Abstract
Objectives
This study used latent growth curve modeling (LGCM) to estimate the independent and joint associations between frailty and depression trajectories and likelihood of nursing home admission and falls resulting in injury.
Methods
Data come from five waves (2004-2012) of the Health and Retirement Study. Community-dwelling individuals age 51 and older (N=13,495) were analyzed using LGCM. Frailty was measured using a Frailty Index consisting of 30 deficits. Depressive symptoms were measured using the 8-item Centers for Epidemiologic Studies Depression scale. Adverse health outcomes included nursing home admissions and falls resulting in injury.
Results
Prevalence of frailty increased over the study period (24.1% to 32.1%) while the prevalence of depression was relatively constant over time (approximately 13%). Parallel process LGCM showed that more rapid increases of frailty and depressive symptoms were associated with higher odds of both nursing home admission and serious falls over time (Frailty: ORNursingHome=1.33, 95% CI: 1.09 to 1.66; ORFall=1.52, 95% CI: 1.12 to 2.08 ; Depression: ORNursingHome=3.63, 95% CI: 1.29 to 9.97; ORFall=1.16, 95% CI: 1.01 to 1.34). Associations between frailty and adverse outcomes were attenuated, and in some cases were no longer statistically significant, after accounting for concurrent depression.
Conclusion
Frailty trajectories may be important indicators of risk for nursing home admissions and falls, independent of baseline frailty status; however, concurrent depression trajectories are associated with adverse outcomes to a similar degree as frailty. Focus should be given to distilling elements of the Frailty Index which confer most risk for poor health outcomes.
Keywords: Depression, frailty, latent growth models
Introduction
Epidemiologic studies indicate that frailty – a state of elevated vulnerability to poor health outcomes – is common in late life. The likelihood of being considered frail increases substantially with age (Collard, Boter, Schoevers, & Oude Voshaar, 2012), and frailty has been linked to greater risk of adverse outcomes such as falls, nursing home admission, hospitalization, higher medical costs, and early mortality (Fassbender, Fainsinger, Carson, & Finegan, 2009; Fried et al., 2001; Klein, Klein, Knudtson, & Lee, 2005; Rockwood et al., 2005). Recent evidence suggests that depression, a common condition among older adults, shares many features and symptoms with frailty (Katz, 2004; Mezuk, Edwards, Lohman, Choi, & Lapane, 2011) and therefore may also play an important role in determining vulnerability to adverse health outcomes. The goal of this study is to investigate and separate the concurrent roles of frailty and depression in predicting risk of adverse health outcomes over time.
Despite a growing body of research, there is no consensus definition of frailty, and various competing conceptual models have emerged (Cigolle, Ofstedal, Tian, & Blaum, 2009; Gobbens, Luijkx, Wijnen Sponselee, & Schols, 2010; Pijpers, Ferreira, Stehouwer, & Nieuwenhuijzen Kruseman, 2012; Rothman, Leo-Summers, & Gill, 2008). Among the most widely cited and analyzed models is the Frailty Index (FI), which views frailty as the sum of various non-specific disorders and deficits, similar to comorbidity (Rockwood & Mitnitski, 2007; Song, Mitnitski, & Rockwood, 2010). In this model of frailty, the accumulation of deficits in multiple physiological systems renders individuals unable to respond to health stressors, making them vulnerable to poor outcomes. The FI thus emphasizes trajectories of gradual deterioration and accumulation of burden over time that may impair ability to maintain homeostasis in response to minor stressors such as infection or medication change (Clegg, Young, Iliffe, Rikkert, & Rockwood, 2013).
An important consideration in the study of frailty as measured by the FI is its relationship to depression. Evidence from multiple fields suggests that frailty and depression are highly related, both as comorbid and conceptually similar conditions (Brown et al., 2013; Katz, 2004; Lohman, Dumenci, & Mezuk, 2015; Mezuk et al., 2011; Paulson & Lichtenberg, 2013b). First, frailty and depression increase risk of similar adverse health outcomes over time. For instance, both frailty and depression are associated with greater risk of mortality among older adults (Hybels, Pieper, & Blazer, 2002; Klein et al., 2005; Rockwood, Song, & Mitnitski, 2011). Second, common instruments used for case ascertainment of frailty and depression lead to similar categorization of afflicted individuals. For instance, previous work has shown that common operationalizations of frailty and depression produce highly overlapping estimates of individuals who are frail and/or depressed (Lohman, Dumenci, & Mezuk, 2014; Lohman et al., 2015; Mezuk, Lohman, Dumenci, & Lapane, 2012). Third, frailty, like depression, is a dynamic condition in which symptoms may manifest and remit over time (Campbell & Buchner, 1997; Rockwood et al., 2011). Accordingly, vulnerability to poor health outcomes conferred by these conditions likely changes over time. Finally, depression may be incorporated as a symptom of frailty within the FI model, making it difficult to determine to what extent these conditions are independently associated with risk of adverse outcomes. Given the potential complexity of frailty indices, depression may provide a more concise measure of risk for adverse outcomes or poor recovery from health stressors.
There are three primary aims of the present study. First, latent growth curve models will be used to estimate trajectories of frailty and depressive symptoms among a population-based sample of older adults. Second, LGCM will be used to assess whether and to what extent frailty and depression growth trajectories are associated with two adverse outcomes in later life: likelihood of nursing home admission and likelihood of experiencing a serious fall. These outcomes are chosen because they are commonly viewed as measures of criterion validity for frailty definitions (Fried et al., 2001; Li et al., 2015; Rockwood, Theou, & Mitnitski, 2015). In other words, the clinical utility of frailty measures is often judged by their ability to predict outcomes such as institutionalization, falls, and injury (Fried et al., 2001; Rockwood, Andrew, & Mitnitski, 2007). The third aim is to determine the extent to which concurrent depression, treated as a time-varying covariate, contributes to the relationship between frailty and these adverse health outcomes. By adjusting for concurrent depressive symptom levels, we aim to disentangle the relative influences of frailty and depression on likelihood of adverse outcomes. We expect that, given the strong association between frailty and depression indicated by prior research, incorporation of depression will substantially diminish the apparent relationship between frailty and the selected outcomes.
