Abstract
Objective
To investigate development of cognitive and motor functions in healthy adolescents and to explore whether hazardous drinking affects the normal developmental course of those functions.
Method
Participants were 831 adolescents recruited across five United States sites of the National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA): 692 met criteria for no/low alcohol exposure, and 139 exceeded drinking thresholds. Cross-sectional, baseline data were collected with computerized and traditional neuropsychological tests assessing eight functional domains expressed as composite scores. General additive modeling evaluated factors potentially modulating performance (age, sex, ethnicity, socioeconomic status, and pubertal developmental stage).
Results
Older no/low-drinking participants achieved better scores than younger ones on five Accuracy composites (General Ability, Abstraction, Attention, Emotion, and Balance). Speeded responses for Attention, Motor Speed, and General Ability were sensitive to age and pubertal development. The exceeds-threshold group (accounting for age, sex, and other demographic factors) performed significantly below the no/low-drinking group on Balance accuracy and on General Ability, Attention, Episodic Memory, Emotion, and Motor speed scores and showed evidence for faster speed at the expense of accuracy. Delay Discounting performance was consistent with poor impulse control in the younger no/low drinkers and in exceeds-threshold drinkers regardless of age.
Conclusions
Higher achievement with older age and pubertal stage in General Ability, Abstraction, Attention, Emotion, and Balance suggests continued functional development through adolescence, possibly supported by concurrently maturing frontal, limbic, and cerebellar brain systems. Whether low scores by the exceeds-threshold group resulted from drinking or from other pre-existing factors requires longitudinal study.
Keywords: adolescent, development, alcohol, cognition, motor speed
Adolescence is a time of significant growth with respect to somatic size, brain structure, sexual maturity, and cognitive, motor, and emotional development (Giedd et al., 2014; Stiles & Jernigan, 2010; Witt, 2010). During their second decade, adolescents are presented with a plethora of options, including increased independence from parents and initiation of high-risk activities. The options of healthy to risky to dangerous activities is vast and poses serious challenges in decision making for teens, whose individual cognitive abilities and emotional maturity may well be at different stages of development. Among the high-risk behaviors adolescents are likely to initiate is drinking alcohol, commonly in binges. One recent study noted that 19% of high school seniors report having consumed five or more drinks in a row (binge episode) at least once in the previous two weeks (Johnston, O’Malley, Miech, Bachman, & Schulenberg, 2015). To investigate how hazardous drinking might affect the normal course of brain structural and functional development, the National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA) has begun a longitudinal study of youth before engaging in heavy drinking compared with adolescents who have already initiated drinking at moderate to heavy levels. Presented herein are results from baseline, cross-sectional testing (Brown et al., 2015).
Cross-sectional studies suggest adolescents with a diagnostically-determined drinking disorder show poorer neuropsychological performance than light and non-drinkers in various cognitive domains, including learning and memory (Brown, Tapert, Granholm, & Delis, 2000; Green et al., 2010; Sneider, Cohen-Gilbert, Crowley, Paul, & Silveri, 2013), executive function (Giancola, Mezzich, & Tarter, 1998; Parada et al., 2012), information processing (Tarter, Mezzich, Hsieh, & Parks, 1995), and language skills (Moss, Kirisci, Gordon, & Tarter, 1994). Longitudinal studies have extended these findings, suggesting that verbal memory (Hanson, Cummins, Tapert, & Brown, 2011; Nguyen-Louie et al., 2015), psychomotor speed (Nguyen-Louie et al., 2015), visuospatial abilities (Hanson et al., 2011; Nguyen-Louie et al., 2015; Squeglia, Spadoni, Infante, Myers, & Tapert, 2009; Tapert & Brown, 1999; Tapert, Granholm, Leedy, & Brown, 2002), and attentional functioning (Squeglia et al., 2009; Tapert et al., 2002) appear to worsen following the initiation (Squeglia et al., 2009) or continuation (Hanson et al., 2011; Tapert et al., 2002) of heavy drinking during adolescence and early adulthood. Untoward effects were also detected in youth who drank alcohol but did not meet diagnostic criteria for alcohol use disorder (Nguyen-Louie et al., 2015; Squeglia et al., 2009). Because many functions continue to mature during adolescence and with pubertal development (e.g., Blakemore, Burnett, & Dahl, 2010; Hedman, van Haren, Schnack, Kahn, & Hulshoff Pol, 2012; Shaw et al., 2008; Sowell, Thompson, & Toga, 2004; Sullivan et al., 2011) (for review, Stiles & Jernigan, 2010), initiation of hazardous drinking in these years of change may have a detrimental effect on the maturing brain.
Sex, socioeconomic status (SES), and ethnicity are factors in addition to age and puberty known to be associated with neuropsychological test performance during normal development and requiring consideration when assessing status of cognitive and motor functions (e.g., Akshoomoff et al., 2014; Noble et al., 2015; Noble, Houston, Kan, & Sowell, 2012). Typically, girls undergo sexual maturity earlier than boys (e.g., Cole, Pan, & Butler, 2014; Tanner, Whitehouse, & Takaishi, 1966) and advance earlier than boys in language skills (Neligan & Prudham, 1969), use of semantic knowledge (Hurks et al., 2010), facial emotion recognition and discrimination (Gur et al., 2012; Lawrence, Campbell, & Skuse, 2015), and components of episodic memory (Gur et al., 2012; Piper et al., 2011). By contrast, boys develop earlier than girls in mental rotation appreciation (Masters & Sanders, 1993; Voyer, Voyer, & Bryden, 1995), fine motor control (but see Denckla, 1973; Denckla, 1974; Piper, 2011), and physical strength (e.g., Dodds et al., 2014; McQuiddy, Scheerer, Lavalley, McGrath, & Lin, 2015). SES also plays a role in development (e.g., Lange, Froimowitz, Bigler, Lainhart, & Brain Development Cooperative, 2010; Noble et al., 2015)—less for motor tasks (Largo et al., 2001) but more so for skills related to language, such as fluency, vocabulary, and reading (e.g., Noble et al., 2012), and executive functioning (Boelema et al., 2014). The contribution of parental education as an index of SES can be distinct from financial status in its relation to focal brain maturation and its effect on specific components of cognitive development, including language, memory, emotional control, and executive functioning (Lawson, Duda, Avants, Wu, & Farah, 2013; Noble et al., 2015; Noble et al., 2012). Compounding these SES-related disparities are known differences in education, nutrition, health care, and safety available to low income, often minority, youth (Coley, Leventhal, Lynch, & Kull, 2013; McLoyd, 1998).
To assemble a sample that is adequately large and nationally representative (cf., Brown et al., 2015) to test the influence of these relevant factors on developmental differences, multisite studies are essential. Further, to assess the constellation of functions potentially affected by alcohol and that are still developing, computerized test batteries provide a means to accomplish this efficiently. Indeed, the utility of computerized test batteries has been demonstrated in a wide variety of settings, including sport head injury (Rahman-Filipiak & Woodard, 2013; Taylor, 2012), active-duty military (Cole et al., 2013), diseases of aging (Canini et al., 2014; Dwolatzky, Dimant, Simon, & Doniger, 2010; Mielke et al., 2014), epilepsy (Martinelli, Cecato, Bartholomeu, & Montiel, 2014), and infectious diseases potentially affecting the brain (Koski et al., 2011). Batteries, such as the CANTAB (Robbins et al., 1994), PhenX Toolkit (McCarty, Berg, et al., 2014; McCarty, Huggins, et al., 2014), NIH Toolbox (Carlozzi et al., 2014; Heaton et al., 2014; Weintraub et al., 2014), and the University of Pennsylvania Web-based Computerized Neurocognitive Battery (WebCNP) (webcnp.med.upenn.edu/) (Gur et al., 2012; Gur et al., 2010), each use multiple measures to assess principal cognitive domains of executive functions, several component processes of declarative memory, visuospatial abilities, emotion discrimination, and emotional control valid for pre-adolescence through senescence and commonly affected in adolescents with alcohol use disorder (for review, Squeglia, Jacobus, & Tapert, 2014). Benefits of most computerized batteries include acquisition of response time for individual trials for every test, thereby enabling assessment of speed of responding and efficiency scores based on speed-accuracy tradeoff (Gur et al., 2010). As these batteries have evolved, the test length relative to the amount of information obtained has become briefer. Another advantage of computer-based testing is automated scoring and data uploading without labor-intensive and error-prone hand scoring, checking, and double entry into a computer database, especially useful in large-scale, multisite studies.
The primary aims of this study were to identify selective cognitive and motor functions showing evidence of continued maturation during adolescence and to distinguish functions spared and those vulnerable to hazardous drinking during this period of functional change (Brown et al., 2015; Winward, Bekman, Hanson, Lejuez, & Brown, 2014). The functions targeted were executive functions of planning, monitoring, mental flexibility, verbal fluency, attention, and inhibition; achievement based on reading, comprehension, math ability; episodic memory for verbal, visual, face, and spatial material; working memory for verbal and nonverbal material; emotion processing and regulation; reward seeking and learning; visual discrimination; and general intelligence. We tested the hypotheses that functions subserved by frontal, superior parietal, and medial temporal cortical regions, which continue to develop into late adolescence (Hedman et al., 2012; Raznahan, Greenstein, Lee, Clasen, & Giedd, 2012; Sowell, Thompson, Leonard, et al., 2004) would exhibit age-related effects, where older adolescents would score higher on accuracy and speed measures of executive functions, emotion processing, episodic memory, and general ability. In exploratory analyses, we tested the hypotheses that adolescents who exceeded a threshold for no/low alcohol or drug exposure would perform more poorly than those who met these criteria on tests of functions commonly compromised in youth with alcohol use disorder (AUD), namely, executive functions, spatial working memory, emotion processing, and balance (e.g., Squeglia, Jacobus, Nguyen-Louie, & Tapert, 2014).
Method
Participants
This report presents the initial, cross-sectional analysis of neuropsychological data collected on 831 adolescents recruited across five sites in the United States (University of California at San Diego, SRI International, Duke University Medical Center, University of Pittsburgh Medical Center, and Oregon Health & Science University) and enrolled in the NCANDA study. Assessment was the same across all sites and used a combination of computerized and traditional neuropsychological tests. The NCANDA study is designed to follow adolescents (age 12 to 21 at entry) annually for four years. Of the total group, 692 met criteria for no-to-low alcohol or drug exposure, and as an initial exploration of the effects of alcohol and drug exposure, an additional 139 adolescents with a history of drinking beyond the age-specific, no/low thresholds were also tested (see Brown et al., 2015).
Informed consent
All participants underwent an informed consent process with a research associate trained in human subject research protocols. Adult participants or the parents of minor participants provided written informed consent before participation in the study. Minor participants provided assent before participation. The Internal Review Boards of each site approved this study, and each site followed this procedure to obtain voluntary informed consent or assent, depending on the age of the participant.
Recruitment strategy
Participants were recruited through local schools and colleges, public notices, and targeted catchment-area calling. Over 7,500 individuals contacted NCANDA sites for screening, and 2,548 target participants (as well as one biological parent per participant) completed a screening interview, ultimately yielding a sample of 831 participants.
A demographic interview inquiring about health and academic functioning, including those associated with initiation of drinking relevant to the adolescents, was completed by youth and one parent to confirm participant eligibility (Anderson, Tomlinson, Robinson, & Brown, 2011; Brown et al., 2008; Zucker, Donovan, Masten, Mattson, & Moss, 2008). Additional inclusion and exclusion criteria were confirmed using a combination of the Semi-Structured Assessment of the Genetics of Alcoholism (SSAGA) (Bucholz et al., 1994; Hesselbrock, Easton, Bucholz, Schuckit, & Hesselbrock, 1999) and the Family History Assessment Module (Rice et al., 1995). For full ascertainment procedures see Brown et al. (Brown et al., 2015).
The majority of participants (83%) had limited exposure to alcohol or other drugs (Supplemental Table 1), which was required, because a primary aim of NCANDA is to determine neurocognitive precursors to, and changes following, the onset of heavy alcohol use. A small portion of the sample (17%) that exceeded criteria for alcohol use was recruited using the same methods and was included to represent a range of drinking for future trajectory analyses. These individuals who exceeded drinking thresholds were also allowed to exceed nicotine and marijuana exposure criteria, but were required to meet all other inclusion criteria (including other drug use; Supplemental Table 1). The exceeds-threshold group included largely the older age ranges, although some younger drinkers were also enrolled (Supplemental Table 2). The exceeds-threshold group did not differ from the larger sample on parental education, sex distribution, or ethnic background (see Brown et al., 2015 for full description of the two samples). The value of recruiting this subsample with more extensive drinking history will be realized in subsequent longitudinal analyses; however, at baseline this group serves as a de facto comparison group to the no/low-drinking group.
Each site contributed 15–26% of the sample. The sample was distributed across age groups and matched for sex with the largest proportion (44%) from the 12–14 year old age group. There were no significant age group or sex differences across sites. The sample is roughly equivalent to reported census numbers (U.S. Census Bureau, 2011) and is reflective of the counties surrounding NCANDA collection sites (see Brown et al., 2015 for comparison with census data). By design, compared with the no/low drinking group, the sample that exceeded drinking thresholds was biased toward the oldest age group with more than 60% over age 18.
Screening was conducted to facilitate oversampling for risk for future alcohol use (e.g., family history of alcohol problems, externalizing disorder symptoms), matching sex within age groups, and meeting enrollment targets for age and racial/ethnic groups. An additional 607 participants met eligibility criteria after screening but were not enrolled in the study as enrollment targets for age, sex, and racial/ethnic categories had already been fulfilled.
Participants were excluded based on age, MRI contraindications, physical limitations, parental availability/consent, substance use history, serious medical conditions, history of traumatic brain injury, ongoing psychotropic medication use, prenatal alcohol/drug exposure, and presence or history of learning disabilities or neurodevelopmental disorders; all of which were confirmed by in-person interviews following initial screening. Specifically, participants were screened for medical conditions that could affect MRI, brain development, or study participation, including diabetes, recurrent migraine, and traumatic brain injury with loss of consciousness >30 minutes. Additionally, participants were screened for neurodevelopmental conditions that could affect brain development or study participation evidenced by history of and persistence in severe learning disorder, pervasive developmental disorder, or other condition requiring repeated or persistent specialized education (e.g., estimated IQ >2 SD below mean). Individuals with a history of mood and anxiety disorders that were not likely to interfere with study participation were not excluded (e.g., major depressive disorder, anxiety/panic [with the exception of claustrophobia], and PTSD). Such disorders were endorsed in the sample commensurate with recent epidemiological reports of these age ranges: 7% (n=50) of the no/low drinking group and 13% (n=18) of the exceeds drinking group endorsing lifetime major depressive disorder, and all other anxiety disorders endorsed by <1% of either sample [Substance Abuse and Mental Health Services Administration (SAMHSA) Center for Behavioral Health Statistics and Quality. (2015). Behavioral health trends in the United States: Results from the 2014 National Survey on Drug Use and Health (HHS Publication No. SMA 15-4927, NSDUH Series H-50]. Inclusion and exclusion criteria were minimized to increase our ability to recruit a more representative sample.
