Abstract
We sought to determine use of any and at least moderate‐intensity statin therapy in a national sample of patients with diabetes mellitus (DM), with the hypothesis that nationwide frequency and facility‐level variation in statin therapy are suboptimal. We sampled patients with DM age 40 to 75 years receiving primary care between October 1, 2012, and September 30, 2013, at 130 parent facilities and associated community‐based outpatient clinics in the Veterans Affairs Health Care System. We examined frequency and facility‐level variation in use of any or at least moderate‐intensity statin therapy (mean daily dose associated with ≥30% low‐density lipoprotein cholesterol lowering). In 911 444 patients with DM, 68.3% and 58.4% were receiving any and moderate‐ to high‐intensity statin therapy, respectively. Patients receiving statin had higher burden of cardiovascular disease, were more likely to be on nonstatin lipid‐lowering therapy and to receive care at a teaching facility, and had more frequent primary‐care visits. Median facility‐level uses of any and at least moderate‐intensity statin therapy were 68.7% (interquartile range, 65.9%–70.8%) and 58.6% (interquartile range, 55.8%–61.4%), respectively. After adjusting for several patient‐related and some facility‐related characteristics, the median rate ratios for any and moderate‐ to high‐intensity statin therapy were 1.20 (95% confidence interval: 1.18‐1.22) and 1.29 (95% confidence interval: 1.24‐1.33) respectively, indicating 20% to 29% variation in statin use between 2 identical patients receiving care at 2 random facilities. Statin use was suboptimal in a national sample of patients with DM with modest facility‐level variation, likely indicating differences in statin‐prescribing patterns.
Introduction
In a meta‐analysis of 18 686 individuals with diabetes mellitus (DM) from 14 randomized trials of statin therapy, moderate‐intensity statin therapy compared with placebo has been shown to reduce the relative risk for cardiovascular disease (CVD) by approximately 27% per 1 mmol/L (38.7 mg/dL) reduction in low‐density lipoprotein cholesterol (LDL‐C).1 Furthermore, there was a 9% and 13% relative reduction in all‐cause and vascular mortality, respectively, per mmol/L reduction in LDL‐C with statin therapy.1 The American Diabetes Association (ADA) recommends moderate‐ to high‐intensity statin therapy in DM patients age 40 to 75 years with or without presence of cardiovascular risk factors and/or disease.2 In addition, the 2013 American College of Cardiology/American Heart Association (ACC/AHA) guideline on the treatment of blood cholesterol also identified DM patients age 40 to 75 years (with or without CVD) to benefit from moderate‐ to high‐intensity statin therapy.3 However, frequency and practice pattern of statin use in a national sample of DM patients is not known. The purpose of this study is to examine the frequency and facility‐level variation in statin use in DM patients age 40 to 75 years receiving care in primary‐care facilities in the entire Veterans Affairs (VA) Health Care System.
Methods
Patient Population
We classified patients as having DM if any of the following were documented: 2 outpatient or 1 inpatient diagnosis code indicating DM, from International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM; 250.xx, 357.2, 366.41; see Supporting Information, Table, in the online version of this article); filled prescription for DM medication; or any fasting glucose ≥126 mg/dL, hemoglobin A1C > 6.5%, or ≥2 outpatient blood glucose readings >200 mg/dL on 2 different days.4, 5, 6 We identified 1 239 260 patients with DM with a primary‐care visits in 130 VA parent facilities and their associated community‐based outpatient clinics between October 1, 2012, and September 30, 2013. After excluding patients with history of metastatic cancer in the 5 years prior or history of hospice care in the preceding year (n = 21 184), we had a total of 1 218 076 patients with DM. Among patients with DM, major US guidelines recommend that those age 40 to 75 years receive moderate‐ to high‐intensity statin therapy,2, 3 and therefore we further excluded individuals age <40 or >75 years (n = 306 632), resulting in 911 444 as the total number of participants included in our final analyses.