Methods
Data come from the Health and Retirement Study (HRS), an ongoing household survey initiated in 1992 in order to study the health and financial dynamics of older Americans. The HRS is a multi-stage probability sample of household units designed to be representative of the non-institutionalized U.S. population of adults over the age of 50 (Heeringa & Connor, 1995). The primary HRS questionnaire is administered by telephone at study entry and at subsequent two-year intervals. The questionnaire asks respondents to report information regarding demographics, health conditions, functional limitations, health insurance and other determinants of health.
The 2004 HRS wave surveyed 20,129 respondents, of whom 94% (n=18,954) were age 51 or older. Respondents were included in analysis if they were at least 51 years of age at study entry. Since nursing home stay was a primary outcome of interest, participants were excluded from analysis if they resided in a nursing home at the time of study entry (n=437) or if they were interviewed via a proxy respondent (n=1,730). Participants with missing information regarding frailty and/or depression symptoms at all five waves were also excluded (n=3,292). These selection criteria resulted in a final analytic sample of N=13,495 respondents. Information for these respondents from the 2004, 2006, 2008, 2010, and 2012 HRS waves was used in the current study.
The HRS is approved by the Institutional Review Board (IRB) at the University of Michigan, and this analysis received exempt status from the IRB at Virginia Commonwealth University. All participants provided informed consent.
Measures
Frailty
The Frailty Index (FI), developed by Rockwood and colleagues, defines frailty as an accumulated burden of diseases, functional disabilities and other health-related deficits and symptoms (Rockwood et al., 2007). The FI is designed to provide a flexible measure of frailty that may be utilized and compared across multiple surveys (Rockwood & Mitnitski, 2007; Searle, Mitnitski, Gahbauer, Gill, & Rockwood, 2008). Therefore, the deficits included in the FI calculation are non-specific provided they satisfy certain inclusion criteria: 1) a deficit must accumulate with age; 2) a deficit must not become universally prevalent at an early age (e.g. presbyopia); 3) a deficit must be related to health status in a biologically plausible way; 4) the deficits considered together must represent a range of bodily systems; 5) the deficits making up a FI must be consistent across time (Searle et al., 2008). While there is no maximum or minimum number of deficits which may be included in a FI, prior studies suggest that frailty indices composed of 30 to 40 deficits have sufficient specificity to predict adverse health outcomes (Rockwood & Mitnitski, 2007; Searle et al., 2008). The current study used a FI consisting of 30 deficits satisfying the inclusion criteria outlined above (Supplemental Table 1), and demonstrating good internal consistency across all waves (Cronbach's α range: 0.80 to 0.83). Individual deficits were not included in the FI if their values were missing for greater than 10% of the sample. Although depression and depressive symptoms may satisfy FI inclusion criteria, we did not include depression or depressive symptoms (as defined by the CESD) as indicators of frailty in order to evaluate growth of frailty independent of depression. In analyses, frailty was considered as a continuous variable measured by the sum of present deficits for each individual at each wave (range: 0-30). For descriptive purposes only, frailty status was dichotomized using cutoff criteria established in prior studies: participants with 25% or more of the possible deficits in the FI were considered to be frail (Cigolle et al., 2009; Rockwood et al., 2007).
Depression
Depressive symptom level (hereafter referred to as depression) was indexed using the 8-item Center for Epidemiological Studies – Depression scale (CESD) (Turvey, Wallace, & Herzog, 1999). The CESD asks respondents to report whether they experienced eight symptoms much of the time during the past week: 1) felt depressed 2) felt activities were efforts, 3) had restless sleep, 4) felt happy, 5) felt lonely, 6) enjoyed life, 7) felt sad, 8) felt unmotivated (could not get going). Positive symptoms of feeling happy and enjoying life were reverse-coded, so that their absence indicated a depressive symptom. The CESD is not a structured interview meant to emulate clinician diagnosis of major depression; however, prior studies have shown that when applying a cutoff of three or more symptoms the CESD has moderate agreement with structured diagnostic instruments such as the Composite International Diagnostic Interview (CIDI) (Steffick, 2000; Turvey et al., 1999). Depression was analyzed as a continuous variable, defined as the sum of present symptoms at each wave (range: 0-8). For descriptive purposes only, we used a cut-off score of 3 or more symptoms to distinguish individuals with elevated depressive symptoms (Andresen, Malmgren, Carter, & Patrick, 1994; Steffick, 2000).
Adverse Outcome Measures
The current study considered frailty and depression trajectories in relation to two adverse health outcomes: nursing home admission and serious falls. Nursing home admission was assessed using a dichotomous variable (1=any nursing home stay, 0=no nursing home stay) indicating whether a respondent had been a patient overnight in a nursing home, convalescent home, or other long-term care facility in the preceding two years. This variable encompassed both short stays (e.g., for rehabilitation after a hospital discharge) as well as longer stays.
We considered ‘serious falls’ as any fall within the past two years which resulted in injury requiring medical treatment as reported by the respondent. Respondents who experienced a fall which did not result in injury were considered not to have experienced a serious fall. A dichotomous variable (1=experienced a serious fall, 0=did not experience a serious fall) was used in analysis.
Time-invariant Covariates
Sociodemographic characteristics and other health related variables were chosen for inclusion as time-invariant covariates through a change-in-estimate procedure using logistic regression of frailty status and the two adverse outcome measures (Maldonado & Greenland, 1993). Variables producing substantial change in the estimate (>10%) of the frailty/outcome relationship were successively included in models until additional covariates no longer produced substantial change.
Based on the change-in-estimate selection procedure, time-invariant covariates considered in analysis were age, sex (male=0; female=1), race (dummy variables for white, black, and other), years of education, primary health insurance provider (dummy variables indicating private, Medicare, and Medicaid insurance), marital status (dummy variables for currently married/partnered, separated/divorced, never married, and widowed), smoking status (0=not current smoker; 1=current smoker), and household poverty-to-income ratio (0=above poverty threshold; below poverty threshold=1).