No/low vs. exceeds-threshold drinking groups
Participants completed the Customary Drinking and Drug use Record (CDDR, Brown et al., 1998) to characterize their past and current alcohol and substance use. By definition, the no/low-drinking group reported no lifetime heavy drinking occasions (i.e., no episodes in which they drank 4 or more drinks for female and 5 or more for male youth); however, 18% of the no/low-drinking group reported some history of drinking. A preponderance of endorsement of drinking history in the no/low-drinking group came from participants over 18 years of age (i.e., 43% of 18 and over participants reported at least one lifetime drink, whereas only 3% of those under age 15 reported the same). In addition, the conservative thresholds for lifetime cigarette, marijuana, and other drug use (Supplemental Table 1) yielded a relatively clean sample with 5% endorsing any nicotine exposure, 9% endorsing marijuana exposure, and 2% endorsing other drug exposure. By contrast, the drinking group that exceeded thresholds endorsed drinking at levels above age-matched national norms (SAMHSA, 2015; see Brown et al., 2015) with 85% reporting a heavy drinking occasion in the last year and 33% in the past month. In addition, 32% endorsed a history of cigarette use, although only 5% (n=7) reported smoking at least once per week and ranged from 1–6 cigarettes smoked per day. Marijuana use was more prevalent in the exceeds group with 68% endorsing lifetime exposure and 12% (n=17) reporting use at least once/week.
Alcohol and drug testing
All participants submitted samples to a 12-panel urine toxicology screen for amphetamine, methamphetamine, cocaine, phencyclidine, benzodiazepines, barbiturates, opiate, oxycodone, propoxyphene, methadone, tricyclic antidepressants, marijuana and a breathalyzer for alcohol to confirm absence of evidence for recent use of drugs of abuse. Positive screens other than marijuana were sent for GC/MS confirmation, and if confirmed, participants were excluded from testing that day. Participants with positive alcohol or drug results were then asked to abstain from alcohol for at least 24 hours and other drugs for 72 hours prior to assessment sessions and were tested again for alcohol and drugs on the return visit. Self-report of recent nicotine, caffeine, and medication use was also obtained at each assessment.
Analysis groups
The first set of analyses focused on neuropsychological data acquired across the five NCANDA recruitment sites in 344 male and 348 female adolescents, ages 12.0 to 21.9 years old (Table 1), who met basic alcohol and drug use criteria for no-to-low exposure (Supplementary Table 1) in the NCANDA study. The second set of analyses compared performance of the no/low-drinking group with an independent group of 139 adolescents (64 male, 75 female) whose alcohol consumption exceeded the thresholds (Supplemental Tables 1–2) and were deemed a moderate/high-drinking group; 9 met lifetime criteria for DSM-IV Alcohol Abuse, and none met criteria for Alcohol Dependence.
Table 1.
NCANDA demographics for neuropsychological test analysis (N=831)
| No/Low Drinker (N=692) | Male vs. Female | Exceeded-Threshold (N=139) | Male vs. Female | No/low vs. Exceeds | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Male | Female | t or χ2 | p | Male | Female | t or χ2 | p | t or χ2 | p | ||
| N | 344 | 348 | 64 | 75 | |||||||
| Age (years) | mean | 15.6 | 15.8 | −0.850 | 0.3954 | 18.4 | 18.5 | −0.305 | 0.7609 | −14.841 | 0.0000 |
| standard deviation | 2.3 | 2.4 | 2.0 | 2.0 | |||||||
| N | 344 | 348 | 64 | 75 | |||||||
| Education (years) | mean | 8.9 | 9.2 | −1.155 | 0.2486 | 11.7 | 11.9 | −0.440 | 0.6607 | −14.524 | 0.0000 |
| standard deviation | 2.3 | 2.5 | 1.9 | 2.0 | |||||||
| N | 342 | 346 | 64 | 74 | |||||||
| Pubertal Development Scale | mean | 2.9 | 3.4 | −10.917 | 0.0000 | 3.5 | 3.8 | −5.635 | 0.0000 | −11.553 | 0.0000 |
| median | 3.0 | 3.6 | 3.6 | 4.0 | |||||||
| standard deviation | 0.7 | 0.6 | 0.5 | 0.2 | |||||||
| N | 339 | 345 | 62 | 73 | |||||||
| Socioeconomic status | mean | 17.0 | 16.6 | 2.057 | 0.0401 | 17.1 | 17.3 | −0.400 | 0.6902 | −1.743 | 0.0832 |
| standard deviation | 2.4 | 2.5 | 2.1 | 2.3 | |||||||
| N | 326 | 326 | 54 | 58 | |||||||
| Handedness | L/R/A | 33/261/49 | 23/290/35 | 5.936 | 0.0514 | 2/53/9 | 7/61/7 | 2.736 | 0.2546 | 0.527 | 0.7685 |
| N | 343 | 348 | 64 | 75 | |||||||
| Family History of Alcoholism | |||||||||||
| negative/positive | 318/26 | 316/32 | 0.410 | 0.5222 | 50/14 | 63/12 | 0.445 | 0.5047 | 12.464 | 0.0004 | |
| Self-declared Ethnicity | N | 344 | 348 | 8.997 | 0.1092 | 64 | 75 | 3.279 | 0.3505 | 5.215 | 0.3902 |
| Caucasian | 251 | 235 | 52 | 57 | |||||||
| African-American | 34 | 53 | 3 | 9 | |||||||
| Asian | 27 | 25 | 6 | 4 | |||||||
| Pacific Islander | 1 | 3 | 0 | 0 | |||||||
| American Indian | 3 | 0 | 0 | 0 | |||||||
| Mixed | 28 | 32 | 3 | 5 | |||||||
N=number
L/R/A=left/right/ambidextrous
Participants were characterized by age, sex, pubertal stage using the self-assessment Pubertal Development Scale (PDS) (Petersen, Crockett, Richards, & Boxer, 1988; Shirtcliff, Dahl, & Pollak, 2009), self-identified ethnicity, and socioeconomic status (SES) determined as the highest level of education achieved by either parent (Akshoomoff et al., 2014) (Table 1). In light of the substantial differences in salaries and incomes across the five geographically-distributed data collection sites, we expressed SES with reference to parental education level, which is less subject than family income to geographical differences in the U.S. Most subjects reported a single self-identified ethnicity (Caucasian, African-American, Asian, Pacific Islander, and Native American) with some reporting mixed heritage. There were adequate numbers of the first three types to assign categorical ethnicity, with dual-heritage identifications assigned to the minority ethnicity group (e.g., Asian-Caucasian was categorized as Asian) (Table 1).
Neuropsychological Tests
Test selection conformed to the requirements of the NIH funding announcement (RFA-AA-12-006), which noted that data collection sites use a common neuropsychological battery, tapping 8 functional domains: 1) executive function (planning/monitoring, mental flexibility, verbal fluency, attention, inhibition); 2) memory (verbal, visual, face, spatial, and working); 3) emotion processing and regulation; 4) reward seeking and learning; 5) handedness and dexterity; 6) visual discrimination; 7) intelligence; and 8) achievement (reading, comprehension, math ability). Other considerations for test selection included recognized validity of domain assessment, validation for age range, reliability, score range, and practice effects. Accordingly, the final test battery comprised selected tests and measures from the WebCNP and traditional neuropsychological tests. Table 2 lists the functional domains, test names, specific cognitive and motor processes assessed, and brain regions reported to support each process. Supplemental Table 2 lists the composite domains, test measures and variable names entered into each composite domain, and scoring procedure for each measure. Delay Discounting (Bickel et al., 2007; Stanger, Budney, & Bickel, 2013; Stanger et al., 2012) was included to examine reward seeking and decision-making and can be considered to provide a measure of impulsive behavior.
Table 2.
Composite domains: Tests, functions, processes, and neural substrates
| Functional Domain | Name of Test | Cognitive or Motor Process | Brain Regions Associated with Process | References |
|---|---|---|---|---|
| Abstraction | Conditional Exclusion Task† Matrix Analysis Test† Logical Reasoning † |
Abstraction and concept
formation Abstraction and mental flexibility Verbal intellectual ability |
Frontal Frontal and posterior parietal Left Temporoparietal |
Kurtz et al., 2004; Gunning-Dixon and Raz,
2003 Lee et al., 2005 Gur et al., 1982, 2000 |
| Attention | Continuous Performance Test - Number Letter Version† | Visual attention and bigilance | Frontoparietal | Kurtz et al., 2001; Ogg et al., 2008 |
| Balance | Walk-a-Line | Postural stability | Frontocerebellar | Fregly et al., 1972; Sullivan et al., 2000, 2006 |
| Emotion | Emotion Recognition Test† Measured Emotion Differentiation† |
Emotion recognition Emotion discrimination |
Temporo-limbic Fronto-amygdala |
Gur et al., 2002a, 2002b Fossati, 2012 |
| Episodic Memory | Facial Memory Test† Word Memory Test† Short Visual Object Learning Test† |
Facel memory (immediate and
delayed) Word memory (immediate and delayed) Visual object learning and memory (immediate and delayed) |
Frontal and bilateral anterior medial
temporal Frontal and bilateral anterior medial temporal Frontal and bilateral anterior medial temporal |
Gur et al., 1997; Jackson and Schacter,
2004 Gur et al., 1997; Jackson and Schacter, 2004 Glahn et al., 1997; Jackson and Schacter, 2004 |
| General Ability | Vocabulary Test† WRAT-4 Math Calculations WRAT-4 Word Reading |
General
knowledge Arithmetic Phonological expression |
Frontotemporoparietal Frontoparietal Temporoparietal |
Lee et al., 2014 Wilkinson and Robertson, 2010; Gruber et al., 2001 Wilkinson and Robertson, 2010; Christodoulou et al., 2014 |
| Working Memory | Short Fractal N-Back Test - 2 Back Version† | Attention and working memory | Dorsolateral prefrontal | Ragland et al., 2002; Rodriguez-Jimenez et al., 2009 |
| Motor Speed | Grooved Pegboard Digit Symbol |
Digit dexterity and psychomotor
speed Psychomotor speed |
Frontal Frontal |
Matthews and
Kløve, 1964; Kochunov et al., 2010 Wechsler, 2008; Gautam et al., 2011 |
Subtests of the WebCNP (Computerized Neuropsychological Testing System, University of Pennsylvania School of Medicine), Gur et al., 2010
Abstraction References
Kurtz MM, Ragland JD, Moberg PJ, Gur RC. The Penn Conditional Exclusion Test: a new measure of executive-function with alternate forms for repeat administration. Archives of Clinical Neuropsychology 2004; 19: 191–201.
Gunning-Dixon FM, Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults: a prospective MRI Study. Neuropsychologia 2003; 41: 1929–1941.
Lee KH, Choi YY, Gray JR, Cho SH, Chae JH, Lee S, Kim K. Neural correlates of superior intelligence: stronger recruitment of posterior parietal cortex. Neuroimage 2006; 29: 578–586.
Gur RC, Gur RE, Obrist WD, Hungerbuhler JP, Younkin D, Rosen AD, Skolnick BE, Reivich M. Sex and handedness differences in cerebral blood flow during rest and cognitive activity. Science 1982; 217: 659–661.
Gur RC, Alsop D, Glahn D, Petty R, Swanson CL, Maldjian JA, Turetsky BI, Detre JA, Gee J, Gur RE. An fMRI study of sex differences in regional activation to a verbal and a spatial task. Brain and Language 2000; 74: 157–170.
Attention References
Kurtz MM, Ragland JD, Bilker W, Gur RC, Gur RE. Comparison of the continuous performance test with and without working memory demands in healthy controls and patients with schizophrenia. Schizophrenia Research 2001; 48: 307–316.
Ogg RJ, Zou P, Allen DN, Hutchins SB, Dutkiewicz RM, Mulhern RK. Neural correlates of a clinical continuous performance test. Magnetic Resonance Imaging 2008; 26: 504–512.
Balance References
Fregly AR, Graybiel A, Smith MJ. Walk on floor eyes closed (WOFEC): a new addition to an ataxia test battery. Aerosp Med, 1972; 43: 395–399.
Sullivan EV, Deshmukh A, Desmond JE, Lim KO, Pfefferbaum A. Cerebellar volume decline in normal aging, alcoholism, and Korsakoff’s syndrome: Relation to ataxia. Neuropsychology 2000; 14:341–352.
Sullivan EV, Rose J, Pfefferbaum A. Effect of vision, touch, and stance on cerebellar vermia-related sway and tremor: a quantitative physiological and MRI study. Cerebral Cortex 2006; 16: 1077–1086.
Emotion References
Gur RC, Sara R, Hagendoorn M, Marom O, Hughett P, Macy L, Turner T, Bajcsy R, Posner A, Gur RE. A method for obtaining 3-dimentional facial expressions and its standardization for use in neurocognitive studies. J Neurosci Methods 2002; 15:137–143.
Gur RC, Schroeder L, Turner T, McGrath C, Chan RM, Turetsky BI, Alsop D, Maldjiian J, Gur RE. Brain activation during facial emotion processing. Neuroimage 2002; 16: 651–662.
Fossati P. Neural correlates of emotion processing: from emotional to social brain. Eur Neuropsychopharmacol 2012; 22 Suppl 3: S487–491.
Episodic Memory References
Gur RC, Ragland JD, Mozley LH, Mozley PD, Smith R, Alavi A, Bilker W, Gur RE. Lateralized changes in regional cerebral blood flow during performance of verbal and facial recognition tasks: Correlations with performance and “Effort.” Brain and Cognition 1997; 33: 388–414.
Glahn DC, Gur RC, Ragland JD, Censits DM, Gur RE. Reliability, performance characteristics, construct validity, and an initial clinical application of a visual object learning test (VOLT). Neuropsychology 1997; 11: 602–612.
Jackson O 3rd, Schacter DL. Encoding activity in anterior medial temporal lobe supports subsequent associative recognition. Neuroimage 2004; 21: 456–462.
General Ability References
Lee NR, Raznahan A, Wallance GL, Alexander-Bloch A, Clasen LS, Lerch JP, Giedd JN. Anatomical coupling among distributed cortical regions in youth varies as a function of individual differences in vocabulary abilities. Human Brain Mapping 2014; 35: 1885–1895.
Gruber O, Indefrey P, Steinmetz H, Kleinschmidt A. Dissociating neural correlates of cognitive components in mental calculations. Cerebral Cortex 2001; 11: 350–359.
Christodoulou JA, Del Tufo SN, Lymberis J, Saxler PK, Ghosh SS, Triantafyllou C, Whitfield-Gabrieli S, Gabrieli JDE. Brain bases of reading fluency in typical reading and impaired fluency in dyslexia. PLOS ONE Vol 9 Issue 7 e100552
Working Memory References
Ragland JD, Turetsky BI, Gur RC, Gunning-Dixon F, Turner T, Schroeder L, Chan R, Gur RE. Working memory for complex figures: an fMRI comparison of letter and fractal n-back tasks. Neuropsychology 2002; 16: 370–379.
