Abstract
Objectives
Primary carcinoma of the Bartholin's gland is a rare malignancy that accounts for approximately 5% of vulvar carcinomas. The objective of this study was to compare the outcomes of women with primary Bartholin's gland carcinoma to those with non-Bartholin's gland related vulvar carcinoma.
Methods/Materials
A retrospective chart review of 429 patients with invasive vulvar carcinoma evaluated at a single institution between 1993 and 2011 was performed. Medical records were reviewed for demographic data, pathologic information, treatment type, and recurrence/outcome information. These variables were compared between patients with primary Bartholin's gland carcinoma and patients with non-Bartholin's gland related vulvar carcinoma.
Results
Thirty-three (7.7%) of the 429 patients with invasive vulvar carcinoma had primary carcinoma of the Bartholin's gland. Twenty-nine patients (87.9%) had squamous cell histology and four (12.1%) had adenocarcinoma. When compared with non-Bartholin's gland related vulvar carcinoma, patients with primary Bartholin's gland carcinoma had a younger age at diagnosis (median 57 vs. 63 years, p=0.045), higher rate of stage III/IV disease (60.6% vs. 35.8%, p=0.008), and were more likely to receive radiation therapy (78.8% vs. 43.9%, p<0.001). However, there were no significant differences between the two groups with regards to histologic subtype, lymphovascular space involvement, perineural invasion, positive margins, recurrence-free survival, or overall survival.
Conclusions
Despite being diagnosed at a more advanced stage, patients with primary carcinoma of the Bartholin's gland appear to have similar oncologic outcomes and survival rates to patients with non-Bartholin's gland related vulvar carcinoma.
Introduction
Vulvar carcinoma is the fourth most common gynecologic cancer in the United States and comprises 5% of cancers of the female genital tract. There is an estimated 5,150 new vulvar cancer cases and 1,080 related deaths in 2015 [1]. Of the vulvar malignancies, primary Bartholin's gland carcinoma (BGC) is exceedingly rare and accounts for fewer than 5% of all vulvar carcinomas. Criteria for the diagnosis of BGC were originally described by Honan in 1897 and subsequently revised by Chamlian and Taylor to include: (1) the tumor involving the area of the Bartholin gland is histologically compatible with the origin from the Bartholin gland; (2) areas of apparent transition from normal elements to neoplastic ones are found in histologic study; and (3) there is no evidence of primary tumor elsewhere [2].
Presentation of primary BGC is usually late as lesions are deep within the vulva and often misdiagnosed as a Bartholin's gland abscess or cyst. BGC is usually a slow growing tumor with a marked propensity for perineural and local invasion. Approximately 50% of BGCs are of squamous histology and are thought to originate in the Bartholin duct, and the remaining 50% include adenocarcinoma and adenoid cystic carcinoma, which mimics behavior of salivary gland carcinoma of the same histology [3-5]. Given the limited data on primary BGC, we describe in this report a single institution experience of 33 patients with primary BGC, and compare their outcomes to patients with vulvar carcinoma not originating in the Bartholin's gland (non-BGC).
Materials & Methods
Following approval from the University of Texas MD Anderson Cancer Center Institutional Review Board, a retrospective chart review of 429 patients with invasive vulvar carcinoma evaluated between 1993 and 2011 was performed. Medical records were reviewed for demographic information, risk factors, stage using the International Federation of Gynecologists and Obstetricians (FIGO) staging system, treatment type, pathologic diagnosis, and recurrence/outcome information. Patients with vulvar melanoma and sarcoma were excluded from the study. All specimens were initially reviewed by a gynecologic pathologist with expertise in vulvar malignancies. The criteria established by Chamlian and Taylor [2] were used for the diagnosis of BCG. Outcomes between patients with BGC were compared to patients with non-BGC vulvar carcinoma.
Demographic and clinical characteristics were summarized using descriptive statistics. Fischer's exact test was used to compare patients with BGC and those with non-BGC. Wilcoxon rank sum test was used to compare the medians of continuous variables between these two groups. Overall survival (OS) and recurrence-free survival (RFS) were estimated using Kaplan and Meier and the log-rank test to compare these two groups of patients for each of these outcomes [6]. Cumulative incidence of recurrence was estimated using the methods of Gooley et al. [7]. Time to recurrence was measured from the date of surgery to the date of last visit or recurrence, and death was considered a competing event. All P values are 2 sided, and were considered significant if <0.05.
Results
There were 429 patients identified with invasive vulvar carcinoma during the study interval. Of these, 33 (7.7%) were identified as primary BGC. Demographic and clinical characteristics are shown in Table 1. Patients with BGC had a younger age at diagnosis compared with non-BGC (median 57 vs. 63 years, p=0.045). A higher proportion of patients with BGC were African American (21.2%) compared with non–BGC vulvar carcinoma (10.1%), p=0.037. There were no significant differences between the two groups with regard to body mass index (BMI) or smoking history.
