Abstract
Background
There are many teaching methods for epidural anesthesia skills. Previous work suggests that there is no difference in skill acquisition whether novice learners engage in low-fidelity versus high-fidelity haptic simulation for epidural anesthesia. However, no study has compared the effect of low-fidelity haptic simulation for epidural anesthesia versus mental imagery training in which no physical practice is attempted. We tested the hypothesis that mental imagery training is superior to low-fidelity haptic simulation training for epidural anesthesia skill acquisition.
Methods
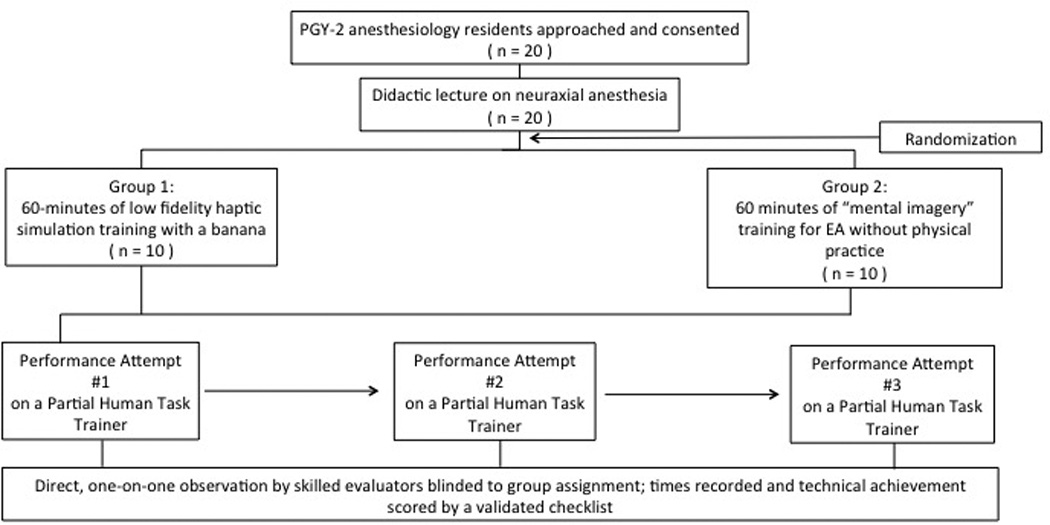
Twenty PGY-2 anesthesiology residents were tested at the beginning of the training year. After a didactic lecture on epidural anesthesia, they were randomized into two groups. Group LF had low-fidelity simulation training for epidural anesthesia using a previously described banana simulation technique. Group MI had guided, scripted mental imagery training in which they were initially oriented to the epidural kit components and epidural anesthesia was described in stepwise detail, followed by individual mental rehearsal; no physical practice was undertaken. Each resident then individually performed epidural anesthesia on a partial-human task trainer on three consecutive occasions under the direct observation of skilled evaluators who were blinded to group assignment. Technical achievement was assessed using a modified validated skills checklist. Scores (0–21) and duration to task completion (minutes) were recorded. A linear mixed-effects model analysis was performed to determine differences in scores and duration between groups and over time.
Results
There was no statistical difference between the two groups for scores and duration to task completion. Both groups showed similarly significant increases (P = 0.0015) in scores over time (estimated mean score [standard error]: Group MI: 15.9 [0.55] to 17.4 [0.55] to 18.6 [0.55]; Group LF: 16.2 [0.55] to 17.7 [0.55] to 18.9 [0.55]). Time to complete the procedure decreased similarly and significantly (P = 0.032) for both groups after the first attempt (estimated mean time [standard error]: Group MI: 16.0 [1.04] minutes to 13.7 [1.04] minutes to 13.3 [1.04] minutes; Group LF: 15.8 [1.04] minutes to 13.4 [1.04] minutes to 13.1 [1.04] minutes).
Conclusions
Mental imagery is not different from low-fidelity simulation training for epidural anesthesia skill acquisition. Education in epidural anesthesia with structured didactics and continual mental imagery training may suffice to prepare novice learners prior to an attempt on human subjects.
INTRODUCTION
Epidural anesthesia has been rated among the most difficult technical skills to acquire during anesthesiology training.1,2 For a wide variety of procedures, simulation education is widely recognized as an effective mechanism for advancing technical skill familiarity in novices prior to participating in clinical care.3–6 For epidural anesthesia skills and other skills, previous work has indicated that there is no difference in skill acquisition and proficiency when novice learners engage in high-fidelity versus low-fidelity simulation training under instructional guidance.7–9 In fact, some studies have suggested that high-fidelity simulation may impede performance among novice learners, which seems to suggest that beginners need to achieve a certain level of automation for new tasks before being able to divert their attention to other components of the task.7, 10, 11
Simulation education for technical skill acquisition is traditionally focused on haptics, from the Greek “haptikos” meaning “to sense or to touch.” This focus has resulted in the creation of simulator platforms aimed at reproducing reality with varying levels of physical fidelity. In contrast, mental imagery, which can also be described in terms of mental practice, mental rehearsal, motor imagery, or mental visualization, is a form of nontechnical simulation rooted in kinesiology. Mental imagery is, “the cognitive rehearsal of a task in the absence of overt physical movement.”12 It is widely used and recognized as effective in the realms of stroke rehabilitation, cognitive behavioral therapy, high-performance athletics, and professional musicianship.13–17
There are many different methods of teaching epidural anesthesia to novice residents, and some methods involve deliberate haptic simulation practice prior to the first epidural attempt in situ. To date, we are unaware of any study that has compared the effect of haptic vs non-haptic simulation training on epidural anesthesia skill acquisition. Non-haptic simulation training, if effective, could relieve educators from the physical, temporal, and financial burdens associated with low-fidelity simulator model design, construction, transportation, and maintenance. Thus, we hypothesized that mental imagery training is more effective than low-fidelity haptic simulation training in achieving technical skills among novice anesthesiology residents. We aimed to test and compare the effectiveness of these two techniques for achieving technical skills in epidural anesthesia.