Covariates
We defined CVD as the presence of acute coronary syndrome, stable or unstable angina, coronary revascularization (percutaneous coronary intervention or coronary artery bypass grafting), ischemic stroke, transient ischemic attacks, peripheral artery disease, or other arterial revascularization. We used ICD‐9‐CM diagnosis and procedure codes, as well as current procedural terminology codes, to identify CVD and other variables, as described in previous studies (see Supporting Information, Table, in the online version of this article).7, 8
We assessed several pertinent patient characteristics that could be related to cardiovascular risk, such as age, race, sex, and history of hypertension and CVD, using the VA administrative database. Race was self reported by patients. We also assessed facility‐level variables, such as receiving care in a teaching vs nonteaching facility and the number of primary‐care visits in the preceding 1 year. We calculated mean Diagnostic Cost Group (DCG) relative risk score (RRS) for each patient to assess the impact of a patient's illness burden on statin adherence. The DCG RSS is a ratio of predicted to the mean cost and has been used as a surrogate of illness burden in prior studies.7, 8 For example, a patient with a score of 1.9 is expected to be 90% more costly than an “average” patient, reflecting 90% incremental illness burden.
We identified the statin and its dose and any nonstatin lipid‐lowering medications using VA administrative pharmacy data sources for each patient. Statin and moderate‐ to high‐intensity statin use was defined as their use within 100 days prior to or 14 days following the most recent primary‐care visit. We studied atorvastatin, rosuvastatin, pravastatin, fluvastatin, lovastatin, simvastatin, and pitavastatin. We defined statin intensity based on the most recent statin fill. Low‐, moderate‐, and high‐intensity statin therapy corrosponded to a daily statin dose that reduces LDL‐C by <30%, 30% to <50%, and ≥50%, respectively, as defined in the 2013 ACC/AHA cholesterol guideline.3 For the purpose of this study, moderate‐ to high‐intensity statin use (or at least moderate‐intensity statin use) was defined as a mean daily dose of atorvastatin ≥10 mg, rosuvastatin ≥5 mg, simvastatin ≥20 mg, pravastatin ≥40 mg, lovastatin ≥40 mg, fluvastatin ≥80 mg, or pitavastatin ≥2 mg. These statin doses would be expected to lead to ≥30% LDL‐C reduction based on the 2013 ACC/AHA cholesterol guideline.3
We compared the levels of various lipid parameters among patients receiving statin therapy and those not receiving statin therapy, and separately among patients receiving at least moderate‐intensity statin therapy and those receiving low‐intensity statin therapy or not receiving any statin therapy. We subtracted high‐density lipoprotein cholesterol (HDL‐C) from total cholesterol to calculate non–HDL‐C. We also evaluated use of nonstatin LDL‐C–lowering medications. These included bile acid binding sequestrants, ezetimibe, and niacin. We excluded fibrates because of their minimal LDL‐C–lowering effects.
Statistical Analysis
Our outcome of interest was to examine use of any statin therapy and at least moderate‐intensity statin therapy in patients with DM and to examine facility‐level variation in the use of any statin and moderate‐ to high‐intensity statin therapy. We compared patient‐, provider‐, and facility‐related characteristics between patients who were on statin therapy and those who were not. We examined categorical variables using the χ2 test and continuous variables with the t test.
To explore facility‐level variation in statin use, we evaluated the median facility‐level use of any statin therapy and of moderate‐ to high‐intensity statin therapy. We then constructed multivariable hierarchical regression models to determine median rate ratio (MRR) to assess the magnitude of facility‐level variation in any statin therapy and moderate‐ to high‐intensity statin therapy. These are 2‐level hierarchical models adjusted for clustering of patients within facilities and modeled individual facility as a random effect and patient characteristics as filter effects within each facility.9 This approach allowed us to control for confounding between facilities to ensure that patients with similar baseline characteristics from different facilities were compared with each other. The MRR can be interpreted as the likelihood that 2 random facilities would differ in treatment of “identical” patients. For example, an MRR of 1 suggests no facility‐level variation, whereas an MRR of 1.50 suggests 50% probability of differing treatment for “identical” DM patients receiving care at 2 random facilities. This methodology to study variation in care has been described before.10, 11 We calculated MRR initially from an unadjusted model, followed by a model adjusting for patient age, sex, race (white vs others), history of hypertension and CVD, DCG RSS, receipt of care at a teaching vs nonteaching facility, and the number of primary‐care visits in the prior 12 months.
We conducted analyses with SAS version 9.1.3 (SAS Institute, Inc., Cary, NC) and Stata version 11 (StataCorp, College Station, TX). The protocol was approved by the institutional review boards at Baylor College of Medicine and the Michael E. DeBakey VA Medical Center, and a waiver for the need of informed consent was approved. Investigations were in accordance with the Declaration of Helsinki.