Analysis
Changes in frailty and adverse health outcomes over time were modeled using latent growth curve modeling (LGCM). LGCM is a statistical procedure built on confirmatory factor analysis (CFA) approaches (Bollen & Curran, 2006; Joreskog, 1969), which estimates underlying latent (unobserved) growth parameters (i.e., intercept and slope) that give rise to correlations among a set of repeated measures over time. In a LGCM framework, the latent intercept and slope are used to describe average trajectories of change over time, as well as individual variability in those growth trajectories (Bollen & Curran, 2006). LGCM is an appropriate approach for modeling longitudinal change in frailty and depression for three reasons: 1) it allows for evaluation of overall model fit using standard criteria, 2) it allows for regression of intercept and slope estimates on other explanatory variables and growth parameters (e.g., does the intercept impact the rate of growth over time?), and 3) it allows straightforward incorporation of time-varying explanatory covariates such as depression (Bollen & Curran, 2006). More detailed discussions of the theoretical bases of LGCM are available elsewhere (Bollen & Curran, 2006).
Latent Growth Models
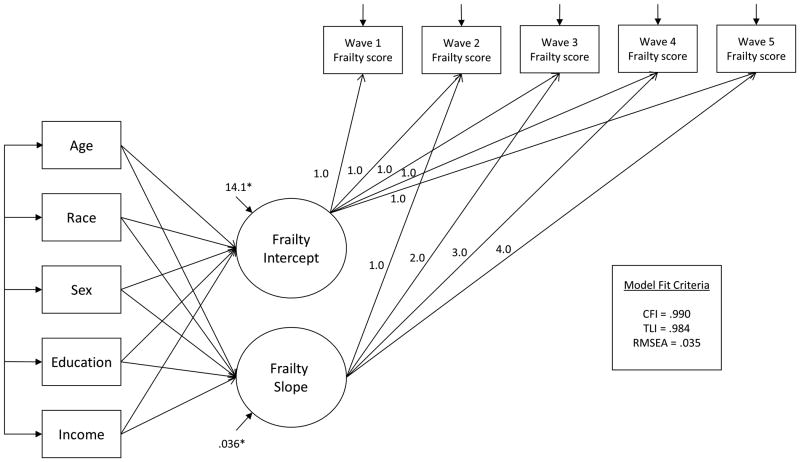
To address the aims of the study, LGC models were built in a hierarchy of increasing complexity. First, unconditional models of frailty, depression, and the two adverse outcomes were fit to assess overall growth in these constructs over time, unadjusted for influence of covariates. Two types of growth, linear and quadratic, were modeled for each outcome and compared in terms of overall model fit and parsimony. Linear growth was specified by constraining the loadings of the latent growth parameters on the observed outcomes to assume incremental change per increase in unit time (Figure 1). By convention, intercept and slope parameters were allowed to co-vary (Bollen & Curran, 2006; Duncan & Duncan, 2004; Tu & Gilthorpe, 2007). Quadratic growth was modeled by the addition of a quadratic latent growth parameter and by fixing factor loadings of the quadratic term to assume exponential change.
Figure 1. Heuristic example of conditional latent growth curve model of frailty.
Notes. Sociodemographic variables are depicted for illustrative purposes and do not represent the full set of model covariates. Curved arrows represent covariances and do not imply reciprocal causal relations.
Next, time-invariant covariates were added to unconditional models to assess influence of these variables on the growth parameters (Figure 1). The growth parameters are continuous variables, and thus estimates were interpreted as linear regression coefficients explaining the expected change in growth parameter (e.g. intercept), associated with each unit change of the covariate.
Parallel Process Latent Growth Models
Building on the conditional LGC models, the next set of models addressed the relationship between trajectories of frailty (and depression) and change in the likelihood of experiencing adverse health outcomes. In these models, two growth processes (e.g. frailty and serious falls), were related through the regression of their growth parameters. The intercept (initial level) and slope (growth) of each adverse health outcome were regressed on the intercept and slope of frailty and depression in separate models.
Lastly, to address the third aim of the current study, depression status was introduced into frailty parallel process models as a time-varying covariate (Figure 2). The magnitude of association between frailty growth parameters and adverse health outcomes after adjusting for depression was interpreted as the influence of frailty trajectories on adverse outcomes net of the influence of concurrent depression (Bollen & Curran, 2006). That is, if the association between frailty and the outcomes is substantially attenuated after adjusting for depression, this indicates that a substantial portion of the observed frailty-outcome relationship instead reflects the association between concurrent depression and the outcome.
Figure 2. Parallel process model adjusted for time-varying depression.
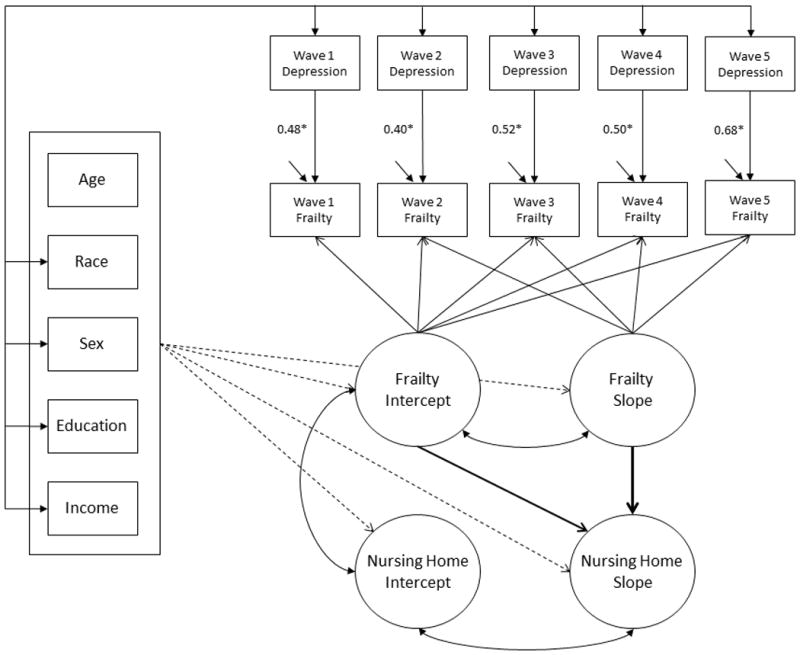
Notes. Sociodemographic variables are depicted for illustrative purposes and do not represent the full set of model covariates. Arrows with square brackets indicate that all pairwise regressions were estimated. All latent variables were adjusted for all model covariates. Curved arrows represent covariances and do not imply reciprocal causal relations. Observed nursing home status variables are implied but not depicted.