Rodriguez-Jimenez R, Avila C, Garcia-Navarro C, Bagney A, Aragon AM, Ventura-Campos N, Martinez-Gras I, forn C, Ponce G, Rubin G, Jimenez-Arriero MA, Palomo T. Differential dorsolateral prefrontal cortex activation during a verbal n-back task according to sensory modality. Behav Brain Res 2009; 205: 299–302.
Motor Speed References
Kochunov P, Coyle T, Lancaster J, Robin DA, Hardies J, Kochunov V, Bartzokis G, Stanley J, Royall D, Schlosser AE, Null M, Fox PT. Processing speed is correlated with cerebral health markers in the frontal lobes as quantified by neurominaging NeuroImage 2010; 49: 1190–1199.
Gautam P, Cherbuin N, Sachdev PS, Wen W, Anstey K. Relationships between cognitive function and frontal grey matter volumes and thickness in middle aged and early old-aged adults: The PATH through life study NeuroImage 2011; 55: 845–855.
Test Procedures
Testing was conducted in quiet rooms by laboratory assistants trained with annual reliability evaluations to criterion and calibrated annually by a centrally-trained psychometrician using procedures established by the NCANDA Data Analysis Component. The battery of tests was administered in the same order across all sites. Scheduled breaks were offered to participants to minimize fatigue. Scoring was completed without intervention for the computer tests via WebCNP, LimeSurvey (www.limesurvey.org/), or Blaise (www.blaise.com); all other tests were double scored and entered into NCANDA-specific forms through the Research Electronic Data Capture (REDCap) system. The total test battery was generally completed in about 3 hours.
WebCNP
We selected 15 WebCNP test, which took approximately 60 min. and was installed on Apple laptop computers (13-inch MacBook Air, OS X 10.8). The battery consisted of computer-administered and computer-scored tests representing 7 of the 8 functional domains, yielding accuracy and speed measures (uncorrected for age, sex, ethnicity, or socioeconomic factors) for all tests used in the current analysis (Table 2 and Supplemental Table 3). Test results were uploaded into the software platform, Scalable Informatics for Biomedical Imaging Studies (SIBIS; Rohlfing, Cummins, Henthorn, Chu, & Nichols, 2014; Nichols & Pohl, 2015) at SRI International. The WebCNP has established construct validity and reliability and was standardized on upwards of 10,000 participants (depending on the measure) with a broad, age range (8–90 years old) (Gur et al., 2010). Descriptions of the 15 WebCNP tests are arranged by functional domains; most tests have both accuracy and speed (response time) measures (Supplemental Table 3). The descriptions are modified from the WebCNP support manual.
Abstraction
Conditional Exclusion measures abstraction and mental flexibility. There are three principles for choosing an object: line thickness, shape, and size. These change as the participant achieves 10 consecutive correct answers for each principle. The participant has 48 trials to make 10 consecutive correct answers for each principle. There is only one principle in effect for any trial, but a response may match more than one principle. The participant is not told what the ruling principle is and must derive the correct principle through feedback. If the participant does not achieve a principle within 48 trials, the test ends.
Matrix Analysis Test, a measure of abstraction and mental flexibility, is a multiple choice task in which the participant must conceptualize spatial, design, and numerical relations that range in difficulty from very easy to increasingly complex. The participant chooses a square that best fits in the missing space of a pattern. Patterns are made up of 2×2, 3×3, and 1×5 arrangements of squares. Each item has five response options.
Logical Reasoning, a measure of verbal intellectual ability, is a multiple-choice task in which the participant must complete verbal analogy problems.
Attention
The Continuous Performance task has two parts: one in which the participant must press the spacebar whenever lines form a complete number, and one whenever lines form a complete letter. Each part lasts 1.5 minutes. Each stimulus flashes for 300 ms followed by a blank page displayed for 700 ms, giving the participant 1 sec to respond to each trial.
Emotion
For Emotion Recognition, participants view a series of 40 faces and indicate what emotion the face is showing: Happy, Sad, Angry, Scared, or No Feeling. There are 4 female faces for each emotion (4 × 5 = 20) and 4 male faces for each emotion (4 × 5 = 20).
Emotion Differentiation measures the ability to detect emotion intensity. The participant views pairs of faces and chooses the face showing greater intensity of emotion (anger, fear, happiness, sadness), or chooses a central button labeled Equal. The stimuli are created using software to morph faces into differing intensities of emotion. There are 36 trials, divided into happy, sad, angry, and fearful faces. Of the 36 trials, 4 show no emotional difference. The remaining 32 trials have emotion differentials in increments of 10% ranging from 10% – 60%, distributed more heavily toward 30% and 40% items. Trials are presented in random order, and the test is a forced-choice task with no time limit per trial.
Episodic Memory
In the Face Memory test, participants are first shown 20 faces that they will be asked to identify later during immediate and delayed recognition trials. During immediate recall, participants view a series of 40 faces; 20 faces are targets for memory and 20 are distractors. Participants decide whether they had been shown the face by choosing one of four buttons, presented in a 4-point scale: “definitely yes,” “probably yes,” “probably no,” and “definitely no” via the mouse. Delayed memory is tested approximately 25 min. after immediate memory.
The Word Memory test is a verbal analogue to Face Memory and follows the same procedure for immediate and delayed recognition.
Visual Object Learning requires participants to view 10 three-dimensional Euclidean shapes that they will be asked to identify for both immediate and delayed recognition in the same manner as Face Memory and Word Memory.
Working Memory
Short Fractal N-back measures attention and working memory. Participants view fractal designs displayed on the computer screen and indicate the “target design.” There are three trial types. During the 0-back, the target design is designated before the trial and the participant responds each time they see it. For the 1-back and 2-back the target design is indicated by the repetition of a design, with the participants responding when they see a design for the first time for 1-back or the second time for 2-back. In all trials, the participant has 2500 ms to respond.
Motor Speed
Motor Praxis is the first WebCNP test in the battery and measures sensorimotor ability by having the participant use the mouse to click on a shrinking box when it moves to a new position on the screen. This test screens a participant’s dexterity, an essential ability to perform the WebCNP tests.
General Ability
Vocabulary comprises five subtests, each containing 10 multiple-choice items with four response choices. The questions in each section are presented in order of increasing difficulty. A section is discontinued if the participant answers 5 questions incorrectly. Each subtest uses a different measure of verbal knowledge. In Part I, the participant chooses a word “closest in meaning” to the target word. In Part II, the participant chooses the word that has a similar meaning to a bolded phrase within a sentence. In Part III, the participant selects the one word that is not a valid English word. In Part IV, the participant selects the word that is opposite in meaning to the target word. In Part V, the participant must choose the correct sentence based on contextual use of a target word.
Traditional tests
Administration and scoring of these “pencil-and-paper tests” follows published instructions. The Wide Range Achievement Test-4 (WRAT4) assesses general ability in word reading (blue form) and math calculation (Wilkinson & Robertson, 2010); these scores were included in the General Ability composite. Grooved pegboard (Lezak, Howieson, & Loring, 2004; Matthews & Kløve, 1964) measures manual dexterity; the score is the number of seconds a participant took to complete insertion of pegs into holes for each hand separately and entered into the Motor Speed composite. The Digit Symbol subtest of the Wechsler Adult Intelligence Scale-IV was administered as prescribed (Wechsler, 2008); only the raw scores were used in analysis in the Motor Speed composite. Postural stability, measured with the modified Fregly-Graybiel Walk-a-Line ataxia test (Fregly, Graybiel, & Smith, 1972; Sullivan, Deshmukh, Desmond, Lim, & Pfefferbaum, 2000), uses 4 conditions and was conducted twice if the first trial was not completed perfectly (arms folded, eyes closed, feet straight on a line of the floor): stand heel-to-toe for 60 sec; stand on one and then the other foot for 30 sec. each; walk heel-to-toe for 10 steps; these scores comprised the Balance composite. Handedness was determined with the Edinburgh Handedness Questionnaire (Oldfield, 1971), visual acuity with the Landolt C test (Bach, 2007), and color vision with the Ishihara Test (Ishihara, 1983).
Cognitive test of reward-seeking and impulsivity
The Delay Discounting task assessed preference for smaller immediate versus larger delayed reward (Stanger et al., 2012). The task was administered and scored by computer (13-inch Dell Inspiron 5323 running Windows 7). Participants are asked to choose between accepting a smaller amount of money today compared to a larger amount of money at varying delays (e.g., 1 day, 1 week, 1 month, or 6 months). The primary outcome variable from the delay discounting is k, which represents the rate of discounting. Since k is positively skewed, the natural log is used (“lnk”) (Mazur, 1987). lnk was determined by fitting the data with a non-linear search function “nls” in R. A steeper rate of discounting is related to greater preference for short-term gains over larger longer-term gains and indicates greater impulsive choice or “impulsivity.” The task was completed for two values ($100 and $1000) at varying delays. The delay rate, lnk, was calculated for each of the 2 values and each of the 4 delays, yielding 8 total variables. Subjects who had an indifference point 20% or larger than the previous point were excluded (Lee, Stanger, & Budney, 2015). Data for the two monetary conditions ($100 and $1000) were first analyzed separately and then those subjects who had valid data for both values were analyzed together to determine the effect of the monetary value.
The computerized Delayed Discounting task implemented here was published by Stanger et al. (Stanger et al., 2012) and has been validated in an adolescent sample. Use of two reward amounts reduces economic context effects across the age and SES range of our sample. Delayed Discounting tasks like this one have shown discriminant validity across a range of substance use disorders, where those who use drugs are more likely to favor immediate rewards than non-users (MacKillop et al., 2011).
Scalable Informatics for Biomedical Imaging Studies (SIBIS)
The informatics infrastructure for collecting data consisted of the Research Electronic Data Capture (REDCap) system (Harris, Taylor, Thielke, Payne, Gonzalez, & Conde, 2009), University of Pennsylvania Web-based Computerized Neurocognitive Battery (WebCNP) (https://webcnp.med.upenn.edu/), LimeSurvey (http://www.limesurvey.org/), and Blaise (http://www.blaise.com). All data collected were automatically merged onto a REDCap server hosted by the NCANDA Data Analysis Component at SRI International. Specifically, test scores not collected directly through entry forms in REDCap were automatically uploaded from the laptop of the collection sites via secure encrypted connections to a Subversion (https://subversion.apache.org/) server, then automatically imported into REDCap. The data used in this manuscript were then organized via a formal, locked data release (VERSION: NCANDA_DATA_00010). Additional information about the NCANDA Data Management System has been published elsewhere (Rohlfing et al., 2014; Nichols & Pohl, 2015).
Data Analysis
The primary independent variable in this cross-sectional analysis was age; the dependent variables were neuropsychological test scores, submitted to empirically-driven data reduction to derive composite scores, reflecting the targeted neuropsychological functions. Covariates of interest were sex, self-described ethnicity, highest parental education achieved as a surrogate for SES, study site, and pubertal development stage.
The primary analysis tools were the General Additive Model (GAM) (Hastie & Tibshirani, 1986, 1990; Wood, 2006, 2011) and analysis of variance (ANOVA) from the “mgcv” package in R Version 3.1.0 [http://www.r-project.org/], testing for the predictive value of the main effect of age with selective covariates. Additional analyses used a General Linear Model (GLM). The initial GAM (Model 1) tested the predictive value of age and 4 covariates—site, ethnicity, SES, and sex—on each performance score.
| Model 1 |
Age was allowed to be a nonlinear smooth effect, implemented via thin plate splines with 3 knots (Wood, 2003). Roughness penalties for the smooth effects were estimated using generalized cross validation (Wood, 2004). Subsequent GAMs replaced age with PDS as the principal variable.
Many scores were modulated by several or all covariates. Therefore, the contributions of the covariates were examined in a step-wise manner with sub-models excluding various covariates and categorical predictions. The first set of analyses focused on the no/low-drinking group, and the second set compared performance by the no/low and exceeds-threshold groups. The sample sizes vary slightly across models tested (noted in the results tables) because not all participants had data for all covariates.
Results
The Results are presented in two main parts. The first part focuses on the 7 accuracy and 7 speed, theoretically-driven, composite scores that represent the functional domains targeted in the NCANDA study, the Delay Discounting task to assess reward seeking and decision making, and pubertal development as a predictor of performance. The second part examines potential performance differences between the no/low-drinking and the exceeds-threshold groups.
Part 1: Performance by the No/low-drinking Group (N=692)
Construction of Composite Scores and Performance on Individual Measures
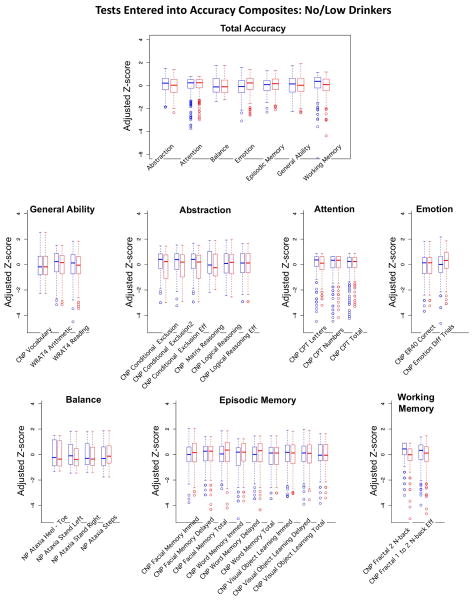
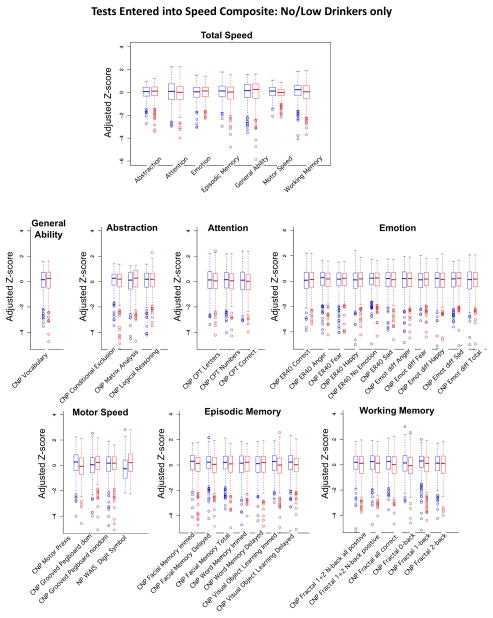
Composite score construction followed three steps (Gur et al., 2012; Sullivan, Shear, Zipursky, Sagar, & Pfefferbaum, 1994). First, each measure was standardized on scores achieved by all male and female adolescents who met NCANDA entry criteria (maximum N=692) and expressed as a Z-score (mean =0±1SD). Not all participants had scores for all measures, typically due to computer failure, participant’s refusal to perform a test, or lack of testing time; Table 3 presents the sample sizes for each composite score. Next, all scores for which a low score signified good performance were transformed by multiplying scores by −1 so that high scores for all measures (i.e., accuracy and speed) were in the direction of good performance (Figures 1–2). Finally, the mean Z-score of all individual measures that comprised a composite was calculated. Accuracy and speed composite scores were calculated separately, are presented in box plots in Figures 1–2, and were used as the dependent measures in testing factors using the GAM.