Results
Baseline Data
In the study cohort of 1 239 260 patients with DM, the final study population was 911 444 patients age 40 to 75 years. Overall, most were men (95.9%); 67.8% were white and 20.2% were black. Race was self reported by patients. Hypertension was present in 84.0%; 37.3% had CVD; and 43.4% were receiving care at a teaching facility.
Compared with patients not receiving statin therapy, statin users were slightly older (Table 1) and more likely to be white and male (P < 0.0001 for all). Statin users, compared with nonusers, had higher prevalence of hypertension (88.0% vs 75.6%) and CVD (43.8% vs 23.3%), and higher illness burden as reflected by a higher DCG RSS (1.9 vs 1.6; P < 0.0001 for all). Statin users had more frequent primary‐care visits in the 1 year prior to the index primary‐care visit and were more likely to receive care in a teaching facility compared with statin nonusers. Compared with statin nonusers, those receiving statins were also more likely to receive nonstatin lipid‐lowering therapy, mainly niacin therapy. Although most of these differences were statistically significant given the large sample size, absolute differences were numerically small for some variables. As expected, patients receiving statin therapy, compared with those not on a statin, had lower on‐treatment levels of LDL‐C (86.9 vs 95.7 mg/dL, respectively) and non–HDL‐C (119.0 vs 127.9 mg/dL, respectively; P < 0.0001 for both). Levels of lipid parameters in moderate‐ to high‐intensity statin users compared with statin nonusers/low‐intensity statin users showed a similar pattern (Table 2).
Table 1.
Baseline Characteristics of the Study Population by Statin Use
| Study Variables | Using Statin, n = 622 503 (68.3%) | Not Using Statin, n = 288 941 (31.7%) | P Value |
|---|---|---|---|
| Age, y | 63.5 (7.0) | 62.4 (7.9) | <0.0001 |
| White race, % | 69.3 | 64.5 | <0.0001 |
| Male sex, % | 96.2 | 95.2 | <0.0001 |
| History of HTN, % | 88.0 | 75.6 | <0.0001 |
| History of CVD, % | 43.8 | 23.3 | <0.0001 |
| DCG RRS | 1.9 (2.9) | 1.6 (2.8) | <0.0001 |
| No. of primary‐care visits in 12 months prior to the index primary‐care visit | 4.6 (5.1) | 3.3 (4.4) | <0.0001 |
| Receiving care in a teaching facility, % | 44.2 | 41.6 | <0.0001 |
| Use of any nonstatin, % | 9.7 | 6.1 | <0.0001 |
| Bile acid sequestrants | 1.0 | 1.6 | <0.0001 |
| Ezetimibe | 1.1 | 0.9 | <0.0001 |
| Niacin | 8.0 | 4.1 | <0.0001 |
| LDL‐C, mg/dL | 86.9 (32.7) | 95.7 (33.3) | <0.0001 |
| HDL‐C, mg/dL | 41.0 (11.3) | 41.9 (12.9) | <0.0001 |
| TG, mg/dL | 146.0 (101.0, 213.0) | 141.0 (96.0, 211.0) | <0.0001 |
| TC, mg/dL | 159.3 (40.7) | 169.3 (40.7) | <0.0001 |
| Non–HDL‐C, mg/dLa | 119.0 (39.0) | 127.9 (39.6) | <0.0001 |
Abbreviations: CVD, cardiovascular disease; DCG RRS, Diagnostic Cost Group relative risk score; HDL‐C, high‐density lipoprotein cholesterol; HTN, hypertension; LDL‐C, low‐density lipoprotein cholesterol; non–HDL‐C, non–high‐density lipoprotein cholesterol; SD, standard deviation; TC, total cholesterol; TG triglycerides.
Data for continuous variables are expressed as mean (SD), except for TG, which is expressed as median (25th, 75th percentiles).
Non–HDL‐C = TC − HDL‐C.
Table 2.