Model estimation and fit criteria
LGC models were estimated using maximum likelihood estimation as implemented in Mplus software version 7 (Muthén & Muthén, Los Angeles, CA). Maximum likelihood estimation uses all available participant information, including participants with incomplete follow-up (Bollen & Curran, 2006; Rubin & Little, 2002). To investigate the influence of missing data on model estimates, we performed secondary missing data analysis using logistic regression.
Model fit was assessed using standard fit criteria: Comparative Fit Index (CFI), Tucker-Lewis Index (TLI), and root mean square error of approximation (RMSEA). Values of CFI >0.95, TLI >0.95, and RMSEA <0.05 were taken to indicate close model fit to the data (Hu & Bentler, 1999). Compared to linear growth models, quadratic growth models did not provide significantly better model fit for frailty, depression, or either of the adverse health outcomes, and so only results from linear growth models are reported. All p-values refer to two-tailed tests.
Results
At baseline the average age of respondents was 65.9 years (range: 51 to 102). Approximately 60% of respondents were female, 83% were white, and 68% were married (Table 1). At baseline (2004 wave), 24.1% of respondents were considered frail, while 19.7% of respondents had depression according to cutoff criteria. On average, frail respondents were older, were more likely to be female, had fewer years of education, were more likely to be widowed or divorced/separated, and were more likely to have a household income below the poverty threshold. Using cutoff scores, the proportion of frail older adults in the sample increased to approximately 32% in the final wave of analysis (2012), while the proportion of depressed individuals ranged between 19.7% and 16.1% over the study period.
Table 1. Baseline sample characteristics by frailty and depression status.
| Characteristic | Frailty | Depression | |||
|---|---|---|---|---|---|
|
| |||||
| Total | Frail1 | Not frail | Depressed2 | Not depressed | |
|
| |||||
| N=13,495 | N=3,252 | N=10,243 | N=2,683 | N=10,812 | |
|
| |||||
| % or Mean (sd) |
% or Mean (sd) |
% or Mean (sd) |
% or Mean (sd) |
% or Mean (sd) |
|
| Age (yrs) | 65.9 (9.3) | 67.4 (9.7) | 65.5 (9.2) | 65.8 (9.7) | 65.9 (9.2) |
| Female | 60.4 | 71.8 | 56.7 | 69.9 | 58.0 |
| Race | |||||
| White | 83.2 | 79.6 | 84.4 | 76.8 | 84.8 |
| Black | 14.2 | 17.4 | 13.1 | 19.7 | 12.8 |
| Other | 2.7 | 3.0 | 2.5 | 3.5 | 2.4 |
| Educations (yrs) | 12.6 (3.2) | 11.5 (3.4) | 12.9 (3.0) | 11.4 (3.5) | 12.9 (3.0) |
| Marital Status | |||||
| Married/partnered | 67.8 | 58.0 | 70.7 | 52.9 | 71.4 |
| Separated/divorced | 12.1 | 15.8 | 11.0 | 19.0 | 10.4 |
| Widowed | 17.0 | 22.8 | 15.2 | 24.2 | 15.2 |
| Never married | 3.1 | 3.4 | 3.1 | 3.8 | 3.0 |
| Household Poverty | 8.3 | 15.8 | 5.9 | 17.1 | 6.1 |
| Health Insurance | |||||
| Medicare | 55.5 | 66.7 | 51.9 | 58.2 | 54.8 |
| Medicaid | 6.7 | 16.1 | 3.7 | 15.2 | 4.5 |
| Private | 18.1 | 18.2 | 18.1 | 15.6 | 18.8 |
| Current smoker | 14.2 | 17.5 | 13.2 | 20.5 | 12.7 |
Frail = Frailty Index score >.25
Depressed = CESD > 3
Of 13,495 participants interviewed at baseline, 3,862 (28.6%) participants were missing measures of frailty, depression, or adverse health outcomes at the final wave of analysis (2012) due to attrition or incomplete interview. The largest source of missing data was incomplete assessment of frailty (N=3,772); however, on average, participants had 3.85 (out of 5 possible) frailty measurements across the study period. The average number of depression measurements across the study period was 4.65. Secondary logistic regression analysis showed that a number of demographic factors were related to greater likelihood of missing data, including greater age (OR: 1.08, 95% Confidence Interval (CI): 1.07 to 1.09), male gender (OR: 1.34, 95% CI: 1.21 to 1.48), and being divorced (OR: 1.20, 95% CI: 1.03 to 1.40) or widowed (OR: 1.24, 95% CI: 1.10 to 1.41) at baseline.
Unconditional and Conditional Latent Growth Models
Parameter estimates from LGC models of frailty and depression are displayed in Table 2. The mean unconditional and conditional slope parameters for frailty were significantly positive (Unconditional β=0.44, 95% CI: 0.42 to 0.45; Conditional β=0.56, 95% CI: 0.49 to 0.63), reflecting an average increase of 0.56 frailty deficits per wave after adjustment for the influence of time-invariant covariates. By contrast, the mean slope for depression was not significantly different from zero (Unconditional β=0.003, 95% CI: -0.01, 0.01; Conditional β= 0.04, 95% CI: 0.00, 0.08), indicating that average number of depressive symptoms did not change substantially over time. Significant variance estimates for intercept and slope of both frailty and depression indicated that there was significant inter-individual variation in both initial level and change of frailty and depression over time not fully explained by the influence of time-invariant characteristics.