Table 3.
Composite scores: Proportion of variance accounted for by the Age Full Model (GAM†) and the Full Model without SES, site, ethnicity, or sex as covariates
| N | FM | FM w/o SES | SES Effect | FM w/o Site | Site Effect | FM w/o Ethnicity | Ethnicity Effect | FM w/o Sex | Sex Effect | Linear | Age w/o covariates | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age x Sex Interaction | R2 | F | p | N | ||||||||||||
| ACCURACY | †† | †† | †† | †† | ||||||||||||
|
| ||||||||||||||||
| Abstraction | 657 | R2 = | 0.167**** | 0.148 | 0.019 | 0.138 | 0.029 | 0.127 | 0.040 | 0.164 | 0.004 | n.s. | 0.039 | 14.410 | 0.0000 | 683 |
| Attention | 664 | R2 = | 0.125**** | 0.111 | 0.014 | 0.117 | 0.008 | 0.120 | 0.005 | 0.124 | 0.001 | n.s. | 0.096 | 36.990 | 0.0000 | 690 |
| Balance | 652 | R2 = | 0.096**** | 0.083 | 0.013 | 0.068 | 0.028 | 0.096 | 0.000 | 0.097 | 0.000 | t=−2.498, p=.0127 | 0.017 | 6.209 | 0.0013 | 676 |
| Emotion | 664 | R2 = | 0.104**** | 0.094 | 0.010 | 0.104 | 0.000 | 0.102 | 0.002 | 0.085 | 0.019 | n.s. | 0.072 | 27.020 | 0.0000 | 690 |
| Episodic Memory | 663 | R2 = | 0.060**** | 0.039 | 0.022 | 0.059 | 0.001 | 0.048 | 0.012 | 0.059 | 0.001 | n.s. | 0.003 | 1.151 | 0.0670 | 689 |
| General Ability | 663 | R2 = | 0.399**** | 0.310 | 0.089 | 0.371 | 0.028 | 0.352 | 0.047 | 0.397 | 0.002 | t=−2.425, p=.0156 | 0.148 | 60.170 | 0.0000 | 689 |
| Working Memory | 661 | R2 = | 0.055**** | 0.048 | 0.007 | 0.052 | 0.003 | 0.030 | 0.025 | 0.053 | 0.002 | n.s. | 0.006 | 2.483 | 0.0516 | 687 |
|
| ||||||||||||||||
| SPEED | ||||||||||||||||
|
| ||||||||||||||||
| Abstraction | 657 | R2 = | 0.025** | 0.024 | 0.001 | 0.030 | 0.000 | 0.015 | 0.010 | 0.026 | 0.000 | n.s. | 0.011 | 4.330 | 0.0060 | 683 |
| Attention | 660 | R2 = | 0.123**** | 0.118 | 0.004 | 0.117 | 0.005 | 0.125 | 0.000 | 0.121 | 0.002 | n.s. | 0.103 | 39.850 | 0.0000 | 686 |
| Emotion | 663 | R2 = | 0.029*** | 0.030 | 0.000 | 0.030 | 0.000 | 0.009 | 0.020 | 0.028 | 0.001 | n.s. | 0.004 | 1.192 | 0.0646 | 689 |
| Episodic Memory | 663 | R2 = | 0.048**** | 0.048 | 0.000 | 0.028 | 0.020 | 0.049 | 0.000 | 0.040 | 0.008 | t=1.981, p=.0480 | 0.020 | 6.733 | 0.0001 | 689 |
| General Ability | 664 | R2 = | 0.047*** | 0.040 | 0.007 | 0.043 | 0.004 | 0.039 | 0.008 | 0.047 | 0.000 | n.s. | 0.017 | 6.087 | 0.0005 | 690 |
| Motor Speed | 653 | R2 = | 0.302**** | 0.290 | 0.012 | 0.293 | 0.009 | 0.255 | 0.047 | 0.295 | 0.007 | n.s. | 0.170 | 67.790 | 0.0000 | 677 |
| Working Memory | 661 | R2 = | 0.008 | 0.010 | 0.000 | 0.002 | 0.006 | 0.011 | 0.000 | 0.002 | 0.006 | n.s. | 0.000 | 0.000 | 0.6600 | 687 |
|
| ||||||||||||||||
| TOTAL ACCURACY AND SPEED | ||||||||||||||||
|
| ||||||||||||||||
| Accuracy | 639 | R2 = | 0.305**** | 0.245 | 0.060 | 0.287 | 0.018 | 0.262 | 0.043 | 0.305 | 0.000 | n.s. | 0.121 | 46.170 | 0.0000 | 663 |
| Speed | 639 | R2 = | 0.085**** | 0.085 | 0.000 | 0.089 | 0.000 | 0.076 | 0.008 | 0.086 | 0.000 | n.s. | 0.066 | 23.890 | 0.0000 | 663 |
| Accuracy-Speed | 632 | R2 = | 0.057*** | 0.029 | 0.028 | 0.051 | 0.006 | 0.051 | 0.006 | 0.055 | 0.002 | t=−2.698, p=.0072 | 0.002 | 0.677 | 0.1270 | 656 |
GAM=general additive model
SES=socioeconomic status
FM=full model
w/o=without
FM=GAM(domain~age + site +SES + ethnicity + sex)
Bold font indicates significant χ2 test of the effect of removing a covariate from the model (p<.05)
p≤.05,
p≤.01,
p=.001,
p≤.0001,
n.s.=not significant
Figure 1.
Accuracy composite scores. Box plots of Z-scores adjusted for site, ethnicity, and SES of the no/low-drinking male (blue) and female (red) participants. The top figure presents the summary scores for each of the 7 composite scores determining the Total Accuracy composite score. The remaining 7 sets of box plots show the individual measures that were entered into each accuracy composite score.
Figure 2.
Speed composite scores. Box plots of Z-scores adjusted for site, ethnicity, and SES of the no/low-drinking male (blue) and female (red) participants. The top figure presents the summary scores for each of the 7 composite scores determining the Total Speed composite score. The remaining 7 sets of box plots show the individual measures that were entered into each speed composite score.
Factors Contributing to Variance of Composite Scores: Age, SES, Site, Ethnicity, and Sex
The initial GAM tested the predictive value of age on each composite score covarying for site, SES, ethnicity, and sex.
Accuracy components
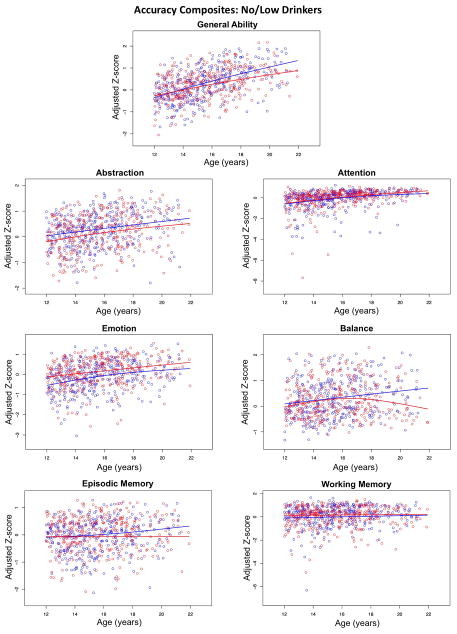
When the full model tested for the contribution of site, SES, ethnicity, and sex, the amount of variance accounted for ranged from a high of 39.9% for General Ability to a low of 5.5% for Working Memory (Table 3). Removing covariates from the full model produced a significant decrease in variance accounted for per composite: SES for all 7 composites (higher parental SES predicted higher scores), site for 4 composites, ethnicity for 4 composites, and sex for 1 composite. When age alone was entered into the model, age was a significant factor (older participants had higher scores) for 5 of the 7 composites, with the exceptions of Episodic and Working Memory (Figure 3). Age varied in its contribution to performance, where the greatest was for General Ability, accounting for 14.8% of the variance, and the least was for Episodic Memory and Working Memory at 0.3% (Table 3). Age-by-sex interactions were identified in the Balance and General Abilities composites; in both cases, older boys performed better than older girls despite lack of differences in the younger ages.
Figure 3.
Accuracy composite scores. Scatterplots of Z-scores adjusted for site, ethnicity, and SES of the no/low-drinking male (blue) and female (red) participants plotted over age.
Speed components
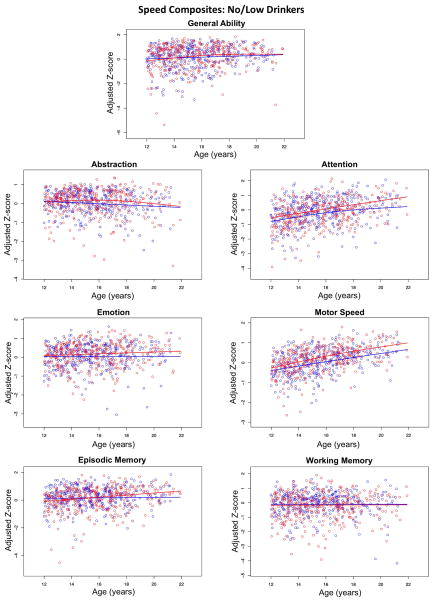
The full model accounted for a high of 30.2% (Motor Speed) to a low of 0.8% (Working Memory). Removing covariates from the full model produced a significant but modest decrease in variance accounted for per composite: SES for 3 composites, site for 2 composites, ethnicity for 4 composites, and sex for 3 composites (Table 3). When age alone was entered into the model, age was a significant factor in 5 of the 7 composites: Abstraction, Attention, Episodic Memory, General Ability, and Motor Speed (Table 3; Figure 4). An age-by-sex interaction was identified for Episodic Memory, such that older girls performed better than older boys despite lack of differences in the younger ages.
Figure 4.
Speed composite scores. Scatterplots of Z-scores adjusted for site, ethnicity, and SES of the no/low-drinking male (blue) and female (red) participants plotted over age.
Total Accuracy, Total Speed, and Accuracy-Speed difference
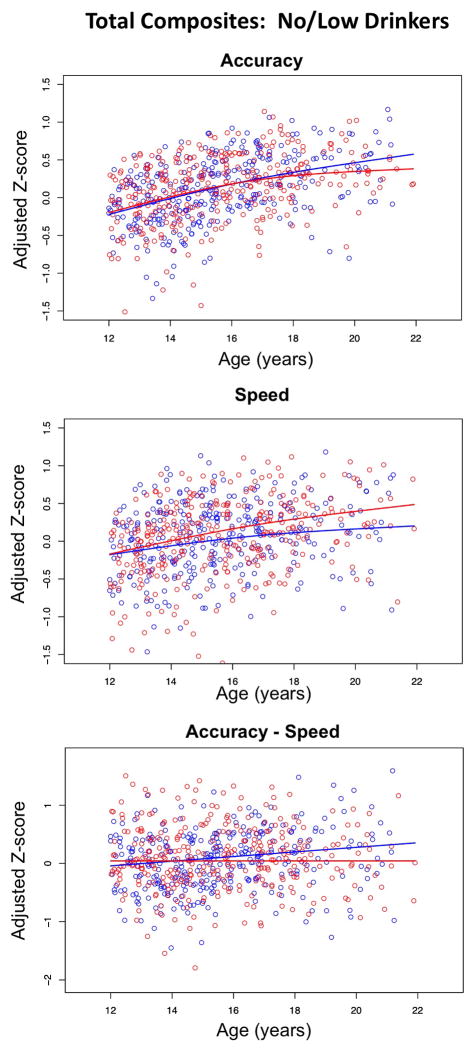
These analyses were based on two composite scores, which were means of all Accuracy composites and of all Speed composites. The full model accounted for 30.5% of the variance for Accuracy but only 8.5% of the variance for Speed. SES, site, and ethnicity were significant contributors to the overall Accuracy variance, but only ethnicity was significant in the model testing Speed. Age alone accounted for 12.1% for Accuracy and 6.6% for Speed variance; older male and female participants achieved higher scores than their younger counterparts. The difference of Accuracy Z-score minus Speed Z-score showed an age-by-sex interaction, where older boys had higher Accuracy-Speed scores than the girls (Figure 5).
Figure 5.
Total composite scores. Scatterplots of Z-scores adjusted for site, ethnicity, and SES of the no/low-drinking male (blue) and female (red) participants plotted over age.
Pubertal Development and Composite Score Variance
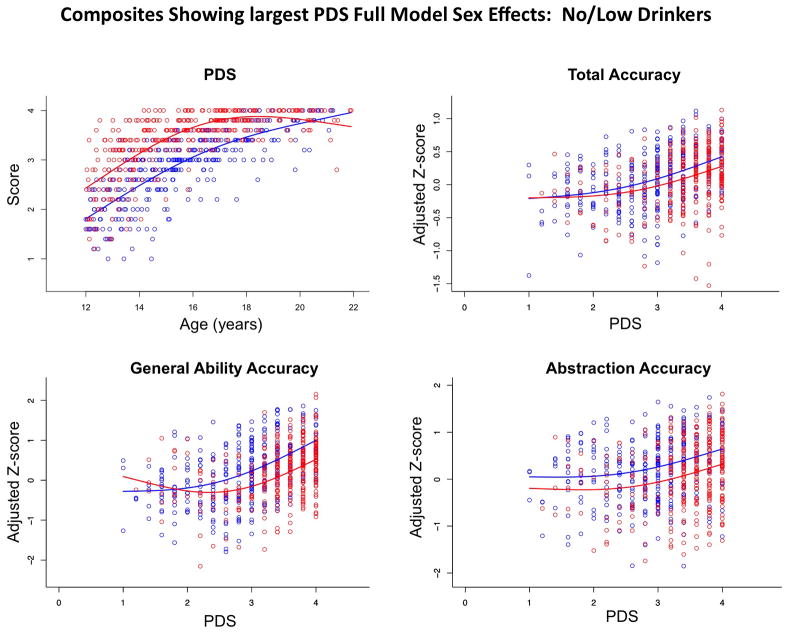
As would be expected, higher PDS scores were highly correlated with older age in both sexes (Figure 6). As with age, these relations were best described by nonlinear functions, where the boys started with lower PDS scores than girls at the younger ages, the girls achieved maximum pubertal status, on average, at age 16 years, and the boys did so in their early 20s. PDS score was then used in place of age as the predictor, keeping sex, SES, site, and ethnicity as covariates. The proportion of variance of the full GAM accounted for ranged from a high of 37.5% (General Ability) to a low of 5.3% (Working Memory) for the Accuracy composites and from 24.4% (Motor Speed) to 0.7% (Working Memory) for the Speed composites (Table 4). When PDS alone was entered into the model, PDS accounted for significant variance in 5 Accuracy and 3 Speed composites (Table 4). Applying the GAM with PDS to the total composite scores revealed that all factors combined accounted for 27.2% of the Accuracy variance but only 5.6% of the Speed variance. Accuracy scores were higher with greater pubertal development in both sexes, although boys achieved higher scores than girls for Abstraction and General Ability Accuracy (Figure 6). Independent contributions of age vs. PDS to performance were not forthcoming, probably because age and PDS were so highly correlated.