Lipid Levels by Intensity of Statin Treatmenta
| Lipid Variables, mg/dL | Moderate‐ to High‐Intensity Statin Use, n = 532 467 (58.4%) | Not Using Statin/Using Low‐Intensity Statin, n = 378 977 (41.6%) | P Value |
|---|---|---|---|
| LDL‐C | 86.5 (32.8) | 94.2 (33.0) | <0.0001 |
| HDL‐C | 40.9 (11.2) | 41.7 (12.7) | <0.0001 |
| TG | 147.0 (101.0, 214.0) | 142.0 (96.0, 211.0) | <0.0001 |
| TC | 158.8 (40.9) | 167.5 (40.5) | <0.0001 |
| Non–HDL‐Cb | 118.5 (39.2) | 126.4 (39.2) | <0.0001 |
Abbreviations: HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; non–HDL‐C, non–high‐density lipoprotein cholesterol; SD, standard deviation; TC, total cholesterol; TG triglycerides.
Data for continuous variables are expressed as mean (SD), except for TG, which is expressed as median (25th, 75th percentiles).
Low‐, moderate‐, and high‐intensity statins correspond to a daily statin dose that reduces LDL‐C by <30%, 30% to <50%, and ≥50%, respectively.
Non–HDL‐C = TC − HDL‐C.
Statin Therapy and Facility‐Level Variation
Approximately 68.3% (n = 622 503) of our study patients were on statin therapy, and 58.4% (n = 532 467) were on at least moderate‐intensity statin therapy (about 85.5% of those receiving any‐intensity statin). Among patients with DM and established CVD, statin therapy was used in 80.2% (moderate to high intensity in 71.3%). Among those with DM but without concomitant CVD, statin therapy was used in 61.2% (moderate to high intensity in 50.8%). The mean (SD) daily doses of individual statin among those receiving statins were as follows: atorvastatin, 40.1 (27.4) mg; fluvastatin, 51.8 (26.0) mg; lovastatin, 38.1 (22.1) mg; pitavastatin, 2.1 (1.2) mg; pravastatin, 40.9 (24.0) mg; rosuvastatin, 21.1 (12.7) mg; and simvastatin, 32.3 (20.5) mg.
The median facility‐level uses of any and moderate‐ to high‐intensity statin therapy were 68.7% (interquartile range [IQR], 65.9%–70.8%; range, 59.9%–82.8%) and 58.6% (IQR, 55.8%–61.4%; range, 47.3%–77.1%), respectively (figures 1 and 2). The unadjusted MRRs for any and moderate‐ to high‐intensity statin therapy were 1.16 (95% confidence interval [CI]: 1.13‐1.17) and 1.28 (95% CI: 1.23‐1.31), respectively. After adjusting for age, sex, race, history of hypertension and CVD, DCG RSS, receipt of care at a teaching vs nonteaching facility, and number of primary‐care visits in the preceding 1 year, there was no attenuation in the MRRs (the MRRs [95% CI] for any and moderate‐ to high‐intensity statin therapy were 1.20 [1.18‐1.22] and 1.29 [1.24‐1.33], respectively).
Figure 1.

Percentage of patients age 40 to 75 years with DM receiving any statin therapy at each of the 130 VA facilities. Abbreviations: DM, diabetes mellitus; VA, Veterans Affairs Health Care System.
Figure 2.
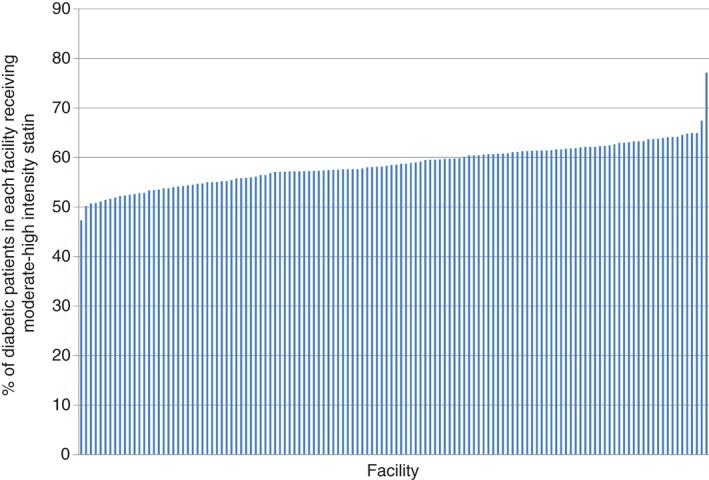
Percentage of patients age 40 to 75 years with DM receiving moderate‐ to high‐intensity statin therapy at each of the 130 VA facilities. Abbreviations: DM, diabetes mellitus; VA, Veterans Affairs Health Care System.