METHODS
After approval from our IRB, (PRO14050455), we approached twenty PGY-2 (CA-1) anesthesiology residents from the University of Pittsburgh Medical Center in July of their training year. The study was disclosed to the residents and written informed consent was obtained. Each resident was asked to provide baseline information about their previous spinal and epidural anesthesia experience, including an approximate number of each technique performed.
Figure 1 shows the study flow diagram. All participating residents received a didactic lecture on epidural anesthesia, delivered by one of two board certified anesthesiologists with obstetric anesthesiology subspecialty training. Following the lecture, the residents were randomized into two groups by a random number generator. (Randomness and Integrity Services Ltd., 1998, Dublin, Ireland, www.random.org, accessed July 11, 2014.)
Figure 1.

Study flow diagram.
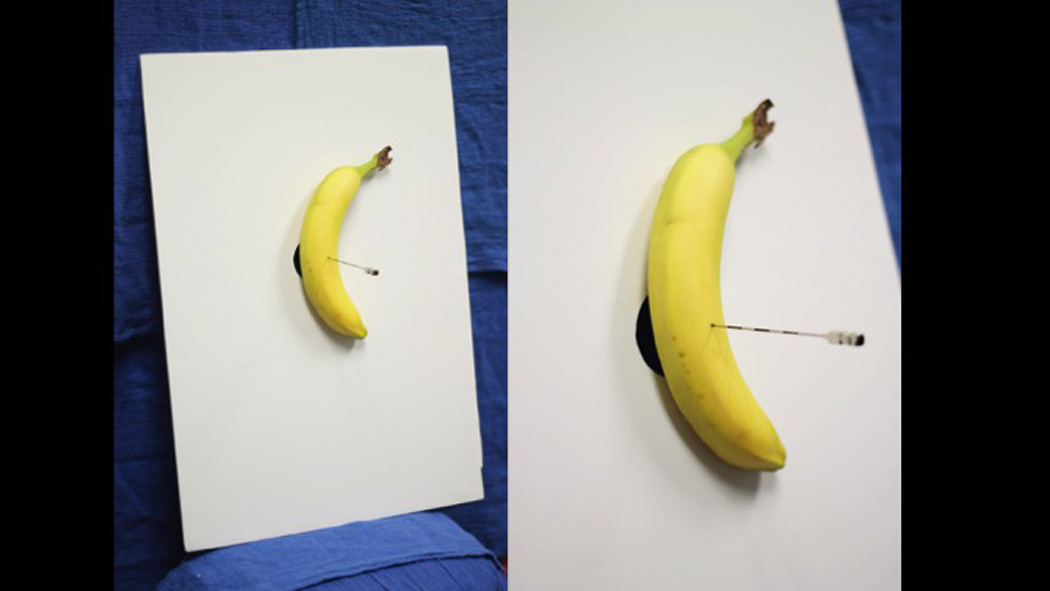
Group LF had 60 minutes of low-fidelity simulation training as a group (N=10) for epidural catheter placement using a banana (Figure 2). This technique has been previously described as successful in achieving the skill for epidural catheter placement among novice learners.18 The banana model has also been shown to have tactile fidelity for loss of resistance compared to other produces.19 In this group, learners were given specific instructions on how to perform the epidural catheter placement technique (Appendix 1). Epidural catheter placement was attempted and supervised by one board certified anesthesiologist with obstetric anesthesiology subspecialty training. Each resident progressed at his or her own pace until epidural catheter insertion, and adjustment was attained at least twice. Residents were permitted to interact with, and ask questions of, the instructing anesthesiologist.
Figure 2.
Low-fidelity epidural anesthesia simulator apparatus.
Group MI had 60 minutes of scripted mental imagery training as a group (N=10) (Appendix 1) under the guidance of another board certified anesthesiologist with obstetric anesthesiology subspecialty training, who was not involved in the instruction of Group LF. Residents were oriented to the parts of the epidural kit, epidural catheter placement was described in stepwise detail, and an anatomical spine model was used as a visual reference. No physical practice was performed. After 20 minutes of scripted guidance through mental rehearsal, the subjects engaged in a mental rehearsal for the task independently. They were permitted to interact with the kit and to ask questions of the instructing anesthesiologist.