Figure 6.
Upper left: Pubertal Development Scale (PDS) scores of the no/low-drinking male (blue) and female (red) participants plotted over age. Upper right and lower left and right: Scatterplots of Z-scores adjusted for site, ethnicity, and SES of the no/low-drinking male (blue) and female (red) participants plotted as a function of PDS at time of testing.
Table 4.
Composite scores: Proportion of variance accounted for by the Pubertal Development Scale (PDS) Full Model (GAM†) with site, SES, ethnicity, and sex as covariates
| N | PDS Full Model | Sex Effect from the PDS Full Model†† | PDS w/o covariates | |||||
|---|---|---|---|---|---|---|---|---|
| R2 | t | p | N | R2 | F | p | ||
| ACCURACY | ||||||||
|
| ||||||||
| Abstraction | 651 | 0.165**** | −4.360 | 0.0000 | 675 | 0.018 | 6.485 | 0.0005 |
| Attention | 658 | 0.097**** | −1.910 | 0.0566 | 682 | 0.059 | 21.640 | 0.0000 |
| Balance | 646 | 0.099**** | −1.946 | 0.0528 | 668 | 0.011 | 3.839 | 0.0036 |
| Emotion | 658 | 0.073**** | 1.441 | 0.5010 | 682 | 0.051 | 18.680 | 0.0000 |
| Episodic Memory | 657 | 0.059**** | 1.308 | 0.1914 | 681 | 0.004 | 1.041 | 0.0805 |
| General Ability | 657 | 0.375**** | −4.905 | 0.0000 | 681 | 0.079 | 29.790 | 0.0000 |
| Working Memory | 656 | 0.053**** | −5.146 | 0.1439 | 680 | 0.000 | 0.000 | 0.7130 |
|
| ||||||||
| SPEED | ||||||||
|
| ||||||||
| Abstraction | 65 | 0.019* | 0.755 | 0.4503 | 675 | 0.003 | 0.907 | 0.0924 |
| Attention | 654 | 0.064**** | −3.343 | 0.0006 | 678 | 0.036 | 12.880 | 0.0000 |
| Emotion | 657 | 0.024** | 0.685 | 0.4935 | 681 | 0.003 | 0.707 | 0.1240 |
| Episodic Memory | 657 | 0.038**** | −3.588 | 0.0004 | 681 | 0.002 | 0.680 | 0.1270 |
| General Ability | 658 | 0.053**** | −0.843 | 0.3998 | 682 | 0.018 | 6.676 | 0.0006 |
| Motor Speed | 647 | 0.244**** | −1.538 | 0.1246 | 669 | 0.108 | 40.740 | 0.0000 |
| Working Memory | 656 | 0.007 | −2.173 | 0.0302 | 680 | 0.000 | 0.000 | 0.4110 |
|
| ||||||||
| TOTAL ACCURACY AND SPEED | ||||||||
|
| ||||||||
| Accuracy | 634 | 0.272**** | −3.123 | 0.0019 | 656 | 0.069 | 24.750 | 0.0000 |
| Speed | 634 | 0.056**** | −2.604 | 0.0094 | 656 | 0.032 | 11.170 | 0.0000 |
| Accuracy-Speed | 627 | 0.059**** | 0.469 | 0.6391 | 649 | 0.005 | 1.592 | 0.0398 |
GAM=general additive model
SES=socioeconomic status
w/o=without
Full model=GAM(domain~PDS + site +SES + ethnicity + sex)
Bold font indicates significant χ2 test of the effect of removing a covariate from the model (p<.05)
p≤.05,
p≤.01,
p=.001,
p≤.0001
Performance on Delay Discounting
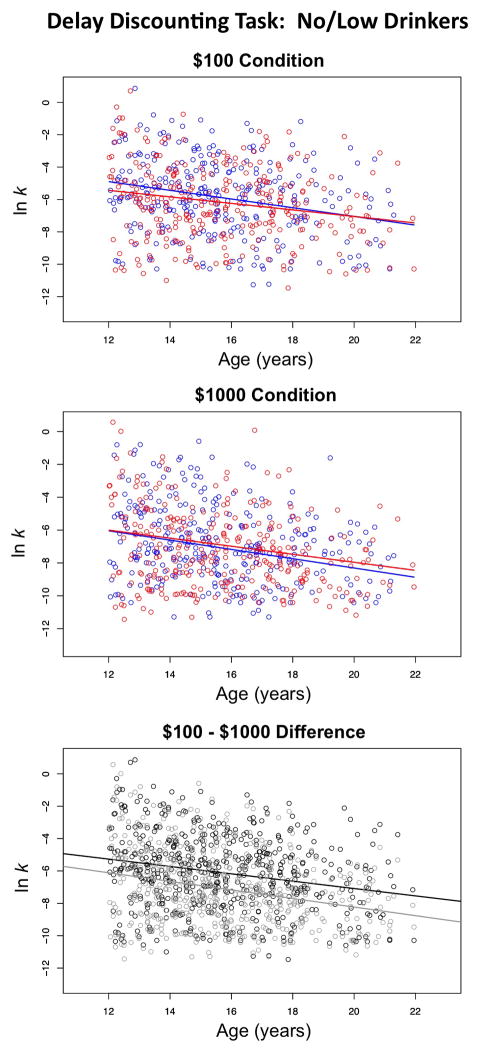
The $100 and the $1000 conditions showed the same pattern of results with respect to influential covariates. For both monetary conditions, age, SES, and ethnicity (but not sex) contributed significantly to performance. For the $100 condition, there was a significant effect of age (t=−5.434, p=.0000), with the full model accounting for 9.1% of the variance. For the $1000 condition, there was a significant effect of age (t=−6.387, p=.0000), with the full model accounting for 9.8% of the variance. In both cases, older adolescents waited a longer time for a larger monetary reward than did younger adolescents. A significant difference between the conditions indicated that adolescents waited longer for greater monetary reward in the $1000 condition relative to the $100 condition (mean difference= 0.966 lnk, paired t(df=559)=14.462, p=.0000) (Figure 7).
Figure 7.
Delay Discounting task scores. Top and middle: Scatterplots of lnk scores adjusted for site, ethnicity, and SES of the no/low-drinking male (blue) and female (red) participants plotted over age. Bottom: Scatterplots of lnk scores adjusted for site, ethnicity, and SES of the no/low-drinking participants plotted as a function of age (black=$100 condition; gray=$1000 condition).
Part 2. Performance Differences: No/Low-Drinking Group vs. Exceeds-Threshold Group
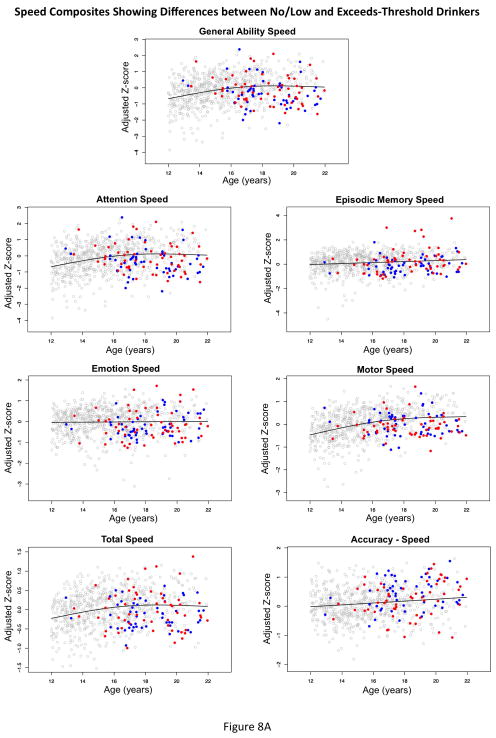
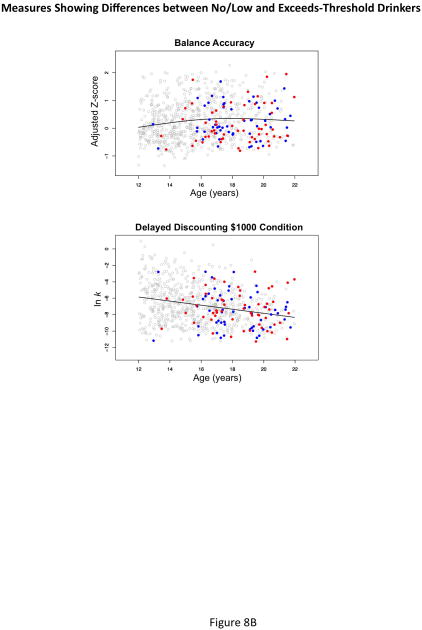
To examine the effects of exceeding exposure criteria, we expanded the GAM to include a dichotomous group covariate. The results indicated that the exceeds group performed more poorly than the no/low-exposure group on 1 Accuracy composite (Balance), 5 Speed composites (Attention, Emotion, Episodic Memory, General Ability, and Motor Speed), and the total Speed composite. Only the Accuracy-Speed score group difference was greater for the exceeds-threshold group than the no/low group (Figure 8 and Table 5). On the Delay Discounting task, the exceeds-threshold group did not wait as long for greater monetary award as did the no/low-drinking group on the $1000 condition (t=2.004, p=0.0455).
Figure 8A and B.
Performance scatterplots (adjusted for site, ethnicity, SES, and sex) showing differences between the 692 no/low-drinking adolescents (open gray circles) and the 139 adolescents who exceeded age-specific thresholds for drinking (filled circles). blue=male; red=female.
Table 5.
Composite scores: No/low vs. exceeds-threshold drinking groups
| Alcohol exposure effect from the Age Full Model† | Alcohol exposure effect from matched Samples†††† | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| N No/low |
N Exceeds-threshold |
t | p | N No/low |
N Exceeds-threshold |
t | p | |
|
|
|
|||||||
| ACCURACY | †† | ††† | †† | |||||
|
|
|
|||||||
| Abstraction | 657 | 110 | 1.119 | 0.2637 | 139 | 136 | 1.473 | 0.1420 |
| Attention | 664 | 111 | 0.058 | 0.9539 | 139 | 138 | 1.171 | 0.2428 |
| Balance | 652 | 111 | −2.322 | 0.0205 | 133 | 138 | −0.539 | 0.5905 |
| Emotion | 664 | 111 | −0.118 | 0.9062 | 139 | 138 | −1.410 | 0.1597 |
| Episodic Memory | 663 | 111 | 0.134 | 0.8927 | 139 | 138 | 0.976 | 0.3300 |
| General Ability | 663 | 111 | −0.655 | 0.5126 | 139 | 138 | 0.799 | 0.4249 |
| Working Memory | 661 | 111 | 1.195 | 0.2323 | 139 | 138 | 2.085 | 0.0381 |
|
|
|
|||||||
| SPEED | ||||||||
|
|
|
|||||||
| Abstraction | 657 | 110 | −0.110 | 0.9227 | 139 | 136 | 0.287 | 0.7743 |
| Attention | 660 | 111 | −3.877 | 0.0001 | 139 | 138 | −4.533 | 0.0000 |
| Emotion | 663 | 110 | −3.427 | 0.0006 | 139 | 137 | −3.245 | 0.0013 |
| Episodic Memory | 663 | 111 | −2.124 | 0.0340 | 139 | 138 | −2.759 | 0.0062 |
| General Ability | 664 | 111 | −2.450 | 0.0145 | 139 | 138 | −3.121 | 0.0020 |
| Motor Speed | 653 | 110 | −5.496 | 0.0000 | 135 | 137 | −6.028 | 0.0000 |
| Working Memory | 661 | 111 | −0.767 | 0.4434 | 139 | 138 | −1.029 | 0.3047 |
|
|
|
|||||||
| TOTAL ACCURACY AND SPEED | ||||||||
|
|
|
|||||||
| Accuracy | 639 | 109 | −0.168 | 0.8666 | 133 | 135 | 0.999 | 0.3188 |
| Speed | 639 | 108 | −4.082 | 0.0000 | 135 | 134 | −4.518 | 0.0000 |
| Accuracy-Speed | 632 | 108 | 3.498 | 0.0005 | 133 | 134 | 4.087 | 0.0000 |
Full model=gam(domain~age + site +SES + ethnicity + sex + exposure)
Note: Negative t-values indicate No/low group>Exceeds-threshold group
Bold font indicates significant χ2 test of the effect of removing a covariate from the model (p<.05)
Welch Two Sample t-tests
As a confirmatory analysis, we constructed a sample matching the exceeds group on sex, age, and ethnicity and compared the two groups with Welch two-sample t-tests. These results (Table 5) showed essentially the same pattern of deficit in the exceeds groups as with the full group GAM comparison, especially in speeded performance. Exceptions were for two accuracy measures, Working Memory and Balance. Although both composite scores were lower in the exceeds than no/low-drinking group, the group difference for Working Memory was significant when based on the matched sample but not the entire sample, whereas Balance showed the opposite pattern of significance. Reasons for these discrepancies include differences in the distributions of the two domain scores over the age ranges examined and, alternatively, chance.
Secondary analyses explored the effects of family history of drug or alcohol use disorders in two ways. First, chi-square analysis of performance by family history positive (FHP) vs. negative (FHN) in no/low vs. exceeds groups revealed a trend for higher incidence of FHP in the exceeds group (27.6%) vs. the no/low group (18.4%) (χ2=3.0165, p=.082). Second, the influence of FHP on performance was added to Model 1 of the GAM, first within the no/low group alone and then in the entire sample (no/low + exceeds). For the no/low group, FHP individuals had lower mean scores on General Ability Accuracy (t=−2.195, p=.0285, N=663) and total Accuracy (t=−1.940, p=.0528, N=639). When the exceeds group was added to the no/low group, the pattern held, with FHP having lower mean scores on General Ability Accuracy (t=−3.258, p=.0012, N=774) and total Accuracy (t=−2.632, p=.0087, N=748). Thus, there was a small effect of FHP on two accuracy measures irrespective of group.
Exploratory analyses examined potential relations between drinking history variables and performance and indicated that poorer scores on two accuracy measures (Abstraction p=.0506; General Ability p=.0530) were marginally related to more binge episodes reported in the past year. In addition, the number of days of alcohol use in a lifetime was included as a factor in the GAM, along with age, sex, site, and ethnicity. These analyses revealed that poorer performance was related to more lifetime days of drinking alcohol on two accuracy measures (Attention: t=−2.507, p=.0135; Episodic Memory: t=−3.132, p=.0022). Although one could interpret these relations to support a dose effect, whereby greater amount of alcohol was associated with lower scores on certain functions, an equally compelling argument could be made that the youth with greater alcohol use had pre-existing differences putting them at risk for low performance. Correlations between amount drunk in a lifetime and performance on these two measures in the exceed group yielded contradictory findings, each supporting one of the two different arguments: for Attention Accuracy, the number of days using alcohol showed little direct correlation with poorer performance (r=+.058, p=.5004); for Episodic Memory Accuracy, the alcohol-performance correlation was only modest (r=−.164, p=.0565).