Discussion
In this nationwide database of 911 444 DM patients from 130 VA primary‐care facilities, about one‐third of patients were not on any statin therapy and about 42% were not on moderate‐ to high‐intensity statin therapy, which is recommended by the most recent guidelines.2, 3 Furthermore, about 39% of DM patients without established CVD and about 20% of DM patients with CVD were not on any statin therapy. There was 20% facility‐level variation in any statin therapy between 2 identical patients receiving care at 2 random facilities and 29% variation for moderate‐ to high‐intensity statin use.
Statin use in patients with DM has been shown to reduce both vascular and all‐cause mortality.1 It has been estimated that after 5 years, 42 fewer DM patients would have major vascular events per 1000 patients allocated to statin therapy.1 The benefit was similar irrespective of whether there was a history of vascular disease or other baseline characteristics.1 The most recent guidelines by the ADA and the ACC/AHA recommend moderate‐ to high‐intensity statin therapy in DM patients age 40 to 75 years with or without CVD.2, 3 Similarly, other major guidelines also recommend statin therapy in patients with DM.12, 13, 14 On the other hand, it has been shown that not using statin therapy in eligible patients, for example from statin nonadherence, could be associated with increased risk of hospitalization for CVD and all‐cause mortality.15 Despite this evidence, in the current study we found that only 58% of eligible patients were on moderate‐ to high‐intensity statin therapy, and about 32% were not on any statin therapy. Accordingly, it is estimated that if the 288 941 patients who were not receiving statin therapy in our study start taking a statin, then after 5 years, there will be on average 12 135 fewer cardiovascular events in these patients.1
There could be several reasons for less‐than‐optimal statin use in our study. Historically, it has been shown that translation of evidence‐based science into routine clinical practice can be suboptimal,16 which may explain our observations. We did not have data on the reasons for not prescribing a statin, such as intolerance to statin therapy. Statin‐related musculoskeletal side effects could be partly responsible for suboptimal statin therapy. Although this is possible, we found that nonstatin lipid‐lowering therapy was higher in statin users compared with statin nonusers, suggesting that statin intolerance cannot completely account for the suboptimal statin therapy, as it is expected that nonstatin lipid‐lowering medication therapy would have been higher in statin nonusers if statin intolerance was a major driver for lower statin therapy. In addition, higher use of nonstatin lipid‐lowering therapy in statin users also suggests that statin‐treated patients are generally more aggressively treated.
Although moderate‐ to high‐intensity statin therapy is recommended in patients with DM per most recent guidelines,2, 3 use of low‐intensity statin therapy could be related to intolerance to high‐intensity statin therapy. However, using the VA database, we have previously shown that there is minimal reduction in statin adherence (of unknown clinical significance) when using high‐intensity compared with low‐ to moderate‐intensity statin therapy.7 Most recent cholesterol guidelines recommend a “treat to risk” approach with a fixed dose using moderate‐ to high‐intensity statin therapy in patients with DM and recommend that there is not enough evidence to use nonstatin lipid‐lowering therapy in most patients.3 Following contemporary lipid guidelines during our study period when the focus was “treat to LDL‐C target” (goal LDL‐C <100 mg/dL in high‐risk patients and <70 mg/dL in very high‐risk patients, such as those with CVD),17, 18, 19, 20 providers may have elected not to use statin therapy in those who were already at their LDL‐C goal, or to use nonstatin lipid‐lowering therapy only. In fact, the mean LDL‐C level in patients not on statin therapy was 96 mg/dL in our study, which could be one of the reasons for the lower level of statin therapy.