Immediately after the 60-minute group training sessions, residents from both groups individually performed epidural catheter placement on a high-fidelity, partial-human task trainer (GENESIS Epidural-Spinal Injection Simulator, Epimed International, Farmers Branch, Texas) for three consecutive trials on the same day, each under the direct observation of one of seven skilled evaluators (Figure 3). We defined a “skilled” individual as an anesthesiologist who has performed at least 90 epidural anesthetics within the previous calendar year.1 All evaluators were blinded to group assignment. They assessed technical achievement using a modified validated skills checklist.20 Scores (0 –21, where 0 is the worst and 21 is the best) and duration (minutes) to task completion were recorded (Appendix 2). All evaluators were trained in the use of the checklist over a 30-minute didactic session held on the same day as the evaluations, immediately before the trials with each resident. The checklist items from the original reference were adjusted to omit items that assessed communication points between the proceduralist and patient, given our use of non-communicative simulators. We also omitted checklist items that included subjective rating points that we felt were not measureable in the context of the designed assessment station (e.g. “optimally positions” and “carefully prepares”). We also eliminated one item, “allows solution to dry,” because the antiseptic solution did not reliably completely dry on the surface of the high-fidelity simulator. We did not include items that measured catheter aspiration and epidural test dose administration, as the simulators would not allow for adequate detection of positive aspiration or a positive test dose. One checklist item was added, “Wears hat and mask” as this is an institutional standard that was thought necessary to educate and to measure.
Figure 3.
Partial human task trainer.
Over the following year, clinical competency for procedural skills was assessed up to 25 times per subject, and compared for average milestone scores between the two groups. We specifically used the Patient Care competency milestone PC-A10, “Technical skills: Regional anesthesia.” Scoring for this competency ranges from Levels 1 to 5, where Level 1 is described as, “Demonstrates sterile technique; Administers infiltrative local anesthetics; Identifies physiologic changes associated with local anesthesia administration and seeks help appropriately,” and Level 5 is aspirational and defined as, “Independently performs peripheral and neuraxial regional anesthesia techniques; Independently manages problems or complications associated with regional anesthesia.” We used PC-A10 as a surrogate for the evaluation of clinical skills for epidural anesthesia because of the lack of a specific milestone that exclusively assesses epidural anesthesia skills in clinical practice.
Descriptive statistics were presented as mean and standard deviation or frequencies and percentages for continuous and categorical data, respectively. Demographic features were compared using Fisher’s exact test or Mann-Whitney’s U test, as appropriate. Average milestone scores between the two groups were compared using the Student t-test. Examination of distributional form for score and time data was determined by box plots. Each box plot indicated minimum value, lower quartile (lowest 25% of data), median, upper quartile (highest 25% of data) and maximum value. Given our repeated measurements of scores and time, linear mixed-effects models were used to test the main effects of time, group and time by group interactions on scores and time separately, and to account for within subject correlation. Raters were included as a random effect in the linear mixed effects model. One between-subjects factor (group) and one within-subjects factor (time) and their interaction were defined as fixed effects, and the subject as random effect. After inspecting the correlation within subjects for scores and time, a compound symmetry structure was assumed. Post hoc comparisons were made using type III tests of fixed effects with Bonferroni correction (significance level: 0.05/3 = 0.017). P-values and adjusted P-values for post-hoc comparisons are shown. We assessed the assumption of normally distributed residuals for the mixed model analysis by Q-Q plots. All statistical analyses were two-sided, and the significance value was P<0.05. All analyses were conducted using SAS, version 9.3 statistical software (SAS Institute Inc., Cary, NC).
RESULTS
Twenty residents were approached, consented to the study and completed the protocol. There was no difference in baseline characteristics between the groups (Table 1).
Table 1.
Baseline characteristics.
| Variable | Group LF n = 10 |
Group MI n = 10 |
P-value |
|---|---|---|---|
| Age (years) | 28.7 ± 2.0 | 28.9 ± 4.4 | 0.38 |
| Sex | |||
| Male | 5 (50.0%) | 6 (60.0%) | 1.00 |
| Female | 5 (50.0%) | 4 (40.0%) | |
| Prior epidural anesthesia experience | |||
| Yes | 2 (20.0%) | 3 (33.3%) | 0.63 |
| Prior spinal anesthesia experience | |||
| Yes | 3 (30.0%) | 2 (22.2%) | 1.00 |
| Degree | |||
| M.D. or D.O. | 9 (90.0%) | 10 (100.0%) | 0.47 |
| M.D., Ph.D. | 1 (10.0%) | 0 (0.0%) | |
Abbreviations: LF, low fidelity; MI, mental imagery.
Note: Data are reported as mean ± SD or frequency (%).
The assumptions of normally distributed residuals and of equal variances were evaluated and not violated. The results of the linear mixed-effects model analysis showed that group effect was not significant. That is, there was no statistically significant difference between the two groups in terms of scores and duration to complete the task. Scores between the two groups were similar and increased over time (Group MI observed score [standard deviation]: 15.5 [2.0] to 17.8 [1.8] to 18.5 [2.6] vs. Group LF observed score: 16.5 [1.8] to 17.2 [2.9] to 19.0 [1.6], P=0.58). Both groups showed a similar time effect for score, in that scores increased significantly (P=0.0015) over time (Group MI estimated score 15.9 to 17.4 to 18.6; Group LF estimated score 16.2 to 17.7 to 18.9; all standard errors 0.55).