We also considered drug consumption as a factor in performance, with the most used drugs being marijuana and nicotine (i.e., cigarettes). The few participants who engaged in either drug, however, precluded formal analysis of potential relations between these drugs and performance: only 9 in the exceeds-threshold group had more than 100 total days of marijuana use in lifetime; 19 had more than 30 total days of marijuana use in lifetime; 5 had smoked more than 100 cigarettes in lifetime; and 10 had smoked more than 30 cigarettes in lifetime.
Discussion
The analysis of these cross-sectional, neuropsychological data on youth, age 12 to 21 years, examined at their baseline visit, used general additive modeling to evaluate factors commonly modulating performance, notably, age, sex, ethnicity, SES, and PDS, and to test potential performance differences between the larger group of 692 no/low drinkers and the smaller group of 139 adolescents who exceeded age-specific, drinking thresholds. The performance metrics were hypothesis-driven composite scores of accuracy and speed derived from multiple measures of selected, cognitive and motor component functions.
Accuracy composite scores, which involved General Ability, Abstraction, Attention, Emotion, and Balance, were more sensitive to age differences than were Speed scores. Nonetheless, composite scores that reflected speeded responses for Attention, Motor Speed, and General Ability were also sensitive to age and pubertal development and comprised a subset of domains showing impairment in the exceeds-threshold group. In support of the study hypotheses, older and more pubertally-advanced adolescents in general achieved higher scores than younger ones on overall accuracy and speed measures. The accuracy domains showing an age effect involved executive functions, emotion processing, and general ability as predicted but not episodic memory, which was also predicted but not forthcoming. Regarding performance of the exceeds-threshold group, the speed composites detected more group differences than did the accuracy composites, with the exceeds-threshold group scoring below the no/low-drinking group. Specific group differences were for Balance accuracy and five speed composites: Attention, Emotion, Episodic Memory, General Ability, and Motor Speed. With the exception of Working Memory performance, the group differences supported the study hypotheses. The Delay Discounting test was successful in detecting age and alcohol history differences, such that younger adolescents in the no/low-drinking group and adolescents in the exceeds-threshold group, regardless of age, exhibited performance consistent with impulsive behavior. This pattern also held for speed/accuracy performance in the exceeds-threshold group, where performance was fast but at the expense of accuracy.
Age and Demographic Factors Contributing to Cognitive and Motor Performance
Overall, the hypothesis-driven functional composites derived from a combination of computerized and traditional neuropsychological tests were adequately sensitive to detect age- and sex-related differences in certain functional domains. Use of composite scores for data reduction has provided useful functional summaries in developmental studies, affording measurement redundancy and robustness for assessment of selective functions (Carlozzi et al., 2014; Gur et al., 2012; Heaton et al., 2014; Nitzburg et al., 2014; Weintraub et al., 2014). In particular, relative to younger ages, the older adolescents in the NCANDA cohort exhibited greater accuracy in tests assessing abstraction, mental flexibility, logical reasoning, and vocabulary. In addition, older adolescents showed greater postural stability and responded faster than younger ones on tests assessing abstraction, attention, episodic memory, mental flexibility, psychomotor speed, and eye-hand coordinated movement. These age-related differences are consistent with performance improvement and efficiency, notable in these processes considered components of executive functions, including delayed gratification, observed over this decade of adolescence. Stage of pubertal development was found to be another factor to consider in neuropsychological studies of adolescents and provided further evidence, albeit cross-sectional, on the relevance of pubertal development on cognitive and motor functioning (cf., Stiles & Jernigan, 2010).
The distributions of several Accuracy and Speed composite scores had adequate variance to detect small differences with age, up to a maximum of 14.8% for General Ability accuracy and 17.0% for Motor Speed. Despite the tight distribution of scores for Attention accuracy relative to the rectangular distribution of scores for Episodic Memory accuracy, the former but not the latter composite exhibited a significant age effect (cf., Gur et al., 2012). Further, the composite scores were differentially modulated by demographic variables, consistent with the assumption that the composites assembled reflected different functions (also see Boelema et al., 2014). Specifically, SES (defined as highest parental education achieved) and self-identified ethnicity exerted the most consistent effects, although accounting for only 1.0% to 4.5% of the variance for a particular Accuracy or Speed composite score. Of note were four instances showing age-by-sex interactions. Older male adolescents had a performance advantage over older female adolescents on two Accuracy measures, Balance and General Ability, but the opposite effect, in favor of the older female adolescents, emerged for speeded responses on the Episodic Memory composite. The interaction involving the Accuracy-Speed difference score indicated that older boys were faster and more accurate in their responses than older girls, despite minimal sex difference in the younger adolescents. The male performance advantage, notable in accuracy measures, was echoed in the comparisons based on pubertal development, such that boys at more advanced pubertal stages performed more accurately and responded more quickly than girls at a comparable pubertal stage, determined with the self-report PDS. A salient sex difference, in favor of the female youth, involved the Emotion composite, which assessed abilities to identify and discriminate facially-expressed emotions, a sex difference that comports with other studies of emotion detection differences between the sexes (Gur et al., 2012; Williams et al., 2009).
Alcohol Consumption and Performance in Adolescents
Even after accounting for age, sex, and other demographic factors, the group with greater drinking experience performed below the no/low-drinking group on 12 of the 15 composite scores. Differences were significant in 6 instances, including Balance accuracy and five speed measures. Slower response times in the exceeds-threshold group suggest a modest immaturity in response levels that would be equivalent to younger participants. Inspection of Figure 8 suggests that some individuals in the exceeds-threshold group were both very fast and very inaccurate. This apparent sacrificing of accuracy for speed is consistent with impulsive behavior, which often characterizes youth who experiment with alcohol and drugs and those who engage in binge drinking (Squeglia, Jacobus, Nguyen-Louie, et al., 2014; Tapert et al., 2002; Wetherill, Squeglia, Yang, & Tapert, 2013). Exploratory quantitative analysis of the number of heavy drinking episodes over the past year, however, did not identify it as a significant covariate of performance on the composite measures in the exceeds-threshold group.
Impulsive behavior, high-risk taking, and questionable decision-making are all considered externalizing behaviors that have the potential of providing a basis for experimenting with alcohol and drugs, providing gateways to addiction (Fein, Di Sclafani, & Finn, 2010; Kendler, Prescott, Myers, & Neale, 2003). Impulsive behavior assessed by the Delay Discounting task showed significant age effects in the no/low-drinking group, where younger adolescents chose the lesser reward ($100) earlier (i.e., showed greater discounting) than the older ones, who opted for a larger reward ($1000) at a longer delay. As observed in the younger, no/low-drinking youth, the exceeds-threshold drinking youth, who were generally in the older age range, exhibited a preference for a smaller, more immediate award at the expense of a larger delayed reward. The pattern of discounting behavior exhibited by the exceeded-threshold youth is typical of heavy drinking adolescents and may convey some ongoing risk for real-world temporal discounting (Isen, Sparks, & Iacono, 2014; Stanger et al., 2013).
Two additional functions that were below par in the exceeds-threshold group deserve note within the context of hazardous and potentially dependent drinking. The first is emotion discrimination and identification, which differentiated the drinking group from the no/low drinkers. Impairment in emotion detection and expression of greater negative emotional states has been documented using several different paradigms in adult alcoholics in recovery (Charlet et al., 2014; Clark, Oscar-Berman, Shagrin, & Pencina, 2007; Kornreich et al., 2013; Maurage, Campanella, Philippot, Martin, & de Timary, 2008; O’Daly et al., 2012; Schulte, Müller-Oehring, Rohlfing, Sullivan, & Pfefferbaum, 2011). Misperception of emotion has been speculated to contribute to misinterpretation of intent of another person, potentially serving as a source, for example, of argument or unwanted advances. Whether this speculation applies to adolescent drinkers with attenuated emotion processing has yet to be determined; however, findings from monitored abstinence of youth suggest that negative affect and poorer distress tolerance are prevalent during early abstinence (Bekman, Winward, Lau, Wagner, & Brown, 2013; Winward, Bekman, et al., 2014).
The second function presenting a challenge for the exceeds-threshold group was is postural stability, which in the current study was measured when participants had not drunk within 48 hours prior to testing. Prior studies examining the effects of acute alcohol on balance reported that nondependent adolescents who showed little sway in response to acute alcohol were more likely to develop alcohol dependence than youth who exhibited excessive sway (Schuckit, 1994), yet without alcohol challenge adolescents who carry familial risk of alcohol use disorder show greater postural sway than non-carriers (Hill, Steinhauer, Locke-Wellman, & Ulrich, 2009). Further, chronic alcohol dependence in adults can result in significant postural instability that remains detectable even in abstinent alcoholics, although sustained sobriety can result in at least partial resolution of imbalance (Smith & Fein, 2012; Sullivan, Rosenbloom, Lim, & Pfefferbaum, 2000). The predictive value of stability testing performance absent acute alcohol challenge awaits longitudinal study.
The small effect of positive family history on General Ability and Total Accuracy present in both the no/low-drinking and the exceeds-threshold groups suggests that low performance can precede initiation of hazardous drinking and that family history carries a liability for compromised neuropsychological performance potentially exacerbated by initiation of substantial drinking or drug consumption. This possibility has been borne out in large cohort studies (e.g., Lovallo et al., 2013; Porjesz & Rangaswamy, 2007) and smaller-scale studies (e.g., Cservenka, Fair, & Nagel, 2014; De Bellis et al., 2008; Herting, Fair, & Nagel, 2011; Hill et al., 2001; Hill et al., 2000; Hill et al., 2009; Jacobus et al., 2009) typically revealing poorer performance or compromised brain structure or function in positive family history than negative family history adolescents, even in adolescents and young adults with similar histories of alcohol drinking.
Limitations
Caution must be taken before drawing conclusions about the direct or indirect role of drinking on performance in the exceeds-threshold group, because the observed group differences could be antecedent to the current assessment and reflect characteristic and familial features of youth at-risk for hazardous drinking (e.g., Begleiter & Porjesz, 1984; Nigg et al., 2004; Nixon & Tivis, 1997; Pulido, Anderson, Armstead, Brown, & Tapert, 2009) (for review, Sher, Grekin, & Williams, 2005), thus highlighting the need for longitudinal study (cf., Squeglia et al., 2009; Tapert et al., 2002). Most (94%) of the adolescents in the higher alcohol consumption group did not meet DSM-IV criteria for Alcohol Abuse or Alcohol Dependence, raising further suspicion that the poorer performance in this group relative to the no/low-drinking group may be pre-existing, given that detection of alcohol-related impairment is typically associated with more chronic alcohol abuse (for review, Squeglia, Jacobus, & Tapert, 2014). It is also critical to recognize that youth exceeding drinking criteria did not show performance impairment in a clinical sense, but rather exhibited statistically lower performance levels than observed in the no/low-drinking group (also see Winward, Hanson, Tapert, & Brown, 2014) and likely not so compromised as occurs in youth in treatment (Brown et al., 2000; Tapert & Brown, 1999; Tapert et al., 2002).
Despite the large sample sizes reported herein, this study has limitations. Firstly, this initial report of NCANDA neuropsychological data presents a cross-sectional view of cognitive and motor development, thus precluding inferences about change, which await longitudinal assessment of this cohort. Secondly, the composites comprised different numbers of tests, likely with differential ability to detect developmental change. Given previous analyses based on the tests that entered the composites, however, we are encouraged that the derived summary scores comprising multiple measures will have the power to detect developmental changes and modulation by different sources of demographic variance. Further, longitudinal analysis will be poised to reveal which tests are most sensitive to change and detection of alcohol and drug use and other mental health and social factors that might change the normal trajectory of development of selective functional processes. Finally, the potential of “ceiling effects” looms in studies of healthy participants. Nonetheless, even tests with ceiling effects can be sensitive to decline in longitudinal testing because of the potential of detecting fall from the ceiling with pathology or other untoward life events.
Conclusion
This cross-sectional analysis provides a baseline report of normal adolescents who have been rigorously screened for psychiatric, substance use, and medical conditions. Even though these neuropsychological tests were typically designed to detect pathology and thus may be less sensitive to variation in non-pathological individuals, the composite scores had adequate power to identify age, pubertal, sex, ethnicity, and SES differences depending on the function examined. We speculate that higher achievement with older age and pubertal stage in General Ability, Abstraction, Attention, Emotion, and Balance suggests continued functional development through adolescence, possibly supported by concurrently maturing frontal, limbic, and cerebellar brain systems (cf., Gogtay et al., 2004; Sowell, Thompson, Leonard, et al., 2004). Regarding alcohol and drug use history, the speed composites detected more group differences than did the accuracy composites, with the exceeds-threshold group performance subpar on Balance accuracy and five speed composites: Attention, Emotion, Episodic Memory, General Ability, and Motor Speed. Determination of whether the performance differences noted between no/low-drinking adolescents and adolescents with greater drinking experience could be attributable to drinking or to other modulating factors requires longitudinal study focused on both groups. Some of the no/low-drinking youth may initiate heavy to hazardous alcohol consumption along with use of other substances during their developmental years in the course of the NCANDA study and may align with family history of alcohol or drug problems. On the other hand, the youth with greater drinking experience may either continue drinking or abstain, affording the opportunity to observe a return to the norm.
Supplementary Material
Acknowledgments
Support: This work was supported by the U.S. National Institute on Alcohol Abuse and Alcoholism with co-funding from the National Institute on Drug Abuse, the National Institute of Mental Health, and the National Institute of Child Health and Human Development [NCANDA grant numbers: AA021697 (AP+KMP), AA021695 (SAB+SFT), AA021692 (SFT+SAB), AA021696 (IMC+FCB), AA021681 (MDDB), AA021690 (DBC), AA021691 (BN); K05 AA017168 (EVS)].
Footnotes
Conflict of interest: None of the authors have conflicts of interest with the reported data or their interpretation.