Table 1. Patient demographic and clinical characteristics.
| Bartholin's Gland Carcinoma (n=33) | Non-Bartholin's Gland Carcinoma (n=396) | p-value | ||
|---|---|---|---|---|
| N (%) | N (%) | |||
| Age (years) | 0.045 | |||
| Mean (SD) | 57 (13) | 63 (15) | ||
| Median | 57 | 63 | ||
| Range | 33 – 83 | 29 – 98 | ||
| Race | 0.037 | |||
| Caucasian | 22 (66.7) | 311 (78.5) | ||
| African American | 7 (21.2) | 40 (10.1) | ||
| Asian | 1 (3.0) | 4 (1.0) | ||
| Hispanic | 2 (6.1) | 39 (9.9) | ||
| Other | 1 (3.0) | 1 (0.3) | ||
| Unknown | 0 (0.0) | 1 (0.3) | ||
| BMI (kg/m2) | 0.236 | |||
| N | 30 | 355 | ||
| Mean (SD) | 28.1 (5.0) | 29.7 (7.0) | ||
| Median | 26.6 | 28.7 | ||
| Range | 20.5 – 40.7 | 16.1 – 52.5 | ||
| Smoking History | 0.193 | |||
| Never | 16 (48.5) | 179 (45.2) | ||
| Past | 11(33.3) | 96 (24.2) | ||
| Current | 5 (15.2) | 114 (28.8) | ||
| Unknown | 1 (3.0) | 7 (1.8) | ||
Surgical and pathologic findings are shown in Table 2. There were no significant differences between the two groups with regard to histologic type: 88% of patients with primary BGC had squamous cell histology and 12% had adenocarcinoma, compared with the non-BGC group where 95% had squamous cell histology, 3% had adenocarcinoma, and 2% had other histology (p=0.169). Patients with BGC had a higher rate of stage III/IV disease compared with patients with non-BGC (60.6% vs. 35.8%, p=0.006). However, there were no significant differences between the two groups with regards to lesion size, lesion focality, tumor grade, lymphovascular space involvement, or perineural invasion.
Table 2. Surgical and pathologic findings.
| Bartholin's Gland Carcinoma (n=33) | Non-Bartholin's Gland Carcinoma (n=396) | p-value | ||
|---|---|---|---|---|
| N (%) | N (%) | |||
| Lesion Size (mm) | 0.718 | |||
| N | 21 | 250 | ||
| Mean (SD) | 38.5 (19.7) | 40.1 (28.0) | ||
| Median | 40 | 30.5 | ||
| Range | 4 – 70 | 1.5 – 200 | ||
| Lesion Focality | 0.316 | |||
| Unifocal | 29 (87.9) | 302 (76.3) | ||
| Multifocal | 1 (3.0) | 46 (11.6) | ||
| Unknown | 3 (9.1) | 48 (12.1) | ||
| Histologic Type | 0.169 | |||
| Squamous | 375 (94.7) | |||
| Adenocarcinoma | 29 (87.9) | 14 (3.5) | ||
| Other | 0 (0) | 7 (1.8) | ||
| FIGO Stage | 0.037 | |||
| I | 8 (34.3) | 177 (44.7) | ||
| II | 4 (12.1) | 60 (15.2) | ||
| III | 16(48.5) | 109 (27.5) | ||
| IV | 4 (12.1) | 33 (8.3) | ||
| Unknown | 1 (3.0) | 17 (4.3) | ||
| LVSI | 0.351 | |||
| No | 6 (18.2) | 143 (36.1) | ||
| Yes | 6 (18.2) | 74 (18.7) | ||
| Unknown | 21 (63.6) | 179 (45.2) | ||
| Tumor Invasion (mm) | 0.752 | |||
| N | 17 | 322 | ||
| Mean (SD) | 5.4 (5.1) | 4.8 (4.7) | ||
| Median | 3.5 | 3.3 | ||
| Range | 1 – 18 | 0.1 – 31 | ||
| Positive Lymph Nodes | 0.091 | |||
| No | 11 (33.3) | 142 (35.9) | ||
| Yes | 14 (42.4) | 88 (22.2) | ||
| Not Performed/Unknown | 8 (24.2) | 166 (42.0) | ||
Treatment modalities used are shown in Table 3. Fewer patients with BGC had primary surgery compared with patients with non-BGC vulvar carcinoma (60.7 vs. 81.6%, p=0.0011). Furthermore, a higher proportion of patients in the BGC group underwent radiation therapy (78.8% vs. 43.9%, p=0.0002). Lymph node assessment was performed in 25 patients (75.8%) in the BCG group and 226 patients in the non-BCG group (57.1%). Sentinel lymph node biopsy alone was performed alone or as part of the full lymphadenectomy in 6 patients (24.0%) in the BCG group and 101 (44.7%) in the non-BCG group.