Time to complete the procedure also decreased similarly and significantly (P=0.032) for both groups after the first attempt (Group MI estimated time 16.0 minutes to 13.7 minutes to 13.3 minutes; Group LF estimated time 15.8 minutes to 13.4 minutes to 13.1 minutes; all standard errors 1.04). Pairwise comparisons of the outcomes among three time points indicated that the significant differences in both scores and duration were between time point 1 and time point 3 with P = 0.0003, adjusted P=0.001 and P = 0.0162, adjusted P=0.049, respectively. Figure 4 shows the differences in scores and time between groups and over time.
Figure 4.
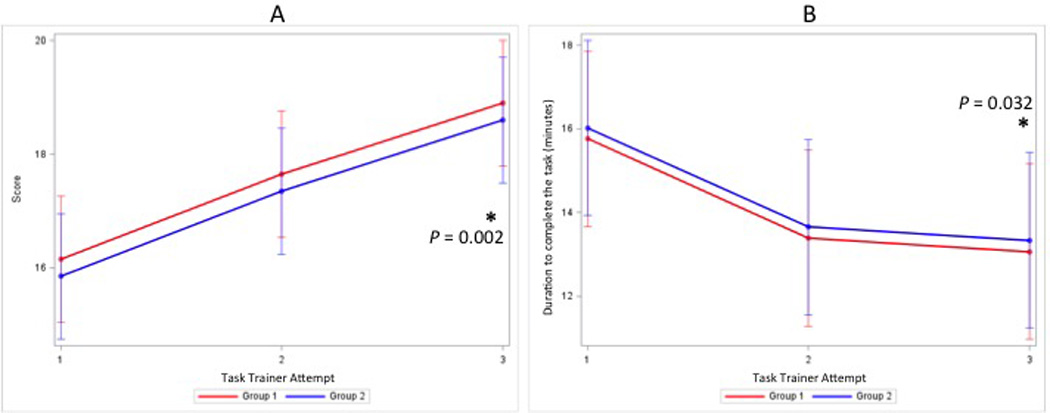
Estimated mean (±SE) score (0 – 21) (A) and duration to complete the task in minutes (B) between groups and over three epidural placement attempts. A mixed model analysis was performed to determine differences in scores and duration between groups and over time. Post hoc comparisons were made using Type III Tests of Fixed Effects with Bonferroni correction. The asterisk (*) denotes a P<0.05 for the third attempt compared to the first attempt within each group.
For the linear mixed model analysis, including rater as a random effect, the interaction between Group and Time was not significant, and therefore was removed from the model. After including the rater as a random effect in the linear mixed model, scores for both groups improved significantly over time (F(2, 50)=13.46, P <0.0001). In terms of differences in time to complete the procedure between groups, the interaction between Group and Time was not significant, and therefore was removed from the model. After including the rater as a random effect in the linear mixed model, time to complete the procedure for both groups improved significantly over time (F(2, 50)=5.59, P=0.006).
Over the following twelve months, there was no significant difference between the two groups in terms of number of evaluations for PC-A10 performed (group LF mean (±SD) 11.3±8.7 evaluations [range 1–25]; group MI mean 13.2±7.0 evaluations [range 4–23]; P = 0.596). The milestone level results for competency PC-A10 were not different between the two groups (Group MI: 2.3±0.2 vs. Group LF: 2.4±0.2, P=0.47). Level 2 on PC-A10 is described as, “Applies appropriate monitors and prepares resuscitative equipment prior to performing regional anesthesia procedures; Performs spinal and epidural anesthesia under direct supervision; Recognizes problems or complications associated with regional anesthesia, and manages them with direct supervision.”
DISCUSSION
The results of our mixed model analysis show no difference between mental imagery and low-fidelity simulation training for epidural anesthesia skill acquisition. Both groups in our study demonstrated improvements in performance when allowed to practice and with the provision of immediate feedback. This lack of a difference appears to support the importance of effective scaffolding, a learning theory concept that describes the process of basic support of a learner through interactions with an expert or other skilled peer, and the gradual adjustment and attenuation of this relationship as the learner progresses toward independence.21, 22
Our findings suggest that mental imagery may be as effective as low-fidelity simulation. Low-fidelity models require a modicum of expenditure of time and money for their design, construction, maintenance, storage, and transportation. In contrast, mental imagery is limited primarily by time and personal effort, and because no physical environment is required, mental imagery can be engaged by the learner any time and any place, at their convenience. Mental imagery, also referred to as "mental practice", “…not only provides a unique opportunity to increase the number of repetitions in a safe and autonomous manner without undue physical fatigue, but it also allows the mental rehearsal of motor tasks when and where the [subject] wants to, or is able to, practice. Furthermore, mental practice enables the rehearsal of more demanding or complex motor tasks… when physical practice is impossible or too difficult.”12
There is some suggestion that, in general, mental practice prior to physical practice for new skills can be more effective than mental practice alone or physical practice alone, but this mental training needs to be intensive.12 Jackson et al. showed that 1,500 mental repetitions of specific foot movements led to significant improvements in physical performance of this task, and that these mental rehearsals correlated with functional neurocortical activities that are similar to what would be observed after physical performance of the task.23 Therefore, mental practice may have direct implications for the implementation of strategies aimed at deliberate practice, which is part of a fundamental educational framework for technical skills learning.24 Future work should focus on methods that integrate mental imagery into deliberate practice for epidural anesthesia skills acquisition in a way that is centered on competency and mastery-level learning.