References
- Akshoomoff N, Newman E, Thompson WK, McCabe C, Bloss CS, Chang L, Jernigan TL. The NIH Toolbox Cognition Battery: results from a large normative developmental sample (PING) Neuropsychology. 2014;28(1):1–10. doi: 10.1037/neu0000001. 2013-38865-001 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KG, Tomlinson K, Robinson JM, Brown SA. Friends or foes: social anxiety, peer affiliation, and drinking in middle school. J Stud Alcohol Drugs. 2011;72(1):61–69. doi: 10.15288/jsad.2011.72.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach M. The Freiburg Visual Acuity Test-variability unchanged by post-hoc re-analysis. Graefes Arch Clin Exp Ophthalmol. 2007;245(7):965–971. doi: 10.1007/s00417-006-0474-4. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. Event-related brain potentials in boys at risk for alcoholism. Science. 1984;225:1493–1496. doi: 10.1126/science.6474187. [DOI] [PubMed] [Google Scholar]
- Bekman NM, Winward JL, Lau LL, Wagner CC, Brown SA. The impact of adolescent binge drinking and sustained abstinence on affective state. Alcohol Clin Exp Res. 2013;37(8):1432–1439. doi: 10.1111/acer.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Miller ML, Yi R, Kowal BP, Lindquist DM, Pitcock JA. Behavioral and neuroeconomics of drug addiction: competing neural systems and temporal discounting processes. Drug Alcohol Depend. 2007;90(Suppl 1):S85–91. doi: 10.1016/j.drugalcdep.2006.09.016. S0376-8716(06)00360-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Burnett S, Dahl RE. The role of puberty in the developing adolescent brain. Hum Brain Mapp. 2010;31(6):926–933. doi: 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelema SR, Harakeh Z, Ormel J, Hartman CA, Vollebergh WA, van Zandvoort MJ. Executive functioning shows differential maturation from early to late adolescence: longitudinal findings from a TRAILS study. Neuropsychology. 2014;28(2):177–187. doi: 10.1037/neu0000049. 2013-44231-001 [pii] [DOI] [PubMed] [Google Scholar]
- Brown SA, Brumback T, Tomlinson K, Cummins K, Thompson WK, Nagel BJ, Tapert SF. The National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA): A multi-site study of adolescent development and substance use. Journal of Studies on Alcohol and Drugs. 2015;76(6):895–908. doi: 10.15288/jsad.2015.76.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, McGue M, Maggs J, Schulenberg J, Hingson R, Swartzwelder S, Murphy S. A developmental perspective on alcohol and youths 16 to 20 years of age. Pediatrics. 2008;121(Suppl 4):S290–310. doi: 10.1542/peds.2007-2243D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Tapert SF, Granholm E, Delis DC. Neurocognitive functioning of adolescents: effects of protracted alcohol use. Alcoholism: Clinical and Experimental Research. 2000;24(2):164–171. [PubMed] [Google Scholar]
- Bucholz K, Cadoret R, Cloninger CR, Dinwiddie SW, Hesselbrock VM, Nurnbueger JI, Schuckit MA. A new semi-structured psychiatric interview for use in genetic linkage studies: A report of the reliability of the SSAGA. Journal of Studies on Alcoholism. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Canini M, Battista P, Della Rosa PA, Catricala E, Salvatore C, Gilardi MC, Castiglioni I. Computerized neuropsychological assessment in aging: testing efficacy and clinical ecology of different interfaces. Comput Math Methods Med. 2014;2014:804723. doi: 10.1155/2014/804723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlozzi NE, Tulsky DS, Chiaravalloti ND, Beaumont JL, Weintraub S, Conway K, Gershon RC. NIH Toolbox Cognitive Battery (NIHTB-CB): the NIHTB Pattern Comparison Processing Speed Test. J Int Neuropsychol Soc. 2014;20(6):630–641. doi: 10.1017/S1355617714000319. S1355617714000319 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlet K, Schlagenhauf F, Richter A, Naundorf K, Dornhof L, Weinfurtner CE, Heinz A. Neural activation during processing of aversive faces predicts treatment outcome in alcoholism. Addict Biol. 2014;19(3):439–451. doi: 10.1111/adb.12045. [DOI] [PubMed] [Google Scholar]
- Clark US, Oscar-Berman M, Shagrin B, Pencina M. Alcoholism and judgments of affective stimuli. Neuropsychology. 2007;21(3):346–362. doi: 10.1037/0894-4105.21.3.346. 2007-06185-009 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole TJ, Pan H, Butler GE. A mixed effects model to estimate timing and intensity of pubertal growth from height and secondary sexual characteristics. Ann Hum Biol. 2014;41(1):76–83. doi: 10.3109/03014460.2013.856472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole WR, Arrieux JP, Schwab K, Ivins BJ, Qashu FM, Lewis SC. Test-retest reliability of four computerized neurocognitive assessment tools in an active duty military population. Arch Clin Neuropsychol. 2013;28(7):732–742. doi: 10.1093/arclin/act040. act040 [pii] [DOI] [PubMed] [Google Scholar]
- Coley RL, Leventhal T, Lynch AD, Kull M. Relations between housing characteristics and the well-being of low-income children and adolescents. Developmental Psychology. 2013;49:1775–1789. doi: 10.1037/a0031033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Fair DA, Nagel BJ. Emotional processing and brain activity in youth at high risk for alcoholism. Alcohol Clin Exp Res. 2014;38(7):1912–1923. doi: 10.1111/acer.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Van Voorhees E, Hooper SR, Gibler N, Nelson L, Hege SG, MacFall J. Diffusion tensor measures of the corpus callosum in adolescents with adolescent onset alcohol use disorders. Alcoholism: Clinical and Experimental Research. 2008;32(3):395–404. doi: 10.1111/j.1530-0277.2007.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denckla MB. Development of speed in repetitive and successive finger-movements in normal children. Dev Med Child Neurol. 1973;15(5):635–645. doi: 10.1111/j.1469-8749.1973.tb05174.x. [DOI] [PubMed] [Google Scholar]
- Denckla MB. Development of motor co-ordination in normal children. Dev Med Child Neurol. 1974;16(6):729–741. doi: 10.1111/j.1469-8749.1974.tb03393.x. [DOI] [PubMed] [Google Scholar]
- Dodds RM, Syddall HE, Cooper R, Benzeval M, Deary IJ, Dennison EM, Sayer AA. Grip strength across the life course: normative data from twelve British studies. PLoS One. 2014;9(12):e113637. doi: 10.1371/journal.pone.0113637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwolatzky T, Dimant L, Simon ES, Doniger GM. Validity of a short computerized assessment battery for moderate cognitive impairment and dementia. Int Psychogeriatr. 2010;22(5):795–803. doi: 10.1017/S1041610210000621. S1041610210000621 [pii] [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Finn P. Sensation seeking in long-term abstinent alcoholics, treatment-naive active alcoholics, and nonalcoholic controls. Alcohol Clin Exp Res. 2010;34(6):1045–1051. doi: 10.1111/j.1530-0277.2010.01179.x. ACER1179 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregly AR, Graybiel A, Smith MS. Walk on floor eyes closed (WOFEC): A new addition to an ataxia test battery. Aerospace Medicine. 1972;43(4):395–399. [PubMed] [Google Scholar]
- Giancola PR, Mezzich AC, Tarter RE. Disruptive, delinquent and aggressive behavior in female adolescents with a psychoactive substance use disorder: relation to executive cognitive functioning. J Stud Alcohol. 1998;59(5):560–567. doi: 10.15288/jsa.1998.59.560. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Raznahan A, Alexander-Bloch A, Schmitt E, Gogtay N, Rapoport JL. Child psychiatry branch of the National Institute of Mental Health longitudinal structural magnetic resonance imaging study of human brain development. Neuropsychopharmacology. 2014;40(1):43–49. doi: 10.1038/npp.2014.236. npp2014236 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101(21):8174–8179. doi: 10.1073/pnas.04026801010402680101. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A, Garrick T, Sheedy D, Blake H, Shores EA, Harper C. The effect of moderate to heavy alcohol consumption on neuropsychological performance as measured by the repeatable battery for the assessment of neuropsychological status. Alcohol Clin Exp Res. 2010;34(3):443–450. doi: 10.1111/j.1530-0277.2009.01108.x. ACER1108 [pii] [DOI] [PubMed] [Google Scholar]
- Gur RC, Richard J, Calkins ME, Chiavacci R, Hansen JA, Bilker WB, Gur RE. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8–21. Neuropsychology. 2012;26(2):251–265. doi: 10.1037/a0026712. 2012-00965-001 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB, Gur RE. A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. J Neurosci Methods. 2010;187(2):254–262. doi: 10.1016/j.jneumeth.2009.11.017. S0165-0270(09)00615-3 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KL, Cummins K, Tapert SF, Brown SA. Changes in neuropsychological functioning over 10 years following adolescent substance abuse treatment. Psychol Addict Behav. 2011;25(1):127–142. doi: 10.1037/a0022350. 2011-05934-002 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie T, Tibshirani R. Generalized additive models (with DIscussion) Statistical Science. 1986;1:297–318. [Google Scholar]
- Hastie T, Tibshirani R. Exploring the nature of covariate effects in the proportional hazards model. Biometrics. 1990;46(4):1005–1016. [PubMed] [Google Scholar]
- Heaton RK, Akshoomoff N, Tulsky D, Mungas D, Weintraub S, Dikmen S, Gershon R. Reliability and validity of composite scores from the NIH Toolbox Cognition Battery in adults. J Int Neuropsychol Soc. 2014;20(6):588–598. doi: 10.1017/S1355617714000241. S1355617714000241 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedman AM, van Haren NE, Schnack HG, Kahn RS, Hulshoff Pol HE. Human brain changes across the life span: a review of 56 longitudinal magnetic resonance imaging studies. Hum Brain Mapp. 2012;33(8):1987–2002. doi: 10.1002/hbm.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Fair D, Nagel BJ. Altered fronto-cerebellar connectivity in alcohol-naive youth with a family history of alcoholism. Neuroimage. 2011;54(4):2582–2589. doi: 10.1016/j.neuroimage.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselbrock M, Easton C, Bucholz KK, Schuckit M, Hesselbrock V. A validity study of the SSAGA--a comparison with the SCAN. Addiction. 1999;94(9):1361–1370. doi: 10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- Hill SY, De Bellis MD, Keshavan MS, Lowers L, Shen S, Hall J, Pitts T. Right amygdala volume in adolescent and young adult offspring from families at high risk for developing alcoholism. Biol Psychiatry. 2001;49(11):894–905. doi: 10.1016/s0006-3223(01)01088-5. [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S, Locke J, Lowers L, Steinhauer S, Konicky C. Developmental changes in postural sway in children at high and low risk for developing alcohol-related disorders. Biological Psychiatry. 2000;47(6):501–511. doi: 10.1016/s0006-3223(99)00175-4. [DOI] [PubMed] [Google Scholar]
- Hill SY, Steinhauer SR, Locke-Wellman J, Ulrich R. Childhood risk factors for young adult substance dependence outcome in offspring from multiplex alcohol dependence families: a prospective study. Biol Psychiatry. 2009;66(8):750–757. doi: 10.1016/j.biopsych.2009.05.030. S0006-3223(09)00702-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurks PP, Schrans D, Meijs C, Wassenberg R, Feron FJ, Jolles J. Developmental changes in semantic verbal fluency: analyses of word productivity as a function of time, clustering, and switching. Child Neuropsychol. 2010;16(4):366–387. doi: 10.1080/09297041003671184. 920952735 [pii] [DOI] [PubMed] [Google Scholar]
- Isen JD, Sparks JC, Iacono WG. Predictive validity of delay discounting behavior in adolescence: a longitudinal twin study. Exp Clin Psychopharmacol. 2014;22(5):434–443. doi: 10.1037/a0037340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara S. Ishihara’s Test for Color Blindness. Tokyo: Kanehara; 1983. [Google Scholar]
- Jacobus J, McQueeny T, Bava S, Schweinsburg BC, Frank LR, Yang TT, Tapert SF. White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicol Teratol. 2009;31(6):349–355. doi: 10.1016/j.ntt.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use: 1975–2014: Overview, key findings on adolescent drug use. Ann Arbor: Institute for Social Research, The University of Michigan; 2015. [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003;60(9):929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kornreich C, Brevers D, Canivet D, Ermer E, Naranjo C, Constant E, Noel X. Impaired processing of emotion in music, faces and voices supports a generalized emotional decoding deficit in alcoholism. Addiction. 2013;108(1):80–88. doi: 10.1111/j.1360-0443.2012.03995.x. [DOI] [PubMed] [Google Scholar]
- Koski L, Brouillette MJ, Lalonde R, Hello B, Wong E, Tsuchida A, Fellows L. Computerized testing augments pencil-and-paper tasks in measuring HIV-associated mild cognitive impairment(*) HIV Med. 2011;12(8):472–480. doi: 10.1111/j.1468-1293.2010.00910.x. [DOI] [PubMed] [Google Scholar]
- Lange N, Froimowitz MP, Bigler ED, Lainhart JE Brain Development Cooperative G. Associations between IQ, total and regional brain volumes, and demography in a large normative sample of healthy children and adolescents. Dev Neuropsychol. 2010;35(3):296–317. doi: 10.1080/87565641003696833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Largo RH, Caflisch JA, Hug F, Muggli K, Molnar AA, Molinari L. Neuromotor development from 5 to 18 years. Part 2: associated movements. Dev Med Child Neurol. 2001;43(7):444–453. doi: 10.1017/s0012162201000822. [DOI] [PubMed] [Google Scholar]
- Lawrence K, Campbell R, Skuse D. Age, gender, and puberty influence the development of facial emotion recognition. Front Psychol. 2015;6:761. doi: 10.3389/fpsyg.2015.00761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson GM, Duda JT, Avants BB, Wu J, Farah MJ. Associations between children’s socioeconomic status and prefrontal cortical thickness. Dev Sci. 2013;16(5):641–652. doi: 10.1111/desc.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DC, Stanger C, Budney AJ. A comparison of delay discounting in adolescents and adults in treatment for cannabis use disorders. Exp Clin Psychopharmacol. 2015;23(2):130–137. doi: 10.1037/a0038792. 2015-04645-001 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4. New York: Oxford University Press; 2004. [Google Scholar]
- Lovallo WR, Farag NH, Sorocco KH, Acheson A, Cohoon AJ, Vincent AS. Early life adversity contributes to impaired cognition and impulsive behavior: studies from the Oklahoma Family Health Patterns Project. Alcohol Clin Exp Res. 2013;37(4):616–623. doi: 10.1111/acer.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Amlung MT, Few LR, Ray LA, Sweet LH, Munafo MR. Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology (Berl) 2011;216(3):305–321. doi: 10.1007/s00213-011-2229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli JE, Cecato JF, Bartholomeu D, Montiel JM. Comparison of the diagnostic accuracy of neuropsychological tests in differentiating Alzheimer’s disease from mild cognitive impairment: can the montreal cognitive assessment be better than the cambridge cognitive examination? Dement Geriatr Cogn Dis Extra. 2014;4(2):113–121. doi: 10.1159/000360279dee-0004-0113. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters MS, Sanders B. Is the gender difference in mental rotation disappearing? Behav Genet. 1993;23(4):337–341. doi: 10.1007/BF01067434. [DOI] [PubMed] [Google Scholar]
- Matthews CG, Kløve H. Instruction manual for the Adult Neuropsychology Test Battery. Madison, WI: University of Wisconsin Medical School; 1964. [Google Scholar]