Table 2. Unconditional and conditional latent growth models of frailty and depression.
| Frailty | Depression | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Unconditional | Conditional1 | Unconditional | Conditional1 | |||||
|
| ||||||||
| Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | |
| Model Fit Statistics | ||||||||
| CFI | 0.989 | --- | 0.990 | --- | 0.997 | --- | 0.996 | --- |
| TLI | 0.989 | --- | 0.984 | --- | 0.997 | --- | 0.994 | --- |
| RMSEA | 0.066 | --- | 0.035 | --- | 0.022 | --- | 0.013 | --- |
| Means | ||||||||
| Slope | 0.44 | (0.42, 0.45) | 0.56 | (0.49, 0.63) | 0.003 | (-0.01, 0.01) | 0.04 | (0.00, 0.08) |
| Intercept | 5.79 | (5.71, 5.86) | 5.26 | (4.95, 5.56) | 1.48 | (1.45, 1.51) | 1.52 | (1.41, 1.64) |
| Variances | ||||||||
| Slope | 0.36 | (0.34, 0.38) | 0.36 | (0.34, 0.38) | 0.06 | (0.06, 0.07) | 0.06 | (0.06, 0.07) |
| Intercept | 15.99 | (15.6, 16.4) | 14.10 | (13.7, 14.5) | 2.37 | (2.30, 2.45) | 2.00 | (1.93, 2.07) |
| Correlation | ||||||||
| Slope with Intercept | -0.07 | (-0.10, -.04) | -0.08 | (-0.11, -0.05) | -0.29 | (-0.33, -0.26) | -0.27 | (-0.31, -0.23) |
Models adjusted for age, race, gender, marital status, education, income, and smoking status
CFI: Comparative Fit Index; TLI: Tucker-Lewis Index; RMSEA: Root-mean-square error of approximation
All p-values are two-tailed
During the study period, 1,062 (7.9%) individuals experienced an admission to a nursing home and 1,937 (14.4%) experienced at least one serious fall. In conditional LGCM of the two adverse health outcomes, the mean slopes for both nursing home stays and serious falls were not significantly different from zero, indicating that, unconditioned on frailty or depression, the likelihood of nursing home admissions and falls did not change for the sample on average.
Parallel Process Models
Further analyses explored associations between frailty trajectories and the parallel growth processes for adverse health outcomes (Table 3). Higher initial level of frailty (intercept) was associated with greater baseline likelihood of admission to a nursing home; however, initial level of frailty was not associated with change in likelihood of nursing home admission over time. Greater rate of frailty deficit accumulation (frailty slope) was associated with greater likelihood of nursing home admission over time, independent of initial levels of frailty (OR=1.33, 95% CI: 1.09 to 1.66). That is, a unit increase in frailty slope was associated with 33% greater odds of nursing home admission per wave. In parallel process models of fall likelihood, initial frailty status was also positively associated with initial likelihood for experiencing a serious fall, but was not associated with rate of change in fall likelihood over time. As with nursing home admissions, increasing frailty was associated with 52% increase in odds of serious falls per wave (OR=1.52, 95% CI: 1.12 to 2.08).
Table 3. Parallel process models adjusting for time invariant covariates and time-varying depression.
| Parameter | Nursing Home Admission | Serious Fall | ||
|---|---|---|---|---|
|
| ||||
| OR | 95% CI | OR | 95% CI | |
| Growth parameter regressions | ||||
| Outcome slope on frailty intercept | 1.02 | (0.99, 1.05) | 1.00 | (0.99, 1.01) |
| Outcome slope on frailty slope | 1.33 | (1.09, 1.66) | 1.52 | (1.12, 2.08) |
| Covariance of intercepts | 1.02 | (0.84, 1.19) | 0.62 | (0.50, 0.75) |
| Growth parameter regressions adjusted for depression | ||||
| Outcome slope on frailty intercept | 0.99 | (0.96, 1.01) | 1.00 | (0.99, 1.01) |
| Outcome slope on frailty slope | 1.16 | (0.99, 1.36) | 1.06 | (1.02, 1.10) |
| Covariance of intercepts | 0.85 | (0.69, 1.02) | 0.36 | (0.25, 0.46) |
| Parameter | β | 95% CI | β | 95% CI |
| Frailty regression on time-varying depression | ||||
| Time 1 | 0.48 | (0.44, 0.52) | 0.48 | (0.44, 0.52) |
| Time 2 | 0.40 | (0.36, 0.44) | 0.40 | (0.36, 0.44) |
| Time 3 | 0.52 | (0.48, 0.56) | 0.52 | (0.48, 0.56) |
| Time 4 | 0.50 | (0.45, 0.54) | 0.50 | (0.45, 0.54) |
| Time 5 | 0.68 | (0.63, 0.72) | 0.68 | (0.63, 0.72) |
All models adjusted for age, race, gender, marital status, education and smoking status
Results of parallel process models relating depression and the two adverse outcomes were similar to those for frailty (Table 4). Higher initial levels of depression were positively associated with higher initial likelihood of both nursing home stay and serious falls. Likewise, greater depression growth over time was associated with increasing likelihood of both nursing home stay and serious falls (ORNursingHome=3.63, 95% CI: 1.29 to 9.97; ORFall=1.16, 95% CI: 1.01 to 1.34).
Table 4. Parallel process models relating depression growth to adverse outcomes.
| Nursing Home Admission | Serious Fall | |||
|---|---|---|---|---|
|
| ||||
| Estimate | Estimate | |||
| Model Fit Statistics | ||||
| CFI | 0.994 | 0.990 | ||
| TLI | 0.990 | 0.986 | ||
| RMSEA | 0.012 | 0.012 | ||
|
| ||||
| Parameter | OR | 95% CI | OR | 95% CI |
|
| ||||
| Growth parameter regressions | ||||
| Outcome slope on depression intercept | 1.02 | (0.97, 1.07) | 0.99 | (0.98, 1.01) |
| Outcome slope on depression slope | 3.63 | (1.29, 9.97) | 1.16 | (1.01, 1.34) |
| Covariance of intercepts | 0.19 | (0.14, 0.25) | 0.22 | (0.17, 0.26) |
All models adjusted for age, race, gender, marital status, education and smoking status
Finally, we evaluated Aim 3 by accounting for depression status as a time-varying covariate in frailty parallel process models. As expected, concurrent depression was significantly associated with frailty status at each wave. Each unit increase in concurrent depression score was associated with between 0.40 and 0.68 more frailty symptoms (Table 3, bottom section). The inclusion of depression into parallel process models also significantly changed estimates of associations between frailty and adverse health outcomes (Table 3). Coefficients describing the association between frailty change over time and change in likelihood of nursing home admission were diminished in magnitude and became non-significant after accounting for depression (ORslopeONslope= 1.16, 95% CI: 0.99 to 1.36), suggesting that depression level explained a significant portion of the relationship between frailty change and change in the likelihood of nursing home admission over time. Similarly, after accounting for depression level, the association between frailty and likelihood of experiencing a serious fall over time was reduced in magnitude although still statistically significant (ORslopeONslope=1.06, 95% CI: 1.02 to 1.10).