Our study has limitations. First, mental imagery was practiced for 60 minutes, whereas 20 to 30 minutes is typically undertaken for mental imagery for other skills.25–27 Our rationale for this duration was to equalize the length of time of exposure to training for both groups, and this time was dictated in part by the amount of time necessary for low-fidelity group to perform the tasks on the low-fidelity models. However, there is also some suggestion that longer periods of mental practice may be more effective than shorter periods in the stroke rehabilitation population.28 Therefore, more research is necessary to identify the ideal period of time for mental practice to be undertaken for this skill. Another limitation is the use of milestones as data for translational outcomes to gauge improvements in patient care practices, otherwise described as T2 of the “3Ts” of the simulation-based education translational research roadmap.5 Currently, there is no specific milestone that evaluates epidural catheterization skills among anesthesiology residents: PC-A10 is the closest technical skill assessment for this procedure, also includes other forms of regional anesthesia in its measurement. Hence, our T2 results should be interpreted with caution. A better design may have been to use a blinded evaluator in situ or video recordings in situ with blinded evaluation; however we were unable to support these proposed designs due to a lack of manpower. Therefore, further research on patient care-related outcomes will clarify the effect of these teaching techniques on long-term clinical performance among our residents. Another potential limitation is that the anesthesiologist who guided the mental imagery group, although experienced and qualified by virtue of extensive training and fellowship, was not a cognitive psychologist specialized in the guidance of mental imagery. Finally, the lack of a control group that did not have any training at all makes it difficult to know if either of these teaching interventions are different from doing nothing for skill acquisition.
In conclusion, our results suggest that there is no difference between mental imagery and low-fidelity haptic simulation for technical performance of epidural anesthesia. Education in epidural anesthesia with structured didactics and mental imagery training may adequately prepare novice learners before an attempt on human subjects. Further research with a larger sample size is necessary to: 1) determine if mental imagery is as effective as low-fidelity simulation training; 2) define the ideal amount of time in which to engage mental imagery training for epidural anesthesia; and 3) determine the effectiveness of epidural anesthesia mental imagery in achieving significant improvements for patient care performance and outcomes.
Acknowledgments
Funding: The project described was supported in part by the National Institutes of Health through Grant Number UL1TR000005. Dr. Lim is supported in part by a grant through the National Institutes of Health, T32GM075770.
Appendix 1
Low-Fidelity Group Curriculum
Learners were instructed to don caps and masks, and to perform hand hygiene. They then palpated the sides of the low-fidelity apparatus to simulate palpation of the iliac crests and spinous processes which were demarcated by marker; the marked the intended site of injection. They were instructed to open the epidural kit without violating the sterile contents, to don sterile gloves without violating the sterile barriers, and then to pour the antiseptic solution without exposing the contents of the epidural kit to the antiseptic. The learners applied antiseptic solution to the low-fidelity apparatus three times. They were then instructed on specific preparation of the remaining kit elements, including needles and syringes with saline and local anesthetic. The learners were guided on placing a sterile drape over the low-fidelity apparatus and adhered to the wall behind the system. They re-palpated the sides of the apparatus and verified their intended site of injection. Local anesthetic was injected using a 22-gauge needle into the “skin” of the produce (i.e. banana peel) and then into deeper “flesh” of the banana in a single pass. The epidural needle was introduced through the same plane, perpendicular to the ground, with the bevel cephalad. Learners were instructed to assess engagement into “ligament” by letting go of the epidural needle and assessing for stability prior to removal of the epidural needle stylet and attachment of the saline-filled loss-of-resistance syringe. Maintenance of the stability of the needle-syringe system was emphasized and assessed by the instructor at this point. The stability of the learner’s non-dominant hand against the low-fidelity apparatus (“patient’s back”) while grasping the epidural needle was taught and assessed by the instructor. Learners were told to then advance the needle-syringe system millimeter by millimeter, and to check resistance on the plunger between each advancement. Once loss of resistance was identified, learners were instructed to immediately release pressure on the plunger. The syringe was removed from the epidural needle, the depth of loss of resistance was measured in centimeters by the markings on the epidural needle, and the catheter was threaded to a depth of 4–5 centimeters. The needle was withdrawn while the learners simultaneously advanced the catheter to maintain correct catheter placement. The catheter was adjusted to the correct depth at the level of the “skin” of the banana.
Mental Imagery Script
Remove all hand and wrist jewelry. Perform hand hygiene. Position the patient with a pillow on the lap to encourage spine flexion. Palpate the iliac crests and spinous processes, and landmark the intended site of injection. Now open your epidural kit in a sterile fashion by carefully peeling open the paper seal, discarding the outer plastic container, discarding the paper inserts, and touching only the corners of blue drape. Put on your sterile gloves. Carefully unfold the packaging and take care not to contaminate yourself. Now, prep the skin by circumferentially expanding the skin prep around the anticipated point of entry. Discard the used prep sticks. Allow the prep to dry as you prepare the rest of the epidural kit. Take the clear plastic drape from the kit, and ensure that the “UP” arrows are pointing up. Remove the sticker backing from the circular hole, and with the adhesive side facing away from you, place the hole over the anticipated entry point. As you unfold the drape, remove the sticker backing from the top, and secure the adhesive to the patient’s skin, making sure not to contaminate yourself.