Table 3. Treatment modalities.
| Bartholin's Gland Carcinoma (n=33) | Non-Bartholin's Gland Carcinoma (n=396) | p-value | ||
|---|---|---|---|---|
| N (%) | N (%) | |||
| Surgical Treatment | ||||
| Wide Local Excision | 2 (6.1) | 86 (21.7) | 0.0087 | |
| Wide Radical Excision | 10 (30.3) | 144 (36.4) | ||
| Vulvectomy | 3 (9.1) | 59 (14.9) | ||
| Hemivulvectomy | 5 (15.2) | 34 (8.6) | ||
| Biopsy | 10 (30.3) | 65 (16.4) | ||
| Other | 3 (9.1) | 8 (2.0) | ||
| Radiation Therapy | 0.0002 | |||
| No | 7 (21.2) | 216 (54.6) | ||
| Yes | 26 (78.8) | 174 (43.9) | ||
| Unknown | 0 (0.0) | 6 (1.5) | ||
| Chemotherapy | 0.0785 | |||
| No | 22 (66.7) | 311 (78.5) | ||
| Yes | 11 (33.3) | 78 (19.7) | ||
| Unknown | 0 (0.0) | 7 (1.8) | ||
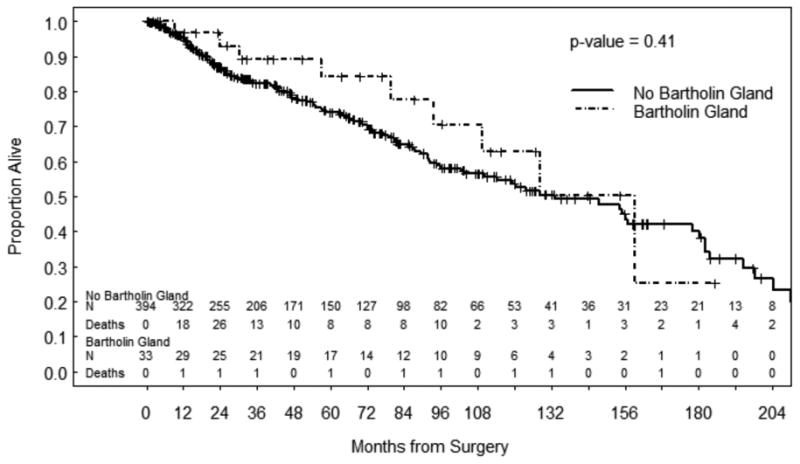
The median follow-up for all patients was 41.9 months (range 0.0 to 259.5). The median follow-up for the 292 patients alive at last contact was 38.9 months (range 0.0 to 248.5). The cumulative incidence of recurrence was similar between groups: 40.9% at 5 years in the BGC group and 44.3% at 5 years in the non-BGC group (p=0.632). Sites of recurrence for the BCG group included the vulva (n=3, 27.3%), groin lymph nodes (n=2, 18.2%), pelvic lymph nodes (n=1, 9.1%) and distant metastatic disease to lung, liver or other sites (n=5, 45.4%). For the non-BCG group, sites of recurrence included the vulva (n=103, 69.1%), groin lymph nodes (n=28, 18.8%), pelvic lymph nodes (n=6, 4.0%) and distant metastatic disease to lung, liver or other sites (n=12, 8.1%). There were no statistically significant differences in RFS or OS between the two groups (Figures 1 and 2).
Figure 1. Recurrence- Free Survival.
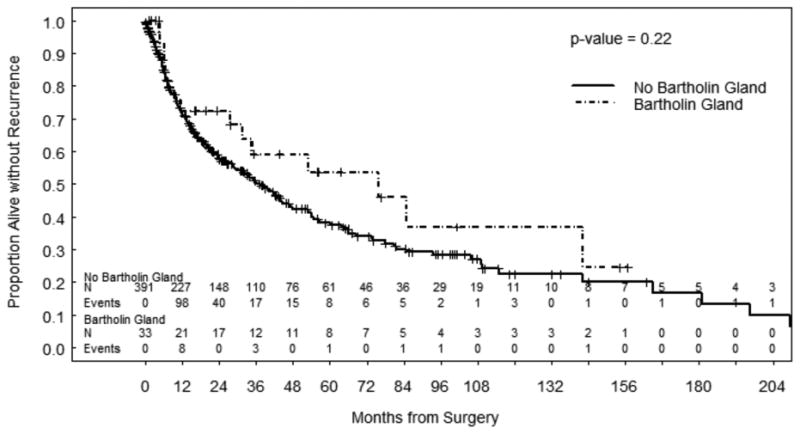
Figure 2. Overall Survival.