Conclusion
There were three primary aims to the current study. First, we used LGCM to empirically estimate the change in frailty and depression status over time among a sample of community-dwelling adults. Second, we used parallel process models to assess the relationship between change in frailty and depression and likelihood of experiencing two adverse and costly health outcomes: nursing home admissions and serious falls. Finally, we examined the degree to which concurrent depression level explained the relationship between frailty and these adverse outcomes over time. Our results indicate that frailty and depression are both related to increased likelihood of adverse outcomes over time. Moreover, when accounting for concurrent depression status, the associations between frailty growth and likelihood of both adverse outcomes was attenuated, suggesting that frailty and depression describe similar vulnerability to these outcomes. These findings highlight the dynamic nature of frailty and its relationship with depression in later life.
Building on prior research, this analysis showed that frailty is a dynamic condition and that the influence of this condition on adverse health outcomes extends beyond frailty status at a single time-point. LGCM showed that, on average, frailty symptoms accumulated over the study period and that there was significant inter-individual variability in both the initial level of and rate of change in frailty over time. While there is an implicit acknowledgement that the signs of frailty may arise over long periods of time (Rockwood et al., 2011), few studies have incorporated frailty growth explicitly in analysis, instead assessing the role of frailty as a static predictor of poor health (Clegg et al., 2013). The present results provide evidence that developmental trajectories of frailty are themselves important predictors of poor outcomes. The more rapid accumulation of symptoms and other indicators of frailty over time may signal increased vulnerability to poor health outcomes, even among individuals who would not meet standard criteria for frailty at a given time.
These results provide further evidence for the hypothesis that frailty and depression are two interrelated sources of vulnerability in late-life (Lohman et al., 2015). Greater number of depressive symptoms were significantly associated with greater number of frailty symptoms at each wave. Furthermore, an increase in depressive symptoms over time was associated with increased likelihood of the two adverse outcomes, and to a similar extent as frailty. When depression was introduced as a time-varying covariate, the associations between frailty and serious falls and nursing home admissions were diminished. These findings indicate that concurrent depression and frailty explain similar variation in which individuals will experience adverse health outcomes over time. The majority of frailty definitions do not explicitly incorporate psychological symptoms or other factors that may overlap biological, social, and psychological domains such as depression (Sternberg, Wershof Schwartz, Karunananthan, Bergman, & Mark Clarfield, 2011). The current results, however, indicate the need for broader consideration of psychosocial factors such as depression in frailty development (Malmstrom & Morley, 2013). As an independent predictor of adverse outcomes, depression may be a more direct and clinically expedient measure of risk compared to the Frailty Index. Furthermore, because depression is a treatable condition which is likely to precede onset of frailty in older adults (Paulson & Lichtenberg, 2013a; Paulson & Lichtenberg, 2013b), depression may be an important target for intervention to prevent frailty and its consequences.
There are a number of potential explanations for these findings. One possibility is that frailty and depression independently lead to similar adverse health outcomes. While frailty and depression are both associated with poor health (Fried et al., 2001; Hybels et al., 2002), it is unlikely, given their pervasive comorbidity and established diagnostic overlap (Lohman et al., 2014; Mezuk et al., 2012), that they are wholly independent of one another. Another possibility is that depression and frailty (as defined by the Frailty Index) are empirically indistinguishable constructs. However, our recent study demonstrated that despite measuring highly overlapping constructs, depression and frailty were distinct (although substantially correlated) concepts as measured in the HRS (Lohman et al., 2015). Furthermore, the conceptual overlap between depression and frailty was similar across multiple common frailty definitions (i.e. not unique to the Frailty Index) and could only be partially explained by shared symptoms (Lohman et al., 2015). Thus, a more likely explanation is that frailty and depression are comparable but distinct expressions of a more general underlying process of decline. For example, age-associated cardiovascular changes have been found to play a role in development of both frailty and depression (Alexopoulos et al., 1997; Hajjar et al., 2009; Newman et al., 2001; Strandberg, Pitkälä, Tilvis, O'Neill, & Erkinjuntti, 2013), and may help to explain comorbidity and common consequences of these two conditions. Consistent with this hypothesis, prior studies by Paulson and colleagues suggest that ‘vascular depression’ may represent a prodromal manifestation of cardiovascular changes which may eventually lead to frailty (Paulson & Lichtenberg, 2013a; Paulson & Lichtenberg, 2013b). Similarly, Hajjar and colleagues have identified an aging phenotype associated with hypertension, diabetes and other cardiovascular disorders characterized by symptoms of both depression and frailty (Hajjar et al., 2009).
Other explanations of comorbidity, for instance that general vulnerability and deterioration are related to inflammatory processes, injury, or other stresses (Katz, 2004), cannot be discounted based on the current analysis. Yet, findings regarding the joint influence of frailty and depression on poor health outcomes signal the need for more comprehensive investigations of geriatric syndromes like frailty. Incorporating rather than excluding depression as a primary measure in frailty research will help to merge two separate lines of investigation from frailty and late-life depression. Joint consideration of frailty and depression may also lead to earlier identification of older adults at higher risk, and may help to tailor treatment to comprehensively address biological, psychological, and social vulnerabilities (Brown et al., 2013).
The primary strength of this study lies in the use of LGCM in a large population-based sample. This is among the first applications of LGCM to understanding change in frailty over time. LGCM allows us to address the question of whether change in frailty status, independent of initial status, is associated with adverse health outcomes over time. Furthermore, we were able to account for a range of characteristics, including change in depression status over time, because of the extensive collection of longitudinal health information and large sample size available in the HRS. Lastly, conceptualization of frailty and depression as continuous rather than dichotomous variables in analysis acknowledges the dimensional nature of these constructs and the lack of clearly defined diagnostic thresholds.
