson DR, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370:1483–1493. doi: 10.1056/NEJMoa1316366. [DOI] [PubMed] [Google Scholar]
- 92.Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878–1887. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 93.Denniston MM, Klevens RM, McQuillan GM, Jiles RB. Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National Health and Nutrition Examination Survey 2001–2008. Hepatology. 2012;55:1652–1661. doi: 10.1002/hep.25556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rein DB, Wittenborn JS, Smith BD, Liffmann DK, Ward JW. The cost-effectiveness, health benefits, and financial costs of new antiviral treatments for hepatitis C virus. Clin Infect Dis. 2015;61:157–168. doi: 10.1093/cid/civ220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ward JW, Mermin JH. Simple, effective, but out of reach? Public health implications of HCV drugs. N Engl J Med. 2015;373:2678–2680. doi: 10.1056/NEJMe1513245. [DOI] [PubMed] [Google Scholar]
- 96.Liebler CA, Halpern-Manners A. A practical approach to using multiple-race response data: a bridging method for public-use microdata. Demography. 2008;45:143–155. doi: 10.1353/dem.2008.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Henry KA, Sherman RL, McDonald K, et al. Associations of census-tract poverty with subsite-specific colorectal cancer incidence rates and stage of disease at diagnosis in the United States [serial online] J Cancer Epidemiol. 2014;2014:823484. doi: 10.1155/2014/823484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pinheiro PS, Sherman RL, Trapido EJ, et al. Cancer incidence in first generation US Hispanics: Cubans, Mexicans, Puerto Ricans, and new Latinos. Cancer Epidemiol Biomarkers Prev. 2009;18:2162–2169. doi: 10.1158/1055-9965.EPI-09-0329. [DOI] [PubMed] [Google Scholar]
- 99.Pinheiro PS, Sherman RL. Why an alternative algorithm for identification of Hispanic subgroups is useful. J Registry Manag. 2009;36:3–4. [PubMed] [Google Scholar]
- 100.Arias E, Schauman WS, Eschbach K, Sorlie PD, Backlund E. The validity of race and Hispanic origin reporting on death certificates in the United States. Vital Health Stat 2. 2008;148:1–23. [PubMed] [Google Scholar]
- 101.Hahn RA, Wetterhall SF, Gay GA, et al. The recording of demographic information on death certificates: a national survey of funeral directors. Public Health Rep. 2002;117:37–43. doi: 10.1016/S0033-3549(04)50106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Murray CJ, Rajaratnam JK, Marcus J, Laakso T, Lopez AD. What can we conclude from death registration? Improved methods for evaluating completeness [serial online] PLoS Med. 2010;7:e1000262. doi: 10.1371/journal.pmed.1000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Adih WK, Selik RM, Hu X. Trends in diseases reported on US death certificates that mentioned HIV infection, 1996–2006. J Int Assoc Physicians AIDS Care (Chic) 2011;10:5–11. doi: 10.1177/1545109710384505. [DOI] [PubMed] [Google Scholar]
- 104.Centers for Disease Control and Prevention (CDC) Hepatocellular carcinoma—United States, 2001–2006. MMWR Morb Mortal Wkly Rep. 2010;59:517–520. [PubMed] [Google Scholar]
- 105.Daniels D, Grytdal S, Wasley A. Surveillance for acute viral hepatitis—United States, 2007. MMWR Surveill Summ. 2009;58:1–27. [PubMed] [Google Scholar]
- 106.Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5 pt 1):329–337. doi: 10.7326/0003-4819-158-5-201303050-00005. [DOI] [PubMed] [Google Scholar]
- 107.van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584–2593. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 108.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 109.National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention; 2014. [Google Scholar]
- 110.Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc. 2008;67:253–256. doi: 10.1017/S002966510800712X. [DOI] [PubMed] [Google Scholar]
- 111.US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 112.US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716–726. doi: 10.7326/0003-4819-151-10-200911170-00008. [DOI] [PubMed] [Google Scholar]
- 113.US Preventive Services Task Force. Recommendations for Primary Care Practice. Rockville, MD: 2014. Available at: http://www.uspreventiveservicestaskforce.org/Page/Name/recommendations. Accessed September 28, 2015. [Google Scholar]
- 114.Henley SJ, King JB, German RR, Richardson LC, Plescia M. Surveillance of screening-detected cancers (colon and rectum, breast, and cervix)—United States, 2004–2006. MMWR Surveill Summ. 2010;59:1–25. [PubMed] [Google Scholar]
- 115.Centers for Disease Control and Prevention (CDC) A National Action Plan for Cancer Survivorship: Advancing Public Health Strategies. Atlanta, GA: CDC; 2004. Available at: http://www.cdc.gov/cancer/survivorship/pdf/plan.pdf. Accessed September 28, 2015. [Google Scholar]
- 116.Cancer survivors: living longer, and now, better. Lancet. 2004;364:2153–2154. doi: 10.1016/S0140-6736(04)17601-0. [DOI] [PubMed] [Google Scholar]
- 117.Bradley CJ, Bednarek HL, Neumark D. Breast cancer and women’s labor supply. Health Serv Res. 2002;37:1309–1328. doi: 10.1111/1475-6773.01041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sasser AC, Rousculp MD, Birnbaum HG, Oster EF, Lufkin E, Mallet D. Economic burden of osteoporosis, breast cancer, and cardiovascular disease among postmenopausal women in an employed population. Womens Health Issues. 2005;15:97–108. doi: 10.1016/j.whi.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 119.Underwood JM, Townsend JS, Stewart SL, et al. Surveillance of demographic characteristics and health behaviors among adult cancer survivors—Behavioral Risk Factor Surveillance System. United States, 2009 MMWR Surveill Summ. 2012;61:1–23. [PubMed] [Google Scholar]
- 120.Yabroff KR, Davis WW, Lamont EB, et al. Patient time costs associated with cancer care. J Natl Cancer Inst. 2007;99:14–23. doi: 10.1093/jnci/djk001. [DOI] [PubMed] [Google Scholar]
- 121.Yabroff KR, Lawrence WF, Clauser S, Davis WW, Brown ML. Burden of illness in cancer survivors: findings from a population-based national sample. J Natl Cancer Inst. 2004;96:1322–1330. doi: 10.1093/jnci/djh255. [DOI] [PubMed] [Google Scholar]
- 122.Ng AK, Travis LB. Subsequent malignant neoplasms in cancer survivors. Cancer J. 2008;14:429–434. doi: 10.1097/PPO.0b013e31818d8779. [DOI] [PubMed] [Google Scholar]
- 123.Sunga AY, Eberl MM, Oeffinger KC, Hudson MM, Mahoney MC. Care of cancer survivors. Am Fam Physician. 2005;71:699–706. [PubMed] [Google Scholar]
- 124.Buehler JW, Centers for Disease Control and Prevention CDC’s vision for public health surveillance in the 21st century. Introduction. MMWR Surveill Summ. 2012;61(suppl):1–2. [PubMed] [Google Scholar]
- 125.Nsubuga P, White E, Thacker SB, et al. Public health surveillance: a tool for targeting and monitoring interventions. Chapter 53. In: Jamison DT, Breman JG, Measham AR, et al., editors. World Bank Group Disease Control Priorities for Developing Countries. 2nd. Washington, DC: World Bank Publishers; 2006. pp. 997–1015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