Now, prepare the epidural kit. Set aside the gauze, the epidural catheter in its plastic bag, and discard the plastic filter. Remove the 10-milliliter glass saline ampule, and discard its blue wrap. Set aside the yellow epidural sticker label, and discard any extraneous paper inserts. Unseal the 18 gauge safety needle and discard its plastic wrapper. Next, draw up lidocaine 1%. Break the ampule and place broken glass into the far right bin. Pick up the 3-milliliter syringe and affix the 18 gauge needle to it. Draw up the lidocaine 1%. Remove the 18 gauge needle, and place it in the red sharps cup. Always place sharps that you are not using into the red sharps cup. Next, affix the 25 gauge needle onto the 3-milliliter syringe, and set the 3-milliliter syringe down on the blue plastic tray. Now locate the 5-milliliter glass syringe. Remove the rubber stop from the glass syringe. Open the saline vial and place broken glass into the far right bin. Rest the saline vial vertically on the blue plastic tray in a designated circular holder. Locate the filter needle. Pick up the glass syringe and draw up 2–3 milliliters of saline using the filter needle. Disengage the filter needle and leave it in the saline vial. Put the glass syringe with saline back down on the blue plastic tray. Now, Pick up and unsheathe the 17 gauge Hustead needle. Place it back on the blue plastic tray. Locate the test dose ampule, which is lidocaine 1.5% with epinephrine. Open the vial and place broken glass into the far right bin. Using the 20-milliliter plastic syringe and filter needle, draw up 3 milliliters of test dose. De-air any entrained air. Now, unsheathe the needle on the 3-milliliter syringe. Palpate the iliac crests and spinous processes, and again verify the site of intended injection. Anesthetize the skin and deeper tissues by creating a skin wheel; this takes about 1 milliliter of local anesthetic. Replace the 25 gauge needle with the 22 gauge needle, and unsheathe it. Proceed to anesthetize deeper tissues by passing the needle through the same entry point, taking care to remain shallow in thin patients. Place 22 gauge needle in the red sharps cup, and set the 3-milliliter syringe down.
Pick up the Hustead needle. Insert the Hustead at the skin wheel, bevel up, and oriented parallel to the ground. Insert to 2 to 3 centimeters, then check for engagement in ligament by letting go of the needle. If it is engaged in ligament, the Hustead will remain still and parallel to the ground. Remove the stylet. Next, bring the glass syringe filled with saline to the Hustead needle and attach it. Bring your left thumb and index finger together, and stabilize the Hustead needle at the patient’s skin. Check for resistance by pushing on the plunger of the glass syringe with your right thumb. Then, with your right thumb and index finger at the head of the Hustead, advance the needle one millimeter. Check for resistance by pushing on the plunger of the glass syringe with your right thumb. Continue to advance, millimeter by millimeter, each time checking for resistance on the glass syringe after each point of advancement. Continue to maintain Hustead needle stability with your left thumb and index finger. Confirm loss of resistance. Remove the glass syringe from the Hustead.
Using both hands, open the epidural catheter from its sterile packaging, taking care to maintain its looped configuration. Leave the catheter cap on the sterile field. Take the tip of the catheter that has the blackened end with your right hand, and insert it into the Hustead and advance the catheter. Unravel the loops with your left hand as you advance the catheter with your right hand. Remember the distance in centimeters at which loss of resistance had been found. Advance the catheter until 4 to 5 cm past that distance. Now, with your left hand, find the other end of the catheter and hold it in your left palm in order to release your left thumb and index finger. Use your left thumb and index finger to withdraw the Hustead while you simultaneously advance the catheter with your right thumb and index finger. Once the tip of the Hustead needle has cleared the skin, continue to slowly remove the Hustead needle completely from the catheter system, taking care not to dislodge the catheter from the skin, and maintaining good control over the catheter end. This can be accomplished by looping the catheter into your left hand. Pick up the blue catheter top. Withdraw the catheter from the skin slowly, until the appropriate depth of 4 to 5 cm in the epidural space has been achieved. Attach the blue catheter top, remembering to squeeze tightly.
APPENDIX 2
Skills Checklist.