- Maurage P, Campanella S, Philippot P, Martin S, de Timary P. Face processing in chronic alcoholism: a specific deficit for emotional features. Alcohol Clin Exp Res. 2008;32(4):600–606. doi: 10.1111/j.1530-0277.2007.00611.x. ACER611 [pii] [DOI] [PubMed] [Google Scholar]
- Mazur JE. An adjusting procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H, editors. Quantitative analysis of behavior. Vol. 5. Hillside, NM: Lawrence Erlbaum Associates; 1987. pp. 55–73. [Google Scholar]
- McCarty CA, Berg R, Rottscheit CM, Waudby CJ, Kitchner T, Brilliant M, Ritchie MD. Validation of PhenX measures in the personalized medicine research project for use in gene/environment studies. BMC Med Genomics. 2014;7:3. doi: 10.1186/1755-8794-7-3. 1755-8794-7-3 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty CA, Huggins W, Aiello AE, Bilder RM, Hariri A, Jernigan TL, Junkins HA. PhenX RISING: real world implementation and sharing of PhenX measures. BMC Med Genomics. 2014;7:16. doi: 10.1186/1755-8794-7-16. 1755-8794-7-16 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoyd VC. Socioeconomic disadvantage and child development. Am Psychol. 1998;53(2):185–204. doi: 10.1037//0003-066x.53.2.185. [DOI] [PubMed] [Google Scholar]
- McQuiddy VA, Scheerer CR, Lavalley R, McGrath T, Lin L. Normative Values for Grip and Pinch Strength for 6- to 19-Year-Olds. Arch Phys Med Rehabil. 2015 doi: 10.1016/j.apmr.2015.03.018. [DOI] [PubMed] [Google Scholar]
- Mielke MM, Weigand SD, Wiste HJ, Vemuri P, Machulda MM, Knopman DS, Petersen RC. Independent comparison of CogState computerized testing and a standard cognitive battery with neuroimaging. Alzheimers Dement. 2014;10(6):779–789. doi: 10.1016/j.jalz.2014.09.001. S1552-5260(14)02821-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss HB, Kirisci L, Gordon HW, Tarter RE. A neuropsychologic profile of adolescent alcoholics. Alcohol Clin Exp Res. 1994;18(1):159–163. doi: 10.1111/j.1530-0277.1994.tb00897.x. [DOI] [PubMed] [Google Scholar]
- Neligan G, Prudham D. Norms for four standard developmental milestones by sex, social class and place in family. Dev Med Child Neurol. 1969;11(4):413–422. doi: 10.1111/j.1469-8749.1969.tb01459.x. [DOI] [PubMed] [Google Scholar]
- Nguyen-Louie TT, Castro N, Matt GE, Squeglia LM, Brumback T, Tapert SF. Effects of emerging alcohol and marijuana use behaviors on adolescents’ neuropsychological functioning over four years. Journal of Studies on Alcohol and Drugs. 2015 doi: 10.15288/jsad.2015.76.738. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols BN, Pohl KM. Neuroinformatics Software Applications Supporting Electronic Data Capture, Management, and Sharing for the Neuroimaging Community. Neuropsychol Rev. 2015;25(3):356–368. doi: 10.1007/s11065-015-9293-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Glass JM, Wong MM, Poon E, Jester JM, Fitzgerald HE, Zucker RA. Neuropsychological executive functioning in children at elevated risk for alcoholism: findings in early adolescence. J Abnorm Psychol. 2004;113(2):302–314. doi: 10.1037/0021-843X.113.2.3022004-13593-013. pii. [DOI] [PubMed] [Google Scholar]
- Nitzburg GC, Derosse P, Burdick KE, Peters BD, Gopin CB, Malhotra AK. MATRICS cognitive consensus battery (MCCB) performance in children, adolescents, and young adults. Schizophr Res. 2014;152(1):223–228. doi: 10.1016/j.schres.2013.11.023. S0920-9964(13)00619-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon SJ, Tivis LJ. Neuropsychological responses in COA’s. Alcohol Health Res World. 1997;21(3):232–236. [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Brito NH, Bartsch H, Kan E, Kuperman JM, Sowell ER. Family income, parental education and brain structure in children and adolescents. Nat Neurosci. 2015;18(5):773–778. doi: 10.1038/nn.3983. nn.3983 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Kan E, Sowell ER. Neural correlates of socioeconomic status in the developing human brain. Dev Sci. 2012;15(4):516–527. doi: 10.1111/j.1467-7687.2012.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Daly OG, Trick L, Scaife J, Marshall J, Ball D, Phillips ML, Duka T. Withdrawal-associated increases and decreases in functional neural connectivity associated with altered emotional regulation in alcoholism. Neuropsychopharmacology. 2012;37(10):2267–2276. doi: 10.1038/npp.2012.77. npp201277 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Parada M, Corral M, Mota N, Crego A, Rodriguez Holguin S, Cadaveira F. Executive functioning and alcohol binge drinking in university students. Addict Behav. 2012;37(2):167–172. doi: 10.1016/j.addbeh.2011.09.015. S0306-4603(11)00316-9 [pii] [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. J Youth Adolesc. 1988;17(2):117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Piper BJ. Age, handedness, and sex contribute to fine motor behavior in children. J Neurosci Methods. 2011;195(1):88–91. doi: 10.1016/j.jneumeth.2010.11.018. S0165-0270(10)00652-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper BJ, Acevedo SF, Edwards KR, Curtiss AB, McGinnis GJ, Raber J. Age, sex, and handedness differentially contribute to neurospatial function on the Memory Island and Novel-Image Novel-Location tests. Physiol Behav. 2011;103(5):513–522. doi: 10.1016/j.physbeh.2011.03.024. S0031-9384(11)00143-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M. Neurophysiological endophenotypes, CNS disinhibition, and risk for alcohol dependence and related disorders. Scientific World Journal. 2007;7:131–141. doi: 10.1100/tsw.2007.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulido C, Anderson KG, Armstead AG, Brown SA, Tapert SF. Family history of alcohol-use disorders and spatial working memory: effects on adolescent alcohol expectancies. J Stud Alcohol Drugs. 2009;70(1):87–91. doi: 10.15288/jsad.2009.70.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman-Filipiak AA, Woodard JL. Administration and environment considerations in computer-based sports-concussion assessment. Neuropsychology Review. 2013;23:314–334. doi: 10.1007/s11065-013-9241-6. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Greenstein D, Lee NR, Clasen LS, Giedd JN. Prenatal growth in humans and postnatal brain maturation into late adolescence. Proc Natl Acad Sci U S A. 2012;109(28):11366–11371. doi: 10.1073/pnas.1203350109. 1203350109 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19(4):1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, Rabbitt P. Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5(5):266–281. doi: 10.1159/000106735. [DOI] [PubMed] [Google Scholar]
- Rohlfing T, Cummins K, Henthorn T, Chu W, Nichols BN. N-CANDA data integration: anatomy of an asynchronous infrastructure for multi-site, multi-instrument longitudinal data capture. J Am Med Inform Assoc. 2014;21(4):758–762. doi: 10.1136/amiajnl-2013-002367. amiajnl-2013-002367 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. American Journal of Psychiatry. 1994;151(2):184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Schulte T, Müller-Oehring EM, Rohlfing T, Sullivan EV, Pfefferbaum A. Disruption of emotion and conflict processing in HIV infection with and without alcoholism comorbidity. Journal of the International Neuropsychological Society. 2011 doi: 10.1017/S1355617711000348. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Wise SP. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28(14):3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. 28/14/3586 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher KJ, Grekin ER, Williams NA. The development of alcohol use disorders. Annu Rev Clin Psychol. 2005;1:493–523. doi: 10.1146/annurev.clinpsy.1.102803.144107. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Dahl RE, Pollak SD. Pubertal development: correspondence between hormonal and physical development. Child Dev. 2009;80(2):327–337. doi: 10.1111/j.1467-8624.2009.01263.x. CDEV1263 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S, Fein G. Persistent but less severe ataxia in long-term versus short-term abstinent alcoholic men and women: a cross-sectional analysis. Alcoholism Clinical and Experimental Research. 2012;35(12):2184–2192. doi: 10.1111/j.1530-0277.2011.01567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneider JT, Cohen-Gilbert JE, Crowley DJ, Paul MD, Silveri MM. Differential effects of binge drinking on learning and memory in emerging adults. J Addict Res Ther. 2013;(Suppl 7) doi: 10.4172/2155-6105.S7-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24(38):8223–8231. doi: 10.1523/JNEUROSCI.1798-04.200424/38/8223. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. Neuroscientist. 2004;10(4):372–392. doi: 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- Squeglia LM, Jacobus J, Nguyen-Louie TT, Tapert SF. Inhibition during early adolescence predicts alcohol and marijuana use by late adolescence. Neuropsychology. 2014;28(5):782–790. doi: 10.1037/neu0000083. 2014-14434-001 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Jacobus J, Tapert SF. The effect of alcohol use on human adolescent brain structures and systems. Handb Clin Neurol. 2014;125:501–510. doi: 10.1016/B978-0-444-62619-6.00028-8. B978-0-444-62619-6.00028-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Spadoni AD, Infante MA, Myers MG, Tapert SF. Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychology of Addictive Behaviors. 2009;23:715–722. doi: 10.1037/a0016516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger C, Budney AJ, Bickel WK. A developmental perspective on neuroeconomic mechanisms of contingency management. Psychol Addict Behav. 2013;27(2):403–415. doi: 10.1037/a0028748. 2012-14756-001 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger C, Ryan SR, Fu H, Landes RD, Jones BA, Bickel WK, Budney AJ. Delay discounting predicts adolescent substance abuse treatment outcome. Exp Clin Psychopharmacol. 2012;20(3):205–212. doi: 10.1037/a0026543. 2011-29376-001 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles J, Jernigan TL. The basics of brain development. Neuropsychol Rev. 2010;20(4):327–348. doi: 10.1007/s11065-010-9148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Deshmukh A, Desmond JE, Lim KO, Pfefferbaum A. Cerebellar volume decline in normal aging, alcoholism, and Korsakoff’s syndrome: Relation to ataxia. Neuropsychology. 2000;14(3):341–352. doi: 10.1037//0894-4105.14.3.341. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A, Rohlfing T, Baker FC, Padilla ML, Colrain IM. Developmental change in regional brain structure over 7 months in early adolescence: Comparison of approaches for longitudinal atlas-based parcellation. NeuroImage. 2011;57:214–224. doi: 10.1016/j.neuroimage.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Lim KO, Pfefferbaum A. Longitudinal changes in cognition, gait, and balance in abstinent and relapsed alcoholic men: Relationships to changes in brain structure. Neuropsychology. 2000;14(2):178–188. [PubMed] [Google Scholar]
- Sullivan EV, Shear PK, Zipursky RB, Sagar HJ, Pfefferbaum A. A deficit profile of executive, memory, and motor functions in schizophrenia. Biological Psychiatry. 1994;36(10):641–653. doi: 10.1016/0006-3223(94)91173-8. [DOI] [PubMed] [Google Scholar]
- Tanner JM, Whitehouse RH, Takaishi M. Standards from birth to maturity for height, weight, height velocity, and weight velocity: British children, 1965. II. Arch Dis Child. 1966;41(220):613–635. doi: 10.1136/adc.41.220.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapert SF, Brown SA. Neuropsychological correlates of adolescent substance abuse: four-year outcomes. Journal of International Neuropsychological Society. 1999;5(6):481–493. doi: 10.1017/s1355617799566010. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Granholm E, Leedy NG, Brown SA. Substance use and withdrawal: Neuropsychological functioning over 8 years in youth. Journal of the International Neuropsychological Society. 2002;8:873–883. doi: 10.1017/s1355617702870011. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Mezzich AC, Hsieh YC, Parks SM. Cognitive capacity in female adolescent substance abusers. Drug Alcohol Depend. 1995;39(1):15–21. doi: 10.1016/0376-8716(95)01129-m. 037687169501129M [pii] [DOI] [PubMed] [Google Scholar]
- Taylor AM. Neuropsychological evaluation and management of sport-related concussion. Curr Opin Pediatr. 2012;24(6):717–723. doi: 10.1097/MOP.0b013e32835a279b. [DOI] [PubMed] [Google Scholar]
- Voyer D, Voyer S, Bryden MP. Magnitude of sex differences in spatial abilities: a meta-analysis and consideration of critical variables. Psychol Bull. 1995;117(2):250–270. doi: 10.1037/0033-2909.117.2.250. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Fourth Edition (WAIS-IV) Pearson Education, Inc; 2008. [Google Scholar]
- Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Slotkin J, Gershon R. The cognition battery of the NIH toolbox for assessment of neurological and behavioral function: validation in an adult sample. J Int Neuropsychol Soc. 2014;20(6):567–578. doi: 10.1017/S1355617714000320. S1355617714000320 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Squeglia LM, Yang TT, Tapert SF. A longitudinal examination of adolescent response inhibition: neural differences before and after the initiation of heavy drinking. Psychopharmacology (Berl) 2013;230(4):663–671. doi: 10.1007/s00213-013-3198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GS, Robertson GJ. Wide Range Achievement Test 4 (WRAT4) 2010. [Google Scholar]
- Williams LM, Mathersul D, Palmer DM, Gur RC, Gur RE, Gordon E. Explicit identification and implicit recognition of facial emotions: I. Age effects in males and females across 10 decades. J Clin Exp Neuropsychol. 2009;31(3):257–277. doi: 10.1080/13803390802255635. 901726529 [pii] [DOI] [PubMed] [Google Scholar]
- Winward JL, Bekman NM, Hanson KL, Lejuez CW, Brown SA. Changes in emotional reactivity and distress tolerance among heavy drinking adolescents during sustained abstinence. Alcohol Clin Exp Res. 2014;38(6):1761–1769. doi: 10.1111/acer.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winward JL, Hanson KL, Tapert SF, Brown SA. Heavy alcohol use, marijuana use, and concomitant use by adolescents are associated with unique and shared cognitive decrements. J Int Neuropsychol Soc. 2014;20(8):784–795. doi: 10.1017/S1355617714000666. S1355617714000666 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt ED. Research on alcohol and adolescent brain development: opportunities and future directions. Alcohol. 2010;44(1):119–124. doi: 10.1016/j.alcohol.2009.08.011. S0741-8329(09)00166-9 [pii] [DOI] [PubMed] [Google Scholar]
- Wood SN. Thin-plate regression splines. Journal of the Royal Statistical Society (B) 2003;65:95–114. [Google Scholar]
- Wood SN. Stable and efficient multiple smoothing parameter estimation for generalized additive models. Journal of the American Statistical Association. 2004;99:673–686. [Google Scholar]
- Wood SN. Low-rank scale-invariant tensor product smooths for generalized additive mixed models. Biometrics. 2006;62(4):1025–1036. doi: 10.1111/j.1541-0420.2006.00574.x. BIOM574 [pii] [DOI] [PubMed] [Google Scholar]
- Wood SN. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear modelsFast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. Journal of the Royal Statistical Society (B) 2011;73(1):3–36. [Google Scholar]
- Zucker RA, Donovan JE, Masten AS, Mattson ME, Moss HB. Early developmental processes and the continuity of risk for underage drinking and problem drinking. Pediatrics. 2008;121(Suppl 4):S252–272. doi: 10.1542/peds.2007-2243B. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.