To our knowledge, only a few studies have evaluated statin use and prescription specifically in patients with DM. In a study of 75 046 DM patients without CVD from the outpatient cardiology practices participating in the Practice Innovation and Clinical Excellence (PINNACLE) registry between January 1, 2008, and December 31, 2012, approximately 56% of patients were not prescribed a statin, which was defined as physician documentation of statin prescription.21 In this study, intensity of statin treatment was not examined. In the current analysis, we found that 39% of 571 506 DM patients without CVD were not on any statin, and about 49% of them were not on moderate‐ to high‐intensity statin therapy. The relatively higher use of statins in the VA health care system, as shown in the current analysis, could reflect a temporal trend with increasing use of statin therapy, as our data represent a more contemporary cohort of DM patients. Unlike the PINNACLE study, where statin use was based on physician documentation of statin prescription,21 the current study is strengthened by the presence of statin fill data from VA administrative pharmacy data sources. We found modest facility‐level variation in statin use, despite the fact that our data were from only 1 health care system. The median facility‐level statin use ranged from 60% to 83% for any statin and 47% to 77% for moderate‐ to high‐intensity statin. Our results showing 20% and 29% facility‐level variation in statin therapy, despite adjusting for several patient‐related and some facility‐level variables, suggest that this random variation is likely due to differences in statin‐prescribing patterns at these facilities by individual practicing providers, rather than patient factors.
Study Limitations
Our study has limitations. Although most VA patients are expected to receive their statins from the VA system where they usually receive their primary‐care service, it is possible that they could have received statins outside the VA system, which we were not able to account for. Although this is possible, we believe its likelihood is low, as all patients included in our study were receiving primary care within the VA health care system. Because our study involved only patients treated in the VA health care system, with a predominantly male population, our findings may not be directly generalizable to other health systems. On the other hand, the strengths include a large patient population from a national sample of DM patients with good ascertainment of several patient‐level characteristics.
Conclusion
This study from the VA health care system shows that statin therapy was not used in 32% (moderate‐ to high‐intensity statin in 42%) of DM patients age 40 to 75 years, and there was modest facility‐level variation, likely indicating differences in statin‐prescribing patterns at these facilities. Achieving concordance with the prevailing guidelines, which recommend using moderate‐ to high‐intensity statin therapy in eligible patients with DM, and minimizing variations in care can potentially lower future cardiovascular events in this high‐risk patient population.
Supporting information
TableS1. Online‐only Supplemental Table. Diagnostic codes
This study was supported by the American Diabetes Association Clinical Science and Epidemiology Award (1‐14‐CE‐44, Dr. Virani), the American Heart Association Beginning Grant‐in‐Aid (14 BGIA20460366, Dr. Virani), the 2015–16 American Medical Association Foundation Seed Grant Award (Dr. Pokharel), and by the Michael E. DeBakey Health Services Research & Development Center for Innovations grant (grant HFP 90‐020). Dr. Pokharel is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number T32HL110837. Dr. Virani is also supported by a Baylor College of Medicine Center for Globalization Grant. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Dr. Nambi serves on the regional advisory board for Sanofi Regeneron, receives grant/research support from Merck (site principal investigator for a study sponsored by them), and has a provisional patent (no. 61721475) titled “Biomarkers to Improve Prediction of Heart Failure Risk” filed by Baylor College of Medicine, Roche. Dr. Virani serves on the steering committee (no financial remuneration) for the Patient and Provider Assessment of Lipid Management (PALM) Registry at the Duke Clinical Research Institute. Dr. Ballantyne receives grant/research support (all paid to the institution, not the individual) from Abbott Diagnostic, Amarin, Amgen, Eli Lilly, Esperion, Novartis, Pfizer, Otsuka, Regeneron, Roche Diagnostic, Sanofi‐Synthelabo, Takeda, the National Institutes of Health, American Heart Association, and the American Diabetes Association; is a consultant for Abbott Diagnostics, Amarin, Amgen, AstraZeneca, Eli Lilly, Esperion, Genzyme, Matinas BioPharma Inc., Merck, Novartis, Pfizer, Regeneron, Roche, and Sanofi‐Synthelabo; and has a provisional patent (no. 61721475) titled “Biomarkers to Improve Prediction of Heart Failure Risk” filed by Baylor College of Medicine, Roche.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Kearney PM, Blackwell L, Collins R, et al; Cholesterol Treatment Trialists' Collaborators . Efficacy of cholesterol‐lowering therapy in 18 686 people with diabetes in 14 randomised trials of statins: a meta‐analysis. Lancet. 2008;371:117–125. [DOI] [PubMed] [Google Scholar]