Study results should also be interpreted in light of potential limitations. First, the measure of depression used in this study, the CESD, is not a substitute for clinician diagnosis of major depression. Nonetheless, the CESD is among the most widely used measures of depressive symptoms in the general community and thus its relationship with common measures of frailty is of research significance. Furthermore, the CESD has moderate diagnostic agreement with structured instruments for the assessment of major depression such as the CIDI (Steffick, 2000; Turvey et al., 1999). Second, there was noteworthy attrition during the study period, as 28.6% of participants were missing measurements for frailty, depression, or adverse health outcomes at the final interview. Because both frailty and depression are related to higher risk of mortality and other common causes of attrition, selective loss to follow-up may have biased results. Despite this concern, 81.3% of participants provided information on both frailty and depression in at least three waves indicating strong study retention. Furthermore, full-information maximum likelihood estimation, as used in this study, incorporates data from participants who did not complete all follow-up interviews. This method helps to minimize bias due to attrition assuming that missing data is explained by covariates included in the model (Bollen & Curran, 2006; Rubin & Little, 2002). Nonetheless, a more comprehensive understanding of this relationship could be gained by future studies accounting for competing risks such as mortality (Xue, Fried, Glass, Laffan, & Chaves, 2008). Lastly, while the Frailty Index model allows for consideration of a broader array of potential frailty symptoms, this flexibility may lead to greater complexity and greater difficulty distinguishing frailty from other conditions like depression. The results of this study can likely be attributed in part to the construct overlap of depression and frailty. Nevertheless, the empirical and conceptual overlap of frailty and depression is common across multiple definitions of frailty (Lohman et al., 2015), and these results highlight the need to consider the influence of depression in all studies of frailty.
In summary, these results provide another step in understanding the multifactorial determinants of functional decline in later life. Our findings suggest that depression plays a substantial role in explaining the risk of poor health conferred by frailty, and moreover depression may be an important feature of what it means to be frail. There is further need for interdisciplinary research to understand the relationships between age-related conditions and to promote health and well-being in later life.
Supplementary Material
Acknowledgments
This work was supported by the National Institute on Aging under grant number F31-AG044974-01A1 and the National Institute of Mental Health under grant number T32-MH073553.
Contributor Information
Matthew C. Lohman, Department of Psychiatry, Institute of Geriatric Psychiatry, Weill Cornell Medical College, White Plains, New York.
Briana Mezuk, Department of Family Medicine and Population Health, Division of Epidemiology, Virginia Commonwealth University School of Medicine, Richmond, Virginia.
Levent Dumenci, Department of Social and Behavioral Health, Virginia Commonwealth University School of Medicine, Richmond, Virginia.
References
- Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. ‘Vascular depression’ hypothesis. Archives of General Psychiatry. 1997;54(10):915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) American Journal of Preventive Medicine. 1994;10(2):77–84. [PubMed] [Google Scholar]
- Bollen KA, Curran PJ. Latent Curve Models: A Structural Equation Perspective. Hoboken, New Jersey: John Wiley & Sons; 2006. [Google Scholar]
- Brown PJ, Roose SP, Fieo R, Liu X, Rantanen T, Sneed JR, Avlund K. Frailty and Depression in Older Adults: A High-Risk Clinical Population. The American Journal of Geriatric Psychiatry. 2013;22(11):1083–95. doi: 10.1016/j.jagp.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell AJ, Buchner DM. Unstable disability and the fluctuations of frailty. Age and Ageing. 1997;26(4):315–318. doi: 10.1093/ageing/26.4.315. [DOI] [PubMed] [Google Scholar]
- Cigolle C, Ofstedal M, Tian Z, Blaum C. Comparing models of frailty: the Health and Retirement Study. Journal of the American Geriatrics Society. 2009;57(5):830–839. doi: 10.1111/j.1532-5415.2009.02225.x. [DOI] [PubMed] [Google Scholar]
- Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collard R, Boter H, Schoevers R, Oude Voshaar R. Prevalence of frailty in community-dwelling older persons: a systematic review. Journal of the American Geriatrics Society. 2012;60(8):1487–1492. doi: 10.1111/j.1532-5415.2012.04054.x. [DOI] [PubMed] [Google Scholar]
- Duncan TE, Duncan SC. An introduction to latent growth curve modeling. Behavior Therapy. 2004;35(2):333–363. doi: 10.1016/S0005-7894(04)80042-X. [DOI] [Google Scholar]
- Fassbender K, Fainsinger RL, Carson M, Finegan BA. Cost Trajectories at the End of Life: The Canadian Experience. Journal of Pain and Symptom Management. 2009;38(1):75–80. doi: 10.1016/j.jpainsymman.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, McBurnie MA. Frailty in older adults: evidence for a phenotype. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences. 2001;56(3):M146–M157. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- Gobbens RJJ, Luijkx K, Wijnen Sponselee M, Schols JMGA. In search of an integral conceptual definition of frailty: opinions of experts. Journal of the American Medical Directors Association. 2010;11(5):338–343. doi: 10.1016/j.jamda.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Hajjar I, Yang F, Sorond F, Jones R, Milberg W, Cupples LA, Lipsitz L. A novel aging phenotype of slow gait, impaired executive function, and depressive symptoms: relationship to blood pressure and other cardiovascular risks. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences. 2009;64(9):994–1001. doi: 10.1093/gerona/glp075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeringa SG, Connor J. Technical Description of the Health and Retirement Study Sample Design. HRS/AHEAD documentation report No. DR-002: Ann Arbor, MI: Institute for Social Research, University of Michigan; 1995. 1995. [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal. 1999;6(1):1–55. doi: 10.1080/10705519909540118. [DOI] [Google Scholar]
- Hybels CF, Pieper CF, Blazer DG. Sex differences in the relationship between subthreshold depression and mortality in a community sample of older adults. The American Journal of Geriatric Psychiatry. 2002;10(3):283–291. doi: 10.1097/00019442-200205000-00007. [DOI] [PubMed] [Google Scholar]