Discussion
The principal findings from our study were that primary BGC was associated with younger age at diagnosis, African American ethnicity, higher rates of advanced stage disease and adjuvant treatment when compared with patients with non–BGC vulvar carcinoma. However there were no significant differences in RFS or OS between the two groups. In our cohort of 429 patients with vulvar carcinoma, 7.7% had primary BGC. This is similar to studies by Leucter et al. [3] as well as Copeland et al. [4] who reported rates of 3.9% and 7%, respectively. Similar to these previous reports, our study found the majority of patients with BCG had squamous cell histology. Given the rarity of this disease and lack of prospective trials, there is currently no consensus on treatment recommendations for BGC. However, general consensus for treatment of vulvar cancer is radical local excision with inguinal femoral lymphadenectomy or sentinel lymph node biopsy. As BGC is a medial and deep vulvar tumor, staging usually includes assessment of the nodes bilaterally. Recommendations have been made to consider preoperative imaging to assess resectability of BCG, specifically in relation to preserving function of bladder, bowel and urethra.
A study by Cardosi et al. [8] reported a 15-year experience of 12 patients with primary BGC. Seven of 12 patients (58.3%) had stage III/IV disease and the majority received adjuvant radiation and/or chemotherapy. The authors reported an overall survival rate of 67%. In a similar study, Copeland et al. [4] retrospectively evaluated 30 years of clinical experience involving 36 patients diagnosed with BGC from 1954 to 1983 at MD Anderson Cancer Center, prior to the cohort included in the current study. They noted that 47% of patients (14/30) had nodal involvement at diagnosis. In addition, 25% of patients (9/36) developed recurrent disease and the five-year overall survival rate was 84%. In the current study, we noted that 42% of patients with BGC presented with nodal involvement and 33% of patients developed recurrent disease. As a result, more patients received radiotherapy compared with patients with non-BGC vulvar cancer. Despite the higher stage at presentation and need for adjuvant therapy, there were no significant differences in survival between the groups.
Our study is limited by its retrospective nature, long study period, missing information for many patients and the limited number of patients with primary BGC. In addition, as a single institutional study there is possible referral bias. However, the strengths of our study include the large number of patients with vulvar carcinoma and the ability for comparison of outcomes between BGC and non-BGC patients. Furthermore, all cases were reviewed by a gynecologic pathologist with expertise in vulvar malignancies. In addition, the median follow-up time for our cohort was long at 41.9 months.
In conclusion, primary BGC is a rare form of vulvar cancer. The diagnosis is often delayed due to the absence of specific symptoms and possible misdiagnosis as a benign Bartholin's cyst. However, the treatment modalities used are similar to other forms of vulvar carcinoma and the outcomes appear to be similar. Additional education for patients and primary providers is needed to avoid the misdiagnosis and improve the early diagnosis of women with BGC and other vulvar malignancies.
Acknowledgments
This research was supported in part by the Raby/Dunaway Family Fund and by the National Institutes of Health through MD Anderson's Cancer Center Support Grant CA016672.
Footnotes
An abstract of this work was presented at the International Gynecologic Cancer Society (IGCS) biannual meeting, November 2014, Melbourne, Australia.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Chamlian DL, Taylor HB. Primary carcinoma of Bartholin's gland. A report of 24 patients Obstet Gynecol. 1972;39(4):489–94. [PubMed] [Google Scholar]
- 3.Leuchter RC, Hacker NF, Voet RL, Berek JS, Townsend DE, Lagasse LD. Primary carcinoma of the Bartholin gland: A report of 14 cases and review of the literature. Obstet Gynecol. 1982;60:361–8. [PubMed] [Google Scholar]
- 4.Copeland LJ, Sneige N, Gershenson DM, McGuffee VB, Abdul Karim F, Rutledga FN. Bartholin gland carcinoma. Obstet Gynecol. 1986;67:794–801. doi: 10.1097/00006250-198606000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Ouldamer L, Chraibi Z, Arbion F, Barillot I, Body G. Bartholin's gland carcinoma: Epidemiology and therapeutic management. Surgical oncology. 2013;22:117–122. doi: 10.1016/j.suronc.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 7.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Statistics in Medicine. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 8.Cardosi RJ, Speights A, Fiorcia JV, Grendys EC, Hakam S, Hoffman MS. Bartholin's gland carcinoma: a 15 year experience. Gynecol Oncol. 2001;82:247–51. doi: 10.1006/gyno.2001.6304. [DOI] [PubMed] [Google Scholar]