- 2. American Diabetes Association . Cardiovascular disease and risk management. Diabetes Care. 2015;38(suppl 1):S49–S57. [DOI] [PubMed] [Google Scholar]
- 3. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in Circulation. 2014;129(25 suppl 2):S46–S48]. Circulation. 2014;129(25 suppl 2):S1–S45. [DOI] [PubMed] [Google Scholar]
- 4. Woodard LD, Landrum CR, Urech TH, et al. Treating chronically ill people with diabetes mellitus with limited life expectancy: implications for performance measurement. J Am Geriatr Soc. 2012;60:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Woodard LD, Landrum CR, Urech TH, et al. Impact of clinical complexity on the quality of diabetes care. Am J Manag Care. 2012;18:508–514. [PMC free article] [PubMed] [Google Scholar]
- 6. Beard AJ, Hofer TP, Downs JR, et al; Diabetes Clinical Action Measures Workgroup . Assessing appropriateness of lipid management among patients with diabetes mellitus: moving from target to treatment. Circ Cardiovasc Qual Outcomes. 2013;6:66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Virani SS, Woodard LD, Akeroyd JM, et al. Is high‐intensity statin therapy associated with lower statin adherence compared with low‐ to moderate‐intensity statin therapy? Implications of the 2013 American College of Cardiology/American Heart Association Cholesterol Management Guidelines. Clin Cardiol. 2014;37:653–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Virani SS, Woodard LD, Ramsey DJ, et al. Gender disparities in evidence‐based statin therapy in patients with cardiovascular disease. Am J Cardiol. 2015;115:21–26. [DOI] [PubMed] [Google Scholar]
- 9. Goldstein H. Multilevel Statistical Models 4th ed. Chichester, UK: John Wiley & Sons, Ltd; 2010. [Google Scholar]
- 10. Hira RS, Kennedy K, Nambi V, et al. Frequency and practice‐level variation in inappropriate aspirin use for the primary prevention of cardiovascular disease: insights from the National Cardiovascular Disease Registry's Practice Innovation and Clinical Excellence registry. J Am Coll Cardiol. 2015;65:111–121. [DOI] [PubMed] [Google Scholar]
- 11. Hira RS, Kennedy K, Jneid H, et al. Frequency and practice‐level variation in inappropriate and nonrecommended prasugrel prescribing: insights from the NCDR PINNACLE registry. J Am Coll Cardiol. 2014;63(25 part A):2876–2877. [DOI] [PubMed] [Google Scholar]
- 12. Jacobson TA, Ito MK, Maki KC, et al. National Lipid Association recommendations for patient‐centered management of dyslipidemia: part 1—executive summary. J Clin Lipidol. 2014;8:473–488. [DOI] [PubMed] [Google Scholar]
- 13. Reiner Z, Catapano AL, De Backer G, et al; European Association for Cardiovascular Prevention and Rehabilitation . ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. 2011;32:1769–1818. [DOI] [PubMed] [Google Scholar]
- 14. Anderson TJ, Grégoire J, Hegele RA, et al. 2012 update of the Canadian Cardiovascular Society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2013;29:151–167. [DOI] [PubMed] [Google Scholar]
- 15. Shin S, Jang S, Lee TJ, et al. Association between non‐adherence to statin and hospitalization for cardiovascular disease and all‐cause mortality in a national cohort. Int J Clin Pharmacol Ther. 2014;52:948–956. [DOI] [PubMed] [Google Scholar]
- 16. Institute of Medicine . Crossing the Quality Chasm: A New Health System for the 21st Century Washington, DC: National Academies Press; 2001. [PubMed] [Google Scholar]
- 17. American Diabetes Association . Executive summary: standards of medical care in diabetes—2012. Diabetes Care. 2012;35(suppl 1):S4–S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. American Diabetes Association . Standards of medical care in diabetes—2013. Diabetes Care. 2013;(36 suppl 1):S11–S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2497. [DOI] [PubMed]
- 20. Grundy SM, Cleeman JI, Merz CN, et al; National Heart, Lung, and Blood Institute, AmericanCollege of Cardiology Foundation, American Heart Association . Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines [published correction appears in Circulation. 2004;110:763]. Circulation. 2004;110:227–239. [DOI] [PubMed] [Google Scholar]
- 21. Maddox TM, Borden WB, Tang F, et al. Implications of the 2013 ACC/AHA cholesterol guidelines for adults in contemporary cardiovascular practice: insights from the NCDR PINNACLE registry. J Am Coll Cardiol. 2014;64:2183–2192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TableS1. Online‐only Supplemental Table. Diagnostic codes


