Brief Introduction
We report a case of variceal hemorrhage in a patient with hepatitis C (HCV) cirrhosis that occurred despite HCV-undetectability for 3.5 years and normalization of liver synthetic function (albumin 2.8 to 3.8 mg/dL).
Presentation of Case
A 64-year old Caucasian male was initially diagnosed with HCV (genotype 3a) when he presented with mild aminotransferase elevations without any evidence of cirrhosis (albumin 4.7 g/dL, platelets 313 ×103/μL) or other etiological factors for chronic liver disease. Ten years later, he was found to have medium-sized esophageal varices and imaging studies compatible with cirrhosis. He had no ascites or encephalopathy with albumin 3.2 g/dL, INR 1.2, bilirubin 0.47 mg/dL, platelets 153 ×103/μL. The patient was started on propranolol and underwent treatment for HCV with pegylated interferon (PEG) and ribavirin (RBV) for 48 weeks with undetectable HCV-RNA at the end of therapy but with relapse after treatment discontinuation. Three months later he was started on a 72-week course of PEG/RBV. Prior to initiating therapy he was still compensated, though albumin was 2.8 g/dL. He achieved SVR (sustained virologic response with HCV-RNA negative 24 weeks after treatment completion). Twelve months after SVR, liver synthetic function was normal with albumin 3.9 g/dL, bilirubin 0.83 mg/dL and INR 1.0.
HCV-had been undetectable for 3.5 years when he presented with massive variceal hemorrhage. Labs revealed albumin 3.8 g/dL, INR 1.1, AST 27 U/L, and a right upper quadrant Doppler was negative for portal vein thrombosis or hepatocellular carcinoma. Liver stiffness by transient elastography was 27 kPa. The patient reported adherence to propranolol with admission heart rate 61. The patient had a body mass index of 26, no evidence of metabolic syndrome, and no history of at risk alcohol use. Variceal hemorrhage responded well to standard therapy and the patient was placed on propranolol plus serial variceal ligation.
Discussion
This case demonstrates that HCV eradication can lead to amelioration of intrahepatic mechanisms leading to liver insufficiency, but in the presence of significant portal hypertension, complications such as variceal hemorrhage may still develop. These findings are aligned with a recent study showing that the main predictor of decompensation in 19 patients with cirrhosis who developed decompensation after SVR was a hepatic venous pressure gradient (HVPG) ≥ 10 mmHg (pressure that defines “clinically significant portal hypertension or CSPH”). Patients with varices have, by definition, CSPH. In a study correlating the presence of CSPH with histological findings in liver biopsies of patients with cirrhosis, thick fibrous septa were found to be the strongest correlate to the presence of CSPH. Thick fibrous septa likely represent septa that have been laid down early in the injury and are the most “mature” (i.e. cross-linked), and even with elimination of the etiologic agent, these thick septa are likely to persist. In this case, fibrosis persisted (very stiff liver) and so did portal hypertension despite normalization of liver function, indicating that these are two separate mechanisms of disease. Normal liver function allowed the patient to recover seamlessly from a massive hemorrhage. It is currently recommended that patients with HCV cirrhosis that achieve SVR should continue monitoring/screening for varices and HCC. This seems to clearly be the case for patients with CSPH, that is, patients with an HVPG ≥10 mmHg, gastroesophageal varices on endoscopy, or collaterals on imaging. With the advent of direct-acting antivirals, experience with larger numbers of patients with HCV cirrhosis achieving SVR will further delineate whether subpopulations can be identified that would not require this follow-up.
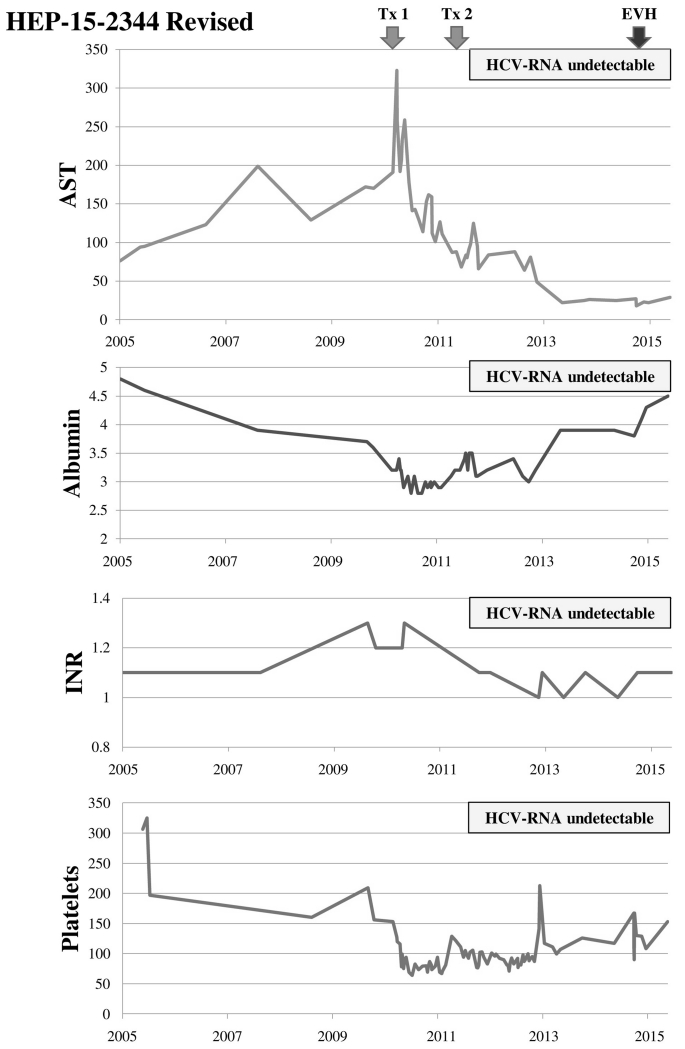
Figure 1.
Timeline of laboratory tests and sequence of events. Albumin (g/dL); AST (U/L); Platelet count (×103/μL); Tx1 = initiation of first course of PEG/RBV (48 weeks); Tx2 = initiation of second course of PEG/RBV (72 weeks); EVH = esophageal variceal hemorrhage. After attaining sustained virological response, liver enzymes normalized as did liver synthetic function (serum albumin, INR). Platelet count decreased with each antiviral therapy but also had a tendency to improve (perhaps also as a reflection of improved liver synthetic function).
Acknowledgments
Grants and Other Financial Support: Grant support: NIH P-30DK 034989
References
- 1).Lens S, Rincon D, Garcia-Retortillo M, et al. Association between severe portal hypertension and risk of liver decompensation in patients with hepatitis C, regardless of response to antiviral therapy. Clin Gastroenterol Hepatol. 2015;13:1846–53. doi: 10.1016/j.cgh.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 2).Nagula S, Jain D, Groszmann RJ, Garcia-Tsao G. Histological-hemodynamic correlation in cirrhosis-a histological classification of the severity of cirrhosis. J Hepatol. 2006;44:111–7. doi: 10.1016/j.jhep.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 3).Garcia-Tsao G, Friedman S, Iredale J, Pinzani M. Now There Are Many (Stages) Where Before There Was One: In Search of a Pathophysiological Classification of Cirrhosis. Hepatology. 2010;51:1445–49. doi: 10.1002/hep.23478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Monitoring Patients Who Are Starting Hepatitis C Treatment, are on Treatment, or have Completed Therapy. AASLD and IDSA guidance recommendations. http://www.hcvguidelines.org.