Wears hat and mask
Performs hand hygiene
Palpates iliac crests and spinous processes, and landmarks intended site of injection
Opens the kit in a sterile fashion
Puts on gloves in a sterile fashion
Pours antiseptic solution without contaminating adjacent non-sterile material
Prepares the skin at the back widely and aseptically (skin prep × 3)
Lays out and prepares all equipment (needles, syringes with saline, local anesthetic)
Places drape over mannequin’s back in a sterile fashion
Re-palpates iliac crests and spinous processes, and verifies site of intended injection
Administers local anesthesia to skin and deeper tissues
Places Tuohy needle with correct positioning of bevel (bevel up)
Inserts epidural needle through skin, subcutaneous tissue, and into ligament before attaching the syringe
Attaches saline-filled syringe to the needle hub with the Tuohy needle controlled well
Braces hand/s holding the Tuohy needle against patient’s back in complete control of the Tuohy needle
Slowly advances needle through supraspinous and interspinous ligaments and into ligamentum flavum while applying and checking pressure on the plunger
Identifies loss-of-resistance and immediately releases pressure on the plunger
Notes depth of needle insertion before threading the catheter
Threads the catheter to a depth of 4–5 cm
Pulls the needle out while maintaining correct catheter placement
Adjusts catheter to appropriate depth
Footnotes
The authors declare no conflicts of interest.
Disclosures
Name: Grace Lim, MD
Contribution: Dr. Lim designed the study, conducted the study, collected the data, analyzed the data, and prepared the manuscript.
Attestation: Dr. Lim approved the final manuscript. Dr. Lim attests to the integrity of the original data and the analysis reported in this manuscript. Dr. Lim is the archival author.
Name: Robert G. Krohner, DO
Contribution: Dr. Krohner designed the study, conducted the study, and prepared the manuscript.
Attestation: Dr. Krohner approved the final manuscript. Dr. Krohner attests to the integrity of the original data and the analysis reported in this manuscript.
Name: David G. Metro, MD
Contribution: Dr. Metro designed the study and prepared the manuscript.
Attestation: Dr. Metro approved the final manuscript.
Name: Bedda L. Rosario, PhD
Contribution: Dr. Rosario analyzed the data and prepared the manuscript.
Attestation: Dr. Rosario approved the final manuscript. Dr. Rosario attests to the integrity of the original data and the analysis reported in this manuscript.
Name: Jong-Hyeon Jeong, PhD
Contribution: Dr. Jeong analyzed the data and prepared the manuscript.
Attestation: Dr. Jeong approved the final manuscript. Dr. Jeong attests to the integrity of the original data and the analysis reported in this manuscript.
Name: Tetsuro Sakai, MD, PhD
Contribution: Dr. Sakai designed the study and prepared the manuscript.
Attestation: Dr. Sakai approved the final manuscript.
This research was presented as an abstract at the Society for Obstetric Anesthesiology and Perinatology (SOAP) 2015 Annual Meeting in Colorado Springs, Colorado, and it won the Research in Education Award.
This manuscript was handled by: Cynthia A. Wong, MD
Contributor Information
Grace Lim, Department of Anesthesiology, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania.
Robert G. Krohner, Department of Anesthesiology, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania.
David G. Metro, Department of Anesthesiology, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania.
Bedda L. Rosario, Department of Epidemiology, University of Pittsburgh Graduate School of Public Health, Pittsburgh, Pennsylvania.
Jong-Hyeon Jeong, Department of Biostatistics, University of Pittsburgh Graduate School of Public Health, Pittsburgh, Pennsylvania.
Tetsuro Sakai, Department of Anesthesiology, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania.
REFERENCES
- 1.Konrad H, Schupfer G, Wietlisbach M, Gerber H. Learning manual skills in anesthesiology: Is there a recommended number of cases for anesthetic procedures? Anesth Analg. 1998;86:635–639. doi: 10.1097/00000539-199803000-00037. [DOI] [PubMed] [Google Scholar]
- 2.Drake EJ, Coghill J, Sneyd JR. Defining competence in obstetric epidural anaesthesia for inexperienced traineesdagger. Br J Anaesth. 2015;114(6):951–957. doi: 10.1093/bja/aev064. [DOI] [PubMed] [Google Scholar]
- 3.Castanelli D. The rise of simulation in technical skills teaching and the implications for training novices in anaesthesia. Anaesth Intensive Care. 2009;37:903–910. doi: 10.1177/0310057X0903700605. [DOI] [PubMed] [Google Scholar]
- 4.McGaghie W, Issenberg S, Cohen E, Barsuk J, Wayne D. Does simulation-based medical education with deliberate practice yield better results than traditional clinical education? A meta-analytic comparative review of the evidence. Acad Med. 2011;86:706–711. doi: 10.1097/ACM.0b013e318217e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGaghie W, Draycott T, Dunn W, Lopez C, Stefanidis D. Evaluating the impact of simulation on translational patient outcomes. Simul Healthc. 2011;6:42–47. doi: 10.1097/SIH.0b013e318222fde9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook D, Brydges R, Zendejas B, Hamstra S, Hatala R. Mastery learning for health professionals using technology-enhanced simulation: a systematic review and meta-analysis. Acad Med. 2013;88:1178–1186. doi: 10.1097/ACM.0b013e31829a365d. [DOI] [PubMed] [Google Scholar]