- Joreskog KG. A general approach to confirmatory maximum likelihood factor analysis. Psychometrika. 1969;34(2):183–202. doi: 10.1007/BF02289343. [DOI] [Google Scholar]
- Katz IR. Depression and frailty: the need for multidisciplinary research. The American Journal of Geriatric Psychiatry. 2004;12(1):1–6. doi: 10.1176/appi.ajgp.12.1.1. [DOI] [PubMed] [Google Scholar]
- Klein BEK, Klein R, Knudtson MD, Lee KE. Frailty, morbidity and survival. Archives of Gerontology and Geriatrics. 2005;41(2):141–149. doi: 10.1016/j.archger.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Li G, Thabane L, Ioannidis G, Kennedy C, Papaioannou A, Adachi JD. Comparison between frailty index of deficit accumulation and phenotypic model to predict risk of falls: data from the global longitudinal study of osteoporosis in women (GLOW) Hamilton cohort. PloS One. 2015;10(3):e0120144. doi: 10.1371/journal.pone.0120144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohman MC, Dumenci L, Mezuk B. Sex Differences in the Construct Overlap of Frailty and Depression: Evidence from the Health and Retirement Study. Journal of the American Geriatrics Society. 2014;62(3):500–5. doi: 10.1111/jgs.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohman MC, Dumenci L, Mezuk B. Depression and Frailty in Late Life: Evidence for a Common Vulnerability. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, [Epub ahead of print] 2015 doi: 10.1093/geronb/gbu180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado G, Greenland S. Simulation study of confounder-selection strategies. American Journal of Epidemiology. 1993;138(11):923–936. doi: 10.1097/00019442-200205000-00007. [DOI] [PubMed] [Google Scholar]
- Malmstrom TK, Morley JE. The Frail Brain. Journal of the American Medical Directors Association. 2013;14(7):453–455. doi: 10.1016/j.jamda.2013.04.010. [DOI] [PubMed] [Google Scholar]
- Mezuk B, Edwards L, Lohman M, Choi M, Lapane K. Depression and frailty in later life: a synthetic review. International Journal of Geriatric Psychiatry. 2011;27(9):879–892. doi: 10.1002/gps.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezuk B, Lohman M, Dumenci L, Lapane K. Are depression and frailty overlapping syndromes in mid- and late-life? A latent variable analysis. American Journal of Geriatric Psychiatry. 2012;21(6):560–569. doi: 10.1097/JGP.0b013e31824afd4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AB, Gottdiener JS, McBurnie MA, Hirsch CH, Kop WJ, Tracy R, Fried LP. Associations of Subclinical Cardiovascular Disease with Frailty. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences. 2001;56(3):M158–M166. doi: 10.1093/gerona/56.3.M158. [DOI] [PubMed] [Google Scholar]
- Paulson D, Lichtenberg PA. Vascular depression and frailty: a compound threat to longevity among older-old women. Aging & Mental Health. 2013a;17(7):901–910. doi: 10.1080/13607863.2013.799115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson D, Lichtenberg PA. Vascular depression: An early warning sign of frailty. Aging & Mental Health. 2013b;17(1):85–93. doi: 10.1080/13607863.2012.692767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijpers E, Ferreira I, Stehouwer CDA, Nieuwenhuijzen Kruseman A. The frailty dilemma. Review of the predictive accuracy of major frailty scores. European Journal of Internal Medicine. 2012;23(2):118–123. doi: 10.1016/j.ejim.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences. 2007;62(7):738–743. doi: 10.1093/gerona/62.7.738. [DOI] [PubMed] [Google Scholar]
- Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences. 2007;62(7):722–727. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A. A global clinical measure of fitness and frailty in elderly people. Canadian Medical Association Journal. 2005;173(5):489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwood K, Song X, Mitnitski A. Changes in relative fitness and frailty across the adult lifespan: evidence from the Canadian National Population Health Survey. Canadian Medical Association Journal. 2011;183(8):E487–494. doi: 10.1503/cmaj.101271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwood K, Theou O, Mitnitski A. What are frailty instruments for? Age and Ageing. 2015;44(4):545–547. doi: 10.1093/ageing/afv043. [DOI] [PubMed] [Google Scholar]
- Rothman MD, Leo-Summers L, Gill TM. Prognostic significance of potential frailty criteria. Journal of the American Geriatrics Society. 2008;56(12):2211–2216. doi: 10.1111/j.1532-5415.2008.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DB, Little RJA. Statistical analysis with missing data. Hoboken, NJ: J WIley & Sons; 2002. [Google Scholar]
- Searle S, Mitnitski A, Gahbauer E, Gill T, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatrics. 2008;8(1):24. doi: 10.1186/1471-2318-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Mitnitski A, Rockwood K. Prevalence and 10-Year Outcomes of Frailty in Older Adults in Relation to Deficit Accumulation. Journal of the American Geriatrics Society. 2010;58(4):681–687. doi: 10.1111/j.1532-5415.2010.02764.x. [DOI] [PubMed] [Google Scholar]
- Steffick DE. Documentation of Affective Functioning Measures in the Health and Retirement Study. Ann Arbor, MI: Survey Research Center; 2000. (HRS/AHEAD Documentation Report No. DR-005) [Google Scholar]
- Sternberg S, Wershof Schwartz A, Karunananthan S, Bergman H, Mark Clarfield A. The identification of frailty: a systematic literature review. Journal of the American Geriatrics Society. 2011;59(11):2129–2138. doi: 10.1111/j.1532-5415.2011.03597.x. [DOI] [PubMed] [Google Scholar]
- Strandberg TE, Pitkälä KH, Tilvis RS, O'Neill D, Erkinjuntti TJ. Geriatric syndromes—vascular disorders? Annals of Medicine. 2013;45(3):265–273. doi: 10.3109/07853890.2012.727022. [DOI] [PubMed] [Google Scholar]
- Tu YK, Gilthorpe MS. Revisiting the relation between change and initial value: a review and evaluation. Statistics in Medicine. 2007;26(2):443–457. doi: 10.1002/sim.2538. [DOI] [PubMed] [Google Scholar]
- Turvey CL, Wallace RB, Herzog R. A revised CES-D measure of depressive symptoms and a DSM-based measure of major depressive episodes in the elderly. International Psychogeriatrics. 1999;11(2):139–148. doi: 10.1017/S1041610299005694. [DOI] [PubMed] [Google Scholar]
- Xue QL, Fried LP, Glass TA, Laffan A, Chaves PH. Life-space constriction, development of frailty, and the competing risk of mortality: the Women's Health And Aging Study I. American Journal of Epidemiology. 2008;167(2):240–248. doi: 10.1093/aje/kwm270. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.