- 7.Friedman Z, Siddiqui N, Katznelson R, Devito I, Bould M, Naik V. Clinical impact of epidural anesthesia simulation on short- and long-term learning curve: High- versus low-fidelity model training. Reg Anesth Pain Med. 2009;34:229–232. doi: 10.1097/AAP.0b013e3181a34345. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto ED, Hamstra SJ, Radomski SB, Cusimano MD. The effect of bench model fidelity on endourological skills: a randomized controlled study. J Urol. 2002;167:1243–1247. [PubMed] [Google Scholar]
- 9.Crabtree NA, Chandra DB, Weiss ID, Joo HS, Naik VN. Fibreoptic airway training: correlation of simulator performance and clinical skill. Can J Anaesth. 2008;55:100–104. doi: 10.1007/BF03016321. [DOI] [PubMed] [Google Scholar]
- 10.Gallagher AG, Ritter EM, Champion H, Higgins G, Fried MP, Moses G, Smith CD, Satava RM. Virtual reality simulation for the operating room: proficiency-based training as a paradigm shift in surgical skills training. Ann Surg. 2005;241:364–372. doi: 10.1097/01.sla.0000151982.85062.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guadagnoli MA, Lee TD. Challenge point: a framework for conceptualizing the effects of various practice conditions in motor learning. J Mot Behav. 2004;36:212–224. doi: 10.3200/JMBR.36.2.212-224. [DOI] [PubMed] [Google Scholar]
- 12.Malouin F, Jackson PL, Richards CL. Towards the integration of mental practice in rehabilitation programs: A critical review. Front Hum Neurosci. 2013;7:576. doi: 10.3389/fnhum.2013.00576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall JC. Imagery practice and the development of surgical skills. Am J Surg. 2002;184:465–470. doi: 10.1016/s0002-9610(02)01007-3. [DOI] [PubMed] [Google Scholar]
- 14.Bathalon S, Dorion D, Darveau S, Martin M. Cognitive skills analysis, kinesiology, and mental imagery in the acquisition of surgical skills. J Otolaryngol. 2005;34:328–332. doi: 10.2310/7070.2005.34506. [DOI] [PubMed] [Google Scholar]
- 15.Rogers RG. Mental practice and acquisition of motor skills: examples from sports training and surgical education. Obstet Gynecol Clin North Am. 2006;33:297–304. doi: 10.1016/j.ogc.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Cocks M, Moulton CA, Luu S, Cil T. What surgeons can learn from athletes: mental practice in sports and surgery. J Surg Educ. 2014;71:262–269. doi: 10.1016/j.jsurg.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Martin J. Mental preparation for the 2014 Winter Paralympic Games. Clin J Sport Med. 2012;22:70–73. doi: 10.1097/JSM.0b013e31824204cc. [DOI] [PubMed] [Google Scholar]
- 18.Leighton B. A greengrocer's model of the epidural space. Anesthesiology. 1989;70:368–369. [PubMed] [Google Scholar]
- 19.Raj D, Williamson RM, Young D, Russell D. A simple epidural simulator: a blinded study assessing the 'feel' of loss of resistance in four fruits. Eur J Anaesthesiol. 2013;30:405–408. doi: 10.1097/EJA.0b013e328361409c. [DOI] [PubMed] [Google Scholar]
- 20.Friedman Z, Katznelson R, Devito I, Siddiqui M, Chan V. Objective assessment of manual skills and proficiency in performing epidural anesthesia--video-assisted validation. Reg Anesth Pain Med. 2006;31:304–310. doi: 10.1016/j.rapm.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Beed P, Hawkins M, Roller C. Moving learners towards independence: the power of scaffolded instruction. Read Teach. 1991;44:648–655. [Google Scholar]
- 22.Palincsar A. The role of dialogue in providing scaffolded instruction. Educ Psychol. 1986;21:73–98. [Google Scholar]
- 23.Jackson PL, Lafleur MF, Malouin F, Richards CL, Doyon J. Functional cerebral reorganization following motor sequence learning through mental practice with motor imagery. Neuroimage. 2003;20:1171–1180. doi: 10.1016/S1053-8119(03)00369-0. [DOI] [PubMed] [Google Scholar]
- 24.Ericsson K, Krampe R, Tesch-Römer C. The role of deliberate practice in the acquisition of expert performance. Psychol Rev. 1993;100:363. [Google Scholar]
- 25.Stevens JA, Stoykov ME. Using motor imagery in the rehabilitation of hemiparesis. Arch Phys Med Rehabil. 2003;84:1090–1092. doi: 10.1016/s0003-9993(03)00042-x. [DOI] [PubMed] [Google Scholar]
- 26.Arora S, Aggarwal R, Sirimanna P, Moran A, Grantcharov T, Kneebone R, Sevdalis N, Darzi A. Mental practice enhances surgical technical skills: a randomized controlled study. Ann Surg. 2011;253:265–270. doi: 10.1097/SLA.0b013e318207a789. [DOI] [PubMed] [Google Scholar]
- 27.Sanders CW, Sadoski M, Bramson R, Wiprud R, Van Walsum K. Comparing the effects of physical practice and mental imagery rehearsal on learning basic surgical skills by medical students. Am J Obstet Gynecol. 2004;191:1811–1814. doi: 10.1016/j.ajog.2004.07.075. [DOI] [PubMed] [Google Scholar]
- 28.Page SJ, Dunning K, Hermann V, Leonard A, Levine P. Longer versus shorter mental practice sessions for affected upper extremity movement after stroke: a randomized controlled trial. Clin Rehabil. 2011;25:627–637. doi: 10.1177/0269215510395793. [DOI] [PMC free article] [PubMed] [Google Scholar]


